Abstract
Objective
To determine whether pattern of skin involvement can predict clinical features, risk of restrictive lung disease, and survival in a large scleroderma (SSc) cohort.
Methods
Demographic and clinical data collected over 30 years from 2,205 SSc patients were retrospectively analyzed after subdividing subjects into four subtypes based on pattern of skin fibrosis: Type-0 (no skin involvement), Type-1 (limited to metacarpophalangeal joints), Type-2 (distal to elbows/knees) and Type-3 (proximal to elbows/knees). Clinical features associated with skin subsets were identified by regression analyses. Kaplan-Meier and Cox proportional hazards models were used to compare time to restrictive lung disease (RLD) and survival across subtypes.
Results
The presence and severity of RLD were positively associated with skin subtype (p<0.001). RLD prevalence incrementally ranged from 51.9% in Type 0 to 76.7% in Type-3 (p<0.001). Type-2 SSc exhibited a distinct phenotype with intermediate risk for RLD relative to Type-1 (higher, p<0.001) and Type-3 (lower, p<0.001), and a unique autoantibody profile, with a prevalence of anti-centromere lower than Type-1 (28.9% vs. 44.1%, p=0.001) and of anti-topoisomerase I similar to Type-3 (p=0.38). These autoantibodies were also found to be significant negative (OR 0.33, p<0.001) and positive (OR 1.6, p=0.01) predictors of RLD risk respectively. Mortality was also intermediate in Type-2 patients relative to Type-3 (p=0.0003) and Type-1 (p=0.066).
Conclusions
These data suggest that the current classification subdividing SSc into the limited and diffuse cutaneous subtypes misclassifies an intermediate group of patients exhibiting unique autoantibody profile, disease course and clinical outcomes.
Keywords: systemic sclerosis, pulmonary fibrosis, scl-70, centromere
Introduction
Systemic sclerosis (scleroderma; SSc) is a chronic multisystem disease characterized by vascular dysfunction, immune activation, and tissue fibrosis.1 These pathogenetic processes contribute to a broad spectrum of clinical phenotypes often associated with substantial morbidity and mortality.2 The name scleroderma derives from the Greek “scleros” and “derma” which literally mean “hard skin”. As a common and early manifestation of SSc, cutaneous fibrosis has been the major focus of diagnostic and classification criteria.
In 1980, preliminary criteria were defined to distinguish scleroderma from other diseases.3 Subsequently, numerous proposals for classification systems that divide scleroderma into disease subtypes based on the extent of skin involvement have been published.4-7 Studies comparing their clinical utility have generated conflicting results, often due to a relatively low number of patients investigated.8-11 For this reason, a dichotomous classification system has prevailed.12,13
Patients exhibiting fibrosis distal to the elbows and knees are included in the limited cutaneous disease (lcSSc) subset.6 In this group, a high prevalence of anti-centromere antibodies (ACA) and an increased risk of developing pulmonary arterial hypertension are recognized.14,15 In contrast, more extensive skin fibrosis involving the proximal portion of the limbs or the trunk is classified as diffuse cutaneous scleroderma (dcSSc). Heterogeneous phenotypes within this subset can be further distinguished by autoantibody status. For example, dcSSc patients with anti-Scl-70 antibodies have an increased risk of restrictive lung disease (RLD), while those with anti-RNA-polymerase III antibodies have an increased risk of scleroderma renal crisis, but develop RLD less frequently.14,16 Although the limited and diffuse classification system may be useful for research purposes, it does not fully account for the heterogeneous nature of SSc skin involvement. In particular, careful characterization of SSc patients suggests that at least 2 other subsets can be consistently observed: an “intermediate” subtype (skin fibrosis involving forearms and legs but not the trunk) and the “sine-scleroderma” group with no skin fibrosis but typical visceral manifestations.17,18 These subtypes are usually “merged” into the lcSSc group, but experientially present with distinct serologic patterns and different clinical outcomes.19
Lung involvement is currently the leading cause of morbidity and mortality in SSc with a prevalence ranging between 30% and 90% of patients.2,16,20 Although it is more common in dcSSc, no reliable association has been found between RLD and the extent or severity of skin involvement.21,22 In addition, the risk for lung disease and related outcomes in subjects with “intermediate” skin phenotype has not been defined.
In this study, we sought to determine in a very large single-center cohort whether subdividing patients into 4 subtypes based on the pattern of skin involvement, in combination with their autoantibody status, can meaningfully stratify the risk of distinct clinical manifestations with particular regard to the development and severity of interstitial lung disease and survival.
Patients and Methods
Patients evaluated at the Johns Hopkins Scleroderma Center from December 1976 to March 2010 have been enrolled into the longitudinal cohort after obtaining written informed consent if they met American College of Rheumatology criteria for SSc, or had at least 3-of-5 features of CREST syndrome (calcinosis, Raynaud's phenomenon, esophageal dysmotility, sclerodactyly, telangectasias), or had definite Raynaud's phenomenon (RP), abnormal scleroderma-pattern nailfold capillaries, and the presence of a SSc-specific autoantibody (anti-Scl-70, ACA or anti-RNA-polymerase III).3 Of total 2348 patients, 100 were excluded from the analysis because missing key data (i.e. lung function tests, skin assessment, and at least one follow-up visit after enrollment), and 43 because developed SSc before age 16. The present study was approved by the Johns Hopkins Institutional Review Board.
