Significance
Drug delivery to pancreatic tumors is impaired by a unique desmoplastic response and poor tumor vascularization. A drug delivery device capable of overcoming these barriers could provide substantial benefit for patients with pancreatic cancer. In this study, we show that local iontophoretic delivery of folinic acid (leucovorin), fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) resulted in better tumor response and tolerability compared with i.v. FOLFIRINOX. Given the low systemic exposure of FOLFIRINOX using iontophoretic delivery, it may be possible to use in combination with systemic delivery to treat micrometastatic disease. Local iontophoretic delivery of cytotoxic agents should be considered as a neoadjuvant approach to improve resection rates and outcome in patients with localized and locally advanced pancreatic cancer.
Keywords: iontophoresis, FOLFIRINOX, pancreatic cancer, device delivery, chemotherapy
Abstract
Poor delivery and systemic toxicity of many cytotoxic agents, such as the recent promising combination chemotherapy regimen of folinic acid (leucovorin), fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX), restrict their full utility in the treatment of pancreatic cancer. Local delivery of chemotherapies has become possible using iontophoretic devices that are implanted directly onto pancreatic tumors. We have fabricated implantable iontophoretic devices and tested the local iontophoretic delivery of FOLFIRINOX for the treatment of pancreatic cancer in an orthotopic patient-derived xenograft model. Iontophoretic delivery of FOLFIRINOX was found to increase tumor exposure by almost an order of magnitude compared with i.v. delivery with substantially lower plasma concentrations. Mice treated for 7 wk with device FOLFIRINOX experienced significantly greater tumor growth inhibition compared with i.v. FOLFIRINOX. A marker of cell proliferation, Ki-67, was stained, showing a significant reduction in tumor cell proliferation. These data capitalize on the unique ability of an implantable iontophoretic device to deliver much higher concentrations of drug to the tumor compared with i.v. delivery. Local iontophoretic delivery of cytotoxic agents should be considered for the treatment of patients with unresectable nonmetastatic disease and for patients with the need for palliation of local symptoms, and may be considered as a neoadjuvant approach to improve resection rates and outcome in patients with localized and locally advanced pancreatic cancer.
Pancreatic cancer is among the most lethal malignancies because of its insidious onset and resistance to therapy. The overall 5-y survival rate for this disease is less than 5%, and estimates indicate that pancreatic cancer will be second only to non-small-cell lung cancer as the leading cause of cancer-related mortality in the United States by 2030 (1–2). Surgical resection is the only curative option, with 15% of patients having resectable disease at presentation. Complete resection of all gross and microscopic disease results in a median survival time of 22–23 mo (3, 4). However, nearly 40% of patients with pancreatic cancer have locally advanced, unresectable disease with a median overall survival time of 9.2–13.5 mo (5). Although the true effect of microscopic positive margins is not fully known, patients destined to have the longest survival are those for whom resection with curative intent is feasible (6).
The efficacy of chemotherapy for pancreatic cancer is impaired by a unique desmoplastic response (7, 8). Pancreatic tumors have a dense desmoplastic stroma with fibrotic connective tissue that surrounds the tumor and may account for >80% of tumor volume (9). This leads to a microenvironment with low blood perfusion and hypoxia, serving as a barrier to diminish the delivery of anticancer drugs (10, 11). A local drug delivery device capable of overcoming this barrier could provide substantial benefit for patients with locally advanced pancreatic cancer.
We have developed an implantable iontophoretic device capable of driving chemotherapies deep into solid tumors. The principle behind iontophoretic drug delivery is the movement of charged species under an applied electric field (12, 13). Iontophoresis has been previously evaluated for oncologic purposes (13–15). An iontophoretic Foley catheter was developed to deliver mitomycin C to bladder tumors. Significant clinical success was achieved using the treatment alone and in combination with Bacillus Calmette-Guérin therapy (15). In addition, Gratieri et al. explored the iontophoretic delivery of 5-fluorouracil and leucovorin in healthy pig buccal tissue for the treatment of head and neck cancer (14).
