Significance
Clinical hematologists have long known that antiinflammatory glucocorticoids such as dexamethasone and blood pressure-supporting catecholamines such as epinephrine cause leukocytes to demarginate from the vascular wall and microvasculature into the main circulation, significantly elevating the effective white blood cell count. Canonically, this has been attributed to down-regulation of adhesion molecules such as selectins, but we show that a purely mechanical phenomenon caused by leukocyte softening plays a major role as well. Our work provides an answer to an old hematological problem and reveals a mechanism in which the immune system simply alters leukocyte stiffness to regulate leukocyte trafficking. This has clinically relevant implications for the inflammatory process overall as well as for hematopoietic stem cell mobilization and homing.
Keywords: cellular mechanics, leukocyte deformability, demargination, microfluidics, atomic force microscopy
Abstract
Leukocytes normally marginate toward the vascular wall in large vessels and within the microvasculature. Reversal of this process, leukocyte demargination, leads to substantial increases in the clinical white blood cell and granulocyte count and is a well-documented effect of glucocorticoid and catecholamine hormones, although the underlying mechanisms remain unclear. Here we show that alterations in granulocyte mechanical properties are the driving force behind glucocorticoid- and catecholamine-induced demargination. First, we found that the proportions of granulocytes from healthy human subjects that traversed and demarginated from microfluidic models of capillary beds and veins, respectively, increased after the subjects ingested glucocorticoids. Also, we show that glucocorticoid and catecholamine exposure reorganizes cellular cortical actin, significantly reducing granulocyte stiffness, as measured with atomic force microscopy. Furthermore, using simple kinetic theory computational modeling, we found that this reduction in stiffness alone is sufficient to cause granulocyte demargination. Taken together, our findings reveal a biomechanical answer to an old hematologic question regarding how glucocorticoids and catecholamines cause leukocyte demargination. In addition, in a broader sense, we have discovered a temporally and energetically efficient mechanism in which the innate immune system can simply alter leukocyte stiffness to fine tune margination/demargination and therefore leukocyte trafficking in general. These observations have broad clinically relevant implications for the inflammatory process overall as well as hematopoietic stem cell mobilization and homing.
Leukocyte margination within the microvasculature and in larger blood vessels is an integral part of the inflammatory process and innate immune system (1, 2). This margination phenomenon is twofold, involving sequestration of leukocytes in the capillary bed (3, 4) as well as movement of leukocytes toward the blood vessel wall (Fig. 1A) (5, 6). Recent experimental and computational data, including our own, indicate that the mechanical properties of leukocytes play a major role in margination and are sufficient to drive leukocytes in whole blood toward the vessel wall (7–12).
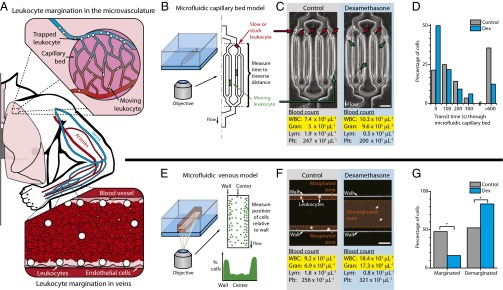
Fig. 1.
A reductionist microfluidic approach to investigate whether alterations in the mechanical properties of leukocytes can cause demargination in the microvasculature and larger blood vessels. (A) Under homeostatic conditions, leukocytes marginate via two main mechanisms: sequestration within the microvascular capillary beds and movement toward the vessel wall in veins. (B) Transit times of leukocytes through a microfluidic capillary bed model were measured to determine the degree of margination within the microvasculature. (C) CBC performed on blood samples obtained from one healthy human subject before and after ingestion of dexamethasone (Dex) showed an expected increase in the WBC and granulocyte (Gran) counts. (D) Leukocytes isolated from the same subject after dexamethasone ingestion have shorter transit times than pretreatment control leukocytes obtained from the same subject, with less obstructed leukocytes within the microfluidic device (P < 0.05 via Mann−Whitney test). (E) Using confocal videomicroscopy and a microfluidic large veins model, the distances of leukocytes from the wall of the microchannel were measured to determine degree of margination. (F) CBCs performed on blood samples obtained from a second healthy human subject also showed the expected WBC and Gran increase after dexamethasone ingestion. (G) Leukocytes within whole-blood samples collected after dexamethasone ingestion demarginated away from the vessel wall in higher proportions compared with pretreatment controls (Ncontrol = 4,894 cells, Ndex6hour = 4,398 cells, P < 0.01 via chi-square test), which correlates to the dexamethasone-induced increases in WBC and granulocyte counts.
What is not known is whether leukocyte softening can cause the reversal of leukocyte margination, which would indicate that leukocyte stiffness may be modulated by the immune system as an additional biophysical means to mediate leukocyte trafficking. To that end, we explored whether leukocyte stiffness alterations play a role in leukocyte demargination induced by glucocorticoid and catecholamine hormones. Although this phenomenon, which causes significant increases in the white blood cell (WBC) count within the clinical complete blood count (CBC) and specifically involves the granulocyte subpopulation of leukocytes, has been well documented from a clinical perspective for decades, the underlying mechanisms remain unclear (13–15). This leukocyte demargination effect can be induced via in vivo ingestion of an exogenous glucocorticoid, such as dexamethasone, or catecholamine, such as epinephrine, both of which are used clinically as an antiinflammatory agent and a vasopressor, respectively. Canonically, glucocorticoid- and catecholamine-induced demargination is attributed to down-regulation of adhesion molecules such as L- and P-selectin (16). However, in humans, although glucocorticoid infusion is associated with decreased leukocyte L-selectin expression, this does not occur until several hours after leukocyte demargination and concurrent increase in leukocyte count has already transpired (17). Furthermore, L- and P-selectin-deficient mice exhibit no abnormalities in leukocyte margination compared with wild type, suggesting additional mechanisms are likely involved (18, 19). Finally, leukocyte margination toward the vessel wall occurs in vitro in the absence of intact endothelium, questioning the need for specific interactions between these ligands and their adhesion molecules during this process (5, 6).
Mechanistically, computational models have determined that cell−cell collisions between leukocytes and softer erythrocytes enhance leukocyte margination (7, 8, 20, 21). Computational research from our own group and others indicate that modulating only the cell mechanical properties, such as stiffness, alters these physical interactions and thus changes in margination, but this has not been validated experimentally (9–12, 20–22). Furthermore, decreases in cell stiffness may also reduce leukocyte sequestration within the capillary bed, as softer cells could deform to release into the circulation. Here we demonstrate that granulocyte softening is the driving force behind glucorticoid- and catecholamine-induced demargination, including leukocyte release both out of the capillary bed and away from the vascular wall of larger vessels, and provides a cellular mechanical mechanism in which the mechanical properties of leukocytes directly contribute to increases in the WBC and granulocyte counts observed clinically. In addition, our findings reveal a biophysical answer to an old hematologic question regarding how glucocorticoids and catecholamines cause leukocyte demargination.
Results
Dexamethasone Ingestion in Human Subjects Leads to Increased Clinical Leukocyte Counts and Ex Vivo Leukocyte Demargination.
Determination of whether physical mechanisms mediate leukocyte demargination requires an experimental system that enables tight control of biofluidic parameters but is devoid of the confounding factors found in vivo such as adhesive endothelial interactions, inflammatory mediators, bone marrow leukocyte release, and channel geometry changes. Accordingly, we used a reductionist approach and used two separate in vitro microfluidic systems. First, we used a microfluidic model of the microvasculature consisting of multiple capillary-sized channels 5. 9 ± 0.08 μm wide, which are smaller than the average leukocyte diameter of ∼7.25 μm, to investigate whether glucocorticoid ingestion leads to changes in the mechanical properties of leukocytes that, in turn, would alter the degree of microvascular leukocyte sequestration (Fig. 1B and Fig. S1) (23). Six hours after dexamethasone ingestion by a healthy human subject, which led to the expected corresponding increase in leukocyte and granulocyte counts within the CBC (Fig. 1C), we found that isolated granulocytes from the subject traversed the microfluidic channels with significantly faster transit times and with a lower proportion of cells becoming stuck within the device compared with granulocytes obtained from the same individual before glucocorticoid ingestion (Fig. 1D). Microchannels are biochemically blocked to prevent nonspecific adhesion, so changes in transit time are due only to alterations in deformability rather than adhesion molecule down-regulation. As such, the reduction in granulocyte entrapment and transit time seen is a reflection of how leukocytes within the marginated microvascular pool in vivo are released into the circulation in response to glucocorticoid ingestion, thereby increasing the clinical WBC count.
