Abstract
BACKGROUND
No studies have examined prescription opioids’ long-term cognitive effects.
OBJECTIVES
To determine whether opioid use is associated with higher dementia risk or greater cognitive decline.
DESIGN
Prospective cohort study
SETTING
Group Health, an integrated healthcare delivery system
PARTICIPANTS
People age 65 and older, community dwelling and nondemented, with at least 10 years of Group Health enrollment at baseline
MEASUREMENTS
The Cognitive Abilities Screening Instrument (CASI) was administered every 2 years. Low scores triggered detailed evaluation, and a multidisciplinary committee assigned dementia diagnoses. From computerized pharmacy data, cumulative opioid exposure was defined as total standardized doses (TSD) dispensed over 10 years (excluding the most recent 1 year because of possible prodromal symptoms). For comparison, we examined use of nonsteroidal anti-inflammatory drugs (NSAIDs), characterized similarly. Analyses of dementia risk used Cox proportional hazards models and of CASI trajectory, linear regression models and generalized estimating equations.
RESULTS
Among 3,434 participants (median age 74), 797 (23%) developed dementia over a mean follow-up of 7.3 years. 637 (19%) had possible or probable Alzheimer’s disease. For cumulative opioid use, the hazard ratios (HRs) for dementia were: 11–30 TSD, HR 1.06 (95% confidence interval [CI] 0.88–1.26); 31–90 TSD, HR 0.88 (0.70–1.09); and 91+ TSD, HR 1.29 (1.02–1.62), compared to 0–10 TSD. A similar pattern was seen for NSAID use. Heavier opioid use was not associated with more rapid cognitive decline.
CONCLUSION
People with the heaviest opioid or NSAID use had slightly higher dementia risk than people with little or no use. These results may reflect an effect of chronic pain on cognition or residual confounding. While opioids convey other risks, we found little evidence of long-term cognitive harm specific to opioids.
Keywords: opioids, non-steroidal anti-inflammatory drugs, dementia, cognitive decline, chronic pain
INTRODUCTION
Prescription opioid medications are widely used by older adults. In 2002, 18% of US adults filled at least one opioid prescription,1 including 7 million people age 65 and older. In 2005, more than 8% of women age 65 and older were using opioids long-term for non-cancer pain.2 Substantial gaps remain in our knowledge about the safety and effectiveness of opioids, particularly for long-term use.3
One unanswered question is whether opioids have long-term effects on cognition.3 Prescription opioid use is a risk factor for delirium,4 which is associated with higher dementia risk,5,6 although the causal sequence is unclear. Opioids reversibly affect cognition by causing sedation. In addition, autopsy studies have shown neuropathologic findings in young drug abusers similar to those seen with Alzheimer’s disease (AD).7,8 Opioids modulate the behavior of microglia,7 immune cells in the brain that mediate inflammation, which may contribute to neurodegenerative diseases including AD.9 Finally, opioids promote apoptosis of microglia10 and neurons.11 All of this evidence suggests that long-term opioid use might contribute to cognitive decline. To our knowledge, no epidemiologic study has examined dementia risk in relation to opioid use.
We analyzed data from the Adult Changes in Thought (ACT) study, a population-based prospective cohort study with data on medication use going back many years. Our objective was to examine the association between prescription opioid use and risk of dementia or cognitive decline. We hypothesized that greater cumulative exposure and more recent use of opioids would be associated with higher risk.
METHODS
Overview
ACT is a population-based, prospective cohort study set within Group Health (GH), an integrated health-care delivery system in the northwest US.12 Participants gave informed consent, and study procedures were approved by GH’s Human Subjects Review Committee.
Population
The ACT study has been described elsewhere.12 In brief, from 1994 through 1996 ACT recruited 2581 community-dwelling adults age 65 and older without dementia from among GH members living in or near Seattle, Washington. An expansion cohort (N=811) was recruited from 2000 through 2003, and in 2004, ACT began continuous enrollment to replace participants who die, drop out, or develop dementia. In all phases, participants were randomly selected. Participants are seen every 2 years, and follow-up continues through the present day. These analyses use data collected through September 30, 2012.