Demographic and clinical data including age, sex, ethnicity, smoking status, disease duration at first visit (defined from onset of first non-RP symptom), scleroderma subtype, specific organ involvement, and autoantibody status were recorded at the initial and follow-up visits. The severity of skin involvement was quantified using the modified Rodnan skin score (MRSS).23 The pattern of skin involvement was defined into four subtypes: Type-0 if no detected cutaneous sclerosis; Type-1 with sclerosis distal to the metacarpophalangeal joints, with or without face involvement; Type-2 with skin changes more proximal but distal to elbows or knees and no trunk involvement; Type-3 with sclerosis extending proximal to the elbows or knees and trunk. Patients were classified by the maximum extent of cutaneous involvement assessed during their clinical visits or as indicated by previous records. A patient's disease subtype designation was not changed if subsequent improvement of skin involvement occurred. The presence and severity of RP, gastrointestinal, heart, and renal organ involvement was assessed using the Medsger severity scale (MSS).24 Severe organ disease was defined as MSS ≥3. Muscle involvement was confirmed by elevated muscle enzymes, abnormal electromyogram, or biopsy proven inflammation. Joint disease was defined by detection of synovitis, effusions, arthralgias or tendon friction rubs. Presence of sicca symptoms was identified by clinical criteria.25 Lung involvement was determined based on abnormal pulmonary function tests (PFT). Measurements of forced vital capacity (FVC) and single breath diffusing capacity for carbon monoxide (DLCO) were calculated as % predicted for an average person of the same height, weight, sex, age, race, and were standardized.26-28 The presence of RLD was defined by an FVC<80% of predicted, with severity categorized as normal (FVC>80%), mild (FVC 70-80%), moderate (FVC 50-69%), or severe (FVC<50%). For the purpose of this study, echocardiographic evidence of pulmonary hypertension (ECHO-PH) was determined based on estimated right ventricular systolic pressure (eRVSP) by Doppler echocardiography ≥45mmHg with no clinical evidence of congestive heart failure or thromboembolic disease.29
Patient demographics and clinical characteristics were compared across scleroderma subtypes, with p-values calculated using chi-squared test for binomial or categorical variables and analysis of variance (ANOVA) for continuous variables. Pairwise comparisons were performed if statistically significant differences among SSc subtypes were present, and Bonferroni correction used to determine significance. For each scleroderma subtype, Kaplan-Meier curves were calculated to visualize the time from SSc onset to development of RLD. Pairs of survival curves were tested for equality using the logrank test. The same analysis was repeated to compare subsets of patients defined by their autoantibody profile. Simple logistic regression was used to estimate the association between different skin subsets and the development of RLD. Multiple logistic regressions adjusting for sex, age at onset, disease duration (from SSc onset to the last visit or death), race, smoking status, muscle disease, anti-Scl-70 and ACA antibody were conducted to further assess the strength of this association.
The cumulative incidence of mortality was estimated by Kaplan-Meier analysis, and logrank test was used to compare the incidence of death based on SSc skin subset and antibody status. The independent contribution of skin subtype to mortality was examined using Cox proportional hazards analyses, with adjustment for sex, age at scleroderma onset, smoking status, and race. Reported p-values are 2-sided with α=0.05. Statistical analyses were performed using Stata 10.0 (Stata Corporation, College Station, TX).
Results
Entry criteria were satisfied by 2205 patients. Table-1 summarizes their sociodemographic and disease characteristics. The proportion of males was highest in the subtype with greater skin involvement (p<0.001). The mean age at onset was similar in the different subtypes, while disease duration at the first visit was significantly shorter in Type-3 SSc patients. African Americans exhibited more frequently Type-3 SSc (205/367, 56%) compared to white subjects (561/1652, 34%) (p<0.001). Broader skin involvement was positively associated with increased prevalence of severe GI disease (p=0.007), muscle inflammation (p<0.001) and joint involvement (p<0.001), especially with tendon friction rubs (p<0.001). Type-0 patients exhibited a pattern of disease manifestations and organ involvement similar to Type-1, but overall with milder expression. Type-2 patients showed a unique clinical phenotype characterized by higher prevalence of calcinosis, sicca symptoms, more severe RP and heart disease. As expected, Type-1 patients showed the highest prevalence of ECHO-PH (33%, p=0.005), followed by Type-2 (29.9%) and Type-3 (24.6%).
Skin involvement and lung disease in SSc
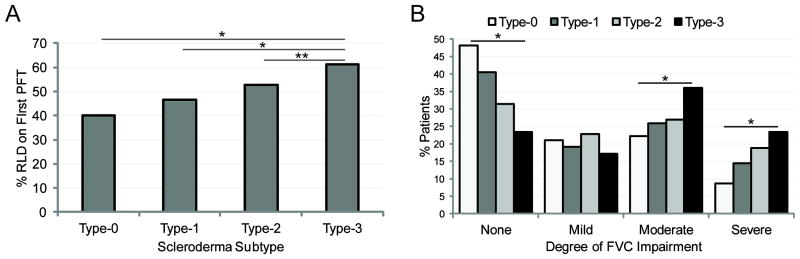
The presence of RLD was positively associated with the pattern of cutaneous involvement, with prevalence increasing of approximately 8% through each subsequent SSc skin subtype (p<0.001) (Table 1). Accordingly, mean minimum DLCO and FVC were lower with increasing category of skin disease (p<0.001). The proportion of patients exhibiting established RLD at the time of their first available PFT incrementally ranged from 40% in Type-0 group to 61% in Type-3 (p<0.001) (Figure 1A). Approximately 41.8% of patients had their first PFT within 6 months of diagnosis and the average time from diagnosis to first PFT was 3.1±6.1 years. The analysis of RLD severity by SSc skin subtype showed that patients in higher categories of skin involvement more frequently developed moderate to severe RLD (p<0.001) (Figure 1B).
Table 1. Patient Demographics and Clinical Characteristics.
