Significance
Understanding the pathophysiological mechanism of central neuropathic pain has attracted much attention, especially because neuropathic pain is often unresponsive to existing medical treatments. In this study, we investigated the role of CaV3.1 T-type Ca2+ channels in the development of trigeminal neuropathic pain (TNP) in mice. Our results show that, intriguingly, CaV3.1 knockout mice had attenuated TNP. Specifically, we demonstrate that increased low-frequency rhythmicity and widely spread noncolumnar activity were present in wild-type TNP mice but not in knockout TNP mice. Moreover, abnormally pronounced coupling between low-frequency and high-frequency rhythms in the thalamocortical network of wild-type mice was absent in CaV3.1 knockout mice. Our results clearly imply that the presence of CaV3.1 channels is a crucial element in the pathophysiology of TNP.
Keywords: central pain, cross-frequency coupling, lateral inhibition, thalamocortical dysrhythmia
Abstract
A crucial pathophysiological issue concerning central neuropathic pain is the modification of sensory processing by abnormally increased low-frequency brain rhythms. Here we explore the molecular mechanisms responsible for such abnormal rhythmicity and its relation to neuropathic pain syndrome. Toward this aim, we investigated the behavioral and electrophysiological consequences of trigeminal neuropathic pain following infraorbital nerve ligations in CaV3.1 T-type Ca2+ channel knockout and wild-type mice. CaV3.1 knockout mice had decreased mechanical hypersensitivity and reduced low-frequency rhythms in the primary somatosensory cortex and related thalamic nuclei than wild-type mice. Lateral inhibition of gamma rhythm in primary somatosensory cortex layer 4, reflecting intact sensory contrast, was present in knockout mice but severely impaired in wild-type mice. Moreover, cross-frequency coupling between low-frequency and gamma rhythms, which may serve in sensory processing, was pronounced in wild-type mice but not in CaV3.1 knockout mice. Our results suggest that the presence of CaV3.1 channels is a key element in the pathophysiology of trigeminal neuropathic pain.
Since 1911, when H. Head and G. M. Holmes first addressed the relevance of the thalamus as a central pattern generator for neuropathic pain (1), many clinical studies have indicated the coexistence of pathophysiological thalamocortical activity and the occurrence of neuropathic pain. Compared with healthy controls, patients with neuropathic pain show increased low-frequency thalamocortical oscillations in magnetoencephalogram (MEG) recordings. Such low-frequency oscillations are a typical thalamocortical dysrhythmia (TCD) syndrome (2). In agreement with such MEG findings, the excess power of low-frequency oscillation was marked in local-field potential (LFP) recordings from the thalamus (3–5) and electroencephalogram (EEG) recordings from the cortex (6, 7) of patients with neuropathic pain. In addition, the presence of thalamic burst firing, which is a well-known underlying mechanism for cortical low-frequency oscillations through the thalamocortical recurrent network (8, 9), has been confirmed in patients with neuropathic pain (10–13). Results from small lesions in the posterior part of the central lateral nucleus of the medial thalamus, which reduce tonic hyperpolarization of thalamic neurons in chronic neuropathic pain patients, have also provided insight into the role of low-frequency thalamic rhythmicity in neuropathic pain. Following such interventions, a marked decrease in low-frequency EEG power was observed as well as pain relief (7, 12), indicating that alteration of thalamocortical rhythms plays a crucial role in the development and/or persistence of neuropathic pain.
Trigeminal neuropathic pain (TNP) is characterized by unilateral chronic facial pain limited to one or more divisions of the trigeminal nerve. There is increasing evidence that TNP is associated with anatomical and biochemical changes in the thalamus (14–16). Moreover, patients with TNP display significant reductions in thalamic volume and neural viability (15), indicating that altered thalamic anatomy, physiology, and biochemistry may result in disturbed thalamocortical oscillatory properties.
Abnormal thalamic activity has been investigated in patients with neuropathic pain (3–7, 10–13, 17, 18), including TNP (14–16). Furthermore, the potential role of thalamic burst firing in abnormally increased low-frequency oscillations has been proposed as a pathophysiological mechanism (2, 12). Because T-type Ca2+ channels are known to underlie thalamic burst firing (8, 9), it is reasonable to propose that pathophysiological low-frequency rhythms, such as those seen in central neuropathic pain, may be mediated by these calcium channels. Nevertheless, this hypothesis has not been directly tested. To determine whether T-type Ca2+ channels play a role in the generation of neuropathic pain, thalamocortical oscillatory properties were examined in mice lacking CaV3.1 channels following induction of TNP through partial ligation of the inferior orbital nerve (IoN). This channel represents the major T-type Ca2+ channel isoform in thalamocortical projection neurons (19). Following IoN ligations, CaV3.1 knockout (KO) mice showed significantly attenuated mechanical hypersensitivity, compared with wild-type (WT) mice. Moreover, spectral analysis of thalamocortical rhythms from CaV3.1 KO mice showed decreased low-frequency rhythm propensity, compared with WT mice. In addition, response to gamma activation and the spatiotemporal patterns of primary somatosensory (S1) cortex activity were altered in WT but not in KO mice after IoN ligation. Moreover, the cross-frequency interactions between low-frequency and gamma rhythms were significantly increased in WT but not in CaV3.1 KO mice. These findings indicate that TNP is associated with altered thalamocortical rhythms, resulting in increased sensitivity to pain as well as pain generation in response to nonnoxious stimuli. In addition, these results indicate that CaV3.1 T-type Ca2+ channels are fundamentally associated with the alteration of thalamocortical rhythms seen in TNP.
Results
Differential Effect of TNP in CaV3.1 KO and WT Mice: Behavioral and Electrophysiological Evaluation.
Hypersensitivity to mechanical stimulation in the TNP mouse model.
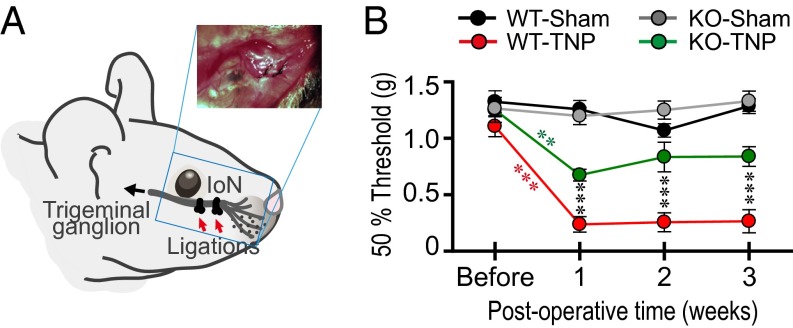
The sensitivity of IoN territory to mechanical stimuli was compared in IoN-ligated (Fig. 1A) and sham-operated WT and KO mice. As shown in Fig. 1B, 1 wk following IoN ligation, both WT (WT-TNP; n = 13) and KO (KO-TNP; n = 13) mice were significantly more sensitive to mechanical stimuli compared with their presurgical response (red asterisks, ***P < 0.001, WT-TNP and green asterisks, **P < 0.01, KO-TNP, by Student’s paired t test). However, the WT-TNP mice were significantly more sensitive to such stimulation than KO-TNP mice at 1, 2, and 3 wk after IoN ligation (Fig. 1B, black asterisks, ***P < 0.001 by two-way repeated-measures ANOVA for genotype and postoperative time in CaV3.1 WT-TNP vs. KO-TNP). Such hypersensitivity was absent in sham-operated WT (WT-sham; n = 11) and KO (KO-sham; n = 12) mice. Thus, this TNP model was successful in inducing pain as measured by the mechanical sensitivity test.
