Significance
Bacteria cooperate by secretion of public good molecules, which benefit the entire community. Such cooperative behaviors are often regulated by cell–cell signaling mechanisms. In many species, these signaling systems are highly diversified in their signal–receptor specificity, but the causal link between the function of signaling and the maintenance of high genetic diversity was unclear. Here we demonstrate experimentally that signaling diversity is maintained by facultative cheating—a minority strain with one signaling system will exploit the public goods production of a majority strain that possesses a different system, but resumes cooperation on its own. Mutual facultative cheating demonstrates the complexity of social strategies attained by bacteria through the regulation of cooperative behaviors and their impact on population genetics parameters.
Keywords: social evolution, sociomicrobiology, Bacillus subtilis, bacteria, quorum sensing
Abstract
Bacterial quorum sensing enables bacteria to cooperate in a density-dependent manner via the group-wide secretion and detection of specific autoinducer molecules. Many bacterial species show high intraspecific diversity of autoinducer–receptor alleles, called pherotypes. The autoinducer produced by one pherotype activates its coencoded receptor, but not the receptor of another pherotype. It is unclear what selection forces drive the maintenance of pherotype diversity. Here, we use the ComQXPA system of Bacillus subtilis as a model system, to show that pherotype diversity can be maintained by facultative cheating—a minority pherotype exploits the majority, but resumes cooperation when its frequency increases. We find that the maintenance of multiple pherotypes by facultative cheating can persist under kin-selection conditions that select against “obligate cheaters” quorum-sensing response null mutants. Our results therefore support a role for facultative cheating and kin selection in the evolution of quorum-sensing diversity.
In many bacteria, a cell–cell signaling mechanism, known as quorum sensing, coordinates the response of a bacterial community in a density-dependent manner. Quorum-sensing bacteria secrete a signal molecule known as an autoinducer and express a specific receptor that binds to it with high affinity, resulting in the activation of a specific cellular response (1). Quorum sensing often regulates the secretion of public goods or other cooperative traits that benefit the community, at a cost to the individual responding cell (2).
The regulation of cooperation by a secreted autoinducer allows for the evolution of cheater genotypes that do not produce the autoinducer or do not respond to it (3, 4). Mutants of the latter type were shown to act as cheaters in a variety of different species (3–7). The elimination of these cheater mutants could occur by kin selection, where cooperation is preferentially directed toward other cooperators (3, 7–9).
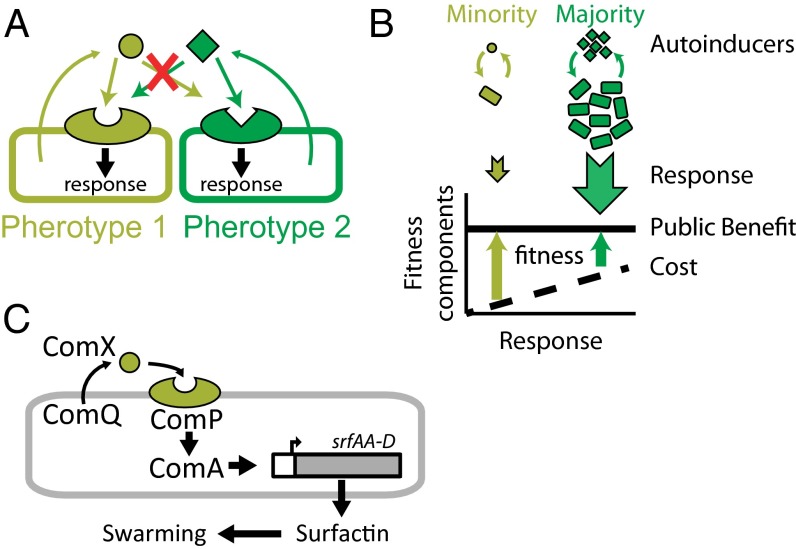
In contrast to the rarity of quorum-sensing response null alleles in wild populations, many species display a high degree of intraspecific genetic variation in functional quorum-sensing alleles, called pherotypes (Fig. 1A). Each allele codes for both receptor and autoinducer genes, where an autoinducer coded by one pherotype will activate its coencoded receptor, but not the receptors encoded by other pherotypes (10–14). Pherotypes differ in their receptor–autoinducer specificity but not in the pathways regulated by the receptor. In addition, many pherotypes show patterns of intraspecific horizontal gene transfer (10, 12) and coexist in the same environment (15, 16).
Fig. 1.
Quorum-sensing pherotypes and the Bacillus Com quorum-sensing system. (A) Pherotype variability is defined when two or more homologous receptor and autoinducer alleles are found in the population. Each allele codes for autoinducer production genes and a receptor gene. The autoinducer produced by a cell carrying one pherotype specifically activates the receptor of the same pherotype, but not the receptor of the other pherotype. (B) Selection for minority pherotype when quorum sensing controls public goods. A minority pherotype (light green) will produce less signal than the majority pherotype (dark green). Consequently the cost of quorum-sensing response of the minority is lower. The benefits of quorum-sensing response are public and shared by all cells. The fitness of the minority pherotype is therefore larger than the fitness of the majority and it will invade into the population. (C) A scheme of the ComQXP pathway. ComQ cleaves and modifies ComX to make the mature secreted autoinducer, which binds the receptor ComP. Bound receptor activates the transcription factor ComA, which regulates production and secretion of surfactin through the srfA operon. Surfactin is necessary for swarming.
The mechanisms that lead to the diversification of pherotypes, to the maintenance of their diversity, and to their rapid horizontal gene transfer are not well understood. We have previously proposed, by analyzing a theoretical model, that if quorum sensing regulates cooperation, novel pherotypes can arise adaptively through sequential selection of a receptor mutation followed by selection for a compensating mutation that changes the autoinducer (17). The model also suggested that different pherotypes will coexist by facultative cheating (18)—each pherotype cheats as a minority and returns to cooperation when its frequency increases (Fig. 1B). This model can thus explain both the observed diversity and the rapid horizontal gene transfer of quorum-sensing alleles.
The Bacillus subtilis ComQXP quorum-sensing system is one of the best-studied systems with multiple characterized pherotypes (Fig. 1C) (19). This system is encoded by a single locus that contains a three-gene operon. The ComX autoinducer production genes (comQ, comX) and the region of comP encoding for the extracellular part of the ComP receptor are highly variable and encode for multiple different pherotypes, which coexist in the soil and undergo rapid horizontal gene transfer (10, 15, 16, 20). The interaction between different comQXP pherotypes also includes cases of asymmetric cross-activation or cross-inhibition, where a ComX autoinducer of one pherotype would activate the receptor of another pherotype, but not vice versa, or when the autoinducer inhibits the receptor of another pherotype (10, 20). The ComQXP quorum-sensing system activates the ComA transcription factor, which regulates a large array of genes, including the srfA operon (19). The srfA operon encodes for the structural enzymes necessary for the production of the surfactant Surfactin (21). Importantly, no quorum-sensing response mutants were observed in natural populations of B. subtilis (16, 22).
In this work, we examine the maintenance of multiple pherotypes by using the B. subtilis ComQXP quorum-sensing system as a model system. First, we verify that this system regulates cooperative swarming behavior. Next, we find that cocultured pherotypes undergo negative frequency-dependent selection by mutual facultative cheating during swarming. Finally, we show that kin selection, brought about by repeated population bottlenecks, maintains pherotype coexistence, while selecting against quorum-sensing response null cheaters. Our results therefore support the importance of facultative cheating and kin selection in the evolution of pherotype diversity.
Results
The ComQXPA Pathway Regulates Cooperation During Swarming Motility.
According to our model, pherotypes will be maintained by facultative cheating if quorum sensing regulates cooperative behaviors, such as the production of secreted molecules [e.g., extracellular enzymes (4) or surfactants (23)]. In B. subtilis, ComA regulates the production of surfactin, which is crucial for swarming motility (24). In addition, exogenous addition of surfactin was shown to rescue the swarming phenotype of strains defective in surfactin production, suggesting that surfactin is a public good (24). Despite the regulation of surfactin by ComA, a quorum-sensing response mutant (∆comP) displayed a moderate swarming phenotype, characterized by slowed swarming, but not a complete lack of it (25). A ΔcomA deletion mutant displayed a similar phenotype, despite the strong effect this mutation had on the expression of the surfactin production operon, srfA (26). We reasoned that previously used swarming conditions led to very high cell densities, allowing the residual surfactin produced by each bacterium to accumulate to high extracellular concentrations and thus maintain the swarming phenotype (25, 27). We therefore studied swarming, using a minimal medium containing a low concentration of glucose (0.005%), compared with the concentration of previously used swarming media. We find that under these conditions the maximal cell density was dramatically reduced, enabling the swarming of the proficient wild type but not of its respective comQXP or comA mutants (Fig. 1 and Figs. S1 and S2). We therefore used the low-glucose conditions in further experiments, as they probably better reflect the strong dependence of swarming on the Com system in naturally relevant low-nutrient conditions.