These analyses were limited to people with at least 10 years of GH membership at ACT enrollment, to ensure adequate data on long-term medication exposure. Analyses of dementia outcomes were limited to people with at least one follow-up visit. For analyses of cognitive trajectory, people who ultimately developed dementia were included up until the time when they became demented. Visits triggering a dementia diagnosis were excluded because cognitive scores at these visits were so low that they would have strongly influenced results, and as a result the cognitive trajectory analysis would have answered essentially the same question as the dementia analysis. Instead, we sought to answer the question: does opioid use lead to more rapid cognitive decline in people who have not yet become demented? Cognitive trajectory analyses also excluded people with an invalid cognitive score at their first visit and, for people with a valid score at their first visit, any follow-up visits with invalid scores. Possible reasons for an invalid score included limited English proficiency or very poor eyesight or hearing.
Exposure
Prescription opioid use was identified from computerized pharmacy data, which include drug name, strength, route of administration, number of pills, and date dispensed. We converted all fills to morphine equivalent doses using conversion factors13,14 and then calculated total standardized doses (TSD), with one TSD being equivalent to 30 mg of morphine.15 For analyses of dementia risk, we constructed a time-varying measure defined as the TSD dispensed over a 10-year window after excluding dispensings in the most recent 1 year, which could have been for prodromal dementia symptoms. Figure 1 illustrates how exposure windows were defined. The 10 year window moved forward in time throughout follow-up. We categorized cumulative opioid use as 0–10, 11–30, 31–90, or 91+ TSD. In clinical terms, a person could reach the highest exposure group by using 30 mg of morphine daily for more than 3 months. Cutpoints were chosen partly based on the population distribution and also to be clinically meaningful.
Figure 1.
Defining exposure windows. The top portion shows exposure windows for analyses of dementia and AD. The “Event” circle at the far right represents the time of dementia onset for an individual or the comparable time for a nondemented participant in the same risk set. The measure of cumulative exposure excludes use in the 1 year immediately prior to the event because of concerns about possible use for prodromal symptoms. Recent exposure is defined as use in the 6 months immediately before the event. The lower half of the figure shows exposure windows for analyses of cognitive decline. The circle at the far right represents a study visit at which the cognitive test was administered. Here, the 1 year immediately prior to a study visit is not excluded from the cumulative use measure because by design, no participants could have been demented at the time of a study visit included in these analyses.
We defined recent opioid use (also time-varying) as filling two or more prescriptions, each with at least 3 TSDs, in the prior 6 months (Figure 1). This measure did not exclude fills in the most recent 1 year because we wanted to explore the short-term effect of opioid exposure.
For the cognitive trajectory analyses, cumulative opioid exposure was defined by summing dispensings over the 10 years prior to the study visit where the CASI was administered. We did not exclude dispensings in the prior 1 year because study visits leading to a dementia diagnosis were excluded, and so no participant could have been demented at the time of a CASI score included in this analysis. Recent opioid use was defined as described above.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used for pain and may be considered as a therapeutic alternative to opioids. Because of potential confounding by indication, we considered NSAID use as a secondary exposure in these analyses. We have previously published analyses of dementia risk in relation to NSAID use using ACT study data.16 As in our past work, we ascertained NSAID use from computerized pharmacy data and converted fills to TSD.16 Variables for cumulative and recent NSAID use were created as described above for opioids (Figure 1). We categorized cumulative NSAID use as 0–60, 61–180, 181–540, or 541+ TSD. In clinical terms, a person could reach the heaviest exposure level by using 1200 mg of ibuprofen daily for about 1.5 years.
Outcomes
Participants underwent cognitive screening at baseline and every 2 years using the Cognitive Abilities Screening Instrument (scale, 0–100).17 People scoring 86 or lower received in-depth evaluation including an examination by a study physician and neuropsychologic testing. Results were reviewed by a multidisciplinary committee that assigned dementia18 and AD19 diagnoses. The date of dementia onset was assigned by convention as the midpoint between the study visit triggering evaluation and the preceding visit. Participants diagnosed with dementia underwent another examination after 1 year to confirm the diagnosis.
Our primary analyses of cognitive trajectory utilized the CASI score. Because of concerns about non-linear scaling, we carried out secondary analyses after applying item response theory (IRT) methods to generate CASI-IRT scores that have linear scaling properties. We used the same methods as we have previously,20 including using the graded response model21,22 in Parscale, edition 4.123 (Scientific Software International Inc., Chicago, Illinois).