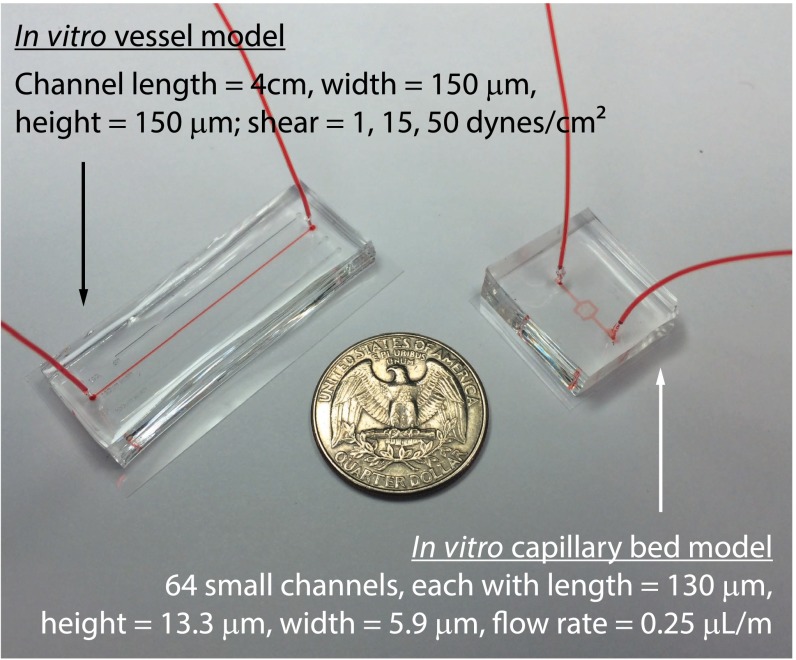
Fig. S1.
Microfluidic models to study larger vessel and capillary bed trafficking in vitro. The vessel device dimensions are optimized for this imaging, with a long center channel for fully developed flow and moderate width and height such that the central plane may still be imaged with a 20× NA 0.8 objective. The capillary bed device dimensions are optimized to capture leukocytes.
Second, we used a microfluidic venous model consisting of a long, straight channel with a cross section of 150 µm by 150 µm to investigate whether glucocorticoid-mediated changes in leukocyte mechanical properties would alter the trafficking and positioning of leukocytes within whole blood with respect to the vessel wall (Fig. 1E and Fig. S1). With this approach, all changes in leukocyte position are the direct result of glucocorticoid or catecholamine effects on leukocytes rather than changes in endothelial adhesion molecules or bone marrow release. Whole blood with fluorescently stained leukocytes was perfused into the system, and individual leukocytes were tracked using confocal videomicroscopy at physiologic venous wall shear stresses (1 dyne/cm2) (24). Six hours after dexamethasone ingestion by a healthy human subject, which again led to the expected corresponding increase in leukocyte and granulocyte count in the CBC (Fig. 1F), higher proportions of leukocytes were observed to travel away from the vessel wall toward the vessel center, the “demarginated zone” of the channel, compared with leukocytes tracked within whole blood samples obtained from the same subject before glucocorticoid ingestion (Fig. 1G). Taken together, the results from these two reductionist vascular microfluidic models show that the underlying mechanisms of how glucocorticoid ingestion in healthy human subjects increases the clinical WBC count and alters leukocyte trafficking in the microvasculature and larger vessels in a manner related to biophysical alterations of the leukocytes themselves and unrelated to the canonical explanation of adhesion molecule down-regulation.
In Vitro Treatment of Isolated Human Leukocytes with Glucocorticoids or Catecholamines Causes Demargination in Microfluidic Systems.
To remove the potential confounding effects of pharmacokinetic variability among different subjects and to confirm that the observed changes in leukocyte trafficking are the direct result of the pharmacologic agents, we performed additional in vitro experiments using the microfluidic models with healthy human granulocytes and/or whole blood exposed, in vitro, to fixed concentrations of dexamethasone and epinephrine for fixed durations of time. Similar to our ex vivo experiments, the microfluidic capillary bed transit times of isolated granulocytes from one human subject directly treated with dexamethasone or epinephrine in vitro were significantly decreased compared with untreated control granulocytes, and a smaller proportion became trapped within the device (Fig. 2 A and B). We then conducted additional microfluidic venous experiments to quantify the degree to which leukocytes tend to marginate or demarginate under physiologic flow conditions, again using a combination of confocal microscopy and a custom-written image processing code (Fig. 2C and Movie S1). Our data reveal that simply incubating whole blood with dexamethasone or epinephrine causes in vitro leukocyte demargination in our microfluidic flow experiments (Fig. 2D). This dexamethasone- or epinephrine-induced leukocyte demargination persists with increasing levels of physiologic shear stress (Fig. S2). These experiments confirm that the effects seen from our ex vivo experiments described in Fig. 1 are directly the result of drug treatment and suggest that leukocyte demargination and sequestration may involve processes related to changes in the mechanical properties of the leukocytes and are external to adhesion molecule down-regulation. In addition, we observed that glucocorticoid and catecholamine-mediated leukocyte demargination occurs in more physiologic microfluidic devices cultured with a confluent endothelial monolayer that spans the entire inner surface area of the device, providing further evidence that this demargination effect likely also occurs in vivo (Fig. S3) (25).
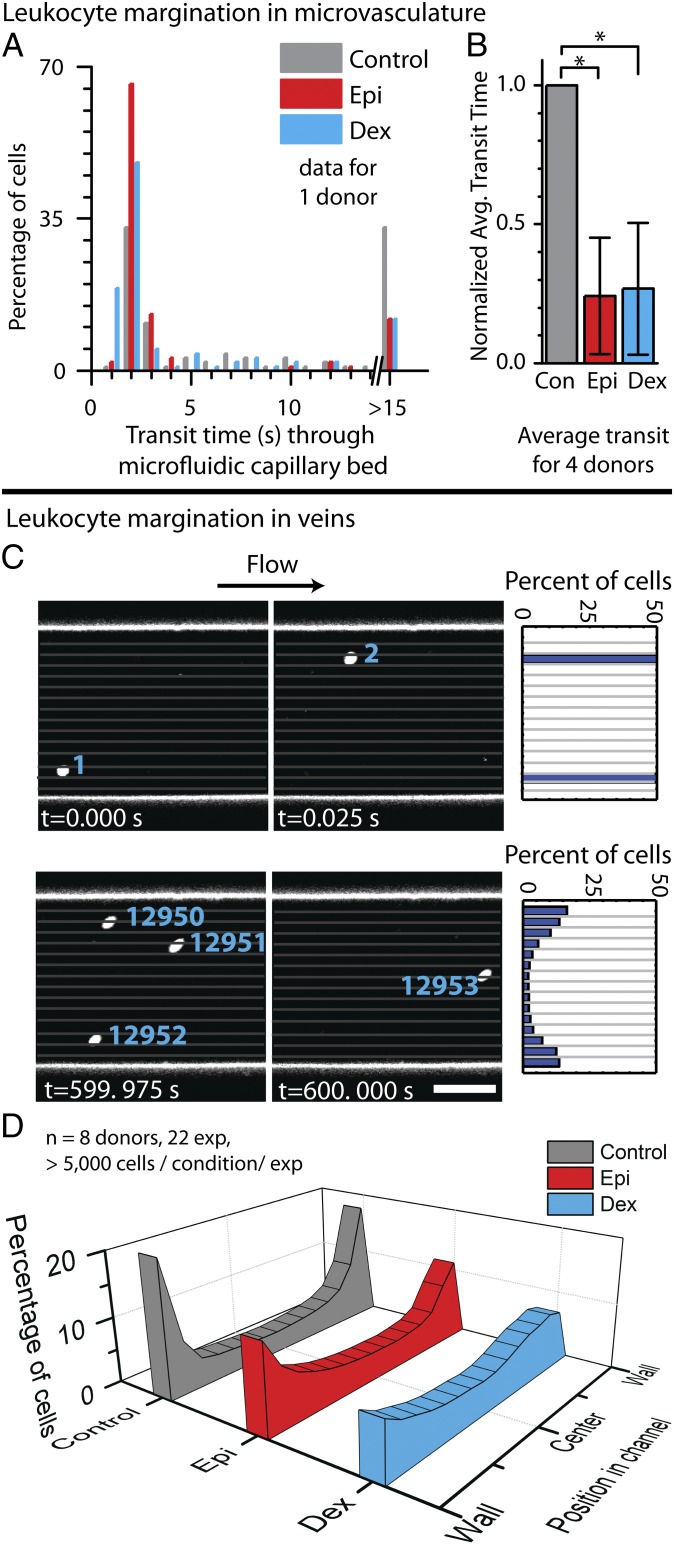
Fig. 2.
In vitro exposure to glucocorticoids or catecholamines alter granulocyte trafficking in microfluidic systems. (A) Compared with untreated controls, isolated leukocytes from one representative healthy donor treated with the glucocorticoid dexamethasone (Dex) or the catecholamine epinephrine (Epi) have shorter transit times with less propensity to cause microchannel obstruction within the microfluidic capillary bed model (P < 1 × 10−5 for each condition via Mann−Whitney test). (B) Aggregate results from four donors further show that leukocytes exposed to dexamethasone or epinephrine have shorter transit times than control leukocytes (P < 1 × 10−6 via Mann−Whitney test). (C) Leukocyte demargination within the microfluidic venous model is tracked by imaging stained leukocytes within whole blood in microfluidics via confocal videomicroscopy. The leukocyte position with respect to the microfluidic wall was calculated using a custom MATLAB imaging processing script. (D) The histogram shows leukocyte locations (perpendicular to flow) pooled from eight donors over 22 experiments, with >5,000 cells analyzed per condition per experiment at physiological venous wall shear stress of 1 dyne/cm2. Compared with untreated controls, leukocytes treated with dexamethasone strongly demarginated, and leukocytes treated with epinephrine showed a similar but less pronounced demargination.