Fig. S1.
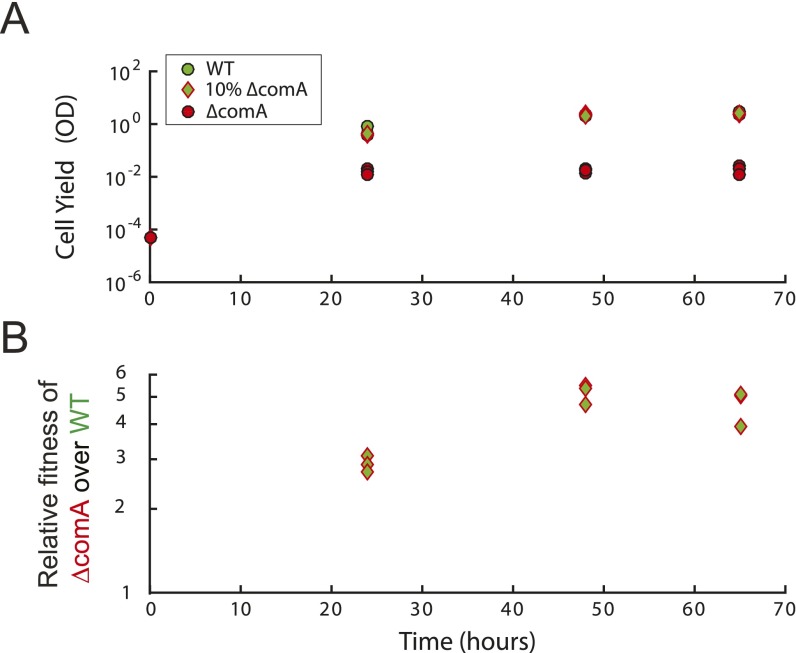
Temporal dynamics of swarming cultures. (A) Average population fitness as a function of time of the wild type (AES2030), ∆comA (AES3002), and a coculture mixture with initial ∆comA frequency of 10% . Average population fitness is measured by the total cell yield of the population in OD units. All populations were inoculated by the same number of cells and allowed to grow under swarming conditions. For all measured time points, the ∆comA pure culture had a severely reduced average fitness, whereas the WT pure culture and a coculture of wild type and ∆comA at a 10% initial ∆comA frequency had a comparable cell yield. (B) Relative fitness of the ∆comA mutant during coculture with the WT strain as a function of time. Three cocultures were initiated with a starting ∆comA frequency of 10%. For each time point, the relative fitness of the ∆comA strain was calculated as described in Methods. The relative fitness of the ∆comA strain over the WT increases between 24 h and 48 h (P value = 7.3846e-04, two-sample t test, n = 6), but remains the same between 48 h and 65 h (P value = 0. 3678, two sample t test, n = 6).
Fig. S2.
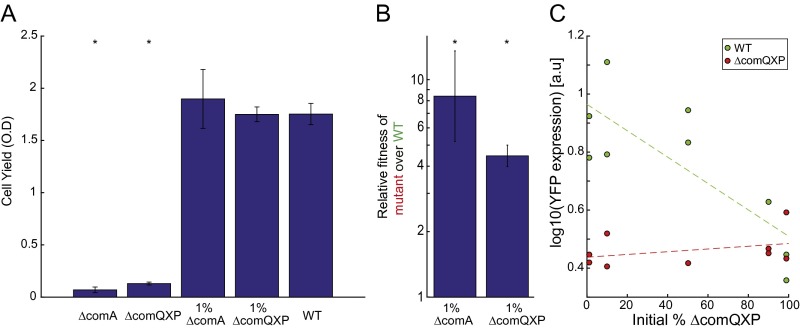
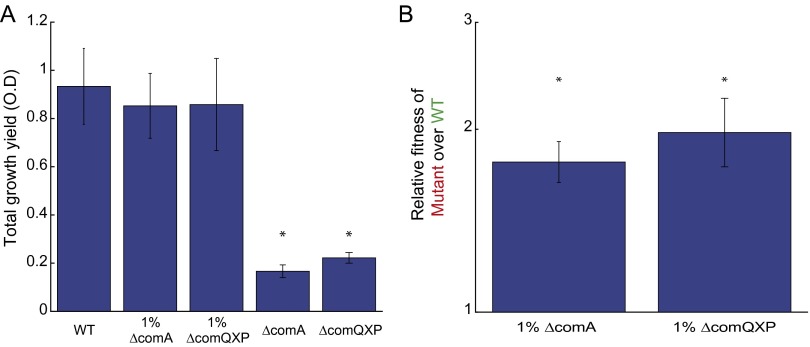
ΔcomQXP is an obligate cheater of the wild type. (A) ΔcomQXP (AES2198) and ΔcomA (AES3002) mutants were grown under swarming conditions either separately or each in coculture with the wild type (AES2030) at an initial mutant frequency of ∼1%. Total cell yield of the mutants is very low whereas both the pure wild-type swarm and the coculture swarms had similarly high yields. Asterisks represent a statistically significant difference from the WT in terms of total cell yield (P < 0.01). (B) Relative fitness of the ΔcomQXP (AES2198) and ΔcomA (AES3002) mutants over the wild type (AES2030) in coculture. Both strains strongly invaded into the wild type. We find that ΔcomA is a stronger cheater than ΔcomQXP, as it has lower yield as pure culture and stronger invasion into the wild type in mixed culture. Asterisks represent a statistically significant relative fitness (P < 0.01). In A and B error bars mark SE (n > 3 in all experiments). (C) YFP gene expression of the PsrfA-YFP reporter measured as a function of ΔcomQXP strain frequency for the ΔcomQXP strain (red, coculture of AES2078 and AES2033) and the wild type (green, coculture of AES2075 and AES2058) . Compare with Fig. 2C of the main text.
It was previously shown that exogenously added surfactin can rescue the swarming phenotype of surfactin production mutants, implying that ComQXPA quorum-sensing response mutants will be able to exploit surfactin-producing strains during coculture. To examine this hypothesis, we cocultured a swarming-proficient derivative of the laboratory strain [swrA+;sfp+ strain (25)] with an isogenic ΔcomA quorum-sensing response mutant at varying initial frequencies of the two strains and monitored their growth (Fig. 2 and Fig. S1). The average growth rate of the culture was determined by measuring the total cell number after 65 h of growth. The relative fitness of the two strains was measured by determining the change in frequency of the two strains during swarming cocultures. Relative fitness of genotype 1 over genotype 2 is defined as the ratio of the frequencies of each strain at the end of the experiment to their ratio at the beginning of the experiment (28). Relative fitness measurement was made possible by introducing different constitutive fluorescent reporters, which allowed us to monitor genotype frequencies before and after swarming (Methods). We find that in contrast to slower, motility-independent, forms of growth, swarming does not lead to significant segregation of genotypes (Fig. S3) (29). Swarming in B. subtilis can therefore be regarded as an unstructured environment to a good approximation (23).
Fig. 2.
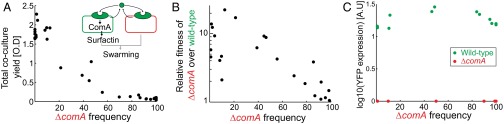
A ΔcomA mutant is an obligate cheater of the wild type during swarming. (A and B) Final cell yield (A) and relative fitness of the ΔcomA strain (B) were measured for different cocultures of ΔcomA (strain AES3001) and wild type (strain AES2137), as a function of the initial frequency of the ΔcomA strain in each coculture (Methods). (C) Average per cell quorum-sensing-dependent gene expression in a swarming coculture, as a function of the initial frequency of the ΔcomA strain in the coculture. Per cell average response was measured by flow cytometry, using a PsrfA-YFP construct inserted either into the wild type [green, in coculture between strains AES2075 (wild type with reporter) and AES3007 (ΔcomA)] or into the ΔcomA [red, in coculture between strains AES2033 (wild type) and AES3008 (ΔcomA with reporter)] strains. Further details of the strains used for A–C are given in Table S1. Each data point in A–C represents a measurement from a different swarming plate. Experiments were done on multiple days.