Covariates
ACT study data include demographics (age, sex, self-reported race, education), measured height and weight, self-reported medical history, health behaviors, self-rated health, and depressive symptoms measured using a validated version of the Center for Epidemiologic Studies Depression (CESD) questionnaire.24 People were asked the number of days per week they participated in various activities for at least 15 minutes. We defined coronary heart disease (CHD) as self-reported history of myocardial infarction, coronary artery bypass grafting, coronary angioplasty or angina. We calculated body mass index (BMI) as weight (kilograms) divided by height squared (meters) and categorized participants as underweight (<18.5), normal (18.5–24.9), overweight (25–29.9), or obese (≥30).25 We defined depression as a CESD score of 10 or more.24
GH databases supplied information about comorbid illnesses and medication use. Stroke was defined as present if it was identified either from self-report or International Classification of Diseases, version 9 (ICD-9) diagnosis codes (430.×, 431.×, 432.×, 434.×, 436.×, or 438.×) in electronic data. Treated diabetes was defined as filling one or more prescriptions for an oral hypoglycemic medication or insulin in the year prior to study enrollment and treated hypertension, two or more antihypertensive medication prescriptions. Information about cancer diagnoses came from Surveillance, Epidemiology and End Results (SEER) cancer registry data.
Variables for CHD, stroke, and cancer were time-varying. All other covariates were defined as of the ACT baseline visit.
Statistical Analysis
Analyses of Dementia and AD
We modeled the association between opioid use and all-cause dementia or possible or probable AD using Cox regression with age as the time scale. We developed separate models for cumulative and recent opioid use and for dementia and AD. Participants entered the analysis at ACT enrollment and were followed until the earliest of dementia onset, GH disenrollment, or last study visit before September 30, 2012. Accordingly, participants who died before developing dementia were censored at the time of their last study visit. Our primary analyses modeled cumulative exposure using categorical terms. In secondary analyses, we used natural cubic splines so that we could examine whether results were influenced by the cutpoints chosen.
Models adjusted for age at study entry, ACT cohort, sex, education (high school or less vs. at least some college), hypertension, diabetes, BMI category, physical activity (< 3 vs. 3+ days/week), stroke, CHD, self-rated health (fair/poor vs. excellent/very good/good), depression, smoking status (current vs. not current), and NSAID use. These models thus allowed us to simultaneously estimate risk of dementia or AD in relation to opioid use adjusted for NSAID use and also risk in relation to NSAID use, adjusted for opioid use. We tested for an interaction between NSAID and opioid exposure with a Wald test, but none was detected, so we present results from models without interaction terms. In analyses examining recent use, we initially tested for an interaction between recent and cumulative opioid use with a Wald test because we hypothesized that the impact of recent exposure might differ depending on history of opioid use. People with longstanding use might have developed tolerance and show little effect from recent use, while new initiators might be more vulnerable. Since no interaction was found, we simply adjusted for cumulative opioid use in these models. Similarly, to keep opioid and NSAID analyses parallel, we tested for an interaction between recent and cumulative NSAID use. We did detect evidence of a potential interaction between recent and cumulative NSAID exposure, so we included those interaction terms in recent use models.
We investigated whether there were differences in the association between opioid use and dementia risk for subgroups defined by age at study entry, sex, baseline BMI, and baseline depression status. Analyses of BMI excluded underweight participants (n=31; 0.9%).
We conducted a complete case analysis. We assessed proportionality of hazards by examining Schoenfeld residuals.
Cognitive Trajectory Analyses
To measure cognitive trajectory, we used CASI scores assessed from ACT study visits. We estimated a linear regression model using generalized estimating equations. We specified an independence working correlation matrix and computed standard errors using the Huber-White sandwich estimator to account for within-subject correlation. The primary predictors in the model were age, opioid use, and an interaction between age and opioid use (to enable estimation of the association between opioid use and rate of cognitive decline). Models adjusted for the same covariates as in the dementia and AD analyses. Analyses of CASI trajectory in relation to recent opioid or NSAID use also adjusted for cumulative use of that medication class, with the time window for cumulative use modified slightly so that these two exposure measures would not overlap. Specifically, in these analyses, recent use included the 6 months immediately prior to the study visit where the CASI was administered, while cumulative use included the period from 6 months to 10 years prior to the visit.