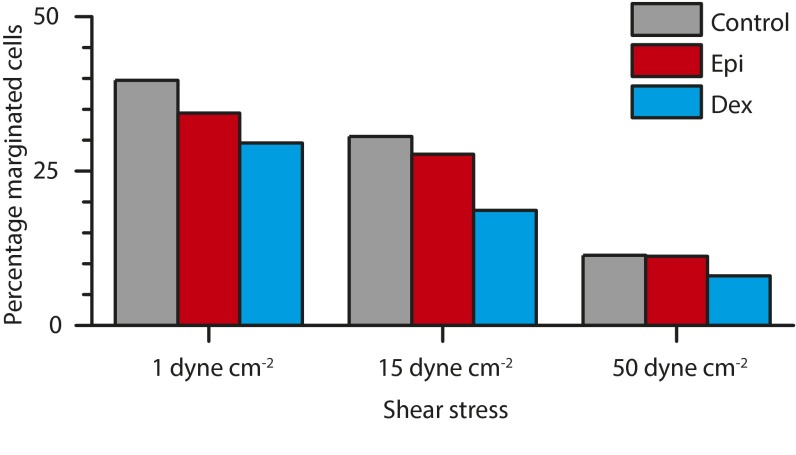
Fig. S2.
Increased shear stress increases overall demargination, but glucocorticoid- and catecholamine-mediated effects persist. Whole blood was perfused through microfluidic devices at flow rates corresponding to 1 dyne/cm2, 15 dynes/cm2, and 50 dynes/cm2. At all rates, epinephrine-treated and dexamethasone-treated leukocytes continued to demarginate compared with control conditions. For all conditions, as shear increased, the number of marginated cells increased. Each condition is statistically different (P < 0.0001) from all other conditions, with the exception of control to epinephrine at 50 dynes/cm2.
Fig. S3.
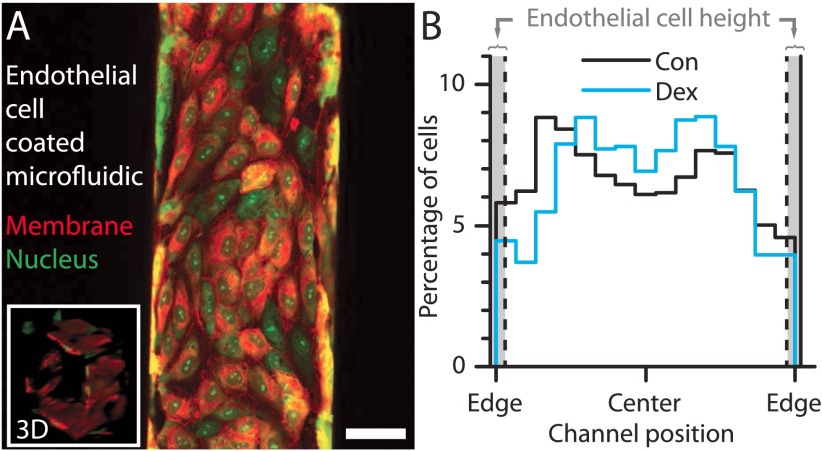
Glucocorticoid-mediated demargination occurs in endothelialized microfluidic channels. (A) Endothelial cells cultured in a 150-μm channel are aligned with the flow, and confluent as shown by Inset. (Scale bar, 50 μm.) (B) Although margination with untreated granulocytes is less pronounced in the endothelial cell-coated microfluidic, demargination with dexamethasone treatment still occurs. The endothelial cells protrude ∼5 μm into the channel, on average.
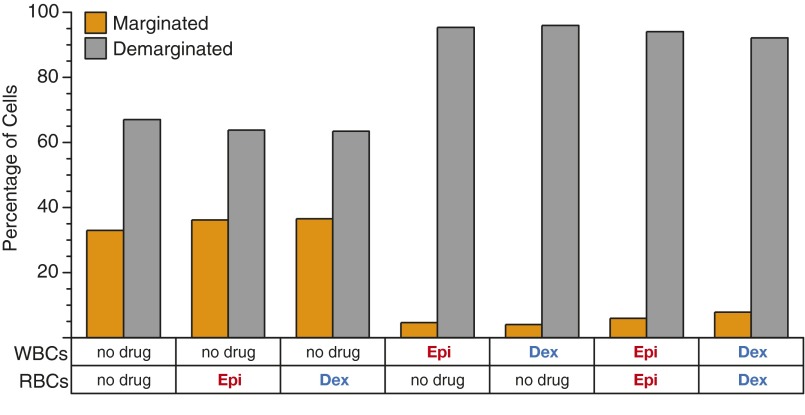
To confirm that the observed demargination phenomenon is due to dexamethasone- or epinephrine-induced changes only to leukocytes and not erythrocytes, erythrocytes or granulocytes were isolated and individually exposed to dexamethasone or epinephrine, recombined to physiologically relevant levels, and examined for margination or demargination. No significant changes in demargination were observed in mixtures of untreated granulocytes and treated erythrocytes compared with an untreated control suspension, whereas changes in demargination comparable to a treated suspension were observed in mixtures of treated granulocytes and untreated erythrocytes (Fig. S4).
Fig. S4.
Exposing only leukocytes or RBCs to glucocorticoids or catecholamines reveals that those hormonal drugs affect only leukocytes, which drive the demargination phenomenon. Suspensions of untreated leukocytes (WBCs) and erythryocytes treated with dexamethasone (Dex) or epinephrine (Epi) show no significant change in percentage of marginated cells compared with suspensions in which no cells were treated. In contrast, suspensions with treated leukocytes and untreated erythryocytes or suspensions with treated leukocytes and treated erythryocytes show no significant change in percentage of marginated cells compared with suspensions where all cells were treated (i.e., whole blood).
Glucocorticoids and Catecholamines Reduce the Mechanical Stiffness of Human Granulocytes via Cytoskeletal Remodeling.
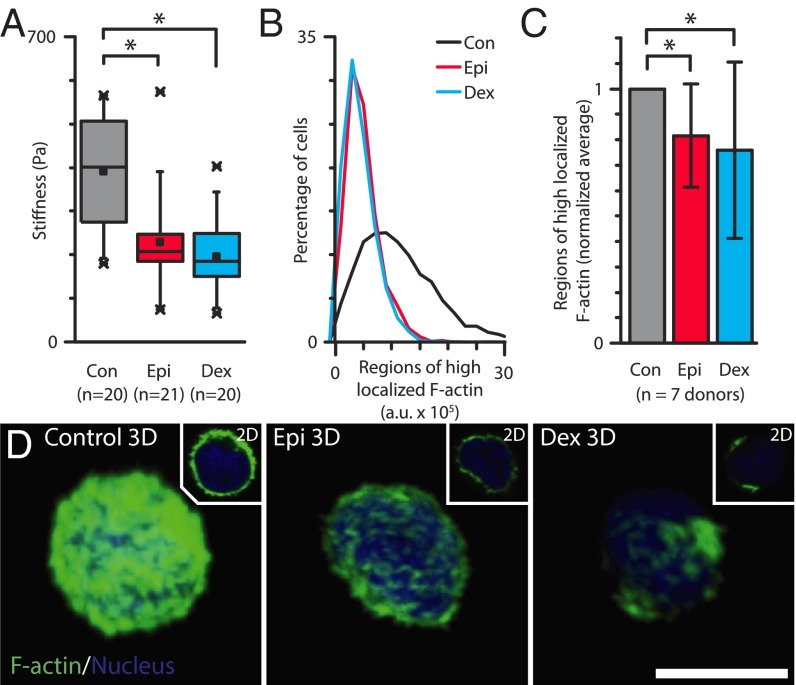
As alterations in the mechanical properties of the leukocytes would lead to the faster transit times seen in the microfluidic capillary model after drug treatment, we hypothesized that glucocorticoids and catecholamines decrease leukocyte stiffness. Atomic force microscopy (AFM) measurements (Fig. 3A) confirmed that epinephrine- and dexamethasone-treated granulocytes were significantly softer than time-controlled untreated granulocytes (P < 1 × 10−4 and 1 × 10−3, respectively). These degrees of reduction in granulocyte stiffness corresponded to the degree of reduction in transit times in the microfluidic capillary bed model and the degree of demargination observed in the microfluidic venous model for both dexamethasone and epinephrine treatment.
Fig. 3.
