Abstract
The role of intercellular communication is increasingly recognized as being critical to tumoral invasion, metastasis, and development of resistance to therapy. The recent discovery of cellular protrusions – tumour microtubes – connecting cancer cells in gliomas, and tunneling nanotubes in several other forms of cancer, sheds light on a novel mechanism for molecular networking. Interrupting and disrupting vital lines of intercellular cross-talk via these membranous cellular tubes has strong potential as a novel form of cancer-directed therapy.
Keywords: tumour microtubes, brain tumor networks, tunneling nanotubes, intercellular communication, glioblastoma, cellular protrusions
Interactions within the tumor microenvironment and intratumoral heterogeneity play major roles in tumor evasion and chemotherapy resistance. Intercellular communication is a critical but underappreciated cellular mechanism of these processes [1]. For many years, there had been a major gap in knowledge regarding how cells communicate in the complex and dense heterogeneous tumor matrix in invasive and difficult-to-treat cancers. This gap has narrowed with recent discoveries of the function of nano-sized exosomes and microvesicles and their role in preparation of the distant tumor niche for cultivation of micrometastases [2]. Discovery of the function of exosomes has cast light on distant tumor cell and tumor-stromal interactions. This is especially critical considering the fact that stromal, non-malignant cells may comprise as much as 80–90% of a given tumor’s volume; in fact, higher proportion of stroma has been associated with a worse prognosis in multiple forms of invasive highly recurrent forms of malignancy. By logical extension, the malignant cells that compose 10–20% or more of the tumor may not be in close enough proximity to exchange cellular information via gap junctions, which are composed of transmembrane connexin proteins. Recognition of tumor-stromal cross-talk and its critical role in tumor development has led to calls for targeting these interactions therapeutically in many cancers, including glioblastomas [3].
The gap in knowledge regarding this distant cell communication has narrowed even further with the recent report by Osswald et al. of intercellular networks formed by brain tumor cells to facilitate cellular invasion and communication in vivo [4]. In this elegant study, researchers made extensive use of intravital imaging with confocal microscopy to elucidate the structure, function and mechanism of long cellular protrusions coined “tumour microtubes.” These unique structures are cytoplasmic F-actin based extensions of astrocytes and oligodendroglioma cells that act as conduits for intercellular transfer of calcium signals, proteins, and – quite remarkably – even of nuclei. These extensions measured as much as 500 μm in length and significantly more than 1 μm in width [4] (Figure 1, panel A). The study brings to the forefront the relatively new field of cellular protrusions as conduits for effective and highly efficient intercellular communication. As the authors correctly state, variants of these structures exist and have been referred to variably in the literature as tunneling nanotubes (TNTs), membrane nanotubes, cytonemes (in Drosophila models), and others [5–8]. A notable difference in the previously reported studies was width of the tubes – often significantly less than 1 μm whereas tumour microtubes in gliomas have notably larger width and are also longer. Many of the microtubes reported in brain tumors were as long as 500 μm in length [4]. Most studies in this field had focused on function and characterizing nano-sized tubes (nanotubes) in non-cancerous cells. In recent years, more studies have examined their potential role in cancer pathophysiology, including demonstration, from our group and others, of nanotube connections in tumors from human patients and animal models [5,9], their ability to transfer mediators of drug resistance [6], and even to modulate the bone marrow tumor microenvironment [7].
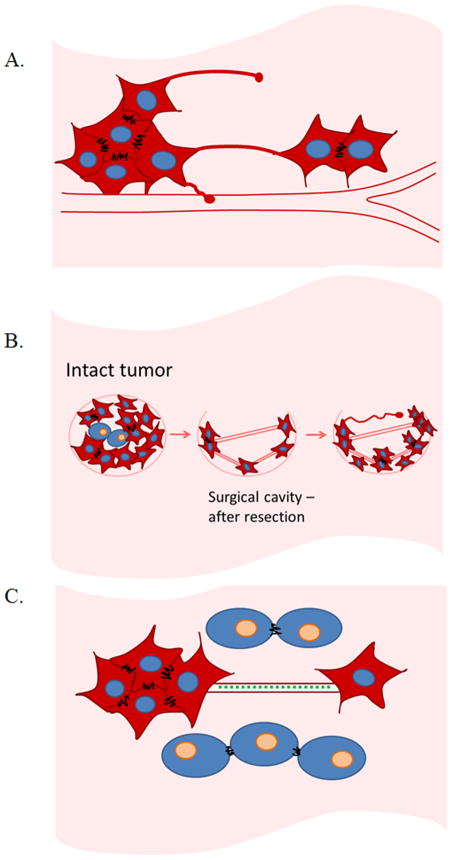
Figure 1. Schematic representations of tumour microtubes/tunneling nanotubes connecting cancer cells, and their potential roles in facilitating tumor cell communication, organization for recurrence and invasion, and also resistance to therapeutic modalities.
A. Tumour microtubes and tunneling nanotubes are F-actin based cellular protrusions extending as much as several hundred microns to form direct connections between cells. These connections facilitate intercellular transport of cargo and signals such as calcium flux, proteins, and mitochondria.
B. Hypothetical representation of tumour microtube/tunneling nanotube formation following surgical resection of invasive malignancies. Intact tumors contain densely packed malignant cells in the context of stroma-filled tumor microenvironment. Surgical resection of tumor leaves behind malignant cells, especially in cancers like glioblastoma or pancreatic cancer and others with poor prognostic features identified on histopathology or by molecular stratification. Residual tumor cells form microtube or nanotube connections to facilitate synchronization and re-organization to re-form a functional unit that manifests as a recurrent malignant tumor.