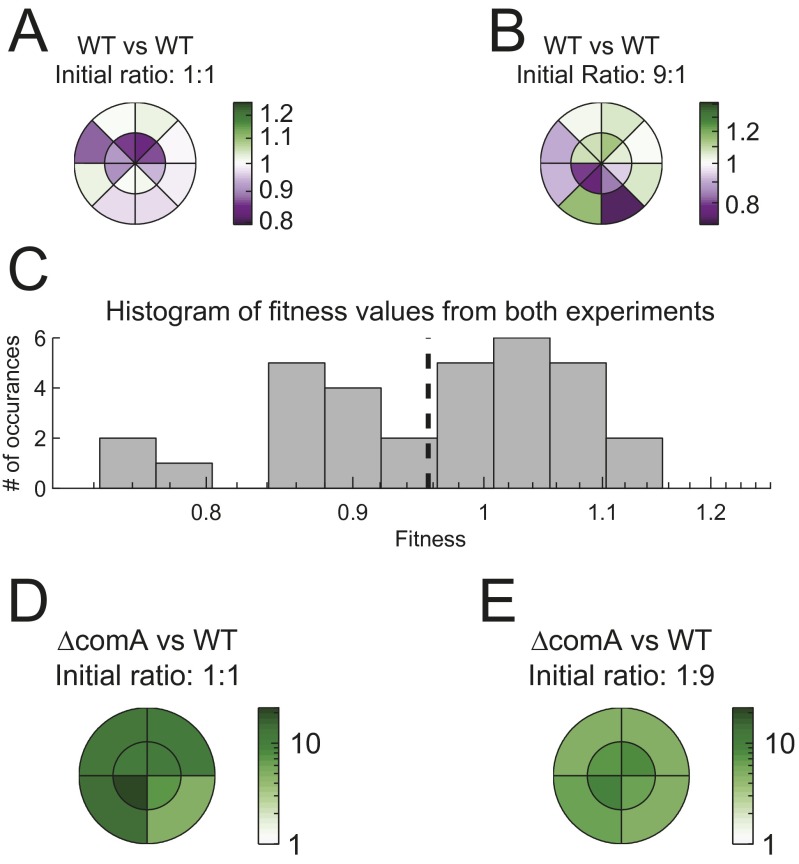
Fig. S3.
Spatial distribution of genotypes during swarming of coculture. (A and B) Two differentially marked wild-type strains (AES2030 and AES2137), harboring both a YFP and an RFP marker or only YFP, are inoculated at (A) 1:1 and (B) 9:1 ratio on separate swarming plates. Sixty-five hours after the initiation of swarming, 16 samples were taken from different positions of the swarm. Samples were taken at two different radii and at eight different angular positions, as marked in A and B. For each position we calculated the apparent relative fitness between strains, which is defined as the local relative frequency in the sample divided by the relative frequency of the inoculum at the beginning of the experiment. The color bar marks the deviation from zero fitness difference. (C) A histogram of all apparent fitness difference measurements. The mean of the histogram is −0.977 ± 0.0186 (mean ± SE), which is not significantly different from 1 difference (t test, P = 0.1 for mean = 1). The SD, σ = 0.048 sets the limit for our ability to distinguish between real selection and apparent selection during swarming. (D and E) The same as in A and B, but for a coculture of ΔcomA (AES3002) and the wild type (AES2030), with an initial ΔcomA frequency of (D) 50% and (E) 10%. ΔcomA has a significant growth advantage over the wild type everywhere.
We find that a quorum-sensing reception mutant (either a ΔcomA mutant or a ΔcomQXP mutant) displayed the signatures of “cheating” behavior (Fig. 2A and Fig. S2). First, the cell yield of the culture was reduced as the frequency of the ∆comA mutant increased (Fig. 2A). Second, the ΔcomA mutant had a pronounced fitness advantage, which led to its invasion into the population (Fig. 2B). At a low initial frequency, the mutant had a >50-fold growth advantage over the wild type. This growth advantage was reduced, but remained positive, as the initial frequency of the ΔcomA mutant increased (Fig. 2B, t test, P value = 1.6e-07, n = 23). The reduction in the relative fitness of the ΔcomA strain can be attributed to the large reduction in the average growth of the coculture.
To further explore the observed cheating behavior, we used a PsrfA-YFP transcriptional reporter to monitor the quorum-sensing response of the wild type and the ΔcomA mutant during swarming coculture (Methods). We find that the average per cell srfA expression level of the wild type was significantly higher than that of the ∆comA mutant (Fig. 2C, two-sample t test, P value = 4.2e-08, n = 12). The expression level of the wild type was constant irrespective of the initial frequency of the two strains [Fig. 2C, linear regression, F(1,10) = 0.237, n = 12, P value = 0.637 for constant model null hypothesis]. This finding supports the obligatory cheating strategy of the ΔcomA mutant. In contrast, when the wild type was cocultured with a ΔcomQXP mutant, the wild-type average per cell quorum-sensing response decreased with the mutant frequency, but was always higher than that of the mutant (Fig. S2, t test, P value = 0.0026, n = 9). This probably reflects the reduction in the total signal level with the increase of the ΔcomQXP mutant frequency (30).
These results were obtained from a swarming-proficient derivative of the domesticated laboratory strain (24, 27). The domestication of the laboratory strain has been accompanied by a multitude of mutations and the loss of additional traits and regulatory circuits in addition to those related to swarming, such as the ability to form biofilms (31). To verify that these additional mutations do not significantly affect the social interactions between ComQXPA variants, we repeated some of the experiments in a biofilm-forming isolate (32). We find that in this genetic background, the ΔcomA and ∆comQXP mutants exploited the wild type, as was observed in the laboratory strain background, albeit with a lower relative fitness (Fig. S4). The reduced relative fitness most likely reflects the lower levels of cooperative investment observed in this genetic background (32). Together, our results suggest that the quorum-sensing response, and specifically the production of surfactin during swarming, is a costly cooperative behavior and that the wild type and the ΔcomA strain behave as a cooperator and an obligate cheater, respectively.
Fig. S4.
Cheating behavior is maintained in a biofilm-forming B. subtilis strain. (A and B) The same measurements as in Fig. S2 A and B were made in a genetic background of strain DS2569—a derivative of the biofilm-forming strain NCIB3610 that lacks the plasmid pBS32 (31, 32). In A, asterisks represent a statistically significant difference from the WT in terms of total growth yield (P < 0.01). In B, asterisks represent a statistically significant difference from zero (P < 0.01). Strains used are AES1822 (WT), AES3450 (ΔcomA), and AES2479 (ΔcomQXP). In A and B error bars mark SE (n > 3 in all experiments).
Different Pherotypes Are Facultative Cheaters of Each Other During Swarming.
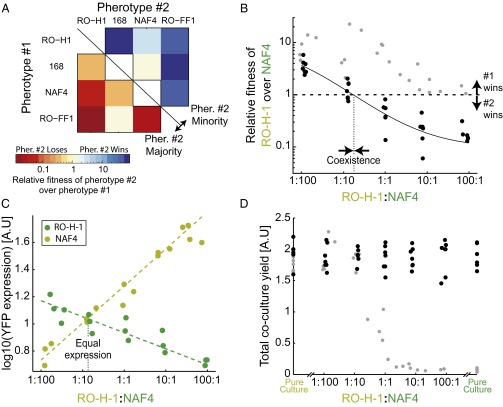
Our model predicts that if quorum sensing regulates cooperative behavior, different pherotypes will perform mutual facultative cheating—a minority pherotype will have reduced cooperative investment compared with the majority, by virtue of its lower density in the population and thus its lower autoinducer concentration. This will lead to exploitation of the majority by the minority pherotype and invasion into the population. In contrast to an obligate cheater, the minority pherotype will resume cooperation as its frequency increases, owing to the increasing autoinducer concentration (17) (Fig. 1B). To test this key prediction in B. subtilis, we studied the interaction between several different pherotypes under swarming conditions. We deleted the endogenous comQXP allele and introduced a comQXP locus of one of four different strains with different pherotypes (168, RO-H-1, NAF4, and RO-FF-1) into an ectopic location (20). We cocultured all possible pairs of strains. For each pair we performed two competitions, where one or the other pherotype is a minority with an initial frequency of ∼1% (Fig. 3A). In almost all cases, a strain had a fitness advantage as a minority and a fitness disadvantage as a majority. We found two exceptions to this trend when either pherotype RO-FF-1 or NAF-4 were cocultured as a minority with pherotype 168. The former case most likely arises from the asymmetric signaling interactions between strains 168 and RO-FF-1, where the former activates the latter, but not vice versa (20).
Fig. 3.
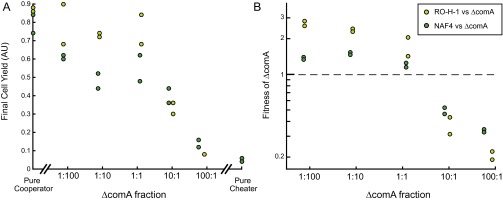
Pherotypes display negative frequency-dependent selection due to facultative cheating under swarming conditions. (A) An interaction matrix between all pairs of nonidentical pherotypes. For each pherotype pair, either pherotype 1 (bottom left) or pherotype 2 (top right) was inoculated as a minority. Each colored square marks the relative fitness of pherotype 2 over pherotype 1, according to the color bar. Strains used are AES3005 (pherotype RO-H-1), AES2355 (pherotype 168), AES3003 (pherotype NAF4), and AES3530 (pherotype RO-FF-1). (B) Shown in black circles are the relative fitness values of the RO-H-1 pherotype over the NAF4 pherotype for varying initial ratios of RO-H-1:NAF4 (strains AES3005 and AES3004). The solid black line serves as a guide to the eye. For comparison, the data of Fig. 2B are redrawn on the same scale and shown as gray circles (note that for these data points, the x axis is the ΔcomA:wild-type frequency ratio). (C) Average per cell YFP gene expression of the PsrfA-YFP reporter inserted into the chromosomes of RO-H-1 (light green, coculture of AES3012 and ASE3009) or NAF4 (dark green, coculture of AES3011 and AES3010) strains. Expression was measured at the end of swarming for cocultures with varying initial ratios of the RO-H-1 and NAF4 strains. (D) Final cell yield of swarming cocultures with varying initial ratios of the RO-H-1 and NAF4 pherotype strains (black circles). Yields were also measured for pure cultures of the two strains. As in B, gray circles are a representation of the data of Fig. 2B on the relative scale and serve for comparison. Each data point in B–D represents a measurement from a different swarming plate. Experiments were done on multiple days.