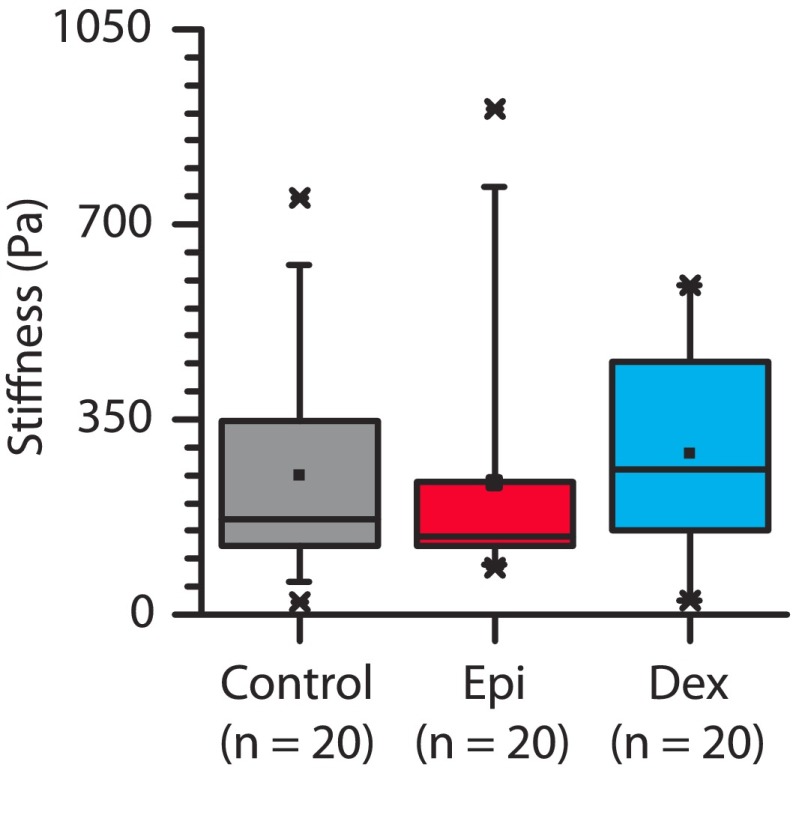
Granulocytes exposed to glucocorticoids and catecholamines are mechanically softer due to reorganization of the cortical actin cytoskeleton. (A) AFM measurements of granulocytes from a single donor exposed to physiologically relevant doses of glucocorticoids (Dex) and catecholamine (Epi) reveal a significant reduction in cellular mechanical stiffness compared with untreated controls (P < 0.0001 and 0.001, respectively.) Box plot denotes 25th and 75th percentile; “x” denotes first and 99th percentile, with central dot representing the mean value. (B) Flow cytometry analysis of phalloidin staining reveals significant reductions in localized, polymerized actin in granulocytes exposed to dexamethasone and epinephrine for one representative donor. (C) Aggregate flow cytometry results from six donors further show reduced localized actin in treated leukocytes compared with untreated leukocytes (P < 0.01 via Mann−Whitney test.) (D) Confocal microscopy images show less polymerized actin in granulocytes after exposure to epinephrine and dexamethasone. (Scale bar, 5 μm.)
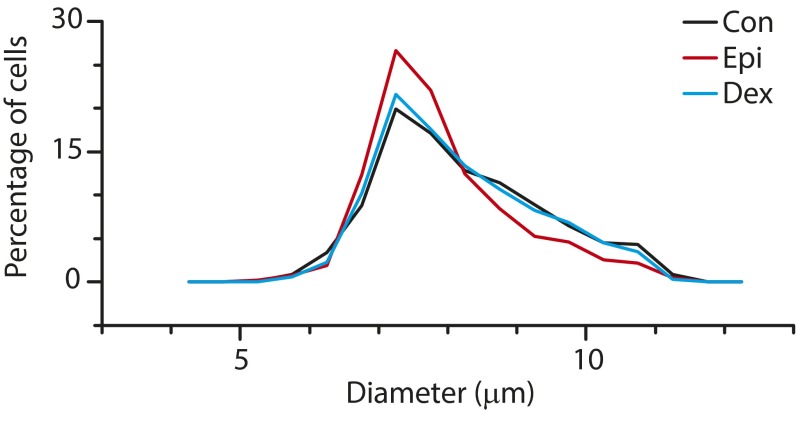
Flow cytometry (Fig. 3 B and C) and confocal microscopy (Fig. 3D) revealed that exposure to dexamethasone and epinephrine reorganize the granulocyte actin cytoskeleton, providing a mechanism for the observed reductions in cell stiffness. A bright, continuous cortical polymerized actin ring and high amounts of localized actin were observed in untreated control granulocytes. In contrast, epinephrine-treated granulocytes revealed a dimmer, but still continuous, polymerized actin ring, and a highly heterogeneous, discontinuous cortical polymerized actin ring was observed in dexamethasone-treated granulocytes (Movie S2). These differential degrees of cortical actin and localized polymerization in epinephrine- and dexamethasone-exposed granulocytes corresponded with the degrees of leukocyte demargination seen in our microfluidic experiments. In addition, no change in the granulocyte size was observed with epinephrine or dexamethasone exposure (Fig. S5). The architecture of intermediate filaments (vimentin), a key part of the leukocyte cytoskeleton, revealed little change after leukocyte exposure to dexamethasone or epinephrine (Fig. S6). Previous studies in other cell types have shown that activation of glucocorticoid receptors and adrenergic receptors (i.e., catecholamine receptors) leads to downstream rearrangements of cytoskeletal actin via multiple pathways including SGK1, ERK, RhoA/ROCK1, and cAMP-dependent protein kinase A signaling (26–31), providing candidate pathways that could provide further insight into our observed phenomenon. Of note, erythrocyte exposure to dexamethasone or epinephrine revealed no measurable changes in red cell concentration (i.e., no aggregation), size, shape, deformability, or margination (Figs. S6 and S7 and Table S1).
Fig. S5.
Glucocorticoid and catecholamine exposure does not induce significant changes in granulocyte size. Control and dexamethasone treatments are nearly overlapping, whereas there is a slight change in the size profile with epinephrine treatment.
Fig. S6.
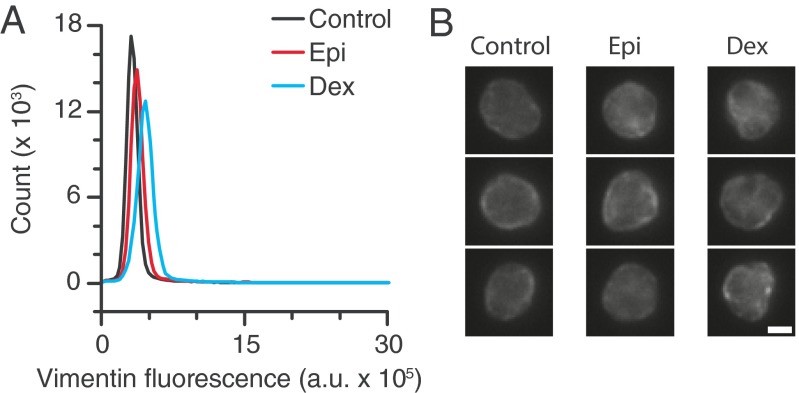
Vimentin is not affected by glucocorticoid or catecholamine treatment. (A) Isolated granulocytes were treated with dexamethasone (Dex) or epinephrine (Epi), fixed, and stained for vimentin. Flow cytometry measurements reveal little change in vimentin fluorescence intensity. (B) Vimentin fluorescent imaging shows little change in the vimentin organization and structure. (Scale bar, 3 μm.)
Fig. S7.
AFM measurements of erythrocytes exposed to physiologically relevant doses of Dex and Epi are not significantly different from untreated, control erythrocytes (P = 0.41 and P = 0.62, respectively). Box plot denotes 25th and 75th percentile; “x” denotes first and 99th percentile, with central dot representing the mean value. Compared with untreated WBCs (Fig. 3A), untreated control, epinephrine-treated, and dexamethasone-treated erythrocytes are also significantly softer (P < 0.005, P < 5 × 10−3, and P < 0.05, respectively.) Erythrocytes from single donor on same day/time.
Table S1.
Comparison of erythrocyte-related metrics from CBC analysis performed on blood samples before and after ingestion of dexamethasone by a healthy human subject
| Measurement | Individual 1 (corresponding to capillary bed experiment) | Individual 2 (corresponding to margination experiment) | ||
| Before dexamethasone | 6 h after dexamethasone | Before dexamethasone | 6 h after dexamethasone | |
| RBC count, × 106/µL | 5.81 | 5.86 | 5.95 | 5.96 |
| Mean cell volume, fL | 79.1 | 79.6 | 79.4 | 78.9 |
| Hemoglobin, g/dL | 17.6 | 17.8 | 17.9 | 18.1 |
RBC count, mean cell volume, and hemoglobin levels are not significantly affected by dexamethasone ingestion.
Mathematical Models Confirm That Leukocyte Mechanical Properties Regulate Margination or Demargination in Large Vessels.