Sensitivity Analyses
For dementia and AD, we performed several sensitivity analyses. To further investigate high cumulative use, we split the heaviest use category for both opioids and NSAIDs into two levels. We adjusted for the Charlson comorbidity index (Deyo adaptation)26 and for cancer diagnoses. Finally, we explored the impact of classifying propoxyphene separately from other opioids, since this drug has been removed from the market and we wanted to understand the impact of currently available opioids on dementia risk. For cognitive trajectory analyses, we explored the impact of adding interactions between age and all covariates. Results changed little, so we present only the primary results.
We used SAS software, version 9.2 (SAS Institute, Inc., Cary, NC) and R, version 2.15.3 (R Foundation for Statistical Computing, Vienna, Austria) for statistical analyses.
RESULTS
There were 3,434 participants eligible for analyses of dementia risk (Figure 2). Median age at study entry was 74, 40% were male and 66% had at least some college education (Table 1). During the study period, 40,843 prescriptions for opioids were dispensed to 3,151 people; 92% of study participants had at least one opioid fill. The most common medications were codeine (39% of fills), oxycodone (26%) and hydrocodone (23%). Table 1 shows participant characteristics by cumulative use at baseline. People with the heaviest opioid use were slightly older, on average, and more likely to be female than people with little to no use. People with heavier opioid use were more likely to have nearly all comorbidities we examined, to be obese, to rate their health as fair or poor, and to report depressive symptoms, and they were less likely to exercise regularly compared to people with little or no use.
Figure 2.
Study sample for dementia and cognitive trajectory analyses. Abbreviations: ACT, Adult Changes in Thought; GH, Group Health; CASI, Cognitive Abilities Screening Instrument.
Table 1.
Characteristics of ACT Participants at Study Entry, Overall and by Cumulative Opioid Use at Baseline*
| All participants | Stratified by cumulative opioid use in the past 10 years† | ||||
|---|---|---|---|---|---|
|
| |||||
| (N=3,434) | 0–10 TSD (N=1852) | 11–30 TSD (N=830) | 31–90 TSD (N=476) | 91+ TSD (N=276) | |
| Median age, years (IQR) | 74 (70–80) | 74 (70–79) | 75 (70–80) | 75 (70–80) | 76 (71–81) |
| Male | 40 | 41 | 41 | 41 | 30 |
| Education: at least some college | 66 | 67 | 66 | 66 | 63 |
| Current smoker | 5 | 5 | 5 | 6 | 5 |
| Treated hypertension‡ | 48 | 43 | 51 | 55 | 63 |
| Treated diabetes‡ | 8 | 7 | 7 | 10 | 11 |
| Prior stroke¶ | 6 | 5 | 6 | 10 | 13 |
| Ischemic heart disease§ | 18 | 15 | 21 | 22 | 29 |
| Cancer** | 11 | 7 | 16 | 15 | 15 |
| Obese | 25 | 22 | 26 | 31 | 38 |
| Regular exercise†† | 72 | 74 | 73 | 68 | 55 |
| Self-rated health fair or poor | 15 | 12 | 15 | 20 | 34 |
| Depression | 10 | 8 | 10 | 12 | 17 |
| Recent NSAID use | 8 | 5 | 8 | 13 | 16 |
| Cumulative NSAID use in past 10 years: 541+ TSD | 15 | 8 | 14 | 25 | 42 |
Abbreviations: ACT, Adult Changes in Thought; NSAID, non-steroidal anti-inflammatory drug; IQR, interquartile range; TSD, Total Standardized Doses.
All results are % unless stated otherwise. Less than 3% of data were missing for each characteristic in all subgroups except for BMI, which was missing in 2.2% overall and in 1.6, 2.2, 2.5 and 5.8% of those with prior opioid use of 0–10, 11–30, 31–90 and 91+ TSD, respectively.
For opioids, 1 TSD = 30 mg oral morphine; see Methods.
Defined from computerized pharmacy data.
Combination of self-report and health plan diagnosis codes.
History of coronary artery bypass grafting, angioplasty, myocardial infarction or angina.
From cancer registry data. Defined as having an incident cancer diagnosis in the 10 years prior to ACT enrollment.
Self-report of exercising for 15 minutes on 3 or more days per week.
Over a total follow-up time of 25,020 person-years (a mean of 7.3 years per person), 797 participants (23%) developed dementia, including 637 with possible or probable AD. The dementia (and AD) cases were distributed across opioid exposure categories as follows: 353 (276) among people with little or no cumulative opioid use (0–10 TSD); 199 (156) among people with 11–30 TSD of cumulative opioid exposure; 120 (109) among people with 31–90 TSD; and, 125 (96) among people with 91+ TSD.