Fig. 1.
Attenuated mechanical hypersensitivity of TNP in CaV3.1 KO mice after IoN ligation. (A) Schematic drawing of IoN ligations performed to induce TNP in mice. (Inset) Picture of two ligatures on IoN. (B) Mechanical sensitivity was robustly increased after the IoN ligations but not after sham operation. Although both genotypes displayed mechanical hypersensitivity (red asterisks, ***P < 0.001 by Student’s paired t test, before vs. 1 wk after IoN ligation in WT-TNP, n = 13; green asterisks, **P < 0.01 by Student’s paired t test, before vs. 1 wk after IoN ligations in KO-TNP, n = 13), CaV3.1 KO-TNP mice had an attenuated mechanical hypersensitivity, following 1-, 2-, and 3-wk IoN ligations, compared with WT (black asterisks, ***P < 0.001 by two-way repeated-measures ANOVA for genotype and postoperative time, WT-TNP vs. KO-TNP). Mechanical hypersensitivity was absent in sham-operated WT (n = 11) and KO (n = 12) mice. All values represent mean ± SEM.
Changes of S1 power spectra in WT but not in KO mice after TNP induction.
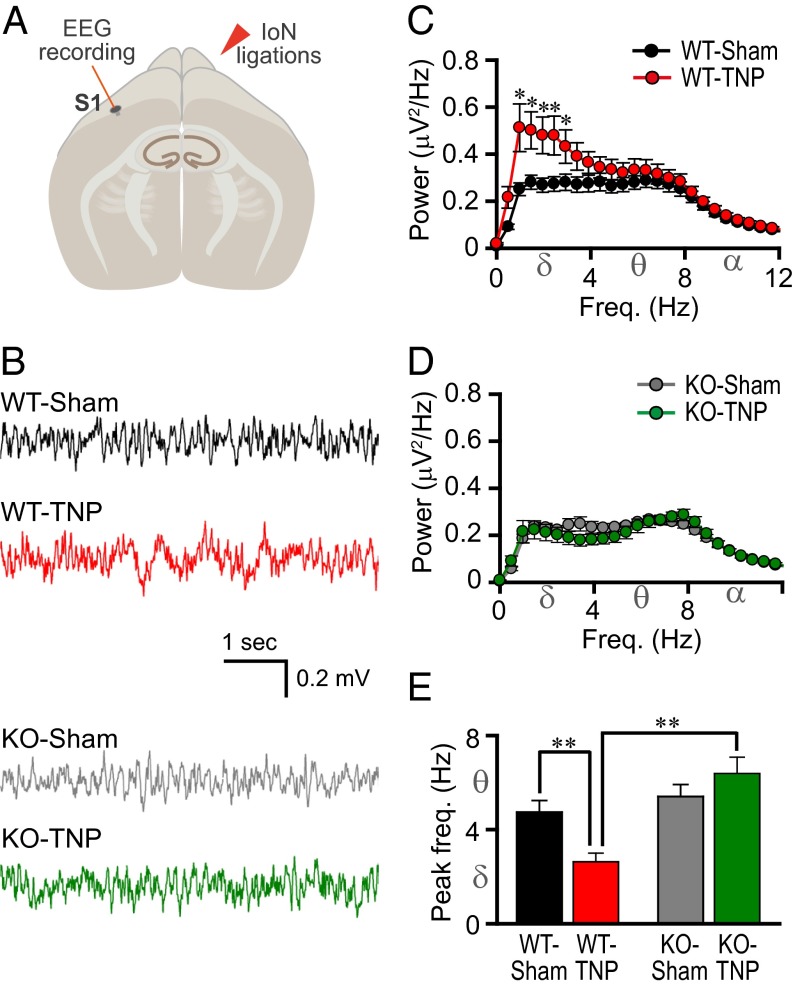
EEG recordings from the S1 cortex (Fig. 2 A and B) and their spectral analysis (Fig. 2 C and D) showed a significantly larger absolute spectral power at low frequencies (1.0–3.0 Hz) in WT-TNP (Fig. 2C, red; n = 13) than in WT-sham mice (Fig. 2C, black; n = 14) (*P < 0.05 by Student’s t test). The spectral power of low-frequency rhythms (1.0–5.0 Hz) in the S1 cortex of KO-TNP (Fig. 2D, green) was significantly lower in mean absolute value, compared with that of WT-TNP (Fig. 2C, red) (P < 0.05 by Student’s t test). There was no difference between KO-TNP (Fig. 2D, green; n = 12) and KO-sham mice (Fig. 2D, gray; n = 13) (P > 0.05 by Student’s t test). The peak EEG frequency is plotted in Fig. 2E. Note that the peak frequency was significantly lower in WT-TNP (Fig. 2E, red) than in sham-operated WT mice (Fig. 2E, black) (**P < 0.01 by Student’s t test). In contrast, there was no difference between the KO-TNP (Fig. 2E, green) and the sham-operated KO mice (Fig. 2E, gray). Notably, the peak frequency was significantly lower in WT-TNP than in KO-TNP mice (Fig. 2E, **P < 0.01 by Mann–Whitney rank-sum test). Thus, induction of TNP led to a reduction in the frequency of the strongest oscillatory brain activity in the S1 cortex in WT but not in KO mice. There were no significant changes in EEG spectral power at frequencies above 12 Hz (Fig. S1A).
Fig. 2.
Reduced S1 cortex low-frequency rhythm in mice lacking CaV3.1 T-type Ca2+ channels after IoN ligation. (A) Schematic drawing of an EEG S1 recording site. (B) Representative EEG traces. (C) Mean absolute spectral power as a function of frequency for WT-sham (n = 14) and WT-TNP (n = 13). (D) Mean absolute spectral power as a function of frequency for KO-sham (n = 13) and KO-TNP (n = 12). (E) Peak EEG frequency in the S1 cortex. The power of low-frequency oscillations in WT-TNP (red) was greater than that in sham-operated WT mice (black) (C, *P < 0.05 by Student’s t test). There was no significant difference between KO-sham (gray) and KO-TNP (green) (D, P > 0.05 by Student’s t test). Note that the power of low-frequency oscillations (1–5 Hz) was significantly lower in IoN-ligated CaV3.1 KO (D, green) than in WT-TNP mice (C, red) (P < 0.05 by Student’s t test). The peak frequency was shifted from theta toward delta in WT after IoN ligations (E, **P < 0.01 by Student’s t test). The peak frequency of CaV3.1 KO-TNP was greater than that of WT-TNP (E, **P < 0.01 by Mann–Whitney rank-sum test; peak in theta rhythm range in KO-TNP and peak in delta rhythm range in WT-TNP). All values represent mean ± SEM.