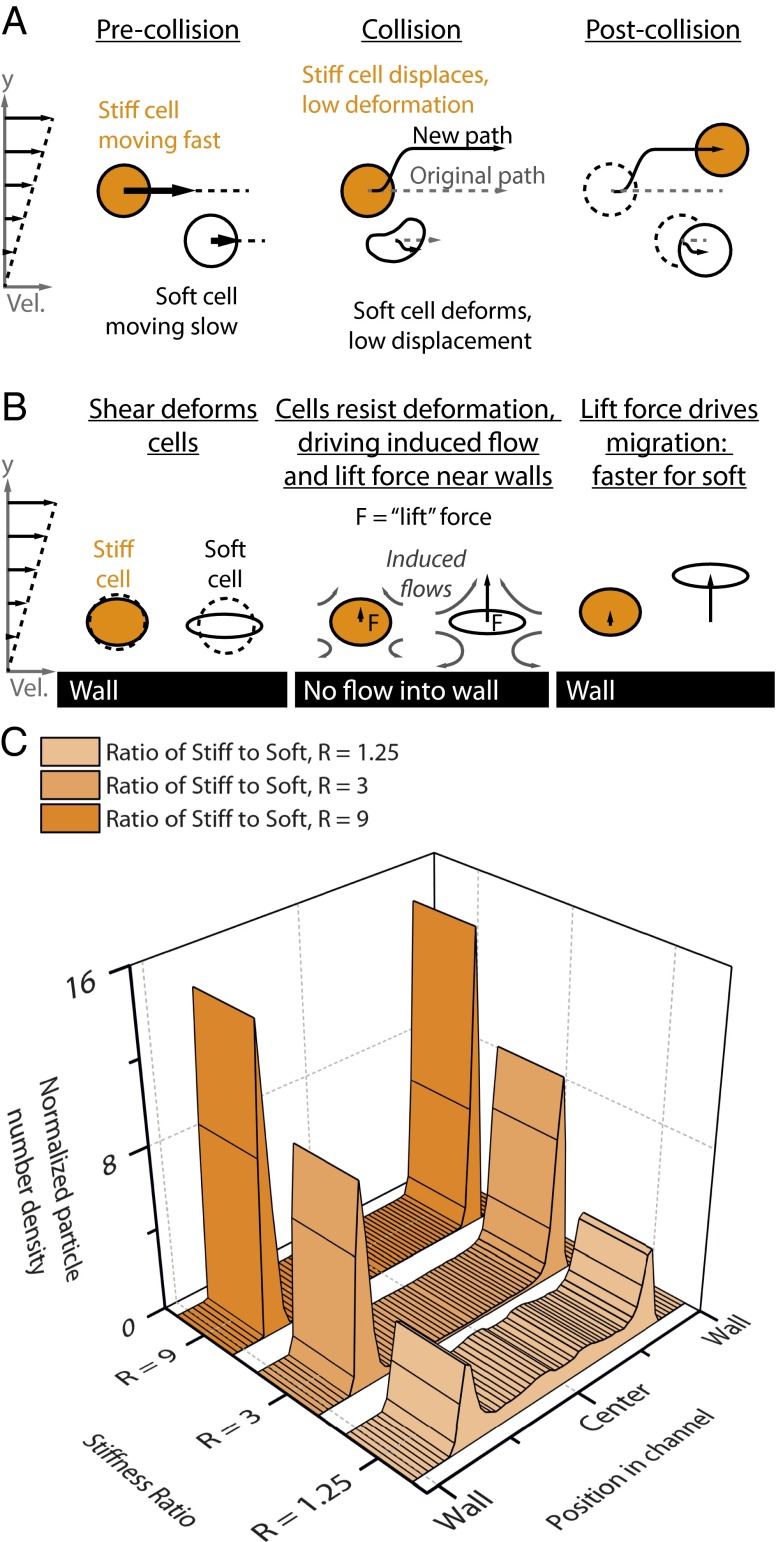
Although reduced mechanical stiffness explains fast transit through a microfluidic capillary bed, the link between cellular stiffness and demargination in larger veins is less clear. Performing a direct cell-level simulation of whole blood with leukocytes of various stiffnesses in a 150-μm channel is computationally infeasible. Nor is it necessary, because the key issue at hand is simply the role of leukocyte flexibility in margination, with all other parameters held constant. To address this issue, we observe that the primary mechanisms for cell transport relative to the vessel walls in blood are (i) collisions between cells as the flow drives them past one another (Fig. 4A) and (ii) movement away from walls due to induced secondary flow around the cells (Fig. 4B). When a stiff cell (i.e., a leukocyte) interacts with a soft cell (i.e., an erythrocyte), the soft one deforms, whereas the stiff one, which does not deform, will displace further from the site of collision (Fig. 4A) (9). Over the course of multiple collisions, stiff cells will reach the vessel wall. Due to the shear near the wall, a softer cell near the wall deforms and orients toward the flow direction more than a stiff one. This cell “pulls back” on the surrounding fluid, generating a secondary flow that drives fluid toward the wall (Fig. 4B). However, as fluid cannot pass through the wall, a lift force on the cell is generated, and, in response, it moves away from the wall. [In theoretical terms, the deformed cell can be idealized as a force dipole; the image of this dipole induced by the presence of the wall generates a flow that drives the cell away from the wall (32).] As a cell becomes softer, this effect becomes more pronounced.
Fig. 4.
Computational modeling of deformable particles reveals that modulating particle stiffness alone is sufficient to cause margination or demargination. (A) The first mediator of particle margination is cross-stream diffusion due to particle−particle collisions in which stiff particles displace whereas soft particles deform but do not displace. (B) The second mediator is a variable lift force near the wall that is dependent on particle stiffness. (C) Simulations reveal that, under typical conditions, stiff particles (leukocytes) marginate toward the vessel wall in the presence of a dominant softer component (erythrocytes). As the stiff particles (leukocytes) soften, demargination occurs, creating a more uniform distribution across the channel. Here, R is the stiffness ratio between stiff particles (leukocytes) and soft particles (erythrocytes).
We now describe two sets of results that integrate these mechanisms and illustrate the qualitative effect of leukocyte stiffness changes on margination. First, we used multiphase flow simulations and model blood as a suspension of two populations of deformable particles, one stiffer (which represents leukocytes) and one softer (which represents erythrocytes), flowing in simple shear between parallel walls. The simulations use a kinetic Monte Carlo method (10, 11). The results show that modulating the relative stiffness between leukocyte and erythrocytes determines the degree of leukocyte margination (Fig. 4C). Although these simulations do not take into account the size and shape differences between cells, due to computational complexity, the overall demargination effect as leukocyte stiffness is decreased should remain relatively unchanged (12).
Finally, these results as well as the experiments are consistent with a recent analytical theory that can be derived as a limiting case from the model used above (12). This theory shows that the tendency toward margination is controlled by a “margination parameter” M. This quantity scales as follows: M ∝ ρm − ρc, where ρm and ρc are the following ratios:
As M increases, the propensity to margination weakens. As the WBCs become softer, ρm increases and ρc decreases; both of these effects increase M, weakening margination in agreement with the experimental observations.
Discussion
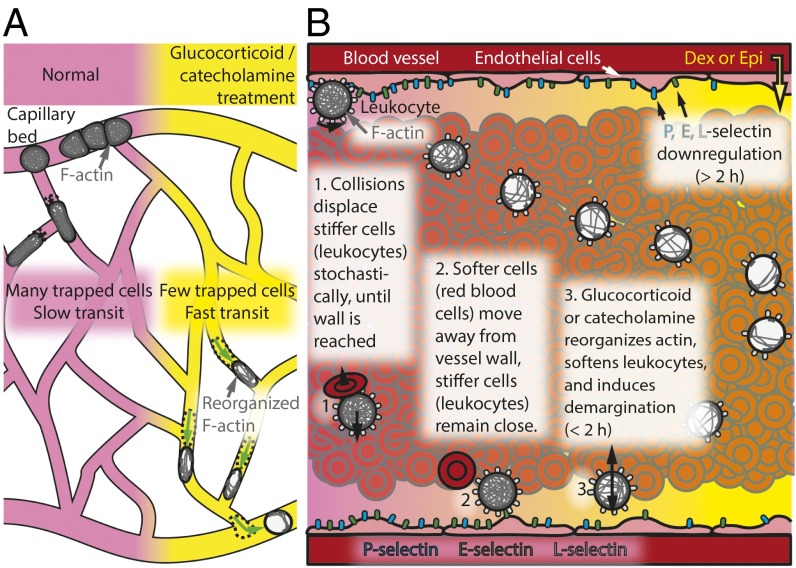
In this work, we show that, upon exposure to glucocorticoids such as dexamethasone or catecholamines such epinephrine, the leukocyte cytoskeleton remodels and softens. As a result of this decrease in cell stiffness, previously trapped or slow-moving leukocytes in the capillary bed more rapidly pass through and enter the circulation (Fig. 5A). Concurrently, marginated leukocytes in the vessels soften, experience a larger lift force, and demarginate to a new equilibrium position farther away from the wall (Fig. 5B). These two phenomena, explained by reduced cellular mechanical stiffness, increase the clinical WBC and granulocyte counts, matching our ex vivo and in vitro experimental results with decades-old clinical observations relating leukocyte demargination as a side effect of treatment with glucocorticoids and catecholamines.
Fig. 5.
Leukocyte softening causes demargination in the microvascular capillary beds and in larger blood vessels. (A) Glucocorticoids (such as dexamethasone) and catecholamines (such as epinephrine) induce actin reorganization, increasing leukocyte deformability. This leads to a decrease in the transit time through the capillary bed and reduces the number of sequestered cells. (B) Through a combination of collisions with softer erythrocytes and reduced wall demargination forces, stiffer leukocytes are normally predominantly marginated along vessel walls. Exposure to glucocorticoids or catecholamines subsequently leads to remodeling of the actin cytoskeleton, which softens the leukocytes, enabling them to dermarginate and move toward the center, creating a more uniform distribution across the vessel.