Table 2 presents risk estimates according to level of cumulative opioid or NSAID use. Compared to people with little or no opioid use, people with the heaviest opioid use (91+ TSD in the past 10 years) had slightly higher risk of all cause dementia (adjusted hazard ratio [HR] 1.29; 95% confidence interval [CI] 1.02–1.62) and possible or probable AD (HR 1.35, 95% CI 1.04–1.76). Moderate opioid exposure was not associated with either outcome. Similarly, people with the heaviest NSAID use (541+ TSD) had modestly higher dementia risk (HR 1.31, 95% CI 1.07–1.62) than people with little to no NSAID use, and moderate NSAID use was not associated with all-cause dementia or AD. When we split the highest opioid use category into two groups, the HR for 91–180 TSD was 1.21 (95% CI 0.89–1.64) and for 181+ TSD, 1.34 (95% CI 1.01–1.79). Findings for AD were similar. There was no statistically significant interaction between cumulative opioid and NSAID use for either outcome.
Table 2.
Risk of Dementia and Alzheimer’s Disease in Relation to Cumulative Opioid or NSAID Exposure
| All-cause dementia | Possible or probable AD | |||
|---|---|---|---|---|
|
| ||||
| Minimally adjusted HR* (95% CI) | Fully adjusted HR† (95% CI) | Minimally adjusted* HR (95% CI) | Fully adjusted HR† (95% CI) | |
| Opioid exposure | ||||
| 0–10 TSD | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 11–30 TSD | 1.09 (0.91, 1.31) | 1.06 (0.88, 1.26) | 1.12 (0.92, 1.37) | 1.10 (0.90, 1.35) |
| 31–90 TSD | 0.93 (0.75, 1.16) | 0.88 (0.70, 1.09) | 1.09 (0.86, 1.38) | 1.07 (0.84, 1.35) |
| 91+ TSD | 1.43 (1.15, 1.79) | 1.29 (1.02, 1.62) | 1.45 (1.13, 1.87) | 1.35 (1.04, 1.76) |
| NSAID exposure | ||||
| 0–60 TSD | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 61–180 TSD | 0.97 (0.79, 1.18) | 0.95 (0.77, 1.16) | 1.00 (0.80, 1.25) | 1.02 (0.81, 1.27) |
| 181–540 TSD | 1.04 (0.83, 1.29) | 1.04 (0.83, 1.30) | 1.02 (0.80, 1.30) | 1.04 (0.81, 1.34) |
| 541+ TSD | 1.28 (1.04, 1.57) | 1.31 (1.07, 1.62) | 1.15 (0.91, 1.45) | 1.21 (0.96, 1.54) |
Abbreviations: ACT, Adult Changes in Thought; AD, Alzheimer’s Disease; CI, confidence interval; NSAID, non-steroidal anti-inflammatory drug; HR, hazard ratio; TSD, Total Standardized Doses
Adjusted for age via the time scale. Models include variables for both opioid and NSAID exposure, and so estimates for opioids are adjusted for cumulative NSAID exposure and vice versa.
Additionally adjusted for ACT study cohort, age at ACT study entry, gender, education, treated hypertension, treated diabetes, smoking, stroke, coronary heart disease, body mass index, exercise, self-rated health, and depression.