Fig. S1.
Mean absolute spectral power as a function of frequency, from 12 to 50 Hz, in WT-sham, WT-TNP, KO-sham, and KO-TNP. (A) Mean absolute spectral power of the S1 cortex in WT-sham (n = 14), WT-TNP (n = 13), KO-sham (n = 13), and KO-TNP (n = 12). (B) Mean absolute spectral power of the VPM thalamus in WT-sham (n = 7), WT-TNP (n = 9), KO-sham (n = 8), and KO-TNP (n = 8). Note that there were no significant changes in EEG spectral power at frequencies above 12 Hz both in the S1 cortex and VPM thalamus. All values represent mean ± SEM.
Changes in delta oscillations in the somatosensory thalamus in WT but not in KO mice.
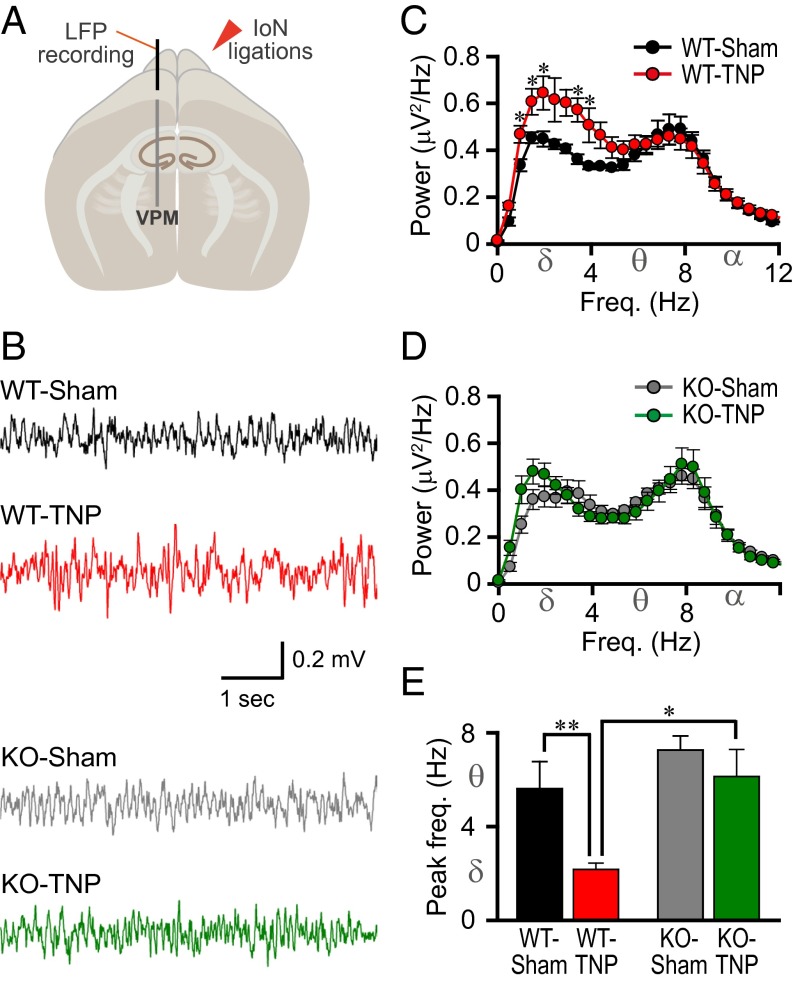
Given the strong projections from the thalamic ventral posteromedial nucleus (VPM) to the S1 cortex and its recurrent connectivity (20), LFPs were recorded from the VPM in CaV3.1 KO and WT mice (Fig. 3 A and B). This allowed us to determine (i) whether the alterations in the rhythms seen in the S1 cortex of WT-TNP mice (Fig. 2 B, C, and E, red) were related to changes at the thalamic level, and (ii) whether these alterations were mediated via CaV3.1 T-type Ca2+ channels. Greater spectral power in the low-frequency range was seen in WT-TNP (Fig. 3C, red; n = 9) than in those from WT-sham–operated mice (Fig. 3C, black; n = 7) (*P < 0.05 by Student’s t test). This difference was not found between KO-TNP (Fig. 3D, green; n = 8) and KO-sham–operated mice (Fig. 3D, black; n = 8) (P > 0.05 by Student’s t test). As seen in the S1 recordings shown in Fig. 2, the amplitude in the delta frequency range was larger in the VPM of WT-TNP mice (Fig. 3C, red) than in KO-TNP (Fig. 3D, green) (P < 0.05 by Student’s t test). There were no significant changes in LFP spectral power at frequencies above 12 Hz (Fig. S1B).
Fig. 3.
Reduced low-frequency rhythms and lack of peak frequency shift in the VPM of the thalamus in CaV3.1 KO mice after IoN ligations. (A) Schematic drawing of an LFP recording from the VPM. (B) Representative traces. (C) Mean absolute spectral power as a function of frequency for WT-sham (black, n = 7) and WT-TNP (red, n = 9). (D) Mean absolute spectral power as a function of frequency for KO-sham (gray, n = 8) and KO-TNP (green, n = 8). (E) Peak LFP frequency in the VPM. The spectral profile in the VPM showed a significantly increased low-frequency rhythm after IoN ligation in the WT (C, *P < 0.05 by Student’s t test, WT-sham vs. WT-TNP). There was no significant difference between KO-sham and KO-TNP (D, P > 0.05 by Student’s t test). The absolute power of low-frequency oscillations (1–4 Hz) was significantly lower in CaV3.1 KO with IoN ligations (KO-TNP, D, green), compared with low-frequency rhythms in WT-TNP (C, red, *P < 0.05 by Student’s t test, WT-TNP vs. KO-TNP). The peak frequency of thalamic LFP in WT-TNP mice was shifted to delta band range, compared with the theta band range of WT-sham (E, **P < 0.01 by Student’s t test). However, the peak frequency of CaV3.1 KO-TNP was in the theta rhythm range, as in the S1 cortex EEG (Fig. 2). All values represent mean ± SEM.