In addition, our findings not only reveal a biophysical solution to an old clinical “mystery” as to how glucocorticoids and catecholamines cause granulocyte demargination but also, in a broader sense, demonstrate that cellular mechanics principles are truly pervasive in human physiology and biomedicine. Specifically, ours is the first work, to our knowledge, to demonstrate that the mechanical properties of blood cells directly mediate the clinical CBC, the most commonly used biomarker/laboratory test in clinical medicine. Our data beg the question of whether other soluble factors, such as inflammatory mediators, would have similar effects on leukocyte margination. For example, the cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-1β are both known to stiffen leukocytes (33, 34). Our results would suggest that, for this reason alone, these cytokines would cause biophysical alterations, in addition to the known biological effects, that lead to increased margination and increased endothelial interactions, which is consistent with the proinflammatory state of TNF-α and IL-1β up-regulation. Noting the rapid time frames involved with the cellular stiffness changes we observed, our results also open up the possibility that changes in leukocyte stiffness may drive the initial steps of leukocyte recruitment during an inflammatory response that work in concert with changes in adhesion molecule expression and bone marrow release of additional leukocytes. Hence, we propose a complementary temporally and energetically efficient biophysical mechanism whereby the innate immune system can simply alter granulocyte stiffness to fine-tune margination/demargination and therefore leukocyte trafficking in general. This, in turn, would have broad clinically relevant implications for the inflammatory process overall and even hematopoietic stem cell mobilization and homing.
Methods
Sample Preparation.
Blood was drawn according to institutional review board protocols approved by Georgia Institute of Technology into sodium citrate (Beckton Dickinson). Microfluidic capillary bed, AFM cell stiffness, flow cytometry, and single-cell imaging used granulocytes isolated with negative magnetic antibody-based selection (Miltenyi Biotec) or Percoll gradient (35) (Sigma-Aldrich). Samples were incubated with physiologically relevant concentrations of 10 µM dexamethasone for 2 h (36) or 50 pM epinephrine (Sigma-Aldrich) for 15 min (37). Incubation times coincide with onset of action for the drugs to cause leukocyte demargination (14, 15).
Microfluidic Experiments.
Confocal microscopy tracked the position of acridine orange-stained leukocytes in whole blood perfused at physiological wall shear of 1 dyne/cm2 through a 150-µm-tall by 150-µm-wide microchannel imaged 3 cm from the inlet of a 4-cm channel. A custom-written MATLAB code (MathWorks) extracted individual leukocyte positions from the series of confocal images. For endothelialized experiments, devices were endothelialized as previously described (25). For the capillary bed experiments, isolated granulocytes in PBS (1–3 × 106 cells per milliliter) were perfused through a microfluidic model of a capillary bed (23) at 0.250–1 µL/min. Cells in transit were recorded using brightfield microscopy and manually calculated.
AFM Cell Stiffness Experiments.
Isolated granulocytes were attached to 0.01% poly-l-lysine (Sigma Aldrich)-treated glass AFM fluorodishes (World Precision Instruments). AFM calibration and methodology to measure cell stiffness were previously described (38).
Modeling of Soft and Stiff Cell Dynamics Within Vessels.
Margination predictions were performed using a kinetic theory (master equation) description of cell dynamics (10) during simple shear flow between parallel walls and simulated using a kinetic Monte Carlo method (10, 11, 32, 39). Around 106 collisions were simulated at a hematocrit of 12%, with 1% of particles being stiff (WBCs) and the remainder soft (RBCs). Stiffer particles were up to 9 times stiffer than soft particles, and particles were of equal size. Additional details are given in Supporting Information.
Computational Model
The predictions of margination were performed using a mathematical model developed by Kumar and coworkers (10, 11) that describes particle cell-free layer formation and margination in confined multicomponent suspensions of deformable particles. The model is formulated for simple shear flow with shear rate in an infinite slit with walls at y = 0 and y = H. Simple shear flow was chosen for three reasons: (i) to isolate the effect of shear on margination, (ii) because the simulation method used here has been shown to quantitatively reproduce large-scale simulation results in this case, and (iii) more broadly, shear and how it mediates hydrodynamic interactions between cells and walls is the dominant effect near blood vessel walls even in the case of pressure-driven flows; only near the channel center, where wall effects cancel out, does the gradient in shear rate present in pressure-driven flow play a substantial role in cell dynamics (9). This has been validated against direct computational fluid dynamics (CFD) results for binary suspensions of deformable particles. In a dilute sheared suspension, the particle interactions can be treated as a sequence of uncorrelated pair collisions (39). If the particles are deformable, they also have a wall-induced cross-stream migration velocity toward the channel center (32). The effect of the pair collisions and the wall-induced particle migration can be described by a kinetic master equation for the number density of each component α,
where δ is the precollision pair offset in the y direction, is the cross-stream displacement of particle of type α after collision with a particle of type β, and the sum is over all of the Ns species in the suspension. The first term on the right-hand side arises from the wall-induced migration, and the integral term arises from the pair collisions. Thus, for a binary suspension of flexible and stiff particles, , , and Ns = 2. See Scheme S1 for a schematic of a pair collision.
Scheme S1.
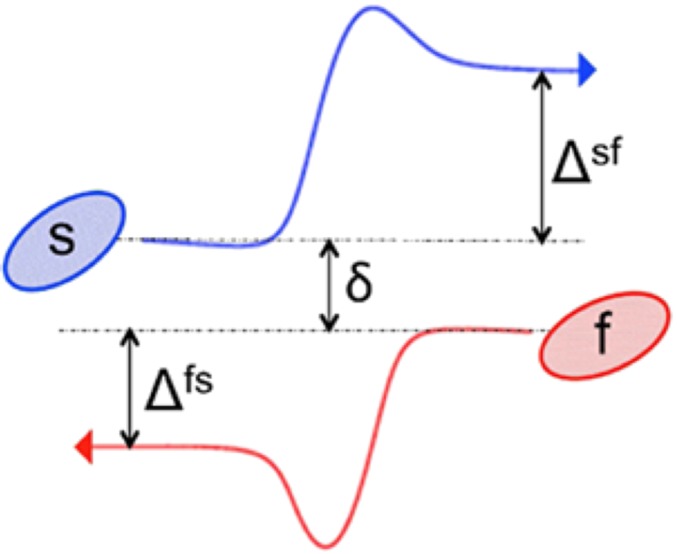
Schematic of a pair collision in simple shear flow between a stiff particle (s) and a flexible one (f). The initial particle offset in the y direction is δ, and the displacements of the stiff and flexible particles due to the collision are Δsf (δ) and Δfs (δ), respectively.
This equation is analogous to the Boltzmann equation for rarefied gases, for which the dynamic simulation Monte Carlo approach is a popular technique to obtain solutions. We developed a similar technique for obtaining steady state solutions from the master equation model, dubbing it the hydrodynamic Monte Carlo (HMC) method. Similar approaches have recently been advanced for single-component suspensions (and thus do not encompass margination) (39).
The HMC method requires as inputs the cross-stream displacements in pair collisions and the wall-induced migration velocity . These are computed by CFD simulation with the boundary integral method, with particles modeled as fluid-filled elastic capsules with spherical rest shapes. The deformability of the capsules is nondimensionally characterized by the capillary number , where μ is the fluid viscosity and a and G are the radius and interfacial elastic modulus of the capsule, respectively. With these inputs, this model was found to very closely match the cross-sectional concentration profiles found in large-scale simulations, faithfully capturing the cell free layer (CFL) and the margination of a dilute concentration of stiff particles in a suspension of mostly flexible particles.
A key observation in studying these collisions is that during “heterogeneous” collisions between different types of particles, there is substantial asymmetry in the final displacements. For size-matched particles of different stiffnesses, the stiffer particle always displaces substantially more than the more flexible one. Indeed, we found that, for simulations of binary suspensions of size-matched particles of different stiffnesses, the differences in migration velocity could be artificially removed with virtually no change in the margination behavior.
In the present work, we took a = 4 μm and G ≈ 2.5⋅10−6 N/m for the flexible particles, to approximate the properties of RBCs. We assigned , corresponding to a flow with . The stiffer particles, which approximate the properties of leukocytes, were taken to be 1% of the total number of particles and were characterized by their stiffness ratio relative to the flexible ones. The ratio of soft to stiff particles was set at 1:100 rather than a physiological ratio of 1:1,000 as this expedites the time for particles to reach steady-state positions without affecting the particle flow profiles. There was no significant difference between results using 1:1,000 or 1:100. The hematocrit was taken to be 12%, which is obviously low compared with the experiments, and was chosen because high-resolution direct simulation results were available at this hematocrit for validation of the kinetic Monte Carlo parameters. The stiffer particles were up to 9 times stiffer than the softer particles (as quantified by the ratio of surface shear moduli), to model the stiffness difference between leukocytes and erythrocytes, and, for simplicity, were of equal size with the softer particles. Our prior work indicates that the main effect of increasing hematocrit on margination is to drive the marginated particles closer to the vessel walls.