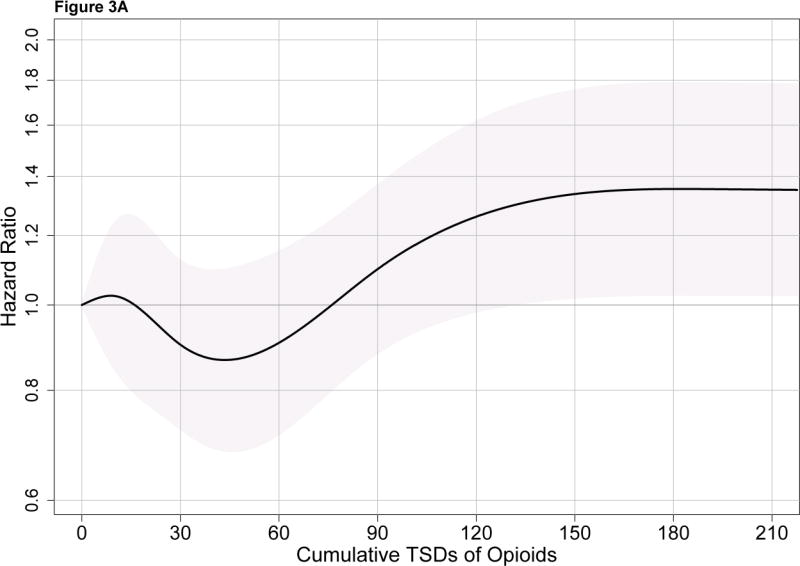
Figure 3A shows the risk of dementia associated with cumulative opioid exposure modeled using a spline. This curve shows the estimated HRs (and 95% CIs) for each level of exposure relative to a referent group with cumulative exposure of 0 TSDs. A modestly elevated HR on the order of 1.3–1.4 is seen with the heaviest levels of opioid use. Figure 3B shows results for cumulative NSAID use.
Figure 3.
Figure 3A. Risk of all-cause dementia in relation to 10-year cumulative opioid exposure. The solid black line shows the hazard ratio for all-cause dementia for each level of cumulative exposure to opioids relative to a reference group with exposure of 0 TSDs, modeled using splines. The gray shaded area corresponds to pointwise 95% confidence intervals. TSD stands for total standardized doses.
Figure 3B. Risk of all-cause dementia in relation to 10-year cumulative nonsteroidal antiinflammatory drug (NSAID) exposure. The solid black line shows the hazard ratio for all-cause dementia for each level of cumulative exposure to NSAIDs relative to a reference NSAID exposure of 0 TSDs, modeled using splines. The gray shaded area corresponds to pointwise 95% confidence intervals. Abbreviations: TSD stands for total standardized doses.
People with recent opioid use had slightly higher dementia risk than people without recent use, adjusted for cumulative exposure, but this association was not statistically significant (HR 1.26, 95% CI 0.99–1.61). There was not a statistically significant interaction between recent and cumulative opioid use (p=0.43 for all-cause dementia and p=0.79 for AD). For NSAID use, models suggested that the association between recent use and all-cause dementia risk was different depending on prior total cumulative use (p=0.03 for interaction; see online Table S1 for results). Among people with a history of moderate to heavy NSAID use (≥181 TSD), recent use (i.e., continued use) tended to be associated with lower dementia risk relative to no recent use (i.e., having discontinued NSAID use.) Among those with little to no prior NSAID use, however, recent users (i.e., apparent new users) had modestly higher dementia risk than non-users. For the outcome of AD, results appear similar to the findings for all-cause dementia.
For the association between opioid use and dementia, we found no effect modification by sex, age at study entry, baseline BMI, or depression status. Adjusting for the Charlson comorbidity score or prior cancer history did not substantially alter results. In analyses that classified propoxyphene use separately, there was no association between propoxyphene use and dementia risk, and the risk for non-propoxyphene opioids changed little from the primary study findings.
The cognitive trajectory analyses included 3,993 people (Figure 1). The median CASI score at baseline was 94 (interquartile range [IQR] 91–97) and did not vary according to prior opioid use. There was no association between cumulative opioid use and the rate of cognitive decline (p=0.14; online Figure S1). The observed differences in slope also were not clinically important. Relative to people with cumulative opioid exposure of 0–10 TSDs, the estimated differences in slopes were −0.04 (95% CI −0.07 to 0.00) for the 11–30 TSD group, 0.00 (95% CI −0.05 to 0.04) for the 31–90 TSD group, and −0.03 (95% CI −0.09 to 0.03) for the 91+ TSD group. Negative values mean the group of interest had a more rapid decline with age than the reference group.
Recent opioid use was not associated with the rate of cognitive decline. The difference in slopes between people with and without recent use was −0.02 (95% CI −0.07 to 0.03). Neither cumulative nor recent NSAID use was associated with the rate of cognitive decline. Results from analyses using the CASI-IRT score were similar.
DISCUSSION
In this population-based cohort study, we observed a slightly higher risk of dementia or AD among people with the highest cumulative use of prescription opioids, compared to people with little to no use (adjusted HR for all cause dementia 1.29, 95% CI 1.02–1.62). We observed a similar pattern for NSAIDs, another widely used class of analgesics (adjusted HR for all cause dementia 1.31, 95% CI 1.07–1.62). Recent opioid use was not associated with markedly higher dementia risk. People with the heaviest opioid use did not demonstrate more rapid decline in cognitive scores with age.