To better understand selection dynamics between strains, we further studied the swarming behavior of strains NAF4 and RO-H-1, as these systems are both orthogonal and exogenous to the 168 background. We cocultured the strains in varying initial frequencies under swarming conditions. We find that the two strains exhibited negative frequency-dependent selection—as a small minority, each strain had a fitness advantage over its cocultured majority pherotype (Fig. 3B). Selection between pherotypes was not symmetrical—the two strains had no relative fitness advantage at a RO-H-1:NAF4 ratio of ∼1:5 (Fig. 3B, x axis intercept at RO-H-1:NAF4 = 0.18, x axis intercept 95% confidence interval [0.11, 0.26], linear slope of line = −0.44725 [linear regression, F(1,26) = 148, n = 28, P value = 3.0644e-12 to a no-selection null hypothesis]). The two strains therefore coinvade each other and coexist at an intermediate frequency.
If negative frequency dependence is due to facultative cheating, the fitness advantage of the invading strain should result from its reduced investment in the quorum-sensing response and in particular in srfA expression. Using the PsrfA-YFP reporter, we measured the average per cell gene expression of each of the strains in a swarming coculture (Methods and Fig. 3C). We find that gene expression patterns correspond well with selection. The minority strain has a lower per cell srfA expression level than the majority strain and the frequency of equal expression corresponded well with the frequency of coexistence (Fig. 3C, compare with Fig. 3B, 95% confidence intervals [0.1, 0.2], P value > 0.05). The gene expression patterns agree with the asymmetry of selection strength between the two pherotypes. We find that the RO-H-1 expression levels as a majority are higher than those of NAF-4 as a majority (Fig. 3C). This asymmetry can stem from higher gene expression of the comQXPRO-H-1 system or a higher affinity between the ComPRO-H-1 receptor and its ComXRO-H-1 autoinducer, compared with the affinity of the NAF4 receptor–autoinducer pair.
We expect that facultative cheating will have only a weak effect on average population fitness, as most cells strongly invest in cooperative activity. We measured the total yield of a swarming coculture containing the two pherotypes (Fig. 3D). In agreement with our expectation, we find the yield to be high and independent of the initial frequency of the two strains [Fig. 3D, linear regression, F(1,47) = 0.0161, n = 49, P value = 0.89971 to a zero slope null hypothesis]. Together, our results suggest that a minority pherotype will invade a majority pherotype to a stable coexistence by facultative cheating, without significantly altering the average fitness of the population.
Kin Selection Maintains Pherotype Diversity While Eliminating Obligate Cheating.
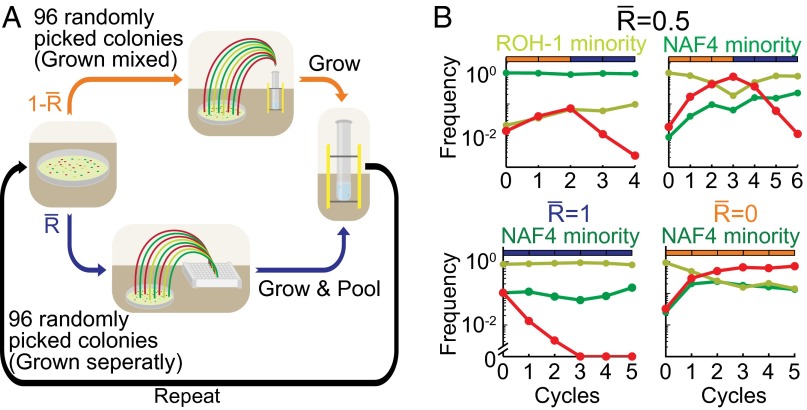
Whereas swarming conditions select for coexistence of pherotypes (Fig. 3), they also select for the invasion and the eventual fixation of quorum-sensing response mutants such as ΔcomA (Fig. 2). Kin-selection theory predicts that cooperation can be maintained if the relatedness between cooperating bacteria is sufficiently high. In bacteria, relatedness can be established if the population goes through growth bottlenecks, and it has been shown that this mechanism is effective in eliminating cheater mutants from the population (3, 33–35). To test for the effect of bottlenecks on pherotype variability and the elimination of quorum-sensing response mutants, we performed three-way competitions between the two pherotypes and the ΔcomA strain in a simple experimental assay, where the population is undergoing repeated cycles of growth and propagation with varying frequencies of growth bottlenecks. At the beginning of each cycle, 96 isolates are randomly chosen from cells of the previous growth cycle and are then propagated either in coculture or in multiple clonal cultures, in a medium requiring quorum-sensing-dependent cooperation for growth (see Fig. 4A legend and Methods for further details). The level of relatedness between cooperating cells is determined by the frequency of cycles that initiates in a growth bottleneck. To facilitate our ability to perform a large number of growth experiments simultaneously, we engineered a synthetic quorum-dependent public good, by placing the amyE gene, coding for secreted α-amylase enzyme, under the regulation of a copy of the srfA promoter (Methods and Fig. S5). This allowed us to use liquid media with starch as the main carbon source for the cooperative growth assay.
Fig. 4.
Pherotypes coexist whereas cheaters are eliminated in the presence of population bottlenecks. (A) A scheme of the selection process. In each cycle, 96 single colonies are either mixed together into a single test tube (no bottlenecks, orange) or used to inoculate 96 separate wells (strong bottlenecks, blue). The frequency of clonal cycles in the process is defined as . Cells are grown in minimal medium containing soluble starch as a main carbon source and express the gene encoding for the starch-degrading exoenzyme α-amylase under the control of the srfA promoter (Methods and main text). (B) Results of different selection schemes with varying . Shown are the absolute frequencies of the ΔcomA (red, AES1341), comQXPRO-H-1 (light green, AES3014), and comQXPNAF4 (dark green, AES3013) genotypes during coculture of the three genotypes, as a function of the cycle. The parameter used in each experiment is marked above each graph as well as the identity of the minority pherotype. Blue and orange rectangles at the top of each graph are used to mark the cycles with and without population bottlenecks, respectively.
Fig. S5.
Competition in starch. Shown is a coculture between each of the strains with different pherotypes [ROH-1 (AES3014), light green; NAF4 (AES3013), dark green] with a ΔcomA mutant strain (AES1341). We note that the mutant strain produces signal of a third type (pherotype 168, which has low impact on the receptors of the other pherotypes). Shown are (A) final yield at the end of growth and (B) relative fitness advantage of the mutant, as a function of mutant frequency. We note that unlike the swarming behavior, in starch there is a snowdrift interaction between the mutant and the two pherotypes—the mutant loses fitness advantage at a 3:1 ratio, where growth is reduced. As discussed in ref. 17, the snowdrift has little effect on the ability of the mutant to invade in a structured population.
We find that at zero relatedness (purely well-mixed cycles), both the ΔcomA strain and the minority pherotype initially invaded into the population, but eventually only the ΔcomA strain prevails (Fig. 4B, Bottom Right). In contrast, at an intermediate level of relatedness (R = 0.5), the ΔcomA strain is selected against after the entire selection scheme is completed, whereas each of the pherotypes invaded from rarity into the population (Fig. 4B, Top). Finally, in pure clonal growth (relatedness of one), the ΔcomA strain is quickly eliminated from the population, whereas the relative frequency of the minority pherotype remains approximately constant (Fig. 4B, Bottom Left). The nonmonotonic dependence of pherotype coexistence on relatedness is due to the combination of two effects—at low relatedness, the cheater mutant overcomes both pherotypes, whereas if relatedness is sufficiently high, it will be eliminated. On the other hand, the invasion rate of the minority pherotype approaches zero as relatedness approaches unity (36).
Discussion
In this work, we showed that comQXP pherotype allelic diversity can be maintained by negative frequency-dependent selection through mutual facultative cheating between strains encoding for different pherotypes. We then demonstrated how kin selection through population bottlenecks could simultaneously explain both the observed standing genetic variation of pherotypes and the rarity of quorum-sensing null mutants. Importantly, the bottleneck structure is a generic mechanism, which illustrates the ability of the structured population to select for pherotype variability. We do not know how well the bottleneck model applies to the natural life history of B. subtilis.