One major benefit of this technology is the ability to deliver highly toxic agents limited by unwanted secondary effects. FOLFIRINOX, a promising mixture of cytotoxic agents including folinic acid (leucovorin), fluorouracil, irinotecan, and oxaliplatin, has limited use in many patients because of its high systemic toxicity (16, 17). Modified FOLFIRINOX regimens have been created to improve tolerability (5, 18). Given the ability of the iontophoretic device to deliver drugs locally with minimal systemic exposure, the iontophoretic delivery of FOLFIRINOX could further enhance the efficacy of this cytotoxic regimen by increasing the local drug concentration and decreasing systemic exposure. The aim of our study was to evaluate the iontophoretic delivery of FOLFIRINOX for the treatment of localized pancreatic cancer. We evaluated this therapy in xenografts derived from patients with pancreatic cancer, which have been shown to reflect recently defined RNA tumor subtypes in patients, mirror patient outcome, and be highly predictive of clinical response to many targeted agents (19, 20). We report the delivery of high levels of the FOLFIRINOX drugs to the tumor, a reduction in systemic exposure of the drugs, and potent tumor regression. This therapy has the potential to improve the resection rates and the outcome for patients with pancreatic cancer.
Results
Implantable Iontophoretic Device.
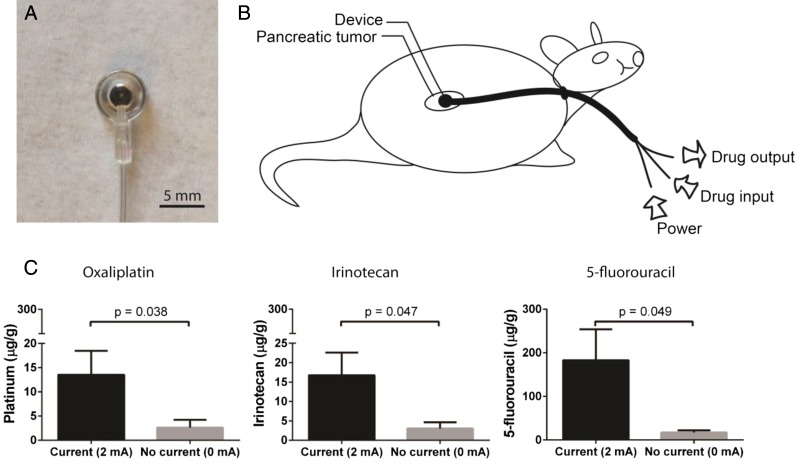
The device was designed for intra-abdominal implantation with external access for power and drug supply (Fig. 1 A and B). A platinum electrode was encased in a polyurethane reservoir covered by a semipermeable membrane, and a multiluminal polyurethane tube connecting the implanted reservoir to the external environment allowed for electrical contact, as well as continuous drug flow into and out of the device. Using this drug flow design, a constant drug concentration was maintained around the electrode and device–tissue interface. Once an electric potential was applied with the device on the tumor, the drugs were actively transported into the tissue.
Fig. 1.
Iontophoretic device used for the local delivery of FOLFIRINOX. (A) Front image of the iontophoretic device. (B) Device treatment setup where the drug is supplied to the device, using a syringe pump and electrical current via a direct current (DC) power supply. The device is connected to the positive lead, and a counter electrode (not shown) placed on the flank is connected to the negative lead. (C) Role of current on drug transport in ex vivo tumor tissue. Oxaliplatin, irinotecan, and 5-fluorouracil transport through PDX tumor tissue was evaluated by applying 2 or 0 mA and comparing drug transport into tumor. Data are means ± SD (n = 3). P values were determined by unpaired t test.
Iontophoretic Drug Delivery Testing in ex Vivo Tumors.
Drug transport studies were conducted using ex vivo pancreatic cancer patient-derived xenograft (PDX) tumors. To test the transport of FOLFIRINOX in the ex vivo PDX tumors, the devices were sutured onto the tumors (Fig. 1 A and B), and the counter electrode was placed on the contralateral side of the tumor. Two or 0 mA of current was applied for two 10-min periods, with a washout period between treatments, and the tumors were subsequently analyzed for the concentrations of the three major cytotoxic drugs in the FOLFIRINOX regimen: 5-fluorouracil, irinotecan, and oxaliplatin. The application of 2 mA constant current resulted in a 5.1-fold increase in oxaliplatin transport (P = 0.024), a 10.8-fold increase in 5-fluorouracil transport (P = 0.018), and a 5.4-fold increase in irinotecan transport (P = 0.015) into the tumor compared with the passive diffusion control (0 mA) (Fig. 1C).
Pharmacokinetics of Cytotoxic Drugs in Mice.