There are several possible explanations for the small elevation in risk seen with heavy use of opioids or NSAIDs. It may be that heavy cumulative use of either medication class truly increases dementia risk. This would presumably require the drugs to act through different mechanisms, since these medications act on different targets and biologic pathways. Alternatively, there could be residual confounding. Measures of poor health were more prevalent among people with heavier opioid or NSAID use than people with little to no use. While we adjusted for many potential confounders including comorbidity, depressive symptoms, physical activity, and smoking status, there could be other factors we did not account for. Importantly, we lacked measures of pain, including pain severity or duration. Studies have shown that chronic pain is associated with structural brain changes, including a global decrease in gray matter volume and density and also decreases in gray matter in specific brain regions, particularly areas related to pain processing.27–32 Some of these areas are also important for memory and executive function.28 Studies have also suggested impairments in cognitive function associated with chronic pain.27,28 These studies have important limitations, however. Most were cross-sectional, making it difficult to determine whether brain changes preceded or were caused by pain. Also, most studies were small and many did not exclude patients using opioids or account for opioid use, so it is difficult to distinguish the effects of pain from those of its treatment.
Strengths of the current study include that it is population based and ascertained outcomes in a rigorous manner. We were able to study subclinical cognitive decline as well as dementia. Detailed pharmacy data going back many years provided a unique opportunity to study long-term medication exposures. Many studies assess medication use through self-report or periodic medication inventory at study visits and thus lack detailed information about cumulative exposure. The ACT study collects data about a wide range of potential confounders including depressive symptoms.
Limitations include that we lacked information about pain, including its chronicity, duration or severity. There could be residual confounding by pain or other characteristics. Relatively few participants had very heavy opioid use. Throughout much of the study period, NSAIDs were available over the counter, and so there could be misclassification of NSAID use. However, GH members often purchase over the counter medications at health plan pharmacies, and these purchases are recorded in the computerized pharmacy database, improving data capture. Most participants were Caucasian, which may limit generalizability.
In conclusion, in this study – the first to examine opioids and dementia risk--there was not convincing evidence that prescription opioid use hastens cognitive decline or increases dementia risk. While we did observe a modestly higher dementia risk among people with the heaviest exposure to opioids, several aspects argue against a causal relationship. A similar pattern was seen for NSAIDs (that is, the finding was not specific to opioids); there was no pattern of increasing risk across categories of greater cumulative opioid exposure; and recent opioid use was not associated with higher risk. Studies of this topic share a common challenge, the difficulty in untangling the impact of pain from the impact of its treatments. Together with the existing literature, our work suggests that while there are other risks associated with opioid use in older adults, the evidence thus far does not suggest that opioid use conveys substantial long-term cognitive harm.
Supplementary Material
Acknowledgments
Funding sources: This work was funded by National Institutes of Health grants R03AG042930 and U01AG006781 and by the Branta Foundation.
We would like to thank Dr. Laura Gibbons, who created the CASI-IRT variables used in this work, and Drs. Susan McCurry, Wayne McCormick and James Bowen, who took part in multidisciplinary consensus committee meetings that determined study participants’ dementia status.
Biography
Dr. Dublin received a Merck/American Geriatrics Society New Investigator Award for unrelated work. Dr. Larson receives royalties from UpToDate. Mr. Walker has received funding as a biostatistician from an unrelated research grant awarded to Group Health Research Institute from Pfizer. Ms. Yu has received funding from unrelated research grants awarded to Group Health Research Institute from Amgen and Bayer.
Footnotes
Prior Presentation: This paper was presented at the 2013 International Conference on Pharmacoepidemiology in Montreal, Canada on August 26, 2013 and as a poster at the 2014 Annual Meeting of the HMO Research Network in Phoenix, Arizona on April 1, 2014.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Sponsor’s Role: The sponsors did not play a role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or in preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Author Contributions: Sascha Dublin, Rod L. Walker, Shelly L. Gray, Rebecca A. Hubbard, Melissa L. Anderson, Onchee Yu, and Eric B. Larson contributed to study conception and design; all authors contributed to acquisition, analysis, or interpretation of data; Sascha Dublin and Rod L. Walker drafted the manuscript; Shelly L. Gray, Rebecca A. Hubbard, Melissa L. Anderson, Onchee Yu, Paul K. Crane, and Eric B. Larson revised the manuscript for critical intellectual content; RLW conducted statistical analyses; Sascha Dublin, Eric B. Larson and Paul K. Crane obtained funding; and Eric B. Larson provided administrative support.