| Total (n= 2205) |
Type-0 (n= 81) |
Type-1 (n= 1070) |
Type-2 (n= 197) |
Type-3 (n= 857) |
P | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Sex | ||||||
| Male | 374 (17.0) | 6 (7.4) | 140 (13.1) | 33 (16.8) | 195 (22.8) | <0.001 |
| Female | 1,831 (83.0) | 75 (92.6) | 930 (86.9) | 164 (83.3) | 662 (77.3) | |
| Age at onset† (years) | 46.2 ± 13.6 | 48.7 ± 15.7 | 46.1 ± 13.9 | 46.6 ± 13.8 | 46.1 ± 13.1 | 0.4 |
| SSc Duration† (years) | 5.6 ± 7.2 | 6.1 ± 6.9 | 7.4 ± 8.2 | 6.0 ± 7.4 | 3.4 ± 4.7 | <0.0001 |
| Follow-up Time† [range, years] |
4.1 ± 4.5 [0 – 26.3] |
1.8 ± 2.6 [0 – 10.8] |
4.4 ± 4.6 [0 – 26.3] |
4.3 ± 4.5 [0 – 17.0] |
3.9 ± 4.4 [0 – 25.6] |
<0.0001 |
| Race | ||||||
| White | 1,652 (74.9) | 62 (76.5) | 875 (81.8) | 154 (78.2) | 561 (65.5) | <0.001 |
| Black | 367 (16.6) | 12 (14.8) | 126 (11.8) | 24 (12.2) | 205 (23.9) | <0.001 |
| Asian | 48 (2.2) | 3 (3.7) | 21 (2.0) | 1 (0.5) | 23 (2.7) | 0.2 |
| Other | 138 (6.3) | 4 (4.9) | 48 (4.5) | 18 (9.1) | 68 (7.9) | 0.005 |
| Smoking status | ||||||
| Never | 1,102 (50.0) | 37 (45.7) | 523 (48.9) | 88 (44.7) | 454 (53.0) | 0.09 |
| Former | 822 (37.3) | 31 (38.3) | 410 (38.3) | 80 (40.6) | 301 (35.1) | 0.4 |
| Current | 251 (11.4) | 11 (13.6) | 121 (11.3) | 26 (13.2) | 93 (10.9) | 0.7 |
| Known Status | 1488 (67.5) | 46 (56.8) | 723 (67.6) | 127 (64.5) | 592 (69.1) | 0.1 |
| Deceased | 550 (37.0) | 24 (52.2) | 236 (32.6) | 48 (37.8) | 242 (40.9) | 0.003 |
| Clinical Characteristics | ||||||
| MRSS† (range, 0-51) | 12.2 ± 11.9 | 0.0 ± 0.0 | 4.7 ± 3.8 | 10.2 ± 5.2 | 23.2 ± 11.7 | <0.0001 |
| Calcinosis | 585 (26.7) | 7 (8.8) | 316 (29.6) | 72 (36.7) | 190 (22.3) | <0.001 |
| Telangiectasias | 1,916 (87.3) | 62 (77.5) | 991 (92.9) | 170 (86.7) | 693 (81.4) | <0.001 |
| Sicca | 1,225 (55.9) | 35 (43.8) | 643 (60.3) | 123 (63.1) | 424 (49.8) | <0.001 |
| Raynaud's | 2,146 (98.1) | 76 (98.7) | 1,064 (99.7) | 188 (97.9) | 818 (96.1) | <0.001 |
| RP Score† (0-4) | 1.8 ± 0.9 | 1.3 ± 0.8 | 1.8 ± 1.0 | 2.0 ± 1.0 | 1.9 ± 0.9 | <0.0001 |
| Score ≥3 | 565 (25.8) | 8 (10.4) | 265 (24.8) | 64 (33.3) | 228 (26.8) | 0.001 |
| GI Disease | 1,993 (90.9) | 67 (83.8) | 963 (90.4) | 180 (91.8) | 783 (91.9) | 0.09 |
| GI Score† (0-4) | 1.4 ± 0.8 | 1.2 ± 0.9 | 1.4 ± 0.8 | 1.5 ± 0.9 | 1.5 ± 0.9 | 0.0001 |
| Score ≥3 | 214 (9.8) | 7 (8.8) | 82 (7.7) | 19 (9.7) | 106 (12.4) | 0.007 |
| Heart Disease | 500 (25.5) | 13 (22.8) | 255 (26.3) | 51 (30.7) | 181 (23.5) | 0.2 |
| Heart Score† (0-4) | 0.8 ± 1.4 | 0.6 ± 1.3 | 0.8 ± 1.5 | 0.9 ± 1.6 | 0.7 ± 1.4 | 0.2 |
| Renal Disease | 352 (17.8) | 8 (14.0) | 165 (17.0) | 29 (16.7) | 150 (19.3) | 0.5 |
| Renal Score† (0-4) | 0.3 ± 0.8 | 0.2 ± 0.5 | 0.3 ± 0.7 | 0.3 ± 0.8 | 0.4 ± 0.9 | 0.04 |
| Muscle disease | 303 (13.9) | 3 (3.8) | 94 (8.8) | 18 (9.3) | 188 (22.2) | <0.001 |
| Joint Disease | 1,527 (69.8) | 30 (38.5) | 686 (64.3) | 136 (69.7) | 675 (79.6) | <0.001 |
| Arthralgias | 1,461 (66.7) | 29 (37.2) | 672 (63.0) | 129 (66.2) | 631 (74.3) | <0.001 |
| Synovitis | 332 (15.2) | 6 (7.7) | 146 (13.7) | 38 (19.5) | 142 (16.8) | 0.02 |
| TFR | 371 (17.0) | 1 (1.3) | 26 (2.4) | 14 (7.2) | 330 (39.2) | <0.001 |
| ECHO-PH | 528 (29.4) | 16 (29.6) | 298 (33.0) | 46 (29.9) | 168 (24.6) | 0.005 |
| RLD | 1,474 (66.9) | 42 (51.9) | 640 (59.8) | 135 (68.5) | 657 (76.7) | <0.001 |
| FVC‡ (% predicted) | 70.2 ± 20.8 | 78.9 ± 21.7 | 73.5 ± 20.8 | 71.0 ± 20.5 | 65.0 ± 19.7 | <0.0001 |
| DLCO‡ (% predicted) | 61.3 ± 25.1 | 68.6 ± 28.9 | 63.6 ± 25.4 | 61.1 ± 23.6 | 57.9 ± 24.2 | <0.0001 |
| Autoantibody Status | ||||||
| ANA | 1,397 (96.2) | 44 (95.7) | 704 (96.4) | 125 (97.7) | 524 (95.6) | 0.7 |
| ACA | 393 (27.5) | 16 (35.6) | 316 (44.1) | 37 (28.9) | 24 (4.4) | <0.001 |
| Anti-RNP | 106 (8.5) | 4 (9.3) | 60 (9.8) | 13 (11.9) | 29 (6.0) | 0.08 |
| Anti-Scl-70 | 304 (23.0) | 5 (12.8) | 108 (17.0) | 39 (32.8) | 152 (28.7) | <0.001 |
All values are given as number (%) unless otherwise specified. Only patients with non-missing data were analyzed for each variable. Supplementary Table S1 is provided in Supplemental Material reporting all the missing values for the socio-demographic and clinical parameters included in the study.