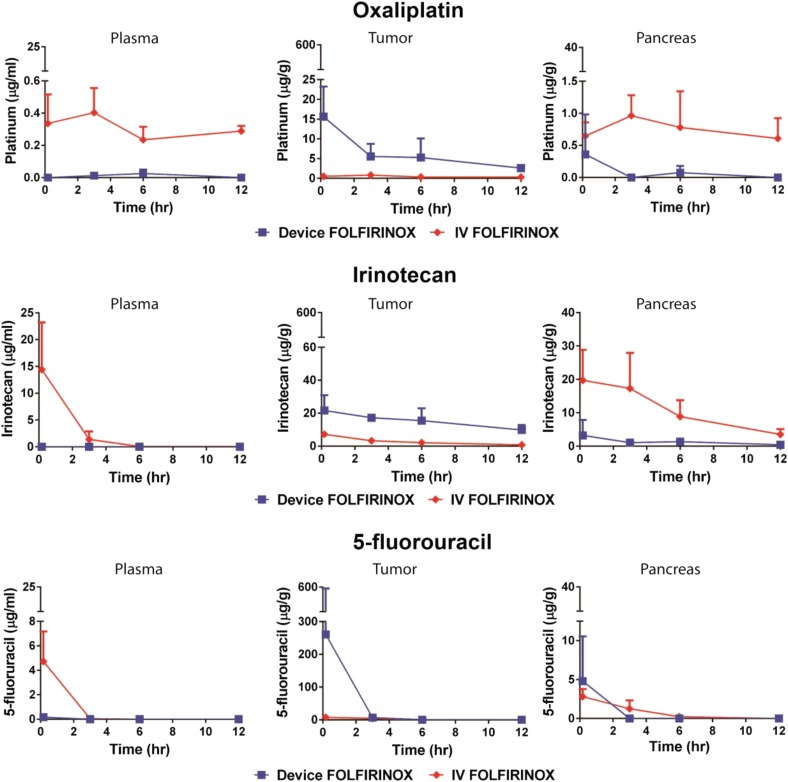
Iontophoretic delivery of the FOLFIRINOX drugs was further characterized with respect to pharmacokinetics (PK), as previously described (12). Briefly, devices were surgically implanted when the tumor reached a median size of 312 mm3 (range, 180–467 mm3). One week after device implantation, a single treatment was administered, tumors were harvested at designated times, and drugs were quantified by tissue (Fig. 2). FOLFIRINOX i.v. dosing was adjusted from clinical dosage in accordance with prior maximum tolerated dose studies (21–23).
Fig. 2.
PK of FOLFIRINOX delivered by device compared with i.v. was evaluated in an orthotopic PDX model of pancreatic cancer. Mice were administered a single treatment of FOLFIRINOX through the device. Organs were collected from each animal at various times, and total drug concentrations were analyzed. Data are means ± SD (n = 3–4 animals per group). Limit of quantitation for oxaliplatin was 1 ng/mL; limits of quantitation for irinotecan and 5-fluorouracil were 30 ng/mL.
The organ exposure to the FOLFIRINOX drugs, as measured by the area under the curve for device versus i.v. delivery, can be found in Table 1. FOLFIRINOX tumor area under the curve for iontophoretic delivery was considerably greater than for i.v. delivery (228.5 vs. 25.4 h*µg/g for 5-fluorouracil, 67.9 vs. 5.5 h*µg/g for oxaliplatin, and 177.75 vs. 30.55 h*µg/g for irinotecan, respectively). The average tumor penetration distances for FOLFIRINOX were not able to be quantified because of the amount of tissue required for measurement of the three cytotoxic drugs. The iontophoretic device delivery of FOLFIRINOX resulted in substantially lower plasma concentrations: a 141.5-fold reduction in 5-fluorouracil concentration, 47.5-fold reduction in oxaliplatin concentration, and 1,340.7-fold reduction in irinotecan concentration compared with i.v. delivery. There was greater exposure of the FOLFIRINOX drugs to the pancreas, kidney, and liver after i.v. delivery compared with device delivery (Table 1).
Table 1.