Effect of Glucocorticoid and Catecholamine Exposure on Erythrocytes
To confirm that only changes in leukocyte stiffness resulting from drug treatment were responsible for the demargination seen in experiments conducted with treated whole blood, we performed several additional experiments recombining physiological proportions of treated and untreated populations of granulocytes and erythrocytes to create a suspension at physiological hematocrit (45% vol/vol) and containing 5 × 106 granulocytes per milliliter. To confirm that the margination effect was predominantly due to changes to leukocytes, we examined margination in suspensions comprised of drug-treated granulocytes and untreated erythrocytes, whereupon we observed no significant difference in margination compared with suspensions in which all cellular components were treated. Similarly, to confirm that alterations in erythrocytes were not responsible for changes in margination, we tested suspensions of untreated granulocytes mixed with treated erythrocytes and observed no significant difference in margination compared with suspensions where no components were treated (Fig. S4). In addition, we also used AFM to measure the mechanical stiffness of erythrocytes after drug treatment and found that there was no significant change in the cell stiffness (Fig. S7). Finally, there were no detectable morphologic changes in erythrocyte shape as confirmed optically via brightfield microscopy at 40× magnification. Collectively, these data indicate that, in our experiments, glucocorticoid and/or catecholamine exposure affects only granulocytes and not red cells and the alterations in demargination due to these hormones/drugs involve only granulocytes and not erythrocytes.
We also examined our CBC data for two individuals before and after ingestion with dexamethasone and did not see a significant change in red cell count, mean cell volume, and hemoglobin content (Table S1), further supporting our conclusion that erythrocyte populations are unaffected by drug treatment and that changes in leukocyte stiffness are responsible for the demargination effect seen.
Full Description of Methods
Sample Preparation.
Blood was drawn according to IRB-approved protocols into sodium citrate (Beckton Dickinson). All microfluidic vessel experiments used whole blood. Microfluidic capillary bed and AFM cell stiffness experiments used granulocytes isolated via negative magnetic antibody-based selection with the MACSxpress neutrophil isolation kit (Miltenyi Biotec). Flow cytometry and single-cell actin imaging experiments used granulocytes isolated with a Percoll (Sigma-Aldrich) gradient (35). Samples were incubated with physiologically relevant concentrations of 10 µM dexamethasone for 2 h (36) or 50 pM epinephrine (Sigma-Aldrich) for 15 min (37). Incubation times coincide with onset of action for the drugs to cause leukocyte demargination (14, 15). For ex vivo experiments, all consenting subjects enrolled in our study obtained CBCs immediately before administration and 6 h after taking 12 mg dexamethasone (40). Whole blood for CBCs was obtained via standard venipuncture procedure and transferred into EDTA-coated tubes before CBC testing. Samples were run in triplicate on a Heska hematology analyzer (Heska). Additional blood was taken and prepared as described above.
Microfluidic Device Fabrication.
A soft lithography mold was created using SU-8 2150 (Microchem), adhering to all manufacturer’s suggestions. Polydimethylsiloxane (PDMS; Dow Corning) was poured into molds and allowed to cure overnight at 60 °C. PDMS was then bonded to #1.5 coverslips (or to thin cured PDMS for endothelialized experiments) using a plasma cleaner (Harrick Plasma).
Imaging the Microfluidic Vessel Model.
Leukocytes within whole blood were stained with 1μL/mL acridine orange [excitation (ex.) 405 nm/emission (em.) 465 nm] (Sigma-Aldrich). The blood was then flowed through the 150-µm-tall by 150-µm-wide microfluidic channel at physiologic shear stresses of 1 dynes/cm2, 15 dynes/cm2, or 50 dynes/cm2 (15) while a camera mounted atop a confocal microscope (LSM 700; Carl Zeiss) with a 20× 0.8 numerical aperture (NA) air objective was used to image the center plane of the channel continuously. The channel was 4 cm long, and imaging was performed ∼3 cm from the inlet to ensure fully developed flow. The sample frame size was set to 100 × 100 pixels. For all experiments, pixel size was set to 1.875 ± 0.2 μm, corresponding to a frame rate of ∼8.3 Hz. Lower resolution was used to accommodate both the speed and depth of focus required to accurately obtain the positions of the leukocytes in the microfluidic. The center plane of the microfluidic was imaged to ensure that video taken was representative of a radial profile. Custom MATLAB code was developed (MathWorks) to extract individual leukocyte positions from the series of confocal images and plotted histograms of their locations, tracking over 10,000 leukocytes per experiment.
Endothelializing the Microfluidic Vessel Model.
Completed microfluidic channels were coated with a 5% fibronectin (Sigma-Aldrich) solution in PBS and incubated at 37 °C for 1 h. Human umbilical vein endothelial cells (HUVECs) (Lonza) were injected into each device at a seeding density of 10,000,000 cells per milliliter and incubated at 37 °C for 1 h to allow the HUVECS to adhere to the walls of the microfluidic channels and spread. Using a syringe pump (Harvard Apparatus), EGM-2 media (Lonza) was infused at a constant rate to achieve physiological shear stress of 1 dyne/cm2 on the HUVECs. Flow was applied for 24 h in order for the HUVECs to become confluent and express normal phenotype. Seeded devices were then used in experiments.
Recombined Granulocyte and Erythrocyte Margination Experiments.
Erythrocytes were isolated by centrifuging whole blood for 5 min at 700 × g. Granulocytes were isolated using negative magnetic antibody-based selection with the MACSxpress neutrophil isolation kit (Miltenyi Biotec). Erythrocyte or granulocyte populations were treated separately with 10 µm dexamethasone for 90 min or 50 pM epinephrine for 15 min according to experimental condition, then recombined in PBS at 45% hematocrit and 5 × 106 granulocytes per milliliter. Imaging was performed as described above and in Methods, Microfluidic Experiments for microfluidic vessel experiments.
Microfluidic Capillary Bed Experiments.
Isolated granulocytes were diluted to 1 or 3 × 106 cells per milliliter in PBS (Calbiochem) and perfused through a microfluidic model of a capillary bed consisting of channels 5.9 ± 0.08 µm wide at 0.250 µL/min or 1.00 µL/min (23). Cells in transit were recorded using brightfield microscopy (Nikon TE2000-U) using a 20× 0.45A air objective, with passage time calculated manually.
AFM Stiffness Measurements of Treated Granulocytes and Erythrocyte.
AFM was used to calculate the deformability of WBCs and erythrocytes. All WBCs were measured in a suspended-like state in which the cells were loosely adhered to the glass substrate but retained a rounded morphology. Following granulocyte or erythrocyte isolation, 3 × 106 cells were removed from each sample to create a cell suspension in 3 mL Dulbecco's PBS (Sigma Aldrich). Glass AFM fluorodishes (World Precision Instruments) were treated with 0.01% poly-l-lysine (Sigma Aldrich) before the addition of the cell solution for enhanced cell plating. The monolayer of poly-l-lysine on the fluorodish enhanced the ability to take AFM measurements by lightly attaching the cells to the glass without altering morphology. Deformability measurements were collected using a molecular force probe 3D (MFP-3D) AFM (Asylum Research) attached to an inverted optical microscope (Nikon Eclipse Ti; Nikon) and a triangular, silicon nitride microlever sharpened contact (MSCT) cantilever (Bruker). The cantilever was calibrated using a thermal vibration method to determine the spring constant (0.03 N/m) and was positioned above the center of a single cell before indentation. Individual granulocytes (n = 20, n = 21, n = 20) or erythrocytes (n = 20, n = 20, n = 19) were indented 0.5 µm by the cantilever. Three force-indentation curves were generated for each measured granulocyte; the slope of each curve was then analyzed using a Hertz contact model for a pyramidal tip to obtain the cell’s Young’s Modulus.
Preparation of Stained Leukocytes and Imaging of Single-Cell Actin or Vimentin Cytoskeletal Structure.
Granulocytes were isolated and fixed with 1% paraformaldehyde (Sigma-Aldrich) solution for 15 min at room temperature. Cells were then permeabilized using Triton X-100 (Calbiochem) for 30 min at room temperature. For actin, granulocytes were stained for 30 min with Alexa Fluor 488 phalloidin (ex. 495 nm/em. 518 nm) (Life Technologies) and Hoescht 33258 (ex. 352 nm/em. 461 nm) (Life Technologies). For vimentin, granulocytes were stained with vimentin primary antibody ab8978 (AbCam) and AF488 anti-mouse secondary antibody (Life Technologies). For single-cell imaging, cells were mounted on glass slides with Fluro-gel mounting media (Electron Microscopy Sciences), covered with #1 glass coverslips, and left to dry overnight. Images were taken at room temperature on the previously described confocal microscope using a 63× 1.4NA oil objective. Measurements of bright detail intensity, a metric of high levels of localized signal, of phalloidin fluorescence of cells (n = 5,000 per treatment) were taken using an Imagestream X Mark II flow cytometer (Amnis).
Supplementary Material
Acknowledgments
The authors wish to thank Andrew Shaw of the Parker H. Petit Institute for Bioengineering and Bioscience at the Georgia Institute of Technology (GT); the Emory University Integrated Cellular Imaging Microscopy and Flow Cytometry Cores of the Children’s Pediatric Research Center; and the GT Institute for Electronics and Nanotechnology cleanroom. Financial support was provided by NIH R01 (HL121264) (to W.A.L.), an American Heart Association Postdoctoral Fellowship (to D.R.M.), and National Science Foundation Grant CBET-1436082 (to M.D.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508920113/-/DCSupplemental.