Facultative cheating was previously defined and identified in the context of fruiting-body forming bacteria and amoebas (18, 37). The complexity of fruiting-body development, however, hinders the elucidation of the molecular mechanisms underlying this behavior (36). In this work, facultative cheating is directly associated with a specific molecular mechanism on the one hand and with observed population genetic diversity patterns on the other. It is important to note that not all bacteria show intraspecific variability in quorum-sensing pherotypes. Pherotype diversity can be constrained by the molecular diversity available to the quorum-sensing signal, by mechanisms that prevent the diversification process (17), or by additional mechanisms that select against exploitation (38).
Pherotypes can be considered as a type of kin-recognition “tags,” whose existence, evolution, and impact on the fate of cooperation have been a focal interest in social evolution and signaling theory (e.g., refs. 39–41). Notably, it has been suggested that variability in several bacterial traits, such as the production of bacteriocins (42), contact-dependent inhibition toxins (43), or colony segregation (44, 45), is maintained through kin-recognition mechanisms. Typically, kin-recognition tags are considered to direct cooperative behavior only among organisms with the same tag or to direct aggressive behavior only toward organisms with a different tag. These interactions naturally lead to positive frequency-dependent selection for the majority tag, which tends to reduce tag variability (39). Under such conditions, tag variability is under a constant threat of elimination and can be maintained only by combining population structure and dynamically complex interactions with tag-bearing cheaters, which tend to eliminate the advantage of the most frequent tag in the population (40–42, 46). In contrast, quorum sensing controls only the decision to cooperate, but not the beneficiaries of cooperation. Under these conditions, interaction between tags (pherotypes) directly leads to negative frequency-dependent selection by mutual facultative cheating. Although a structured population is still required in that case to eliminate obligate cheaters, the additional complications are avoided and tag variability is directly favored.
An alternative explanation for the maintenance of pherotype diversity [and kin tags in general (41)] is that it can arise from apostatic selection (47) between the signaling bacteria and another organism that uses the quorum-sensing system to identify the bacteria and attack them. We find this to be unlikely due to the cytoplasmic location of multiple receptors with diverging pherotypes (more discussion in Supporting Information).
From an ecological perspective, facultative interactions can occur between different species in a multispecies population, and these may explain some of the diversity found in microbial communities (48). Recent work has demonstrated the ecological richness that can be attained by the chemical diversity of antibiotic production and degradation (49, 50). Our work demonstrates that a similar ecological richness may arise from the diversity of chemical signaling.
Methods
Detailed information on growth media and strain construction is given in SI Methods and Table S2.
Table S2.
Primer list
| Primer | Sequence* |
| ComA del P1 | AAGTTGGACCGGACTGGAAT |
| ComA del P2 | TTTTCTAATGTCACTAACCTGCCAAACTGTTCGCTCGGTTCAG |
| ComA del P3 | AGTAATCCGCCCGACGGTATAGCGGTCCATTGAATACAGC |
| ComA del P4 | GGTGAGCCGGTGATGTTTAC |
| Psrf F | ATGGGGAATTCCGTTGTAAGACGCTC |
| Psrf R | AGGTGGCTAGCTTTATAAGCAGTGAACAT |
| Psrf-AmyE P1 | GTTCTGGCGAAGCCGTTGTATTTATTCC |
| Psrf-AmyE P2 | ATAAACCCTTGCATAGGGGGTCAAAACAACTTGGCAGAGTGAATACAAATCAATGT |
| Psrf-AmyE P3 | ATGTTCACTGCTTATAAAAAGCGTTAACAAAATTCTCCAGTCTTCACATCGG |
| Psrf-AmyE P4 | ATATTCGACACGCCCAGATTACGATTCTT |
| sfp P1 | CCGCCATCCTCACCGGACTT |
| sfp P2 | ATTATGTCTTTTGCGCAGTCGGCTTCCTCAGGATCTGCCCGCC |
| sfp P3 | CATTCAATTTTGAGGGTTGCCAGGTGCATACAGGGTGCCTGCC |
| sfp P4 | GCAGGAGCTGGAAAAGCGCC |
| swrA seq | TGCATCGACCTTTTTATATCTGTCAG |
| ComQXP-P1 | TAAAGACCGTATCCACTTCATGCCG |
| ComQXP-P2 | TGCCCGCAGCTGTGACAACCCCCTCCCATTCCATTTTACT |
| ComQXP-P3 | GTAGCGCGGTGGTCCCACGGCTTTAGATGGGCGCCT |
| ComQXP-P4 | GGTTGGCGTTAATCTCCAAACCAAC |
| ComQXP (RO-H-1) F | AGCTGAATTCAGTCGTTTCCGTTATAAAACCATTACA |
| ComQXP (RO-H-1) R | AGCTGGATCCACAAAAGCATTGATCAGCTCGA |
| ComQXP (NAF-4) F | AGCTGGATCCACAATTATGCAATGAAAATTTCGTGA |
| ComQXP (NAF-4) R | AGCTGAATTCTGTGCCAAGTCGTTTCCGTT |
| ComQXP (RO-FF-1) F | AGCTGAATTCTCATTACGAAACATTAACAAAAGCATTGATCA |
| ComQXP (RO-FF-1) R | AGCTGGATCCACTTGGCCTGTGCCAAGTCGTTT |
| ComQXP (168) F | AGCGGATCCCAGATTCATTACGAAACATT |
| ComQXP (168) R | CGAGAATTCTTACAATTCCATTTCAATATC |
Restriction sites used are underlined.
Swarming Assays.
Swarm plates were made of Spizizen minimal media (SMM) containing 0.005% glucose (wt/vol) and supplemented with 0.7% (wt/vol) Bacto-agar. Briefly, cells were grown for 1 d in minimal media before their inoculation and then placed in a humid incubator set to 30 °C for the designated time. Cells were then collected from the plates into a fixed volume of 5 mL. Cell yield was measured using optical density whereas population proportions were measured using a flow cytometer. Further details are in SI Methods. Relative fitness of genotype 1 over genotype 2 is determined as , where are the frequencies of genotype 1 at the end and beginning of the experiment, correspondingly.
Structured Population Assay.
Growth was conducted in liquid SMM media with soluble starch as the major carbon source (0.2% wt/vol with addition of 0.01% glucose). Each cycle of growth was either clonal or mixed, where the overall frequency of each mode of growth over the entire experiment is determined by the average R parameter. At the beginning of each growth cycle, 96 colonies are randomly picked from a plate and subsequently are either mixed into a single test tube (no bottleneck cycle) or clonally grown in separate wells of a 2-mL 96-well plate (bottleneck cycle). Cells were allowed to grow for 24 h and then pooled and plated on an LB plate, to initiate the next growth cycle.
SI Methods
Growth Media and Conditions.
Growth and swarming were performed in Luria–Bertani (LB) broth and Spizizen minimal media (SMM) supplemented with trace elements as detailed in the text (51). Antibiotic concentrations used were as follows: macrolides, lincosamides, and streptogramins (MLS) (1 µg⋅mL−1 erythromycin, 25 µg⋅mL−1 lincomycin); spectinomycin (100 µg⋅mL−1); tetracycline (10 µg⋅mL−1); chloramphenicol (5 µg⋅mL−1); and kanamycin (15 µg⋅mL−1). A total of 0.01 M phosphate-buffered saline (PBS) (pH 7.4) was used for dilution and suspension of cells.
Strain Construction.
All of the strains used in this study are listed in Table S1. Table S1 also details which strains were used in which figure in the main text. Molecular cloning and integration into the B. subtilis genome were done using standard protocols (51). Deletion mutations and their replacement with the indicated antibiotic resistance cassette were performed using the long flanking homology PCR method (52). One-kilobase fragments corresponding to regions upstream and downstream of the target gene were amplified by PCR. The 5′ end of the reverse primer for the upstream region and the 3′ end of the forward primer for the downstream region contained a short overhang sequence homologous to an antibiotic resistance cassette. A second PCR was then performed using the PCR products of the first reaction as primers and the antibiotic cassette as a template. The final product was transformed to B. subtilis PY79. Integration of the construct to the genomic DNA was confirmed by PCR. Accordingly, comA and comQXP were deleted from the PY79 chromosome, using the primers comA-P1-P4 and comQXP-P1-P4 (Table S2). The same method was used for replacing the native amyE promoter with the quorum-sensing regulated srfA promoter (using primers Psrf-amyE-P1-P4) and for constructing the sfp3610 allele (using primers sfp-P1-P4), where in the latter, the B. subtilis NCIB3610 genome was used as template for PCR (Table S2).
Table S1.