RP, GI, renal, and heart severity scores are reported as previously defined by Medsger TA et al.24
Mean ± SD.
Minimum recorded value for the percent of predicted ± SD. SSc = systemic sclerosis; MRSS = modified Rodnan skin thickness score; RP = Raynaud's Phenomenon; GI = gastrointestinal; TFR = tendon friction rubs; ECHO-PH = pulmonary hypertension; DLCO = diffusion capacity for carbon monoxide; RLD = restrictive lung disease; FVC = forced vital capacity; ANA = anti-nuclear antibody; ACA = anti-centromere antibody; RNP = ribonucleoprotein; Scl-70 = topoisomerase I
Figure 1.
A, Percentage of patients with Type-0 (n=81), Type-1 (n=1070), Type-2 (n=197), and Type-3 (n=857) scleroderma with evidence of RLD (FVC<80%) at their first pulmonary function test (*p<0.001, **p=0.03). B, Percentage of patients in each subtype analyzed by severity of restrictive lung disease: none (FVC>80% predicted), mild (FVC 70-80% predicted), moderate (FVC 50-69% predicted), or severe (FVC<50% predicted) (*p<0.001). RLD = restrictive lung disease; PFT = pulmonary function test; FVC = forced vital capacity (% of predicted)
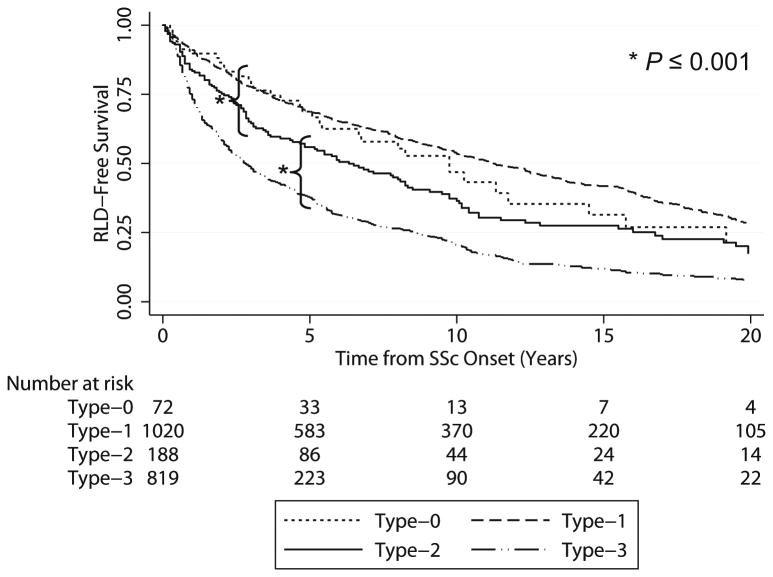
The risk of developing RLD from the time of scleroderma onset is analyzed in Figure 2. Type-2 SSc patients showed an intermediate and statistically distinct incidence of RLD over time compared to Type-1 (p<0.0001) and Type-3 (p<0.0001). We then evaluated the relevance of other variables potentially associated with increased odds of developing lung disease (Table 2). In unadjusted analyses, skin subtype was significantly associated with RLD risk. Relative to Type-2, the odds of developing RLD for Type-0 and Type-1 patients were significantly reduced (OR 0.5, p=0.009 and OR 0.7, p=0.02 respectively), while for Type-3 were significantly higher (OR 1.5, p=0.018). Among all predictors tested, increase risk for RLD was positively associated with anti-Scl-70 antibodies (OR 2.2, p<0.001) and muscle disease (OR 2.8, p<0.001), while ACA exhibited a protective effect (OR 0.27, p<0.001). After adjusting for the main demographic features (Table 2), the regression model confirmed a lower risk for RLD in Type-1 and Type-0 relative to Type-2 patients (OR 0.6, p=0.01 and OR 0.5, p=0.009), while no difference was detected between Type-2 and Type-3 (OR 1.3, p=0.2).
Figure 2.
Kaplan-Meier curves of the association between scleroderma subtype and the detection of restrictive lung disease (RLD). All available pulmonary function tests were analyzed from the time of scleroderma (SSc) onset (first non-Raynaud's symptom) and patients were considered to have RLD when their forced vital capacity dropped below 80% of predicted for an average person of the same height, weight, sex, age, and race. The logrank test for Type-2 patients vs. Type-1 and Type-3 patients indicated significant differences in both comparisons (*p≤0.0001).
Table 2. Predictors of Restrictive Lung Disease in SSc Skin Subtypes.
| Unadjusted* | Adjusted Logistic Models** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | Anti-Scl-70 Status | ACA Status | All Predictors | |||||||
|
| ||||||||||
| Type§ | OR 95% CI |
P | OR 95% CI |
P | OR | P | OR | P | OR | P |
| 0 vs. 2 | 0.5 [0.3, 0.8] | 0.009 | 0.5 [0.3, 0.8] | 0.009 | 0.9 [0.4, 2.1] | 0.8 | 0.7 [0.3, 1.5] | 0.3 | 0.8 [0.3, 2.1] | 0.7 |
| 1 vs. 2 | 0.7 [0.5, 0.9] | 0.02 | 0.6 [0.4, 0.9] | 0.01 | 0.6 [0.4, 1.0] | 0.045 | 0.7 [0.4, 1.1] | 0.09 | 0.7 [0.4, 1.1] | 0.09 |
| 3 vs. 2 | 1.5 [1.1, 2.1] | 0.02 | 1.3 [0.9, 1.8] | 0.2 | 1.1 [0.7, 1.8] | 0.7 | 0.8 [0.5, 1.3] | 0.3 | 0.8 [0.4, 1.3] | 0.3 |
| Scl-70 | 2.2 [1.6, 3.0] | <0.001 | 2.1 [1.5, 2.9] | <0.001 | NA | NA | 1.4 [1.0, 2.0] | 0.045 | 1.6 [1.1, 2.2] | 0.01 |
| ACA | 0.2 [0.2, 0.3] | <0.001 | 0.3 [0.2, 0.4] | <0.001 | 0.3 [0.2, 0.4] | <0.001 | NA | NA | 0.3 [0.2, 0.5] | <0.001 |
| Muscle Disease | 2.8 [2.0, 3.8] | <0.001 | 2.5 [1.8, 3.5] | <0.001 | 2.6 [1.8, 4.0] | <0.001 | 1.8 [1.2, 2.6] | 0.006 | 1.9 [1.2, 2.9] | 0.003 |
Simple logistic regression model used to determine the association of each predictor with the odds of developing RLD
Multiple logistic regression models controlling for demographics (including sex, age at onset, disease duration, race, and smoking status), Scl-70, ACA status or all predictors together.