Organ exposure (hr*μg/g) to FOLFIRINOX after device or i.v. treatment
| Specimen | Device FOLFIRINOX | i.v. FOLFIRINOX | ||||
| 5-fluorouracil | Oxaliplatin | Irinotecan | 5-fluorouracil | Oxaliplatin | Irinotecan | |
| Tumor | 228.5 | 67.9 | 177.75 | 25.4 | 5.55 | 30.55 |
| Plasma | 0.027 | 0.076 | 0.015 | 3.82 | 3.61 | 20.11 |
| Pancreas | 0.4 | 0.65 | 14.42 | 7.54 | 9.09 | 126.86 |
| Kidney | 0.007 | 3.11 | 1.39 | 15.47 | 15.12 | 83.61 |
| Liver | 0.24 | 0.31 | 3.93 | 1.99 | 10.78 | 181.39 |
Device Delivery of FOLFIRNOX Promotes Tumor Regression.
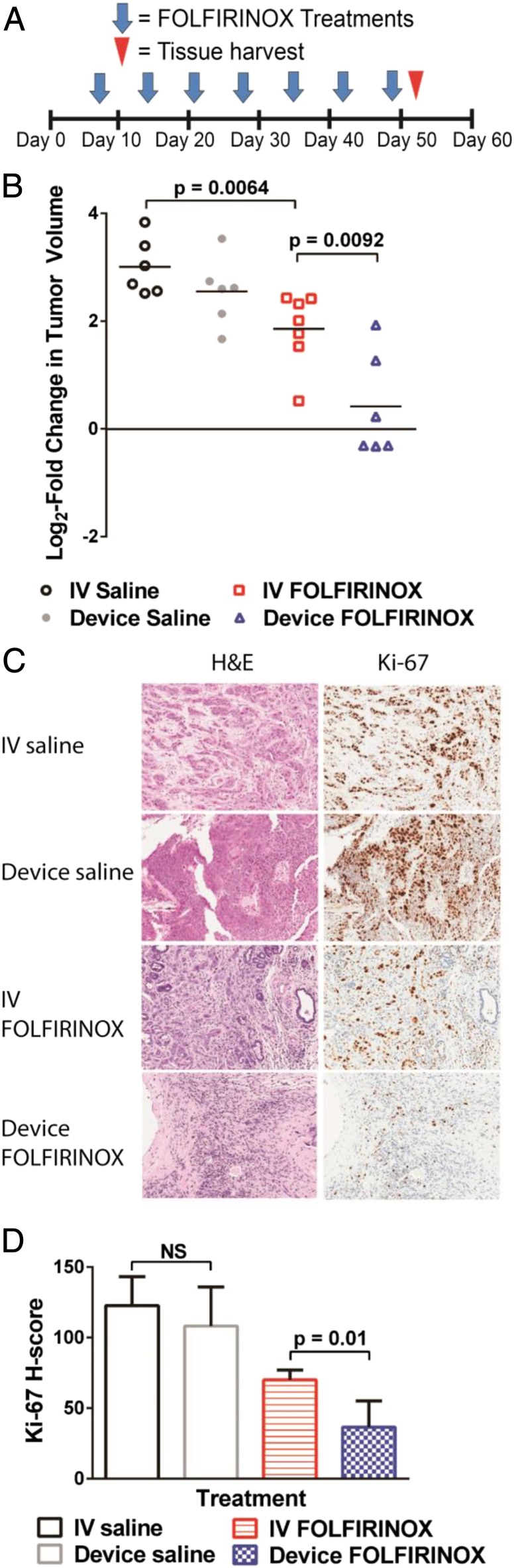
To evaluate the antitumor activity of FOLFIRINOX delivered by the iontophoretic devices, we performed efficacy studies in the same orthotopic PDX model of pancreatic cancer used in the PK studies. Devices were surgically implanted onto orthotopic pancreatic tumors when their size reached a median of 260 mm3 (range, 117–433 mm3). Mice were treated once per week for 7 wk with device FOLFIRINOX, i.v. FOLFIRINOX, device saline (0.9% NaCl), or i.v. saline. Tumor volumes were measured only after completion of the treatment, as implanted devices impaired ultrasound imaging.
Device FOLFIRINOX resulted in tumor regression in three of six mice and stable disease in another mouse, outperforming i.v. FOLFIRINOX and the control groups of i.v. and device saline during the 7-wk period of the study (Fig. 3A). Mice treated with device FOLFIRINOX had a mean log2-fold change in tumor volume of 0.4 compared with a mean log2-fold change in tumor volume of 1.9 for i.v. FOLFIRINOX (P = 0.0092), 3.0 for i.v. saline (P = 0.0002), and 2.6 for device saline (P = 0.0011) groups. No difference in tumor volume was seen in mice treated with device saline compared with i.v. saline.