Strain list
| Strain name | Genotype | Source | Figure |
| B. subtilis* | |||
| AES101 | B. subtilis PY79 wild type | Bacillus Genetic Stock Center | |
| AES3319 | NCIB 3610 | Bacillus Genetic Stock Center | |
| DS2569 | NCIB 3610; plasmid cured | (31) | |
| AES522 | zba-88::(Tn917::pTV21∆2::pD177.1::pD179.1 kan cm) | (53) | |
| AES1619 | zba-88::(Psrf-3xYFP spec cm kan) | AES945→AES522 | |
| AES2026 | amyE::(Psrf-amyE Sp) | This study (LFH-PCR) | |
| AES1403 | ∆comA::Cm | This study (LFH-PCR) | |
| AES1650 | sfp::(sfp3610 Sp) swrA::swrA3610 | This study (LFH-PCR) | |
| AES2026 | amyE::(Psrf-3xYFP Sp) | This study | |
| AES2663 | ppsB::(PtrpE-mCherry Ph) | AEC375→AES101 | |
| AES2135 | ∆comQXP::Tet | This study (LFH-PCR) | |
| AES2001 | lacA::([P43-synthRBS-BFP]x2) | AES814→AES101 | |
| AES2030 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::(P43-YFP mls) | AEC767→AES1650 | Fig. 2 A and B and Figs. S1, S2 A and B, and S3 |
| AES2137 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::(P43-YFP mls) ppsB:: (PtrpE-mCherry Ph) | AEC375→AES2030 | Fig. 2 A and B and Figs. S1, S2 A and B, and S3 |
| AES3001 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::(P43-YFP mls) ∆comA::Cm | AES1403→AES2030 | Fig. 2 A and B and Figs. S1, S2 A and B, and S3 |
| AES3002 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::(P43-YFP mls) ppsb::(PtrpE-mCherry Ph) ∆comA::Cm | AES1403→AES2030 | Fig. 2 A and B and Figs. S1, S2 A and B, and S3 |
| AES2119 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::(P43-YFP mls) ∆comQXP::Tet | AES2135→AES2030 | Fig. S2 A and B |
| AES2198 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::(P43-YFP mls) ppsB:: (PtrpE-mCherry Ph) ∆comQXP::Tet | AES2135→AES2137 | Fig. S2 A and B |
| AES3003 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::(P43-YFP mls) ∆comQXP::Tet sacA::(comQXPNAF4 cm) | AEC951→AES2119 | Fig. 3 A, B, and D |
| AES3004 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::(P43-YFP mls) ppsB:: (PtrpE-mCherry Ph) sacA::(comQXPNAF4 cm) | AEC951→AES2198 | Fig. 3 A, B, and D |
| AES3005 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::(P43-YFP mls) ∆comQXP::Tet sacA::(comQXPRO-H-1 cm) | AEC1018→AES2119 | Fig. 3 A, B, and D |
| AES3006 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::(P43-YFP mls) ppsB:: (PtrpE-mCherry Ph) sacA::(comQXPRO-H-1 cm) | AEC1018→AES2198 | Fig. 3 A, B, and D |
| AES3530 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::(P43-YFP mls) ∆comQXP::Tet sacA::(comQXPRO-FF-1 cm) | AEC1136→AES2119 | Fig. 3A |
| AES3514 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::(P43-YFP mls) ppsB:: (PtrpE-mCherry Ph) sacA::(comQXPRO-FF-1 cm) | AEC1136→AES2198 | Fig. 3A |
| AES2355 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::(P43-YFP mls) ∆comQXP::Tet sacA::(comQXP168 cm) | AEC959→AES2119 | Fig. 3A |
| AES2033 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::([P43-synthRBS-BFP]x2) | AEC814→AES1650 | Fig. 2C and Fig. S3C |
| AES2058 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::([P43-synthRBS-BFP]x2) ∆comQXP::Tet | AES2135→AES2033 | Fig. S2C |
| AES2078 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::([P43-synthRBS-BFP]x2) ∆comQXP::Tet zba-88::(Psrf-3xYFP spec cm kan) | AES1619→AES2058 | Fig. S2C |
| AES3007 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::([P43-synthRBS-BFP]x2) ∆comA::Cm | AES1403→AES2033 | Fig. 2C |
| AES2075 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::([P43-synthRBS-BFP]x2) zba-88::(Psrf-3xYFP spec cm kan) | AES1619→AES2033 | Fig. 2C and Fig. S2C |
| AES3008 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::([P43-synthRBS-BFP]x2) ∆comA::Cm zba-88::(Psrf-3xYFP spec cm kan) | AES1619→AES3007 | Fig. 2C |
| AES3009 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::([P43-synthRBS-BFP]x2) ∆comQXP::Tet sacA::(comQXPNAF4 Cm) | AEC951→AES2058 | Fig. 3C |
| AES3010 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::([P43-synthRBS-BFP]x2) ∆comQXP::Tet sacA::(comQXPRO-H-1 Cm) | AEC1018→AES2058 | Fig. 3C |
| AES3011 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::([P43-synthRBS-BFP]x2) ∆comQXP::Tet sacA::(comQXPNAF4 Cm) zba-88::(Psrf-3xYFP spec cm kan) | AES1619→AES3009 | Fig. 3C |
| AES3012 | sfp::(sfp3610 Sp) swrA::swrA3610 lacA::([P43-synthRBS-BFP]x2) ∆comQXP::Tet sacA::(comQXPRO-H-1 Cm) zba-88::(Psrf-3xYFP spec cm kan) | AES1619→AES3010 | Fig. 3C |
| AES2128 | amyE::(Psrf-amyE Sp) ppsB::(PtrpE-mCherry Ph) | AEC375→AES2026 | |
| AES2126 | amyE::(Psrf-amyE Sp) lacA::(P43-YFP mls) | AEC767→AES2026 | |
| AES2139 | amyE::(Psrf-amyE Sp) lacA::(P43-YFP mls) ppsB::(PtrpE-mCherry Ph) | AEC767→AES2126 | |
| AES1341 | amyE::(Psrf-amyE Sp) ppsB::(PtrpE-mCherry Ph) ∆comA::Cm | AES1403→AES2128 | Fig. 4B and Fig. S5 |
| AES2133 | amyE::(Psrf-amyE Sp) lacA::(P43-YFP mls) ∆comQXP::Tet | AES2135→AES2126 | |
| AES2153 | amyE::(Psrf-amyE Sp) lacA::(P43-YFP mls) ppsB::(PtrpE-mCherry Ph) ∆comQXP::Tet | AES2135→AES2139 | |
| AES3013 | amyE::(Psrf-amyE Sp) lacA::(P43-YFP mls) ∆comQXP::Tet sacA::(comQXPNAF4 Cm) | AEC951→AES2133 | Fig. 4B and Fig. S5 |
| AES3014 | amyE::(Psrf-amyE Sp) lacA::(P43-YFP mls) ppsB::(PtrpE-mCherry Ph) ∆comQXP::Tet sacA::(comQXPRO-H-1 Cm) | AEC1018→AES2153 | Fig. 4B and Fig. S5 |
| AES1822 | NCIB 3610 plasmid cured lacA::(P43-YFP mls) | AEC767→DS2569 | Fig. S4 |
| AES3537 | NCIB 3610 plasmid cured lacA::(P43-YFP mls) ppsB:: (PtrpE-mCherry Ph) | AEC375→DS2569 | Fig. S4 |
| AES2441 | NCIB 3610 plasmid cured lacA::(P43-YFP mls) ∆comQXP::Tet | AES2135→AES1822 | Fig. S4 |
| AES2479 | NCIB 3610 plasmid cured lacA::(P43-YFP mls) ppsB:: (PtrpE-mCherry Ph) ∆comQXP::Tet | AES2135→AES3537 | Fig. S4 |
| AES3450 | NCIB 3610 plasmid cured lacA::(P43-YFP mls) ppsB:: (PtrpE-mCherry Ph) ∆comA::Cm | AES1403→AES3537 | Fig. S4 |
| Escherichia coli† | |||
| AEC808 | DH12 | ||
| AEC777 | pDR111 | (57) | |
| AEC945 | pDL30::Psrf-3xYFP | (32) | |
| AEC310 | ECE174 | Bacillus Genetic Stock Center | |
| AEC548 | ECE137 | Bacillus Genetic Stock Center | |
| AEC375 | ppsB::PtrpE-mCherry | (32) | |
| AEC767 | ECE137::P43-YFP | This study | |
| AEC814 | ECE137::[P43-BFP]x2 | This study | |
| AEC951 | ECE174::comQXPNAF4 | This study | |
| AEC1018 | ECE174::comQXPRO-H-1 | This study | |
| AEC959 | ECE174::comQXP168 | This study | |
| AEC1136 | ECE174::comQXPRO-FF-1 | This study | |
All strains were constructed in strain PY79 background (AES101) unless otherwise noted.
All plasmids are in strain DH-12 background (AEC808).