Type 2 patients are used as a reference for to assessment of the relationship between SSc skin disease type and RLD risk. OR = odds ratio; 95% CI = 95% confidence interval; Scl-70 = topoisomerase I; ACA = anti-centromere; NA = not applicable
In order to determine how the presence of SSc-specific autoantibodies may influence the association between skin subtype and RLD, the multivariate regression model was further adjusted for anti-Scl-70 or ACA separately. After adjusting for anti-Scl-70, the risk for RLD was still reduced for Type-1 patients relative to Type-2 (OR 0.6, p=0.045), but not for Type-0 compared to Type-2 (OR 0.9, p=0.8). When controlling for ACA status, no difference for ILD risk was found among skin subtypes. When all significant predictors were included in the model, skin subtype was not significantly associated with the development of RLD. In contrast, anti-Scl-70 positivity was associated with 60% increased odds of developing RLD (p=0.01) and ACA with 70% reduction (p <0.001).
Autoantibody associations with scleroderma subtypes and RLD
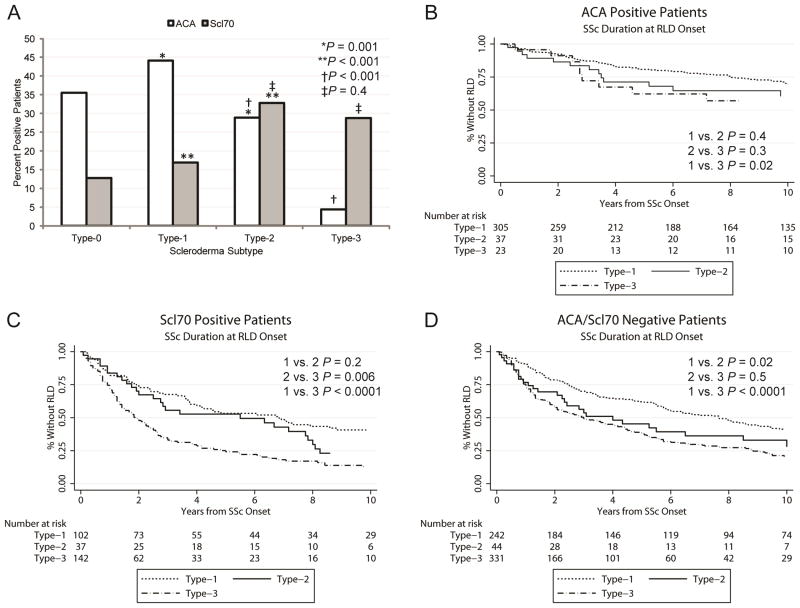
We next analyzed the autoantibody profile of the different skin subsets (Figure 3A). The prevalence of ACA was significantly lower in Type-2 patients compared to Type-1 (28.9% vs. 44.1%, p=0.001), while that of anti-Scl-70 was higher (17% vs. 32.8%, p<0.001). Interestingly, Type-2 patients also differed significantly from Type-3 with regard to ACA prevalence (28.9% vs. 4.4%, p<0.001), but not anti-Scl-70 (32.8% vs. 28.7%, p=0.38).
Figure 3.
Scleroderma (SSc) specific autoantibodies association with skin involvement subtypes and the risk of restrictive lung disease (RLD). A, Percentage of patients who are positive for anti-centromere (ACA, n=393) and anti-topoisomerase I (Scl-70, n=304) antibodies in each of the four skin subtypes. * ACA Type-1 vs. Type-2; ** anti-Scl-70 Type-1 vs. Type-2; † ACA Type-2 vs. Type-3; ‡ anti-Scl-70 Type-2 vs. Type-3. B-D, Kaplan-Meier curves showing the association between scleroderma subtypes and RLD among patients with the same autoantibody status: ACA positive (B), anti-Scl-70 positive (C) and patients negative for both ACA and anti-Scl-70 antibodies (D).
To further determine the relevance of SSc-related autoantibodies as predictors of RLD, we stratified SSc patients by autoantibody status (Figure 3B-D). Type-0 patients were excluded from this analysis due to their low number after stratification. Among ACA positive patients (n=365) (Figure 3B), those with Type-2 SSc had an intermediate risk for RLD that was not significantly different from that of Type-1 (p=0.4) or Type-3 (p=0.3), although the latter had an increased RLD risk compared to Type-1 (p=0.02). Among anti-Scl-70 positive patients (n=281) (Figure 3C), Type-1 and Type-2 showed similar risk of RLD (p=0.2), but significantly lower than Type-3 (p<0.0001 and p=0.006 respectively). Finally, in patients negative for both ACA and anti-Scl-70 (n=617) (Figure 3D), the Type-2 group exhibited an RLD risk similar to that of Type-3 (p=0.5), which was greater than Type-1 (p=0.018 and p<0.001 respectively). The same analyses conducted from the time of RP onset showed even stronger differences across skin subtypes (data not shown). Taken together, these data confirm that the pattern of skin involvement is an independent risk factor for RLD.