Fig. 3.
Therapeutic effect of FOLFIRINOX delivered iontophoretically in an orthotopic PDX model of pancreatic cancer. (A) Treatment schedule and (B) the efficacy of device FOLFIRINOX, i.v. FOLFIRINOX, device saline, and i.v. saline in PDX mice treated for 7 wk. Data are log2-fold change in tumor volume (n = 6–7). (C) Histological staining of representative tumors for H&E and Ki-67. (D) Ki-67 staining was quantified according to H-score. P values were determined by unpaired t test. Data are means ± SD (n = 6–7).
Device Delivery of FOLFIRNOX Inhibits Cancer Cell Proliferation.
Tumors from mice treated with device FOLFIRINOX for 7 wk showed a significant decrease in Ki-67, a marker of cell proliferation, compared with tumors from mice that received i.v. FOLFIRINOX (P = 0.01) (Fig. 3 B and C). No difference in Ki-67 was seen between device saline-treated and i.v. saline-treated tumors. Of note, Ki-67 staining of tumors from the two nonresponders treated with device FOLFIRINOX resulted in H-scores (62.3 and 57.3) that were closer to the average i.v. FOLFIRINOX H-score (70.1 ± 6.8) than the average device FOLFIRINOX H-score (36.6 ± 18.6).
Device Delivery of FOLFIRNOX Does Not Cause Obvious Evidence of Tissue Toxicity.
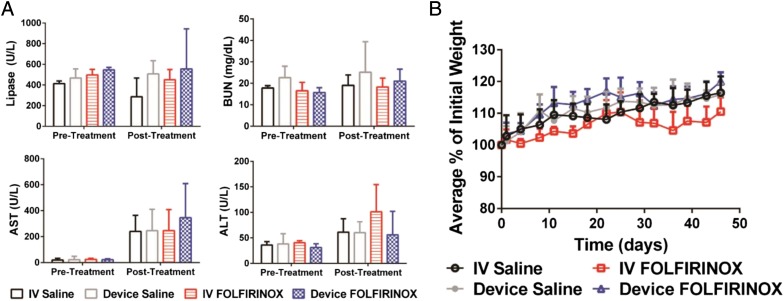
No difference in alanine transaminase, aspartate transaminase, blood urea nitrogen, and lipase levels was noted for the different delivery routes (Fig. 4A). Mice treated with device FOLFIRINOX showed better or at least equivalent treatment tolerance based on a greater body weight gain compared with i.v. FOLFIRINOX (Fig. 4B).
Fig. 4.
Clinical chemistry values and body weight changes in response to chemotherapy. (A) Evaluation of pertinent laboratory values for FOLFIRINOX treatments in an orthotopic PDX model of human pancreatic cancer. Mice were given saline or FOLFIRINOX. Pretreatment laboratories were collected 10 d before the treatments were started. Posttreatment laboratories were collected 3 d after the last treatment. Data are means ± SD (n = 6–7). (B) Body weight changes in response to therapy. Data are mean body weight changes as a percentage of initial weight ± SD (n = 6–7).
Discussion
Here, we show that iontophoretic delivery of FOLFIRINOX substantially increases intratumoral drug concentrations while limiting systemic exposure. The iontophoretic delivery of FOLFIRINOX resulted in better tumor response and tolerability, as defined by body weight gain, compared with i.v. FOLFIRINOX. Pancreatic PDX models such as the one used in this study have been shown to be highly predictive of relapse after surgery, reflective of patient outcome, genetics, and RNA tumor subtypes (19, 20, 24). These results suggest that the iontophoretic delivery of FOLFIRINOX may translate to improved clinical outcomes by enhancing the therapeutic index of this drug mixture, allowing for resection of localized and locally advanced pancreatic cancer.
As the role of chemoradiotherapy is increasingly being questioned for use in locally advanced pancreatic cancer, there is a need for new treatments to improve resection rates (25, 26). Our device has the ability to overcome the “wall-like” barrier of pancreatic tumors and improve responses to neoadjuvant chemotherapy. The iontophoretic device is able to increase intratumoral drug concentrations well above current i.v. administration while maintaining low systemic exposure. Through better delivery of an agent that has shown promise, we may not only increase the number of patients who may be resectable but also improve overall rates of margin-negative resections, and thus improve long-term patient outcomes. With concern for early micrometastatic disease, the low plasma exposure of the device delivery of FOLFIRINOX allows for concomitant device and potentially full-dose systemic administration of additional agents.