The peak frequencies of the VPM LFP and S1 EEG were similar (compare Figs. 2E and 3E). The peak frequency in WT-TNP (Fig. 3E, red) was lower than that in WT-sham (Fig. 3E, black) mice (**P < 0.01 by Student’s t test). This difference was not present in the two groups of KO mice (Fig. 3E, KO-sham, gray and KO-TNP, green). The peak frequency of the WT-TNP was significantly lower than that in KO-TNP mice (Fig. 3E, red and green, respectively; 2.2 ± 0.3 Hz in WT-TNP and 6.1 ± 1.1 Hz in KO-TNP, *P < 0.05 by Mann–Whitney rank-sum test). These findings indicate that the changes seen in the S1 cortex after TNP induction were related to similar changes at the thalamic level. Further, the differences observed between WT and KO mice indicate that such changes were indeed mediated via CaV3.1 T-type Ca2+ channels.
Abolished Lateral Inhibition in the S1 Cortex of IoN-Ligated WT but Not in CaV3.1 KO or Sham-Operated Mice: Optical Imaging Evaluation.
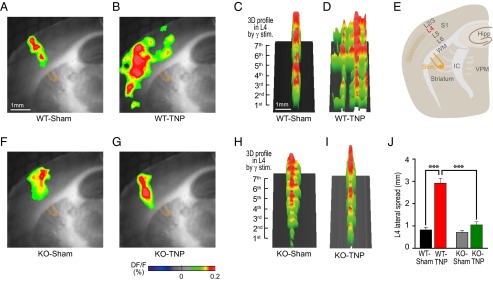
High-frequency (>35 Hz, gamma range) white matter stimulation has been shown to elicit a spatially restricted columnar activation in the cortex (21). Columnar activation is an essential step in cortical sensory processing because it allows the formation of temporal coherence with spatially segregated neuronal activity clusters (21–23). A key factor in shaping the cortical activity distribution is the frequency of the thalamic input to the cortex (24). To investigate the effect of gamma input on the distribution of cortical activity, voltage-sensitive dye imaging (VSDI) was used in thalamocortical slices from WT-sham, WT-TNP, KO-sham, and KO-TNP mice (Fig. 4E). Because the functional connectivity between the thalamus and cortex is preserved in these slices, VSDI allowed direct visualization of the spatiotemporal dynamics between the S1 cortex and thalamus. As previously demonstrated (21, 24), white matter stimulation at gamma frequency activated spatially restricted fluorescence changes [DF/F, (F − F0)/F, where F0 is the base fluorescence level] in layer 4 of the S1 cortex near the region of the stimulating electrode (Fig. 4A). In contrast, a more extensive noncolumnar pattern of cortical activity was seen in WT-TNP mice after white matter stimulation (Fig. 4B). Three-dimensional profiles of layer 4 activity clearly illustrate this difference between WT-sham (Fig. 4C) and WT-TNP (Fig. 4D) mice. There was no significant difference in the pattern of cortical activity in KO-sham (Fig. 4 F and H) and KO-TNP (Fig. 4 G and I) mice. The lateral spread of cortical activity in layer 4 is plotted for the four groups of animals in Fig. 4J. The lateral spread of activity was clearly greater in WT-TNP mice (red; n = 8) than in WT-sham–operated (black; n = 5) or KO-TNP mice (green; n = 6) (***P < 0.001 by Student’s t test). There was no difference between KO-TNP and KO-sham–operated mice (n = 6) (P > 0.05 by Student’s t test).
Fig. 4.
Abolished lateral inhibition of cortical gamma activity in a thalamocortical slice of WT following IoN ligation. (A–D) Voltage-sensitive dye imaging of spatiotemporal patterns in the S1 cortex resulting from a sequence of seven 40-Hz white matter (WM) electrical stimulus trains shown in 2D (A and B) and 3D (C and D) images in WT mice. Note the spreading of cortical activity in WT-TNP (B and D), in contrast to the columnar pattern in WT-sham (A and C) mice. (E) Schematic drawing of a thalamocortical slice illustrating the S1 cortex, interconnected thalamic nuclei (VPM), and bipolar stimulation electrode. IC, internal capsule. (F–I) Same as in A–D for KO mice. Columnar pattern of cortical activation in KO mice. Note the restrained columnar cortical activity in KO-TNP (G and I), in contrast to the widely propagated cortical activity in WT-TNP (B and D) mice. (J) Plot of lateral spread of activity in each animal group. There was significantly increased L4 lateral spread in CaV3.1 WT-TNP (red) compared with the other animal groups (***P < 0.001 by Student’s t test). All values represent mean ± SEM.
Increased Phase–Amplitude Cross-Frequency Coupling within the S1 Cortex of IoN-Ligated WT Mice but Not in CaV3.1 KO Mice.
In TCD, abnormal low-frequency thalamic oscillations have been observed at the cortical level (2). Moreover, low-frequency firing of inhibitory interneurons reduces lateral inhibition, resulting in high-frequency firing of neighboring regions. This has been termed the “edge effect” (24), and was initially reported in the retina (25). This edge effect has been proposed to underlie positive symptoms in TCD, including allodynia (2, 12, 18, 26). In the present study, increased low-frequency thalamic (Fig. 3) and cortical (Fig. 2) rhythmicity and altered high-frequency oscillatory activity (Fig. 4) have been seen in WT-TNP but not in KO-TNP or sham-operated mice.
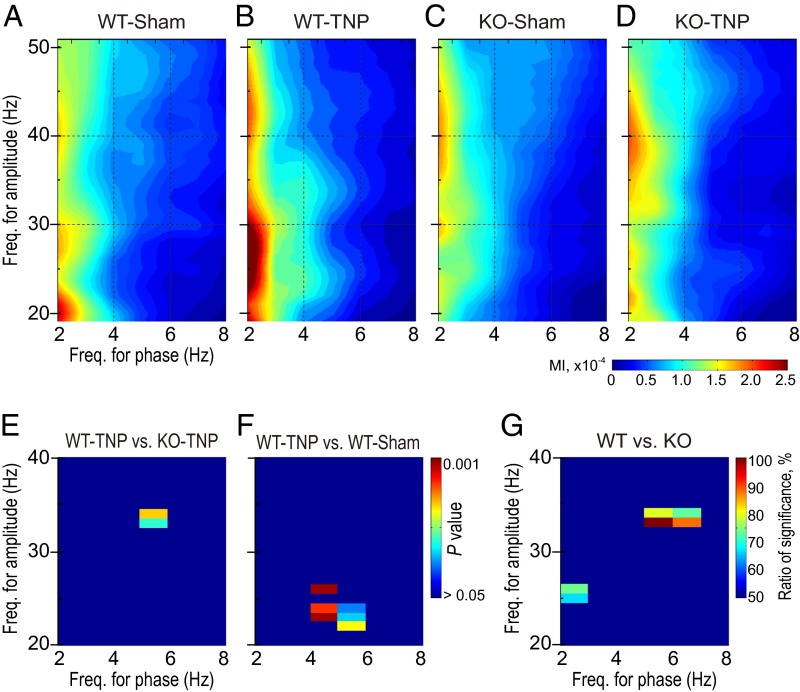
To find whether there was a correlation between the delta/theta and beta/gamma range activity in the S1 cortex, phase–amplitude cross-frequency coupling (CFC) was determined. CFC gives a measure of the degree to which the phase of the low-frequency component modulates the amplitude of the high-frequency component and the coordination of oscillations at different frequencies. Fig. 5 A–D shows the modulation index (MI) between the phase of low-frequency oscillations (abscissa, 2–8 Hz) and the amplitude of high-frequency oscillations (ordinate, 20–50 Hz) in the four groups of mice. The CFC showed a stronger modulation of beta/gamma (modulated amplitude) by delta (modulating frequency) in WT-TNP mice (Fig. 5B) than in the other three groups of mice (Fig. 5 A, C, and D; n = 12 for each group).