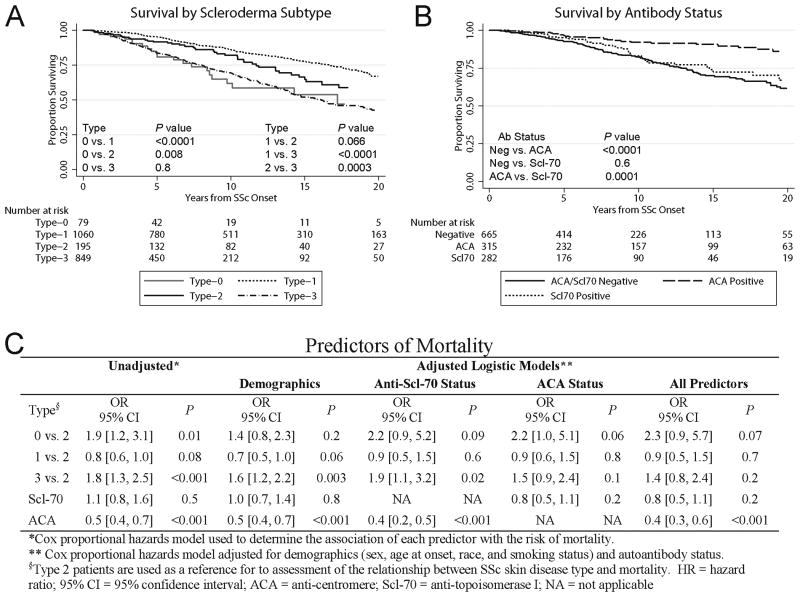
Survival differences among scleroderma subtypes
At the time of this analysis, 25% of patients were known to be deceased. Survival analysis by SSc subtype is shown in Figure 4A. Overall mortality risk was increased in patients with Type-3 SSc compared the Type-2 and Type-1 (p=0.0003 and p<0.0001 respectively). The survival curve for Type-2 and Type-1 subjects was similar during early phases of SSc, but over time Type-2 patients showed an overall trend towards worse outcome (p = 0.066). Surprisingly, the survival of patients with Type-0 SSc was similar to that of Type-3 (p=0.8).
Figure 4.
Mortality of scleroderma (SSc) patients based on skin involvement subtype (A) and antibody status (B). Kaplan-Meier survival curves were analyzed from the time of SSc onset (first non-Raynaud's symptom). (C) Risk of mortality analyzed by Cox proportional hazards models.
Survival analysis by antibody status (Figure 4B) showed that patients positive for ACA have significantly decreased mortality rates compared to those positive for anti-Scl-70 (p=0.0001) and to those negative for both autoantibodies (p<0.0001). The proportional hazards models (Figure 4C) did not show a significant association between skin subtype and survival. Also, anti-Scl-70 was not a significant predictor of mortality.
Discussion
This large longitudinal cohort study provides evidence that the current division of scleroderma into two subtypes (limited and diffuse) may misclassify a group of patients with intermediate degree of skin involvement, who presents a unique serological profile and distinct clinical outcomes. This study also highlights the prognostic value of defining the pattern of skin involvement together with the autoantibody status to predict risk and severity for RLD as well as overall survival in SSc.
We found that Type-2 patients show an “intermediate” clinical phenotype compared to Type-1 (milder) and Type-3 (worse) in terms of RLD prevalence, severity and time of onset. In addition, the risk of developing RLD is increased in Type-2 patients compared to Type-0 or Type-1, suggesting that combining these groups in the lcSSc subset may not be appropriate. The detection of higher prevalence of anti-Scl-70 in Type-2 SSc patients, may partially explain the clinical features and outcomes detected in this intermediate subset.
The debate among SSc experts regarding which nosological system may provide the best clinical and prognostic value for SSc patients has been relatively quiet for the past two decades.9-11,18,30-32 While some studies demonstrated the presence of an intermediate skin phenotype with unique clinical outcomes and survival rates not captured in the lcSSc/dcSSc classification, other investigations conducted on small cohorts have not shown significant differences using two or more skin subsets.11,18,33 Thus, a 1988 editorial co-signed by leading experts in the field has resulted in the subsequent general use of the limited and diffuse SSc subsets based largely on prevailing opinion.6
While some authors have shown an increase risk of RLD in diffuse compared to limited SSc patients, others did not find any difference.14,34-38 Based on our findings, it is possible that this may be due in part to the inclusion of a variable number of patients with intermediate skin phenotype in the lcSSc group. Mixed results have also been reported in studies analyzing patients based on their MRSS, a scoring system assessing the severity of skin disease rather than the specific pattern of cutaneous involvement.22,39 The main challenge in using the MRSS is the subjective physician variability in examining the skin and the difficulty of uniformly measuring disease at the time of its maximal severity.
Our investigation confirms previous findings that a higher degree of skin disease is more prevalent among males and African Americans. It also reinforces the fact that RLD is an early and prevalent manifestation in scleroderma, as we found that a high number of patients who obtained their PFTs within 6 months from diagnosis showed presence of lung involvement.40-44 As previously shown, the value of FVC at the time of the first PFTs is an important predictor of RLD progression and severity.45,46 Importantly, our study shows that progression to RLD after several years from disease onset can still be observed in all subtypes.
Scleroderma is associated with increased mortality and this investigation shows that the degree of skin involvement in association with autoantibody status can be an early predictor of survival. We found that patients negative for ACA and with Type-3 skin disease have the greatest mortality risk, and that survival in Type-2 patients tends to decline faster compared to Type-1. Our data also suggest that Type-0 patients have mortality rates similar to Type-3 despite their milder clinical phenotype. The interpretation of these findings is limited by the low number of Type-0 patients and the lack of knowledge about causes of death. There are several other limitations. This study is retrospective. One quarter of our patients are non-white, which may limit the applicability of our findings to populations with different racial distribution. A referral bias toward more severely affected SSc patients is possible. Data on other scleroderma-associated autoantibodies, including anti-RNA-polymerase III, anti-Pm/Scl, anti-Ku, anti-phospholipid, and anti-ribonucleoprotein (Th/To, U1, U3, U11/U12) antibodies are not available.47,48 Imaging studies to confirm the definite presence of ILD have not been recorded in our database and therefore there is the possibility that underlying muscle disease may contribute to lower lung volumes. Also, we cannot fully exclude a lead-time bias, as patients with more extensive SSc skin disease may have develop RLD earlier and not more frequently compared to other subjects. With regards to patient's allocation into skin subtypes, a very small number of subjects with limited follow-up may have been misclassified because they did not reach their peak skin involvement. Finally, the multivariate regression models showed lack of association between SSc subtypes and specific outcomes (i.e. development of RLD and survival). This may be in part explained by the effects of collinearity since a well-established association is recognized between skin subtypes and specific autoantibodies such as anti-Scl-70 and ACA in SSc. The strengths of this study include the availability of a very large number of SSc patients followed at a single tertiary referral center and the refined characterization of their clinical phenotype over a long period of time (30 years) by physicians expert in the field of scleroderma.
