Abstract
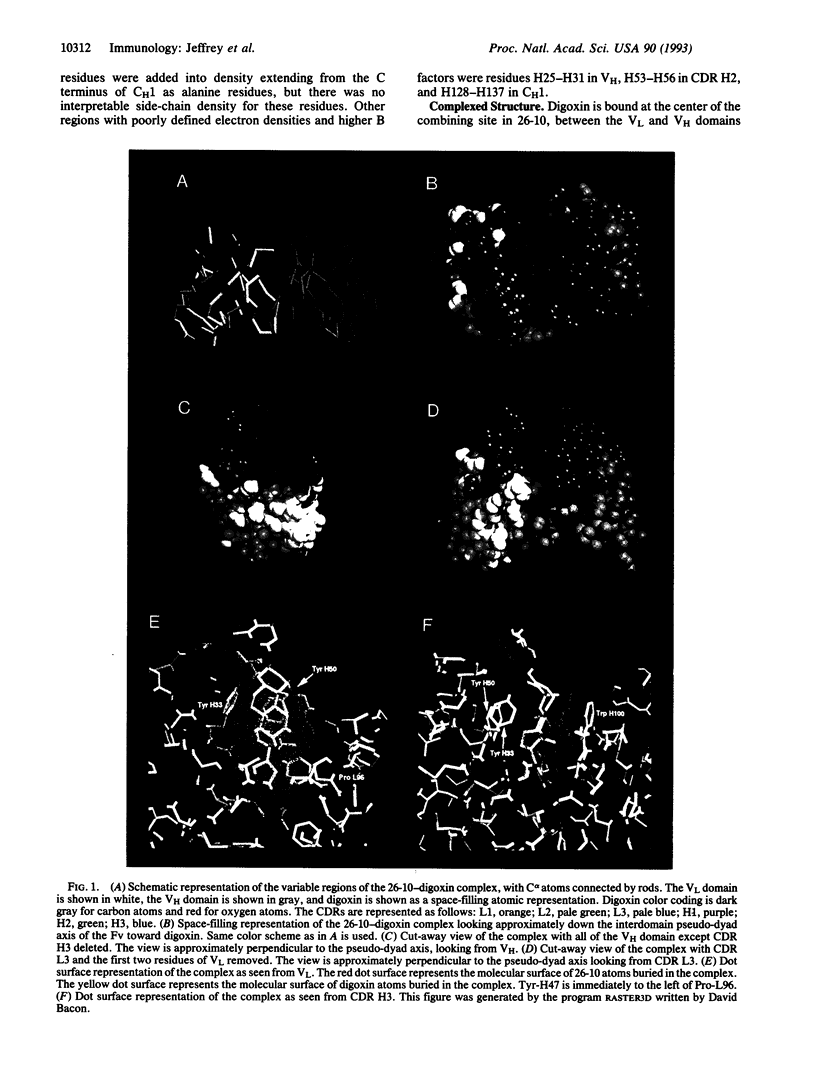
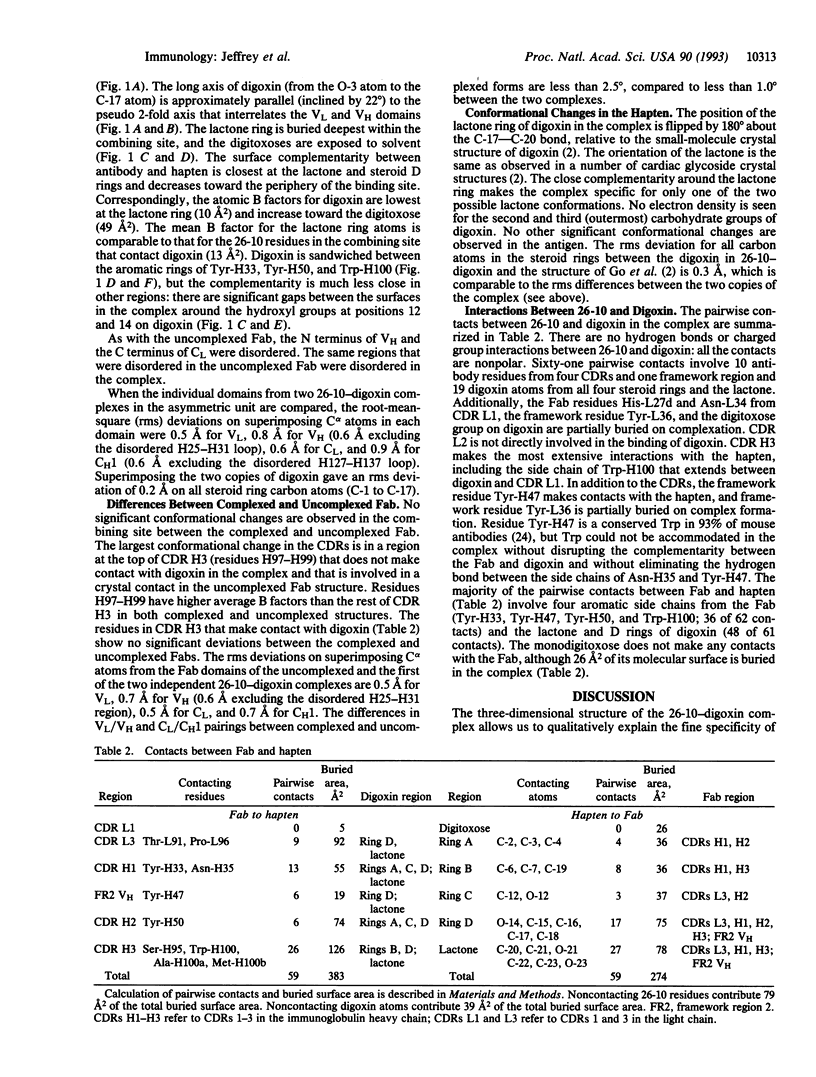
We have determined the three-dimensional structures of the antigen-binding fragment of the anti-digoxin monoclonal antibody 26-10 in the uncomplexed state at 2.7 A resolution and as a complex with digoxin at 2.5 A resolution. Neither the antibody nor digoxin undergoes any significant conformational changes upon forming the complex. Digoxin interacts primarily with the antibody heavy chain and is oriented such that the carbohydrate groups are exposed to solvent and the lactone ring is buried in a deep pocket at the bottom of the combining site. Despite extensive interactions between antibody and antigen, no hydrogen bonds or salt links are formed between 26-10 and digoxin. Thus the 26-10-digoxin complex is unique among the known three-dimensional structures of antibody-antigen complexes in that specificity and high affinity arise primarily from shape complementarity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Brünger A. T. Solution of a Fab (26-10)/digoxin complex by generalized molecular replacement. Acta Crystallogr A. 1991 May 1;47(Pt 3):195–204. doi: 10.1107/s0108767390011795. [DOI] [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Side-chain torsional potentials: effect of dipeptide, protein, and solvent environment. Biochemistry. 1979 Apr 3;18(7):1256–1268. doi: 10.1021/bi00574a022. [DOI] [PubMed] [Google Scholar]
- Hunter M. M., Margolies M. N., Ju A., Haber E. High-affinity monoclonal antibodies to the cardiac glycoside, digoxin. J Immunol. 1982 Sep;129(3):1165–1172. [PubMed] [Google Scholar]
- Lechat P., Mudgett-Hunter M., Margolies M. N., Haber E., Smith T. W. Reversal of lethal digoxin toxicity in guinea pigs using monoclonal antibodies and Fab fragments. J Pharmacol Exp Ther. 1984 Apr;229(1):210–213. [PubMed] [Google Scholar]
- Mudgett-Hunter M., Anderson W., Haber E., Margolies M. N. Binding and structural diversity among high-affinity monoclonal anti-digoxin antibodies. Mol Immunol. 1985 Apr;22(4):477–488. doi: 10.1016/0161-5890(85)90132-4. [DOI] [PubMed] [Google Scholar]