Fig. 5.
CFC in the EEG from the S1 cortex. (A–D) Grand average of the cross-frequency coupling showing interactions under the four conditions studied: WT-sham (A), WT-TNP (B), KO-sham (C), and KO-TNP (D) (n = 12 for each condition). The magnitude of this coupling (modulation index; MI) was calculated for pairs of phases of delta/theta (2–8 Hz, abscissa) and amplitudes of beta/gamma oscillations (20–50 Hz, ordinate). Warm colors indicate higher values of phase–amplitude coupling. In WT-TNP (B), the phase of delta/theta rhythms strongly entrained the amplitude of the beta/gamma rhythm, revealing a predominant low-frequency rhythm–to–high-frequency coupling. (E and F) Statistical significance of the difference between CaV3.1 WT-TNP and KO-TNP (E) or between WT-TNP and WT-sham mice (F) using the Mann–Whitney rank-sum test with Bonferroni correction. Cross-frequency bins in which the CFC increased significantly (P < 0.05) were found in low-frequency rhythm–to–gamma CFC in WT-TNP, compared with KO-TNP (E) and WT-sham (F). (G) Statistical significance of the difference between CaV3.1 WT and KO using random sampling comparison with Friedman’s two-way ANOVA. Warm colors indicate the cross-frequency coupling bins showing statistical significance (P < 0.05 with more than 50% of the 100 repetitive random sampling trials).
These CFC patterns were compared for pairs of two groups of mice using the Mann–Whitney rank-sum test with Bonferroni correction for multiple comparisons. There was a distinct modulation of coupling between 30–40 Hz (gamma) and 4–6 Hz (theta) in WT-TNP mice compared with the KO-TNP (Fig. 5E). On the other hand, coupling between 20–30 Hz (beta/gamma) and 4–6 Hz (theta) frequency was enhanced in WT-TNP compared with WT-sham (Fig. 5F). In contrast, there was no difference in coupling between the frequencies in WT-sham and KO-sham mice (Fig. S2A) or KO-sham and KO-TNP mice (Fig. S2B).
Fig. S2.
Direct comparison of CFC patterns between WT-sham and KO-sham (A) or KO-sham and KO-TNP (B) using the Mann–Whitney rank-sum test with Bonferroni correction (n = 12 for each condition). Note that the CFC difference was absent between WT-sham and CaV3.1 KO-sham or KO-sham and KO-TNP.
Finally, we further examined a possible role for CaV3.1 T-type Ca2+ channels in the pathophysiological development of TNP by comparing the enhanced frequency coupling between each genotype using Friedman’s two-way ANOVA. Comparison was made between both groups of WT and both groups of KO mice with repetitive random sampling (after adjusting for a difference in variability). As shown in Fig. 5G, we found significantly enhanced cross-frequency coupling in WT (criterion P <0.05 with >50% of the 100 repetitive random sampling trials) in the range of 2–3 Hz (modulating phase) with near 25 Hz (modulated amplitude) and 5–7 Hz with 35 Hz. Note that the bin showing relatively strong coupling, 5–7 Hz (modulating phase), with 30–40 Hz (modulated amplitude) is similar to that seen when WT-TNP and KO-TNP mice were compared (Fig. 5E).
Discussion
Thalamic burst firing, supported by deinactivation of CaV3.1 T-type Ca2+ channels, has long been proposed as the underlying mechanism for cortical low-frequency oscillations (8, 9). Such thalamic activity has been observed in patients with neuropathic pain (10–13). Burst firing has also been observed in rodents following a spinal cord lesion (27–29). Thus, we proposed that a CaV3.1 T-type Ca2+ channel-mediated alteration of thalamocortical rhythms plays a crucial role in the development and/or persistence of neuropathic pain. In the present study, KO mice lacking CaV3.1 T-type Ca2+ channels were studied to evaluate the contribution of this channel to the behavioral and electrophysiological characteristics of mice with chronic pain following trigeminal neuropathy.
The present results clearly indicate that the hypersensitivity that is usually associated with neuropathy after IoN ligation was markedly reduced in the CaV3.1 KO mice, compared with WT mice (Fig. 1). Consistent with these behavioral data, there was an increased probability of low-frequency thalamocortical oscillations in WT mice after IoN ligation that was not found in CaV3.1 KO mice under the same conditions (Figs. 2 and 3). Such findings indicate that the attenuated mechanical hypersensitivity in KO-TNP mice is related to their reduction in pathophysiological low-frequency rhythms. We also found that the alteration of normal sensory perception in WT mice following IoN ligation may be related to reduced lateral inhibition in the S1 cortex (Fig. 4). In addition, an increased CFC between the phase of low-frequency oscillations and the amplitude of gamma oscillations was also presented in WT-TNP mice (Fig. 5), demonstrating that cross-frequency thalamocortical coupling accompanies chronic pain.
From a neuronal perspective, low-frequency oscillations are mostly related to the hyperpolarization-induced deinactivation of T-type Ca2+ channels and oscillatory thalamic burst firing (30). The increased thalamic burst firing and its cortical consequence (low-frequency firing with an edge effect), which have been proposed to underlie the pain response seen in WT, were absent in KO mice. Although it must be studied further, it is quite likely that the intrinsic electrophysiological properties of thalamic neurons were modified in animals with TNP following IoN neuropathy. Further, significant enhancement of T-type Ca2+ current in sensory neurons has been reported in rats with diabetic neuropathy (31).
A critical aspect of the altered thalamocortical rhythm in TNP, where projections from the thalamus entrain an increase of low-frequency rhythm in the cortex, is the loss of lateral inhibition (Fig. 4). In S1 cortical layer 4, loss of lateral inhibition, seen as an abnormal spread of cortical columnar activation, is due to the lower rate of cortical firing (21, 24). Deafferentation induces hyperpolarization of thalamic neurons, leading to the deinactivation of T-type Ca2+ channels (32) and enhancement of inward rectifying current (33), thus enforcing gamma oscillation in adjacent cortical areas by lateral disinhibition (24). This asymmetric lateral inhibition has also been seen in central chronic pain patients (4, 18).