In summary, this study suggests that a more refined phenotyping of SSc cutaneous involvement allows a classification of SSc patients showing a greater power to predict clinical outcomes and survival compared to the traditional subdivision into limited and diffuse SSc. The improved prognostic value of more definite skin subsets together with their SSc-specific autoantibody patterns could facilitate both the investigation and the management of this complex and highly heterogeneous disease.
Supplementary Material
Acknowledgments
We thank Adrianne Woods for database assistance, the Johns Hopkins Bayview Biostatistics, Epidemiology and Data Management (BEAD) Core for statistical help, and the Scleroderma Research Foundation and the National Institute of Health (F.B. NIH grant AR-055667) for their support.
Footnotes
Contributorship: All authors actively participated in the design, data analysis and writing of this manuscript and approved the submitted version.
Financial disclosure: No benefit from any commercial sources has been obtained to support the work reported on this manuscript. None of the authors have financial benefits or conflict of interest to disclose relevant to this investigation.
Contributor Information
Tricia R. Cottrell, Johns Hopkins University School of Medicine, Baltimore, MD
Robert A. Wise, Professor of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD
Fredrick M. Wigley, Professor of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD
Francesco Boin, Assistant Professor of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD
References
- 1.Boin F, Wigley FM. Clinical Features and Treatment of Scleroderma. In: Firestein GS, Budd RC, Gabriel SE, McInnes IB, O'Dell JR, editors. Kelley's Textbook of Rheumatology, 9th Edition. Philadelphia, PA: Elsevier; pp. 2012pp. 1366–1403. [Google Scholar]
- 2.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66:940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masi AT, Rodnan GP, Medsger TA, et al. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 4.Barnett AJ. Classification of Systemic Sclerosis (Scleroderma) In: Black CM, Myers AR, editors. Current Topics in Rheumatology: Systemic Sclerosis (Scleroderma) Vol. 1985. New York, NY: Gower Medical Publishing; p. 18. [Google Scholar]
- 5.Giordano M, Valentini G, Migliaresi S, et al. Different antibody patterns and different prognoses in patients with scleroderma with various extent of skin sclerosis. J Rheumatol. 1986;13:911–916. [PubMed] [Google Scholar]
- 6.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 7.Nishimagi E, Kawaguchi Y, Tanaka E, et al. Classification of systemic sclerosis in the Japanese population based on rapid progression of skin thickening. Mod Rheumatol. 2004;14:216–221. doi: 10.1007/s10165-004-0294-5. [DOI] [PubMed] [Google Scholar]
- 8.Barnett AJ, Miller MH, Littlejohn GO. A survival study of patients with scleroderma diagnosed over 30 years (1953-1983): the value of a simple cutaneous classification in the early stages of the disease. J Rheumatol. 1988;15:276–283. [PubMed] [Google Scholar]
- 9.Ferri C, Bernini L, Cecchetti R, et al. Cutaneous and serologic subsets of systemic sclerosis. J Rheumatol. 1991;18:1826–1832. [PubMed] [Google Scholar]
- 10.Vayssairat M, Baudot N, Abuaf N, et al. Long-term follow-up study of 164 patients with definite systemic sclerosis: classification considerations. Clin Rheumatol. 1992;11:356–363. doi: 10.1007/BF02207193. [DOI] [PubMed] [Google Scholar]
- 11.Scussel-Lonzetti L, Joyal F, Raynauld JP, et al. Predicting mortality in systemic sclerosis: analysis of a cohort of 309 French Canadian patients with emphasis on features at diagnosis as predictive factors for survival. Medicine (Baltimore) 2002;81:154–167. doi: 10.1097/00005792-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Wollheim FA. Classification of systemic sclerosis. Visions and reality Rheumatology (Oxford) 2005;44:1212–1216. doi: 10.1093/rheumatology/keh671. [DOI] [PubMed] [Google Scholar]
- 13.Johnson SR, Feldman BM, Hawker GA. Classification criteria for systemic sclerosis subsets. J Rheumatol. 2007;34:1855–1863. [PubMed] [Google Scholar]
- 14.Walker UA, Tyndall A, Czirjak L, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials And Research group database. Ann Rheum Dis. 2007;66:754–763. doi: 10.1136/ard.2006.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stupi AM, Steen VD, Owens GR, et al. Pulmonary hypertension in the CREST syndrome variant of systemic sclerosis. Arthritis Rheum. 1986;29:515–524. doi: 10.1002/art.1780290409. [DOI] [PubMed] [Google Scholar]
- 16.Ioannidis JP, Vlachoyiannopoulos PG, Haidich AB, et al. Mortality in systemic sclerosis: an international meta-analysis of individual patient data. Am J Med. 2005;118:2–10. doi: 10.1016/j.amjmed.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Poormoghim H, Lucas M, Fertig N, et al. Systemic sclerosis sine scleroderma: demographic, clinical, and serologic features and survival in forty-eight patients. Arthritis Rheum. 2000;43:444–451. doi: 10.1002/1529-0131(200002)43:2<444::AID-ANR27>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 18.Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002;81:139–153. doi: 10.1097/00005792-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Masi AT. Classification of systemic sclerosis (scleroderma): relationship of cutaneous subgroups in early disease to outcome and serologic reactivity. J Rheumatol. 1988;15:894–898. [PubMed] [Google Scholar]
- 20.Baron M, Sutton E, Hudson M, et al. The relationship of dyspnoea to function and quality of life in systemic sclerosis. Ann Rheum Dis. 2008;67:644–650. doi: 10.1136/ard.2007.075721. [DOI] [PubMed] [Google Scholar]
- 21.Steen VD, Conte C, Owens GR, et al. Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994;37:1283–1289. doi: 10.1002/art.1780370903. [DOI] [PubMed] [Google Scholar]