C. Tumour microtubes/tunneling nanotubes form long-range connections between distant malignant cells in the tumor matrix. Factors expressed as a form of molecular stress response to radiation or drug therapy may be propagated from cell-to-cell via these conduits to induce resistance via this novel form of intercellular transportation.
Osswald et al. provide the first evidence of intercellular tube physiology as well as function in an in vivo tumor model, and further correlate their activity to established prognostic features of malignant brain tumors, including deletions of portions of chromosomes 1 and 19 in oligodendrogliomas [4]. A relevant and translational approach taken by this group included examination of data gleaned from The Cancer Genome Atlas (TCGA) dataset (http://cancergenome.nih.gov/) to identify dysregulated cellular structures or components potentially relevant to cell invasion and motility and associated with poor prognosis in patients. This interrogation of existing datasets identified the role of connexin 43 in tumour microtube formation and activity, providing further evidence that elements of cell-cell communication are not mutually exclusive but rather quite complementary. This is in fact consistent with previous studies demonstrating synergistic effects of exosomes on tunneling nanotubes in cancer [10].
The concept of intercellular networks facilitating synchronization and reactive response for mediating resistance to radiotherapy – and also potentially to chemotherapy – is highly significant. This is especially true for difficult-to-treat cancers such as glioblastoma, a highly invasive brain cancer for which effective treatment remains a difficult puzzle to solve and prognosis generally remains poor. More recent studies have correlated connexin communication channels (e.g. connexin 43) with worse prognostic outcome, and negative association with efficacy of temozolomide, an alkylating agent in common use for treatment of high-grade gliomas. Identification of tumour microtubes in gliomas may provide a missing link to ‘connect the dots’, so to speak, between modes of cellular communication among nearby and distant cells, both malignant and stromal. Otherwise stated, we should no longer look to examine gap junctions alone, or exosomes, or microtubes/nanotubes, as they may all interact seamlessly to facilitate progression of tumours. This is especially likely considering gap junctions have been found to be localized at the junction of some tunneling nanotubes connecting cells [8], as well as the fact that exosomes and microvesicles can take advantage of nanotube structures as highways for intercellular transport [10].
As cellular communication is vital to tumor formation, progression and recurrence, it is important to comprehensively characterize various mechanisms of interactions at the cellular level. If these interactions are critical to tumor ‘molecular networking’ and coordination of players in the tumor matrix, then disrupting or ‘cutting off’ lines of communication would represent an important and perhaps underdeveloped strategy for selective and more effective therapies. To provide just one clinical scenario, therapeutically treating these cells in the peri-operative setting in order to prevent microtube or nanotube formation presents a feasible and rational translational application of this work (Figure 2, panel B). Furthermore, if TNTs mediate intercellular transfer of chemoresistant factors and/or invasion, disrupting their formation and maintenance via pharmacologic or other methods would also represent a novel approach to treatment (Figure 3, panel C). The finding of microtubes/nanotubes is not limited to any specific cancer type – and in fact not just to cancer at all – but as evidence does support their upregulation in cancer, this opens doors to a new strategic approach for cancer therapy. Further studies will require more in-depth investigation regarding function and mechanism of these conduits, and determination of common versus variable properties between different cancers. The work by Osswald et al. is a major step forward in understanding how brain tumors communicate shared signals and form a functionally invasive unit. Even more broadly for the field of cellular oncology, this work also provides supportive evidence of intercellular tumour microtubes and nanotubes as a viable target for cancer therapy. Moving forward, we are at the intersection of cellular and molecular oncology with clinically relevant genomics and implicated prognostic factors. Pharmacologic approaches targeting these cellular structures should be considered a new strategic approach for rational design of future clinical trials in cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ruckert F, Grutzmann R, Pilarsky C. Feedback within the inter-cellular communication and tumorigenesis in carcinomas. PLoS One. 2012;7:e36719. doi: 10.1371/journal.pone.0036719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie Q, Mittal S, Berens ME. Targeting adaptive glioblastoma: an overview of proliferation and invasion. Neuro Oncol. 2014;16:1575–1584. doi: 10.1093/neuonc/nou147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015 doi: 10.1038/nature16071. [DOI] [PubMed] [Google Scholar]
- 5.Lou E, Fujisawa S, Morozov A, Barlas A, Romin Y, et al. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PLoS One. 2012;7:e33093. doi: 10.1371/journal.pone.0033093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasquier J, Guerrouahen BS, Al Thawadi H, Ghiabi P, Maleki M, et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med. 2013;11:94. doi: 10.1186/1479-5876-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polak R, de Rooij B, Pieters R, den Boer ML. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood. 2015;126:2404–2414. doi: 10.1182/blood-2015-03-634238. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Veruki ML, Bukoreshtliev NV, Hartveit E, Gerdes HH. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc Natl Acad Sci U S A. 2010;107:17194–17199. doi: 10.1073/pnas.1006785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thayanithy V, Dickson EL, Steer C, Subramanian S, Lou E. Tumor-stromal cross talk: direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Transl Res. 2014 doi: 10.1016/j.trsl.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thayanithy V, Babatunde V, Dickson EL, Wong P, Oh S, et al. Tumor exosomes induce tunneling nanotubes in lipid raft-enriched regions of human mesothelioma cells. Exp Cell Res. 2014;323:178–188. doi: 10.1016/j.yexcr.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]