Intriguingly, this alteration of lateral inhibition in thalamocortical slices from WT mice with trigeminal neuropathy indicates that the abnormal electrophysiological properties in the thalamocortical network, which are seen in TNP, persisted even in the absence of a pain signal from peripheral primary sensory neurons. This result suggested that the alteration in thalamocortical activity seen in neuropathic pain is not a secondary response to primary changes at the level of peripheral sensory input. It is consistent with the pathophysiological changes in thalamic activity seen in diabetic neuropathic pain (34, 35) and with chronic constriction injury of the sciatic nerve (36–38), supporting the presence of a central neuropathic mechanism.
The present results are also in agreement with previous studies demonstrating attenuated neuropathic pain behaviors in CaV3.1 KO mice following spinal nerve ligation (39). Our results are consistent with those behavioral observations, suggesting that the CaV3.1 T-type Ca2+ channels could contribute to the alteration of thalamocortical dynamics seen in spinal neuropathy and trigeminal neuropathy chronic pain. Furthermore, chronic neuropathic pain, in contrast to nonneuropathic pain, has been shown to be associated with significant biochemical, physiological, and anatomical changes in the thalamus (15), suggesting that these two conditions are fundamentally different. It is likely, therefore, that the finding of no difference in tests of acute pain following direct activation of pain receptors in CaV3.1 KO mice (40, 41) does not address central pain sensitization but rather a physiologically evoked pain response.
Compared with the synchronization at single frequencies, CFC allows the analysis of complex interactions at different frequency bands (42, 43) and phase–amplitude. Indeed, CFC has been proposed to be an effective mechanism in combining network activity with sensory processing (44–46). Significantly different CFC patterns between low-frequency rhythms and gamma rhythm were found within the S1 cortex following IoN ligations in WT, compared with KO mice (Fig. 5). The S1 cortex is involved in the sensory processing of nociceptive information, and activity in gamma rhythm is obligatory to sensory perception (47). Therefore, it is reasonable to infer that the increased coupling between the phase of low-frequency oscillation and the amplitude of gamma oscillation underlies mechanical hypersensitivity in TNP. This CFC finding suggests that low-frequency rhythms strongly modulate high-frequency rhythms in the S1 cortex of TNP mice, resulting in a coherent system of tightly coupled oscillations associated with the perception of pain itself. Our results further suggest that coupling between low- and high-frequency oscillations is involved in the generation and/or persistence of neuropathic pain, and that CaV3.1 T-type Ca2+ channels are implicated in this pathophysiological change in TNP.
In conclusion, the present findings indicate that the CaV3.1 T-type Ca2+ channel in the central nervous system is involved in the development of mechanical hypersensitivity, known as allodynia. This is in addition to previous studies showing a role for T-type Ca2+ channels in hyperexcitability and ectopic discharges within damaged sensory neurons and their axons in the peripheral nervous system (31, 48). Our findings address new implications concerning the pathophysiological mechanism underlying chronic neuropathic pain. These data also suggest that inhibition of CaV3.1 channels in the thalamocortical network may effectively reduce mechanical hypersensitivity in TNP, making this channel a potential target for the development of neuropathic pain analgesics.
Materials and Methods
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committees of the New York University School of Medicine and the Marine Biological Laboratory, and were in accordance with the guidelines of the International Association for the Study of Pain (49). All observations were performed in a blind fashion. The mice and experiments for this study are described in detail in SI Materials and Methods.
SI Materials and Methods
Animals.
CaV3.1 null KO mice and their littermate WT controls were generated by mating heterozygotes of CaV3.1 on a background of C57BL/6J from the RIKEN BioResource Center. Littermates or age-matched mice of each genotype and gender, 8–12 wk of age, and 20–30 g body weight were used for all experiments. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committees of the New York University School of Medicine and the Marine Biological Laboratory, and were in accordance with the guidelines of the International Association for the Study of Pain (49). All observations were performed in a blind fashion.
Model of TNP.
To induce TNP in mice, unilateral ligation to the right IoN was performed following protocols from a previous study with some modifications (50). In brief, 8- to 10-wk-old mice were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine (10 mL/kg i.p. injection). The head of the mouse was fixed on the stereotaxic frame. After shaving the hair on top of the snout, a midline scalp incision was made to expose the skull and nasal bone. The right IoN was dissected and exposed using blunt dissection tools. The IoN was gently isolated using fine forceps without damaging facial nerve branches. Two silk suture (7-0) ligatures were loosely tied to reduce the diameter of the IoN to 1/3–1/2 of its original diameter. After ligation, the incision was closed using silk suture (5-0). In sham-operated mice, the IoN was exposed and isolated using the same procedure but no ligation was implemented.
Behavioral Assessment.
To determine the IoN ligation-induced mechanical hypersensitivity, the 50% withdrawal threshold of IoN territory (near the vibrissal pad center on the hairy skin surrounding the mystacial vibrissae) was examined using a set of von Frey filaments (0.04, 0.07, 0.16, 0.4, 0.6, 1, 1.4, and 2.0 g; North Coast Medical), according to the up–down method and formula (51). Animals were placed in a plastic restrainer with a 15-min habituation time. Mice first received a stimulus using the 0.4-g filament. In the presence of a clear withdrawal response, the next-smallest filament was used. When there was no initial response, the next-largest filament was used. Clear withdrawal responses are as follows: (i) the mouse avoids prolonged contact with the filament; (ii) the mouse actively attacks the stimulating object; or (iii) the mouse turns its head rapidly away, swiping the stimulated area.
EEG and LFP Recordings.
Differential EEG or LFP recordings were performed in 10- to 12-wk-old mice. After IoN ligations or sham operation in 8- to 10-wk-old mice, screw-type electrodes (Plastics One) were implanted over the contralateral S1 cortex (anteroposterior: −1.5 and medial lateral: +3.0 mm) for EEG recording. LFP recording was carried out via a parylene-coated tungsten electrode (A-M Systems) implanted in the VPM thalamic region (anteroposterior: −1.7 and medial lateral: +1.7, −3.5 mm below the brain surface) using a stereotaxic device (David Kopf Instruments). The reference electrode was implanted in the occipital region of the skull. A head mount was secured by dental cement, and the mice were allowed to recover for at least 2 wk. Spontaneous EEG or LFP signals were recorded at a bandwidth of a 0.1- to 500-Hz filter (model 1700; A-M Systems) and digitized at a 1-kHz sampling frequency. Data were acquired using the pCLAMP 10.2 program (Molecular Devices). Spectral power was calculated in 0.5-Hz bins (fast Fourier transform with Hamming window) using Clampfit software (version 10.2; Molecular Devices) from artifact-free 10-min EEG or LFP recordings made from each animal. EEG or LFP signals were low-pass–filtered at 50 Hz and high-pass–filtered at 0.1 Hz for spectral analysis. The recording positions for thalamic LFP were identified by postmortem histology.