- 22.Morelli S, Barbieri C, Sgreccia A, et al. Relationship between cutaneous and pulmonary involvement in systemic sclerosis. J Rheumatol. 1997;24:81–85. [PubMed] [Google Scholar]
- 23.Clements P, Lachenbruch P, Siebold J, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–1285. [PubMed] [Google Scholar]
- 24.Medsger TA, Jr, Silman AJ, Steen VD, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999;26:2159–2167. [PubMed] [Google Scholar]
- 25.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knudson RJ, Kaltenborn WT, Knudson DE, et al. The single-breath carbon monoxide diffusing capacity. Reference equations derived from a healthy nonsmoking population and effects of hematocrit Am Rev Respir Dis. 1987;135:805–811. doi: 10.1164/arrd.1987.135.4.805. [DOI] [PubMed] [Google Scholar]
- 27.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 28.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 29.Mukerjee D, St George D, Knight C, et al. Echocardiography and pulmonary function as screening tests for pulmonary arterial hypertension in systemic sclerosis. Rheumatology (Oxford) 2004;43:461–466. doi: 10.1093/rheumatology/keh067. [DOI] [PubMed] [Google Scholar]
- 30.Maricq HR, Valter I. A working classification of scleroderma spectrum disorders: a proposal and the results of testing on a sample of patients. Clin Exp Rheumatol. 2004;22:S5–13. [PubMed] [Google Scholar]
- 31.Nadashkevich O, Davis P, Fritzler MJ. A proposal of criteria for the classification of systemic sclerosis. Med Sci Monit. 2004;10:CR615–21. [PubMed] [Google Scholar]
- 32.Hudson M, Fritzler MJ, Baron M Canadian Scleroderma Research Group (CSRG) Systemic sclerosis: establishing diagnostic criteria. Medicine (Baltimore) 2010;89:159–165. doi: 10.1097/MD.0b013e3181dde28d. [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen S, Halberg P, Ullman S. Mortality and causes of death of 344 Danish patients with systemic sclerosis (scleroderma) Br J Rheumatol. 1998;37:750–755. doi: 10.1093/rheumatology/37.7.750. [DOI] [PubMed] [Google Scholar]
- 34.Nihtyanova SI, Tang EC, Coghlan JG, et al. Improved survival in systemic sclerosis is associated with better ascertainment of internal organ disease: a retrospective cohort study. QJM. 2010;103:109–115. doi: 10.1093/qjmed/hcp174. [DOI] [PubMed] [Google Scholar]
- 35.Gilson M, Zerkak D, Wipff J, et al. Prognostic factors for lung function in systemic sclerosis: prospective study of 105 cases. Eur Respir J. 2010;35:112–117. doi: 10.1183/09031936.00060209. [DOI] [PubMed] [Google Scholar]
- 36.Clements PJ, Roth MD, Elashoff R, et al. Scleroderma lung study (SLS): differences in the presentation and course of patients with limited versus diffuse systemic sclerosis. Ann Rheum Dis. 2007;66:1641–1647. doi: 10.1136/ard.2007.069518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kane GC, Varga J, Conant EF, et al. Lung involvement in systemic sclerosis (scleroderma): relation to classification based on extent of skin involvement or autoantibody status. Respir Med. 1996;90:223–230. doi: 10.1016/s0954-6111(96)90291-7. [DOI] [PubMed] [Google Scholar]
- 38.Morgan C, Knight C, Lunt M, et al. Predictors of end stage lung disease in a cohort of patients with scleroderma. Ann Rheum Dis. 2003;62:146–150. doi: 10.1136/ard.62.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanitsch LG, Burmester GR, Witt C, et al. Skin sclerosis is only of limited value to identify SSc patients with severe manifestations--an analysis of a distinct patient subgroup of the German Systemic Sclerosis Network (DNSS) Register. Rheumatology (Oxford) 2009;48:70–73. doi: 10.1093/rheumatology/ken408. [DOI] [PubMed] [Google Scholar]
- 40.McNearney TA, Reveille JD, Fischbach M, et al. Pulmonary involvement in systemic sclerosis: associations with genetic, serologic, sociodemographic, and behavioral factors. Arthritis Rheum. 2007;57:318–326. doi: 10.1002/art.22532. [DOI] [PubMed] [Google Scholar]
- 41.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 42.Laing TJ, Gillespie BW, Toth MB, et al. Racial differences in scleroderma among women in Michigan. Arthritis Rheum. 1997;40:734–742. doi: 10.1002/art.1780400421. [DOI] [PubMed] [Google Scholar]
- 43.Reveille JD, Fischbach M, McNearney T, et al. Systemic sclerosis in 3 US ethnic groups: a comparison of clinical, sociodemographic, serologic, and immunogenetic determinants. Semin Arthritis Rheum. 2001;30:332–346. doi: 10.1053/sarh.2001.20268. [DOI] [PubMed] [Google Scholar]
- 44.Assassi S, Sharif R, Lasky RE, et al. Predictors of interstitial lung disease in early systemic sclerosis: a prospective longitudinal study of the GENISOS cohort. Arthritis Res Ther. 2010;12:R166. doi: 10.1186/ar3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plastiras SC, Karadimitrakis SP, Ziakas PD, et al. Scleroderma lung: initial forced vital capacity as predictor of pulmonary function decline. Arthritis Rheum. 2006;55:598–602. doi: 10.1002/art.22099. [DOI] [PubMed] [Google Scholar]
- 46.Hasegawa M, Asano Y, Endo H, et al. Investigation of prognostic factors for skin sclerosis and lung function in Japanese patients with early systemic sclerosis: a multicentre prospective observational study. Rheumatology (Oxford) 2012;51:129–133. doi: 10.1093/rheumatology/ker333. [DOI] [PubMed] [Google Scholar]
- 47.Graf SW, Hakendorf P, Lester S, et al. South Australian Scleroderma Register: autoantibodies as predictive biomarkers of phenotype and outcome. Int J Rheum Dis. 2012;15:102–109. doi: 10.1111/j.1756-185X.2011.01688.x. [DOI] [PubMed] [Google Scholar]
- 48.Nihtyanova SI, Denton CP. Autoantibodies as predictive tools in systemic sclerosis. Nat Rev Rheumatol. 2010;6:112–116. doi: 10.1038/nrrheum.2009.238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.