We hypothesize that the two nonresponders treated with device FOLFIRINOX may be the result of inadequate drug delivery secondary to device placement or damage. Further device design revisions may be warranted to improve the functionality and durability of the device. Furthermore, the efficacy of the device FOLFIRINOX treatment may be further improved by modifying the dosing schedule and optimizing the FOLFIRINOX formulations. Because of the low level of systemic exposure after a single treatment, the frequency of device FOLFIRINOX treatments may be increased to more than once weekly. Combining the drugs into the same solution may affect the overall mobility of each drug within the system, and further optimization of the formulation is warranted to improve the delivery (13). In addition, seeing that irinotecan is the water-soluble prodrug of SN-38, it may be beneficial to deliver SN-38 or a more active form of irinotecan (27).
Looking beyond pancreatic cancer, this iontophoretic delivery of FOLFIRINOX may be applied to a variety of other cancers, including sarcomas, gastroesophageal, head and neck, and recurrent rectal cancers. The minimization of systemic exposure by iontophoresis will allow more patients to tolerate therapy and will allow new systemic regimens to be evaluated in combination with iontophoresis. This device treatment has tremendous potential to improve surgical outcomes in challenging cancers.
Methods
Study Design.
Device fabrication and tumor implantation into the pancreas followed previously published methods (12). The ex vivo work was performed to evaluate the transport of the FOLFIRINOX drugs into tumor tissue under an applied electric potential. The three major cytotoxic drugs of the FOLFIRINOX regimen, 5-fluorouracil, irinotecan, and oxaliplatin, were evaluated for drug transport and PK analysis. For PK and efficacy studies, PDX mice with pancreatic tumors were randomly assigned into the experimental and control groups. The study endpoint for efficacy was an interval of 7 wk. For histological analysis, tumors were annotated and the number of positively stained cells according to stain intensity was quantified. H-scores for the Ki-67 stains were calculated from the stain intensity and percentage stain positivity by the formula 3 × percentage of strongly staining nuclei + 2 × percentage of moderately staining nuclei + percentage of weakly staining nuclei, giving a range of 0–300. The H-score is an established method of assessing the extent of nuclear immunoreactivity (12, 28). No outlying data points were excluded in the studies.
Device Fabrication.
A 5-mm platinum (Strem Chemicals) disk was soldered to a stainless steel cable wire and embedded in a polyurethane (Hapco Steralloy 2056) reservoir. The polyurethane was allowed to crosslink for at least 2 h at 60 °C after use. The steel wire was then threaded through custom-made multiluminal tubing, and the tubing/reservoir interface was encased in heat-shrink tubing with extra polyurethane to secure the tubing. A semipermeable 14K cellulose membrane (Fisher Scientific) was adhered to the reservoir for enclosure using polyurethane.
Drug Formulations.
The drugs were combined into two separate solutions: 50 mg of leucovorin dissolved with a 5 mg/mL oxaliplatin solution to final concentrations of 10 mg/mL leucovorin and 5 mg/mL oxaliplatin at a final pH of 5.5, and 10 mg/mL irinotecan solution added to 50 mg/mL 5-fluorouracil solution to create a final concentration of 14.4 mg/mL irinotecan and 14.0 mg/mL 5-fluorouracil at a pH of 6.0, using hydrochloric acid to limit conversion of irinotecan to its inactive form (29).
For i.v. delivery of FOLFIRINOX, the drugs were delivered via tail vein injection and dosed at 100 mg/kg leucovorin, 5 mg/kg oxaliplatin, 50 mg/kg 5-fluorouracil, and 50 mg/kg irinotecan.
Animal Studies.
All studies involving animals were approved by the appropriate Institutional Animal Care and Use Committee before initiation. All animals used in PK and efficacy studies were allowed to acclimate for at least 1 wk in the animal facilities before experimentation. Animals were exposed to a 12-h light/dark cycle and received food and water ad libitum through the studies.
Ex Vivo Studies.