References
- 1.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res. 1996;32(4):733–742. [PubMed] [Google Scholar]
- 2.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 3.Wiggs BR, et al. Contributions of capillary pathway size and neutrophil deformability to neutrophil transit through rabbit lungs. J Appl Physiol (1985) 1994;77(1):463–470. doi: 10.1152/jappl.1994.77.1.463. [DOI] [PubMed] [Google Scholar]
- 4.Hogg JC, Doerschuk CM. Leukocyte traffic in the lung. Annu Rev Physiol. 1995;57:97–114. doi: 10.1146/annurev.ph.57.030195.000525. [DOI] [PubMed] [Google Scholar]
- 5.Goldsmith HL, Spain S. Radial distribution of white cells in tube flow. Kroc Found Ser. 1984;16:131–146. [PubMed] [Google Scholar]
- 6.Nobis U, Pries AR, Cokelet GR, Gaehtgens P. Radial distribution of white cells during blood flow in small tubes. Microvasc Res. 1985;29(3):295–304. doi: 10.1016/0026-2862(85)90020-2. [DOI] [PubMed] [Google Scholar]
- 7.Jain A, Munn LL. Determinants of leukocyte margination in rectangular microchannels. PLoS One. 2009;4(9):e7104. doi: 10.1371/journal.pone.0007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munn LL, Melder RJ, Jain RK. Role of erythrocytes in leukocyte-endothelial interactions: Mathematical model and experimental validation. Biophys J. 1996;71(1):466–478. doi: 10.1016/S0006-3495(96)79248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A, Graham MD. Segregation by membrane rigidity in flowing binary suspensions of elastic capsules. Phys Rev E Stat Nonlin Soft Matter Phys. 2011;84(6 Pt 2):066316. doi: 10.1103/PhysRevE.84.066316. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Graham MD. Mechanism of margination in confined flows of blood and other multicomponent suspensions. Phys Rev Lett. 2012;109(10):108102. doi: 10.1103/PhysRevLett.109.108102. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Henríquez Rivera RG, Graham MD. Flow-induced segregation in confined multicomponent suspensions: Effects of particle size and rigidity. J Fluid Mech. 2014;738:423–462. [Google Scholar]
- 12.Henríquez Rivera RG, Sinha K, Graham MD. Margination regimes and drainage transition in confined multicomponent suspensions. Phys Rev Lett. 2015;114(18):188101. doi: 10.1103/PhysRevLett.114.188101. [DOI] [PubMed] [Google Scholar]
- 13.Athens JW, et al. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest. 1961;40(6):989–995. doi: 10.1172/JCI104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.BIERMAN HR, et al. The release of leukocytes and platelets from the pulmonary circulation by epinephrine. Blood. 1952;7(7):683–692. [PubMed] [Google Scholar]
- 15.Nakagawa M, et al. Glucocorticoid-induced granulocytosis: Contribution of marrow release and demargination of intravascular granulocytes. Circulation. 1998;98(21):2307–2313. doi: 10.1161/01.cir.98.21.2307. [DOI] [PubMed] [Google Scholar]
- 16.Weber PS, et al. Mechanisms of glucocorticoid-induced down-regulation of neutrophil L-selectin in cattle: Evidence for effects at the gene-expression level and primarily on blood neutrophils. J Leukoc Biol. 2004;75(5):815–827. doi: 10.1189/jlb.1003505. [DOI] [PubMed] [Google Scholar]
- 17.Jilma B, et al. Dexamethasone down-regulates the expression of L-selectin on the surface of neutrophils and lymphocytes in humans. Clin Pharmacol Ther. 1997;62(5):562–568. doi: 10.1016/S0009-9236(97)90052-7. [DOI] [PubMed] [Google Scholar]
- 18.Doyle NA, et al. Neutrophil margination, sequestration, and emigration in the lungs of L-selectin-deficient mice. J Clin Invest. 1997;99(3):526–533. doi: 10.1172/JCI119189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson RC, et al. Blood cell dynamics in P-selectin-deficient mice. Blood. 1995;86(3):1106–1114. [PubMed] [Google Scholar]
- 20.Sun C, Munn LL. Influence of erythrocyte aggregation on leukocyte margination in postcapillary expansions: A lattice Boltzmann analysis. Physica A. 2006;362(1):191–196. [Google Scholar]
- 21.Sun C, Munn LL. Lattice Boltzmann simulation of blood flow in digitized vessel networks. Comput Math Appl. 2008;55(7):1594–1600. doi: 10.1016/j.camwa.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Migliorini C, et al. Red blood cells augment leukocyte rolling in a virtual blood vessel. Biophys J. 2002;83(4):1834–1841. doi: 10.1016/S0006-3495(02)73948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenbluth MJ, Lam WA, Fletcher DA. Analyzing cell mechanics in hematologic diseases with microfluidic biophysical flow cytometry. Lab Chip. 2008;8(7):1062–1070. doi: 10.1039/b802931h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fung YC. Biomechanics: Circulation. 2nd Ed Springer; New York: 1997. [Google Scholar]
- 25.Myers DR, et al. Endothelialized microfluidics for studying microvascular interactions in hematologic diseases. J Vis Exp. 2012;(64):3958. doi: 10.3791/3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark R, et al. Comparative genomic and proteomic analysis of cytoskeletal changes in dexamethasone-treated trabecular meshwork cells. Mol Cell Proteomics. 2013;12(1):194–206. doi: 10.1074/mcp.M112.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirshman CA, Zhu D, Panettieri RA, Emala CW. Actin depolymerization via the β-adrenoceptor in airway smooth muscle cells: A novel PKA-independent pathway. Am J Physiol Cell Physiol. 2001;281(5):C1468–C1476. doi: 10.1152/ajpcell.2001.281.5.C1468. [DOI] [PubMed] [Google Scholar]
- 28.Jafari M, Seese RR, Babayan AH, Gall CM, Lauterborn JC. Glucocorticoid receptors are localized to dendritic spines and influence local actin signaling. Mol Neurobiol. 2012;46(2):304–315. doi: 10.1007/s12035-012-8288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HK, et al. Beta adrenergic overstimulation impaired vascular contractility via actin-cytoskeleton disorganization in rabbit cerebral artery. PLoS One. 2012;7(8):e43884. doi: 10.1371/journal.pone.0043884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmid E, et al. Serum- and glucocorticoid-inducible kinase SGK1 regulates reorganization of actin cytoskeleton in mast cells upon degranulation. Am J Physiol Cell Physiol. 2013;304(1):C49–C55. doi: 10.1152/ajpcell.00179.2012. [DOI] [PubMed] [Google Scholar]
- 31.Rubenstein NM, Guan Y, Woo PL, Firestone GL. Glucocorticoid down-regulation of RhoA is required for the steroid-induced organization of the junctional complex and tight junction formation in rat mammary epithelial tumor cells. J Biol Chem. 2003;278(12):10353–10360. doi: 10.1074/jbc.M213121200. [DOI] [PubMed] [Google Scholar]
- 32.Smart JR, Leighton DT. Measurement of the drift of a droplet due to the presence of a plane. Phys Fluids A Fluid Dyn. 1991;3(1):21–28. [Google Scholar]
- 33.Lavkan AH, Astiz ME, Rackow EC. Effects of proinflammatory cytokines and bacterial toxins on neutrophil rheologic properties. Crit Care Med. 1998;26(10):1677–1682. doi: 10.1097/00003246-199810000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Betticher DC, Keller H, Maly FE, Reinhart WH. The effect of endotoxin and tumour necrosis factor on erythrocyte and leucocyte deformability in vitro. Br J Haematol. 1993;83(1):130–137. doi: 10.1111/j.1365-2141.1993.tb04643.x. [DOI] [PubMed] [Google Scholar]
- 35.Brinkmann V, Laube B, Abu Abed U, Goosmann C, Zychlinsky A. Neutrophil extracellular traps: how to generate and visualize them. J Vis Exp. 2010;36:1724. doi: 10.3791/1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schleimer RP, Freeland HS, Peters SP, Brown KE, Derse CP. An assessment of the effects of glucocorticoids on degranulation, chemotaxis, binding to vascular endothelium and formation of leukotriene B4 by purified human neutrophils. J Pharmacol Exp Ther. 1989;250(2):598–605. [PubMed] [Google Scholar]
- 37.Deitch EA, Bridges RM. Stress hormones modulate neutrophil and lymphocyte activity in vitro. J Trauma. 1987;27(10):1146–1154. doi: 10.1097/00005373-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Xu W, et al. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS One. 2012;7(10):e46609. doi: 10.1371/journal.pone.0046609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.daCunha FR, Hinch EJ. Shear-induced dispersion in a dilute suspension of rough spheres. J Fluid Mech. 1996;309:211–223. [Google Scholar]
- 40.Mishler JM, Emerson PM. Development of Neutrophilia by serially increasing doses of dexamethasone. Br J Haematol. 1977;36(2):249–257. doi: 10.1111/j.1365-2141.1977.tb00646.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.