References
- 1.Williams RE, Sampson TJ, Kalilani L, et al. Epidemiology of opioid pharmacy claims in the United States. J Opioid Manag. 2008;4:145–52. doi: 10.5055/jom.2008.0019. [DOI] [PubMed] [Google Scholar]
- 2.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18:1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid MC, Bennett DA, Chen WG, et al. Improving the pharmacologic management of pain in older adults: Identifying the research gaps and methods to address them. Pain Med. 2011;12:1336–1357. doi: 10.1111/j.1526-4637.2011.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisani MA, Murphy TE, Araujo KL, et al. Factors associated with persistent delirium after intensive care unit admission in an older medical patient population. J Crit Care. 2010;25:540, e1–7. doi: 10.1016/j.jcrc.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCusker J, Cole M, Dendukuri N, et al. Delirium in older medical inpatients and subsequent cognitive and functional status: A prospective study. CMAJ. 2001;165:575–583. [PMC free article] [PubMed] [Google Scholar]
- 6.Rockwood K, Cosway S, Carver D, et al. The risk of dementia and death after delirium. Age Ageing. 1999;28:551–556. doi: 10.1093/ageing/28.6.551. [DOI] [PubMed] [Google Scholar]
- 7.Anthony IC, Norrby KE, Dingwall T, et al. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain. 2010;133(Pt 12):3685–98. doi: 10.1093/brain/awq263. [DOI] [PubMed] [Google Scholar]
- 8.Ramage SN, Anthony IC, Carnie FW, et al. Hyperphosphorylated tau and amyloid precursor protein deposition is increased in the brains of young drug abusers. Neuropathol Appl Neurobiol. 2005;31:439–448. doi: 10.1111/j.1365-2990.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 9.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 10.He L, Li H, Chen L, et al. Toll-like receptor 9 is required for opioid-induced microglia apoptosis. PLoS One. 2011;6:e18190. doi: 10.1371/journal.pone.0018190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu S, Sheng WS, Lokensgard JR, et al. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 12.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: A prospective cohort study. Arch Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 13.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dublin S, Walker RL, Jackson ML, et al. Use of opioids or benzodiazepines and risk of pneumonia in older adults: A population-based case-control study. J Am Geriatr Soc. 2011;59:1899–1907. doi: 10.1111/j.1532-5415.2011.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ATC/DDD Index. [Accessed Mar 18, 2014];World Health Organization Collaborating Centre for Drug Statistics Methodology (online) 2014 Available at: http://www.whocc.no/atc_ddd_index/
- 16.Breitner JC, Haneuse SJ, Walker R, et al. Risk of dementia and AD with prior exposure to NSAIDs in an elderly community-based cohort. Neurology. 2009;72:1899–1905. doi: 10.1212/WNL.0b013e3181a18691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samejima F. Estimation of latent ability using a response pattern of graded scores. Psychometrika. 1969;34:129–301. [Google Scholar]
- 22.Samejima F. Graded response model. In: van der Linden WJ, Hambleton RK, editors. Handbook of modern item response theory. New York, NY: Springer-Verlag; 1997. pp. 85–100. [Google Scholar]
- 23.Parscale for Windows (computer program). 4.1 ed. Chicago, IL: Scientific Software International; 2003. [Google Scholar]
- 24.Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 25.World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 26.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 27.Block C, Cianfrini L. Neuropsychological and neuroanatomical sequelae of chronic non-malignant pain and opioid analgesia. NeuroRehabil. 2013;33:343–366. doi: 10.3233/NRE-130965. [DOI] [PubMed] [Google Scholar]
- 28.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog Neurobiol. 2011;93:385–404. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 29.May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 30.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuchinad A, Schweinhardt P, Seminowicz DA, et al. Accelerated brain gray matter loss in fibromyalgia patients: Premature aging of the brain? J Neurosci. 2007;27:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blankstein U, Chen J, Diamant NE, et al. Altered brain structure in irritable bowel syndrome: Potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.