- Rose D. R., Seaton B. A., Petsko G. A., Novotný J., Margolies M. N., Locke E., Haber E. Crystallization of the Fab fragment of a monoclonal anti-digoxin antibody and its complex with digoxin. J Mol Biol. 1983 Mar 25;165(1):203–206. doi: 10.1016/s0022-2836(83)80252-6. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Potter M., Segal D. M., Padlan E. A., Davies D. R. Crystals of phosphorylcholine-binding Fab-fragments from mouse myeloma proteins: preparation and x-ray analysis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3689–3692. doi: 10.1073/pnas.69.12.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildbach J. F., Panka D. J., Parks D. R., Jager G. C., Novotny J., Herzenberg L. A., Mudgett-Hunter M., Bruccoleri R. E., Haber E., Margolies M. N. Altered hapten recognition by two anti-digoxin hybridoma variants due to variable region point mutations. J Biol Chem. 1991 Mar 5;266(7):4640–4647. [PubMed] [Google Scholar]
- Schillbach J. F., Near R. I., Bruccoleri R. E., Haber E., Jeffrey P. D., Novotny J., Sheriff S., Margolies M. N. Modulation of antibody affinity by a non-contact residue. Protein Sci. 1993 Feb;2(2):206–214. doi: 10.1002/pro.5560020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff S., Hendrickson W. A., Smith J. L. Structure of myohemerythrin in the azidomet state at 1.7/1.3 A resolution. J Mol Biol. 1987 Sep 20;197(2):273–296. doi: 10.1016/0022-2836(87)90124-0. [DOI] [PubMed] [Google Scholar]
- Smith T. W. Digitalis. Mechanisms of action and clinical use. N Engl J Med. 1988 Feb 11;318(6):358–365. doi: 10.1056/NEJM198802113180606. [DOI] [PubMed] [Google Scholar]
- Smith T. W., Haber E., Yeatman L., Butler V. P., Jr Reversal of advanced digoxin intoxication with Fab fragments of digoxin-specific antibodies. N Engl J Med. 1976 Apr 8;294(15):797–800. doi: 10.1056/NEJM197604082941501. [DOI] [PubMed] [Google Scholar]