VSDI in Thalamocortical Slices.
Two weeks after IoN ligations or sham operation, animals were deeply anesthetized with isoflurane and decapitated after loss of the limb-withdrawal reflex. The brain was isolated and sectioned using a vibratome (VT1000 S; Leica Microsystems) in chilled low-sodium/high-sucrose artificial cerebrospinal fluid (ACSF) containing 248 mM sucrose, 26 mM NaHCO3, 1.25 mM Na2HPO4, 5 mM KCl, 2 mM MgCl2, 0.5 mM CaCl2, and 10 mM glucose, and aerated with 95% O2/5% CO2 to a final pH of 7.4. Thalamocortical slices (350-μm) were obtained as previously described (52). Slices were recovered for at least 1 h (at ∼25 °C) in a chamber containing a continuously oxygenated combination of 1:1 (vol/vol) low-sodium/high-sucrose ACSF and normal ACSF (124 mM NaCl, 5 mM KCl, 1.25 mM KH2PO4, 26 mM NaHCO3, 2.0 mM MgCl2, 2.0 mM CaCl2, and 10 mM glucose, pH 7.4) before use. The VSDI apparatus comprised a 12-V halogen light source, 515 ± 35-nm filter, dichroic mirror, and 5× objective lens mounted on an upright microscope (BX50WI; Olympus). Thalamocortical slices were transferred to an interface-type chamber (at 34 °C) superfused with normal ACSF and stained with 0.2 mM di-4-ANEPPS, 4-(2-(6-(Dibutylamino)-2-naphthalenyl)ethenyl)-1-(3-sulfopropyl)pyridinium hydroxide inner salt (Molecular Probes). The bipolar electrical stimulation (at twice threshold, 0.5–2.0 mA, 200 μs) was delivered to white matter for the study of cortical responses. The emitted fluorescence was filtered (>590 nm), monitored with a CCD camera (MiCAM ULTIMA; BrainVision), and collected every 1 ms for 512 ms. Optical recordings were analyzed off-line using BrainVision analyzer software (version 13.12.20). Changes in cortical activities were evaluated as DF/F, (F − F0)/F, where F0 is the base fluorescence level.
CFC in the Primary Somatosensory Cortex.
Cross-frequency phase–amplitude coupling between delta/theta and gamma rhythms was calculated as described in the work of Tort et al. (42, 43, 46). The modulation index (MI), which measures how well the phase of low-frequency rhythm modulates the amplitude of high-frequency rhythm, was evaluated between the phase of delta/theta (1–8 Hz) and the amplitude of gamma (20–50 Hz) rhythms in every 1-Hz frequency bin. Instantaneous phase and amplitude time series were calculated by Hilbert transformation of band-pass–filtered EEG signals (zero-phase filtering with a finite impulse response (FIR) filter of order 60; 3- and 2-Hz bandwidths for phase and amplitude frequencies, respectively). The distribution of amplitude with respect to the phase was obtained, and the MI was defined as the normalized entropy of the amplitude distribution. The MI is zero when the amplitude distribution is uniform, indicating no phase–amplitude dependence, and has a larger value with the stronger amplitude modulation by the phase.
Statistical Analysis.
All data values in the text and figures are presented as means ± SEM. Statistics were conducted using SigmaPlot-SigmaStat (version 12.0; Systat Software, Inc.). Student’s t test, Mann–Whitney rank-sum test, or two-way ANOVA with Tukey’s post hoc comparison test or Bonferroni adjustments for pairwise comparison were used to assess statistical significance between WT and KO mice. Student’s paired t tests were used for two-sample comparisons within the same genotype. Differences were considered significant if at least P <0.05.
Acknowledgments
We are grateful to Dr. Kerry D. Walton for helpful suggestions regarding the manuscript, Dr. Kenji Sakimura for providing CaV3.1 heterozygous mice, and Daniel Johnson for mouse care. We also thank Ms. Mary Clarke for excellent research administration. This work was supported by a grant from the National Institutes of Health (NS13742/NS/NINDS/NIH HHS) (to R.R.L.), a Postdoctoral Program Research grant from the University of Science and Technology in the Republic of Korea (UST Post-Doctoral Research Program) (to S.C.), a National Research Foundation of Korea grant funded by the Korean Government (NRF-2009-352-C00111) (to E.Y.), and a Flagship grant of the Korea Institute of Science and Technology (2E25472) and a grant of the Global Frontier R&D Program (NRF-M1AXA003-2011-0031525) (to E.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600418113/-/DCSupplemental.
References
- 1.Head H, Holmes GM. Sensory disturbances from cerebral lesions. Brain. 1911;34(2-3):102–254. [Google Scholar]
- 2.Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96(26):15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gücer G, Niedermeyer E, Long DM. Thalamic EEG recordings in patients with chronic pain. J Neurol. 1978;219(1):47–61. doi: 10.1007/BF00313368. [DOI] [PubMed] [Google Scholar]
- 4.Sarnthein J, Jeanmonod D. High thalamocortical theta coherence in patients with neurogenic pain. Neuroimage. 2008;39(4):1910–1917. doi: 10.1016/j.neuroimage.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Stern J, Jeanmonod D, Sarnthein J. Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. Neuroimage. 2006;31(2):721–731. doi: 10.1016/j.neuroimage.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 6.Boord P, et al. Electroencephalographic slowing and reduced reactivity in neuropathic pain following spinal cord injury. Spinal Cord. 2008;46(2):118–123. doi: 10.1038/sj.sc.3102077. [DOI] [PubMed] [Google Scholar]
- 7.Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129(Pt 1):55–64. doi: 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- 8.Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: Insights into central nervous system function. Science. 1988;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 9.Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 10.Gorecki J, Hirayama T, Dostrovsky JO, Tasker RR, Lenz FA. Thalamic stimulation and recording in patients with deafferentation and central pain. Stereotact Funct Neurosurg. 1989;52(2-4):219–226. doi: 10.1159/000099504. [DOI] [PubMed] [Google Scholar]
- 11.Hirayama T, Dostrovsky JO, Gorecki J, Tasker RR, Lenz FA. Recordings of abnormal activity in patients with deafferentation and central pain. Stereotact Funct Neurosurg. 1989;52(2-4):120–126. doi: 10.1159/000099492. [DOI] [PubMed] [Google Scholar]
- 12.Jeanmonod D, Magnin M, Morel A. Thalamus and neurogenic pain: Physiological, anatomical and clinical data. Neuroreport. 1993;4(5):475–478. doi: 10.1097/00001756-199305000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR. Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain. Brain Res. 1989;496(1-2):357–360. doi: 10.1016/0006-8993(89)91088-3. [DOI] [PubMed] [Google Scholar]