De-identified tumors from patients with pancreatic cancer were grafted and passaged in athymic nude mice, as described previously (12). After pancreatic (orthotopic) implantation, tumor volumes were tracked by weekly abdominal ultrasound imaging. Upon reaching ∼400 mm3 (range, 323–484 mm3), the PDX tumors were directly removed and placed into Petri dishes filled with 10 mL saline. Devices were sutured directly onto the tumor, using 6–0 prolene, followed by either no current (control) or 2 mA of current for 10 min with each drug solution and a 5-min drug washout period with saline between drug solutions. The drug solutions were flowed at 50 µL/min through the device. After treatment, the tumors were snap frozen, using liquid nitrogen, and stored at −80 °C before drug extraction and quantification. Drug quantification by liquid chromatography (LC)-MS and inductively coupled plasma (ICP)-MS followed previously published methods (30–32).
PK Studies.
Devices were surgically implanted onto the pancreatic tumors when they reached a median volume of 312 mm3 (range, 180–467 mm3). Treatment was started 1 wk postimplantation to allow for epithelialization around the device. A constant DC power supply was used to provide the electric potential gradient. The positive lead was connected to the device wire, and the negative lead was connected to a silver chloride electrode placed on the mouse’s skin with electrolyte gel. The device treatments were identical to the ex vivo studies. When the constant voltage was applied, drug was driven from the device reservoir into the tumor tissue. Upon completion of the treatment, the device was emptied of drug solution. Terminal bleeds and organs were removed at their designated times. The tissues were snap frozen using liquid nitrogen and stored at −80 °C before drug extraction and quantification.
Efficacy Studies.
The technical protocols for surgical implantation of the device and device treatments were identical to the ex vivo and PK studies. Devices were surgically implanted onto orthotopic pancreatic tumors when their size reached a median size of 260 mm3 (range, 117–433 mm3). Mice were treated once weekly for 7 wk, using device FOLFIRINOX or i.v. FOLFIRNOX. Data for mice treated with device saline (0.9% NaCl) and i.v. saline for 7 wk were derived from our previous study (12). Ultrasound imaging was not used during the treatment interval because of device interference with tumor volume quantification. After completion of the treatments, mice were killed, and the tumors were extracted. The tumor volumes were quantified by volume displacement, for which there was good correlation between ultrasound imaging and volume displacement (R2 = 0.83).
PK and Statistical Analyses.
Data are expressed as means ± SD. The PKs of all drugs in plasma and tissues were analyzed by noncompartmental analysis using WinNonlin Professional Edition version 6.2 (Pharsight Corp.). Unpaired t tests were used to make comparisons of continuous values between groups. Unadjusted P values are reported for pairwise comparisons when an overall difference was detected.
Acknowledgments
We thank X. Wang, C. Santos, S. Herrera-Loeza, the University of North Carolina (UNC) Animal Studies Core, the patient-derived xenograft (PDX) Program, the Tissue Procurement Facility, the Translational Pathology Laboratory, and the Center for Gastrointestinal Biology Disease Histology Core for their contributions to this work. This research was funded by the University Cancer Research Fund at UNC. J.D.B. was supported by a National Defense Science and Engineering Graduate Fellowship, UNC Medical Scientists Training Program NIGMS-2-T32-GM008719, and PhRMA Foundation Fellowship.
Footnotes
Conflict of interest statement: J.M.D. and J.J.Y. have personal financial interests in the early-stage device company, Advanced Chemotherapy Technologies Inc., which was founded based on prior published research that the research described here builds on.