Construction of sacA::(comQXPRO-H-1 Cm) and sacA::(comQXPNAF4 Cm) was performed by PCR amplification of the comQXP from strain Bacillus mojavensis RO-H-1 or from strain B. subtilis (natto) NAF4, using either the comQXP-ROH1-F and comQXP-ROH1-R or the comQXP-NAF4-F and comQXP-NAF4-R primer pairs. The PCR products were digested with restriction enzymes BamHI and EcoRI and ligated to ece174 plasmid (Tables S1 and S2). The resulting vectors were integrated into the sacA site on the chromosome, using chloramphenicol resistance for selection.
All of the mutations and constructs were transferred to PY79 by natural transformation (51). Integration of amyE integration plasmids into the zba8::amyEΩ CmKan (53) was done in two steps. First, the plasmid was integrated into a PY79 strain carrying the zjd89 construct and was screened for an Amy+ phenotype. A genomic prep of the resulting strain was then inserted into other strains with selection for either Kan or Cm, depending of the genetic background of the integrated genome.
The swrA+ mutation allele is a spontaneous revertant that was selected for by plating swrA−sfp+ cells on 0.7% LB agar plates and selecting motile variants, as was done previously (25). The reconstituted swrA+ allele was verified by sequencing.
The constitutive fluorescent construct P43-yfp was synthesized by genewiz and subcloned into ece137, using BamHI and EcoRI restriction enzymes.
Premeasurement Growth Protocol.
Before all assays, an overnight LB colony was inoculated in 1 mL SMM liquid medium and grown for 7 h until an OD600 of 0.1–0.3 was reached. The cultures were then diluted by a factor of 106 and grown overnight at 37 °C. We find that this long incubation in minimal medium both reduced the effects of quorum sensing before growth and reduced the arbitrary difference in growth between two cocultured wild-type colonies. For coculture experiments, cells of different strains were mixed in appropriate ratios after the overnight growth in SMM, based on relative optical density. The exact ratios were then measured using flow cytometry.
Swarming Assay.
Swarm plates were made of SMM media containing 0.005% glucose (wt/vol) and supplemented with 0.7% (wt/vol) Bacto-agar. Following autoclave, 25 mL was pipetted into each Petri dish after which the plates were allowed to dry for 1 h before cell inoculation and for an additional 5 min for drying in a laminar flow hood following inoculation. After drying, the plates were placed in an incubator set to 30 °C for 65 h or the indicated time. Humidity inside the incubator was kept high by placing two open 1-L water baths in the in the incubator during the experiment. Swarm cell yields were measured by collecting cells from the plate surface and homogenizing them in 5 mL of PBS. Frequency of the different genotypes was measured using flow cytometry.
Flow Cytometry.
All samples were run in a Beckman-Coulter Gallios flow cytometer equipped with four lasers (405 nm, 488 nm colinear with 561 nm, 638 nm). The emission filters used were as follows: BFP, 450/50; YFP, 525/40; and mCherry, 620/30. All samples were run at the “low” acquisition rate to reduce intrasample variability. Events were discriminated upon the forward-scatter parameter. For each run, discrimination was such that a single, well-defined population appeared in the forward-scatter (FS) by side-scatter plot. Gating on the fluorescent populations and inspection of the undiscriminated forward- by side-scatter plot indicated that over 99.9% of the fluorescent cells are found in the discriminated population. In all analyzed samples, only single cells were considered by gating on correlated time-of-flight and FS events. Gating of the different fluorescent populations was done by inspection of the log–log FLx-by-FLy plots (where x and y represent the appropriate filter number for each fluorescent marker), where two distinct populations are clearly visible, resulting in type I and type II errors of less than 0.05%. For each run, at least 105 cells were analyzed and the total events analyzed were such that the minority population was never below 1,000 events.
In Figs. 2 and 3, cocultures contained strains marked with either YFP or YFP+RFP reporters in a single copy in their genome, driven by strong constitutive promoters. The presence of these markers allowed us to identify the proportions of each genotype in the coculture. We have found that our YFP reporter carries a small fitness cost, so the presence of the YFP reporter in both cocultured strains allowed us to balance this cost. In Figs. 2C and 3B all cocultured strains carried a BFP reporter to identify the bacteria in the FACS, and additionally one strain carried the PsrfA-3xYFP quorum-sensing reporter. In all cocultures of Figs. 2 and 3, the RFP reporter was swapped between genotypes with no effect on total yield or relative growth difference. In Fig. 4, the three strains were marked by RFP (ΔcomA), YFP (NAF4), and RFP+YFP (RO-H-1).
SI Discussion—Selection of Pherotypes by Apostatic Interactions
An alternative explanation for the maintenance of pherotype diversity is that it can arise from apostatic selection (47) between the signaling bacteria and another organism that uses the quorum-sensing system to identify the bacteria and attack them. Apostatic interactions will lead to frequency-dependent selection for the minority without invoking intraspecific sociality. There are two likely variants to this explanation. First, it may be that an external factor [such as an antibody (54) or a phage (55)] can identify the quorum-sensing receptor. We find this to be an unlikely general mechanism, as many quorum-sensing systems with multiple pherotypes code for cytoplasmic receptors and the signal is imported into the cell by a nonspecific transporter (e.g., the PlcR and NprR quorum-sensing receptors of Bacillus cereus) (12, 56). Second, the quorum-sensing signal can be eavesdropped by another organism that will then modify its behavior to reduce the fitness of the quorum-sensing bacteria. Signals, however, are diffusible in the environment and therefore the response to them will not be directed toward a specific bacterium, but to all of the community. A cell encoding a different pherotype but that is otherwise isogenic to the majority pherotype would also be affected by such a nondirected response and would therefore not have a fitness advantage.
Acknowledgments
We thank Naama Barkai for comments on the manuscript and helpful discussions. This work was supported by funding from the European Research Council Grant 281301.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 1968.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520615113/-/DCSupplemental.
References
- 1.Waters CM, Bassler BL. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21(1):319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 2.Schuster M, Sexton DJ, Diggle SP, Greenberg EP. Acyl-homoserine lactone quorum sensing: From evolution to application. Annu Rev Microbiol. 2013;67:43–63. doi: 10.1146/annurev-micro-092412-155635. [DOI] [PubMed] [Google Scholar]
- 3.Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450(7168):411–414. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 4.Sandoz KM, Mitzimberg SM, Schuster M. Social cheating in Pseudomonas aeruginosa quorum sensing. Proc Natl Acad Sci USA. 2007;104(40):15876–15881. doi: 10.1073/pnas.0705653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katzianer DS, Wang H, Carey RM, Zhu J. Quorum non-sensing: Social cheating and deception in Vibrio cholerae. Appl Environ Microbiol. 2015;81(11):3856–3862. doi: 10.1128/AEM.00586-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollitt EJ, West SA, Crusz SA, Burton-Chellew MN, Diggle SP. Cooperation, quorum sensing, and evolution of virulence in Staphylococcus aureus. Infect Immun. 2014;82(3):1045–1051. doi: 10.1128/IAI.01216-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou L, Slamti L, Nielsen-LeRoux C, Lereclus D, Raymond B. The social biology of quorum sensing in a naturalistic host pathogen system. Curr Biol. 2014;24(20):2417–2422. doi: 10.1016/j.cub.2014.08.049. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton WD. The genetical evolution of social behaviour. I. J Theor Biol. 1964;7(1):1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 9.Drescher K, Nadell CD, Stone HA, Wingreen NS, Bassler BL. Solutions to the public goods dilemma in bacterial biofilms. Curr Biol. 2014;24(1):50–55. doi: 10.1016/j.cub.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansaldi M, Marolt D, Stebe T, Mandic-Mulec I, Dubnau D. Specific activation of the Bacillus quorum-sensing systems by isoprenylated pheromone variants. Mol Microbiol. 2002;44(6):1561–1573. doi: 10.1046/j.1365-2958.2002.02977.x. [DOI] [PubMed] [Google Scholar]
- 11.Iannelli F, Oggioni MR, Pozzi G. Sensor domain of histidine kinase ComD confers competence pherotype specificity in Streptoccoccus pneumoniae. FEMS Microbiol Lett. 2005;252(2):321–326. doi: 10.1016/j.femsle.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Slamti L, Lereclus D. Specificity and polymorphism of the PlcR-PapR quorum-sensing system in the Bacillus cereus group. J Bacteriol. 2005;187(3):1182–1187. doi: 10.1128/JB.187.3.1182-1187.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji G, Beavis R, Novick RP. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276(5321):2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 14.Morohoshi T, Kato M, Fukamachi K, Kato N, Ikeda T. N-acylhomoserine lactone regulates violacein production in Chromobacterium violaceum type strain ATCC 12472. FEMS Microbiol Lett. 2008;279(1):124–130. doi: 10.1111/j.1574-6968.2007.01016.x. [DOI] [PubMed] [Google Scholar]