- 14.Desouza DD, Moayedi M, Chen DQ, Davis KD, Hodaie M. Sensorimotor and pain modulation brain abnormalities in trigeminal neuralgia: A paroxysmal, sensory-triggered neuropathic pain. PLoS One. 2013;8(6):e66340. doi: 10.1371/journal.pone.0066340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gustin SM, et al. Different pain, different brain: Thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J Neurosci. 2011;31(16):5956–5964. doi: 10.1523/JNEUROSCI.5980-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youssef AM, et al. Differential brain activity in subjects with painful trigeminal neuropathy and painful temporomandibular disorder. Pain. 2014;155(3):467–475. doi: 10.1016/j.pain.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Gustin SM, et al. Thalamic activity and biochemical changes in individuals with neuropathic pain after spinal cord injury. Pain. 2014;155(5):1027–1036. doi: 10.1016/j.pain.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walton KD, Dubois M, Llinás RR. Abnormal thalamocortical activity in patients with complex regional pain syndrome (CRPS) type I. Pain. 2010;150(1):41–51. doi: 10.1016/j.pain.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Talley EM, et al. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19(6):1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh SW, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508(7495):207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contreras D, Llinás R. Voltage-sensitive dye imaging of neocortical spatiotemporal dynamics to afferent activation frequency. J Neurosci. 2001;21(23):9403–9413. doi: 10.1523/JNEUROSCI.21-23-09403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA. 1989;86(5):1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steriade M, Amzica F, Contreras D. Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. J Neurosci. 1996;16(1):392–417. doi: 10.1523/JNEUROSCI.16-01-00392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llinás R, Urbano FJ, Leznik E, Ramírez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28(6):325–333. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- 26.Jones EG. Thalamocortical dysrhythmia and chronic pain. Pain. 2010;150(1):4–5. doi: 10.1016/j.pain.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Gerke MB, Duggan AW, Xu L, Siddall PJ. Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neuroscience. 2003;117(3):715–722. doi: 10.1016/s0306-4522(02)00961-2. [DOI] [PubMed] [Google Scholar]
- 28.Hains BC, Saab CY, Waxman SG. Alterations in burst firing of thalamic VPL neurons and reversal by Na(v)1.3 antisense after spinal cord injury. J Neurophysiol. 2006;95(6):3343–3352. doi: 10.1152/jn.01009.2005. [DOI] [PubMed] [Google Scholar]
- 29.Saab CY. Pain-related changes in the brain: Diagnostic and therapeutic potentials. Trends Neurosci. 2012;35(10):629–637. doi: 10.1016/j.tins.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Llinás R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297(5865):406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- 31.Messinger RB, et al. In vivo silencing of the Ca(V)3.2 T-type calcium channels in sensory neurons alleviates hyperalgesia in rats with streptozocin-induced diabetic neuropathy. Pain. 2009;145(1-2):184–195. doi: 10.1016/j.pain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steriade M, Domich L, Oakson G, Deschênes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57(1):260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- 33.Soltesz I, et al. Two inward currents and the transformation of low-frequency oscillations of rat and cat thalamocortical cells. J Physiol. 1991;441(1):175–197. doi: 10.1113/jphysiol.1991.sp018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer TZ, Tan AM, Waxman SG. Thalamic neuron hyperexcitability and enlarged receptive fields in the STZ model of diabetic pain. Brain Res. 2009;1268:154–161. doi: 10.1016/j.brainres.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 35.Fischer TZ, Waxman SG. Neuropathic pain in diabetes—Evidence for a central mechanism. Nat Rev Neurol. 2010;6(8):462–466. doi: 10.1038/nrneurol.2010.90. [DOI] [PubMed] [Google Scholar]
- 36.Guilbaud G, Benoist JM, Jazat F, Gautron M. Neuronal responsiveness in the ventrobasal thalamic complex of rats with an experimental peripheral mononeuropathy. J Neurophysiol. 1990;64(5):1537–1554. doi: 10.1152/jn.1990.64.5.1537. [DOI] [PubMed] [Google Scholar]
- 37.Leblanc BW, Lii TR, Silverman AE, Alleyne RT, Saab CY. Cortical theta is increased while thalamocortical coherence is decreased in rat models of acute and chronic pain. Pain. 2014;155(4):773–782. doi: 10.1016/j.pain.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Zhao P, Waxman SG, Hains BC. Sodium channel expression in the ventral posterolateral nucleus of the thalamus after peripheral nerve injury. Mol Pain. 2006;2:27. doi: 10.1186/1744-8069-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Na HS, Choi S, Kim J, Park J, Shin HS. Attenuated neuropathic pain in Ca(V)3.1 null mice. Mol Cells. 2008;25(2):242–246. [PubMed] [Google Scholar]
- 40.Kim D, et al. Thalamic control of visceral nociception mediated by T-type Ca2+ channels. Science. 2003;302(5642):117–119. doi: 10.1126/science.1088886. [DOI] [PubMed] [Google Scholar]
- 41.Shin HS, Cheong EJ, Choi S, Lee J, Na HS. T-type Ca2+ channels as therapeutic targets in the nervous system. Curr Opin Pharmacol. 2008;8(1):33–41. doi: 10.1016/j.coph.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Tort AB, et al. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci USA. 2008;105(51):20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tort AB, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104(2):1195–1210. doi: 10.1152/jn.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adamchic I, Langguth B, Hauptmann C, Tass PA. Abnormal cross-frequency coupling in the tinnitus network. Front Neurosci. 2014;8:284. doi: 10.3389/fnins.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho RY, et al. Development of sensory gamma oscillations and cross-frequency coupling from childhood to early adulthood. Cereb Cortex. 2015;25(6):1509–1518. doi: 10.1093/cercor/bht341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tort AB, et al. Cortical networks produce three distinct 7–12 Hz rhythms during single sensory responses in the awake rat. J Neurosci. 2010;30(12):4315–4324. doi: 10.1523/JNEUROSCI.6051-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Todorovic SM, Jevtovic-Todorovic V. T-type voltage-gated calcium channels as targets for the development of novel pain therapies. Br J Pharmacol. 2011;163(3):484–495. doi: 10.1111/j.1476-5381.2011.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 50.Xu M, Aita M, Chavkin C. Partial infraorbital nerve ligation as a model of trigeminal nerve injury in the mouse: Behavioral, neural, and glial reactions. J Pain. 2008;9(11):1036–1048. doi: 10.1016/j.jpain.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dixon WJ. Staircase bioassay: The up-and-down method. Neurosci Biobehav Rev. 1991;15(1):47–50. doi: 10.1016/s0149-7634(05)80090-9. [DOI] [PubMed] [Google Scholar]
- 52.Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41(2-3):365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]