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Kleeff J, et al. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 2007;245(4):566–572. doi: 10.1097/01.sla.0000245845.06772.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oettle H, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA. 2007;297(3):267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 5.Blazer M, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. 2015;22(4):1153–1159. doi: 10.1245/s10434-014-4225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mian OY, Ram AN, Tuli R, Herman JM. Management options in locally advanced pancreatic cancer. Curr Oncol Rep. 2014;16(6):388. doi: 10.1007/s11912-014-0388-y. [DOI] [PubMed] [Google Scholar]
- 7.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tannock IF, Lee CM, Tunggal JK, Cowan DS, Egorin MJ. Limited penetration of anticancer drugs through tumor tissue: A potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res. 2002;8(3):878–884. [PubMed] [Google Scholar]
- 9.Erkan M, Reiser-Erkan C, Michalski CW, Kleeff J. Tumor microenvironment and progression of pancreatic cancer. Exp Oncol. 2010;32(3):128–131. [PubMed] [Google Scholar]
- 10.Neesse A, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60(6):861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 11.Yeh JJ, Kim WY. Targeting tumor hypoxia with hypoxia-activated prodrugs. J Clin Oncol. 2015;33(13):1505–1508. doi: 10.1200/JCO.2014.60.0759. [DOI] [PubMed] [Google Scholar]
- 12.Byrne JD, et al. Local iontophoretic administration of cytotoxic therapies to solid tumors. Sci Transl Med. 2015;7(273):273ra14. doi: 10.1126/scitranslmed.3009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56(5):619–658. doi: 10.1016/j.addr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Gratieri T, Kalia YN. Targeted local simultaneous iontophoresis of chemotherapeutics for topical therapy of head and neck cancers. Int J Pharm. 2014;460(1-2):24–27. doi: 10.1016/j.ijpharm.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 15.Di Stasi SM, et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: A randomised controlled trial. Lancet Oncol. 2006;7(1):43–51. doi: 10.1016/S1470-2045(05)70472-1. [DOI] [PubMed] [Google Scholar]
- 16.Conroy T, et al. Groupe Tumeurs Digestives of Unicancer PRODIGE Intergroup FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 17.Garrido-Laguna I, Hidalgo M. Pancreatic cancer: From state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12(6):319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- 18.Mahaseth H, et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas. 2013;42(8):1311–1315. doi: 10.1097/MPA.0b013e31829e2006. [DOI] [PubMed] [Google Scholar]
- 19.Moffitt RA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47(10):1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao H, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21(11):1318–1325. doi: 10.1038/nm.3954. [DOI] [PubMed] [Google Scholar]
- 21.Na YS, et al. Effects of the HDAC inhibitor CG2 in combination with irinotecan, 5-fluorouracil, or oxaliplatin on HCT116 colon cancer cells and xenografts. Oncol Rep. 2010;24(6):1509–1514. doi: 10.3892/or_00001012. [DOI] [PubMed] [Google Scholar]
- 22.Azrak RG, et al. Therapeutic synergy between irinotecan and 5-fluorouracil against human tumor xenografts. Clin Cancer Res. 2004;10(3):1121–1129. doi: 10.1158/1078-0432.ccr-0913-3. [DOI] [PubMed] [Google Scholar]
- 23.Louvet C, et al. Synergistic antitumoral activity of combined UFT, folinic acid and oxaliplatin against human colorectal HT29 cell xenografts in athymic nude mice. Anticancer Drugs. 2000;11(7):579–582. doi: 10.1097/00001813-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Thomas RM, et al. The canary in the coal mine: The growth of patient-derived tumorgrafts in mice predicts clinical recurrence after surgical resection of pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2015;22(6):1884–1892. doi: 10.1245/s10434-014-4241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammel P, et al. Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: Final results of the international phase III LAP 07 study. J Clin Oncol. 2013;31:LBA4003. [Google Scholar]
- 26.Chauffert B, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19(9):1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 27.Kunimoto T, et al. Antitumor activity of 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxy-camptothec in, a novel water-soluble derivative of camptothecin, against murine tumors. Cancer Res. 1987;47(22):5944–5947. [PubMed] [Google Scholar]
- 28.Bajetta E, et al. Effects of short-term pre-operative tamoxifen on steroid receptor and Ki-67 expression in primary breast cancer: An immunocytochemical study. Int J Oncol. 1998;12(4):853–858. doi: 10.3892/ijo.12.4.853. [DOI] [PubMed] [Google Scholar]
- 29.Li WY, Koda RT. Stability of irinotecan hydrochloride in aqueous solutions. Am J Health Syst Pharm. 2002;59(6):539–544. doi: 10.1093/ajhp/59.6.539. [DOI] [PubMed] [Google Scholar]
- 30.Wu H, et al. Factors affecting the pharmacokinetics and pharmacodynamics of PEGylated liposomal irinotecan (IHL-305) in patients with advanced solid tumors. Int J Nanomedicine. 2015;10:1201–1209. doi: 10.2147/IJN.S62911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kai MP, et al. Evaluation of drug loading, pharmacokinetic behavior, and toxicity of a cisplatin-containing hydrogel nanoparticle. J Control Release. 2015;204:70–77. doi: 10.1016/j.jconrel.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosovec JE, Egorin MJ, Gjurich S, Beumer JH. Quantitation of 5-fluorouracil (5-FU) in human plasma by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(2):224–230. doi: 10.1002/rcm.3362. [DOI] [PubMed] [Google Scholar]