- 15.Stefanic P, Mandic-Mulec I. Social interactions and distribution of Bacillus subtilis pherotypes at microscale. J Bacteriol. 2009;191(6):1756–1764. doi: 10.1128/JB.01290-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefanic P, et al. The quorum sensing diversity within and between ecotypes of Bacillus subtilis. Environ Microbiol. 2012;14(6):1378–1389. doi: 10.1111/j.1462-2920.2012.02717.x. [DOI] [PubMed] [Google Scholar]
- 17.Eldar A. Social conflict drives the evolutionary divergence of quorum sensing. Proc Natl Acad Sci USA. 2011;108(33):13635–13640. doi: 10.1073/pnas.1102923108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santorelli LA, et al. Facultative cheater mutants reveal the genetic complexity of cooperation in social amoebae. Nature. 2008;451(7182):1107–1110. doi: 10.1038/nature06558. [DOI] [PubMed] [Google Scholar]
- 19.Grossman AD. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annu Rev Genet. 1995;29(1):477–508. doi: 10.1146/annurev.ge.29.120195.002401. [DOI] [PubMed] [Google Scholar]
- 20.Tortosa P, et al. Specificity and genetic polymorphism of the Bacillus competence quorum-sensing system. J Bacteriol. 2001;183(2):451–460. doi: 10.1128/JB.183.2.451-460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano MM, et al. srfA is an operon required for surfactin production, competence development, and efficient sporulation in Bacillus subtilis. J Bacteriol. 1991;173(5):1770–1778. doi: 10.1128/jb.173.5.1770-1778.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dogsa I, Oslizlo A, Stefanic P, Mandic-Mulec I. Social interactions and biofilm formation in Bacillus subtilis. Food Technol Biotechnol. 2014;52(2):149–157. [Google Scholar]
- 23.Xavier JB, Kim W, Foster KR. A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa. Mol Microbiol. 2011;79(1):166–179. doi: 10.1111/j.1365-2958.2010.07436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kearns DB, Losick R. Swarming motility in undomesticated Bacillus subtilis. Mol Microbiol. 2003;49(3):581–590. doi: 10.1046/j.1365-2958.2003.03584.x. [DOI] [PubMed] [Google Scholar]
- 25.Kearns DB, Chu F, Rudner R, Losick R. Genes governing swarming in Bacillus subtilis and evidence for a phase variation mechanism controlling surface motility. Mol Microbiol. 2004;52(2):357–369. doi: 10.1111/j.1365-2958.2004.03996.x. [DOI] [PubMed] [Google Scholar]
- 26.D’Souza C, Nakano MM, Zuber P. Identification of comS, a gene of the srfA operon that regulates the establishment of genetic competence in Bacillus subtilis. Proc Natl Acad Sci USA. 1994;91(20):9397–9401. doi: 10.1073/pnas.91.20.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Julkowska D, Obuchowski M, Holland IB, Séror SJ. Comparative analysis of the development of swarming communities of Bacillus subtilis 168 and a natural wild type: Critical effects of surfactin and the composition of the medium. J Bacteriol. 2005;187(1):65–76. doi: 10.1128/JB.187.1.65-76.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross-Gillespie A, Gardner A, West SA, Griffin AS. Frequency dependence and cooperation: Theory and a test with bacteria. Am Nat. 2007;170(3):331–342. doi: 10.1086/519860. [DOI] [PubMed] [Google Scholar]
- 29.Hallatschek O, Hersen P, Ramanathan S, Nelson DR. Genetic drift at expanding frontiers promotes gene segregation. Proc Natl Acad Sci USA. 2007;104(50):19926–19930. doi: 10.1073/pnas.0710150104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oslizlo A, Stefanic P, Dogsa I, Mandic-Mulec I. Private link between signal and response in Bacillus subtilis quorum sensing. Proc Natl Acad Sci USA. 2014;111(4):1586–1591. doi: 10.1073/pnas.1316283111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLoon AL, Guttenplan SB, Kearns DB, Kolter R, Losick R. Tracing the domestication of a biofilm-forming bacterium. J Bacteriol. 2011;193(8):2027–2034. doi: 10.1128/JB.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omer Bendori S, Pollak S, Hizi D, Eldar A. The RapP-PhrP quorum-sensing system of Bacillus subtilis strain NCIB3610 affects biofilm formation through multiple targets, due to an atypical signal-insensitive allele of RapP. J Bacteriol. 2015;197(3):592–602. doi: 10.1128/JB.02382-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430(7003):1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 34.Brockhurst MA, Svensson E. Population bottlenecks promote cooperation in bacterial biofilms. PLoS One. 2007;2(7):e634–e634. doi: 10.1371/journal.pone.0000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diard M, et al. Stabilization of cooperative virulence by the expression of an avirulent phenotype. Nature. 2013;494(7437):353–356. doi: 10.1038/nature11913. [DOI] [PubMed] [Google Scholar]
- 36.Smith J, Van Dyken JD, Velicer GJ. Nonadaptive processes can create the appearance of facultative cheating in microbes. Evolution. 2014;68(3):816–826. doi: 10.1111/evo.12306. [DOI] [PubMed] [Google Scholar]
- 37.Fiegna F, Velicer GJ. Exploitative and hierarchical antagonism in a cooperative bacterium. PLoS Biol. 2005;3(11):e370. doi: 10.1371/journal.pbio.0030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dandekar AA, Chugani S, Greenberg EP. Bacterial quorum sensing and metabolic incentives to cooperate. Science. 2012;338(6104):264–266. doi: 10.1126/science.1227289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crozier R. Genetic clonal recognition abilities in marine invertebrates must be maintained by selection for something else. Evolution. 1986;40(5):1100–1101. doi: 10.1111/j.1558-5646.1986.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 40.Gardner A, West SA. Social evolution: The decline and fall of genetic kin recognition. Curr Biol. 2007;17(18):R810–R812. doi: 10.1016/j.cub.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 41.Rousset F, Roze D. Constraints on the origin and maintenance of genetic kin recognition. Evolution. 2007;61(10):2320–2330. doi: 10.1111/j.1558-5646.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 42.Biernaskie JM, Gardner A, West SA. Multicoloured greenbeards, bacteriocin diversity and the rock-paper-scissors game. J Evol Biol. 2013;26(10):2081–2094. doi: 10.1111/jeb.12222. [DOI] [PubMed] [Google Scholar]
- 43.Rendueles O, Amherd M, Velicer GJ. Positively frequency-dependent interference competition maintains diversity and pervades a natural population of cooperative microbes. Curr Biol. 2015;25(13):1673–1681. doi: 10.1016/j.cub.2015.04.057. [DOI] [PubMed] [Google Scholar]
- 44.Gibbs KA, Urbanowski ML, Greenberg EP. Genetic determinants of self identity and social recognition in bacteria. Science. 2008;321(5886):256–259. doi: 10.1126/science.1160033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stefanic P, Kraigher B, Lyons NA, Kolter R, Mandic-Mulec I. 2015. Kin discrimination between sympatric Bacillus subtilis isolates. Proc Natl Acad Sci USA 112(45):14042–14047.
- 46.Grafen A. Do animals really recognize kin? Anim Behav. 1990;39(1):42–54. [Google Scholar]
- 47.Ayala FJ, Campbell CA. Frequency-dependent selection. Annu Rev Ecol Syst. 1974;5(1):115–138. [Google Scholar]
- 48.Ke X, Miller LC, Bassler BL. Determinants governing ligand specificity of the Vibrio harveyi LuxN quorum-sensing receptor. Mol Microbiol. 2015;95(1):127–142. doi: 10.1111/mmi.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vetsigian K, Jajoo R, Kishony R. Structure and evolution of Streptomyces interaction networks in soil and in silico. PLoS Biol. 2011;9(10):e1001184. doi: 10.1371/journal.pbio.1001184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelsic ED, Zhao J, Vetsigian K, Kishony R. Counteraction of antibiotic production and degradation stabilizes microbial communities. Nature. 2015;521(7553):516–519. doi: 10.1038/nature14485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harwood CR, Cutting SM. Molecular Biological Methods for Bacillus. Wiley; New York: 1990. [Google Scholar]
- 52.Wach A. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast. 1996;12(3):259–265. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C259::AID-YEA901%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 53.Dworkin J, Losick R. Differential gene expression governed by chromosomal spatial asymmetry. Cell. 2001;107(3):339–346. doi: 10.1016/s0092-8674(01)00528-1. [DOI] [PubMed] [Google Scholar]
- 54.Andrews TD, Gojobori T. Strong positive selection and recombination drive the antigenic variation of the PilE protein of the human pathogen Neisseria meningitidis. Genetics. 2004;166(1):25–32. doi: 10.1534/genetics.166.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gómez P, Buckling A. Bacteria-phage antagonistic coevolution in soil. Science. 2011;332(6025):106–109. doi: 10.1126/science.1198767. [DOI] [PubMed] [Google Scholar]
- 56.Perchat S, et al. A cell-cell communication system regulates protease production during sporulation in bacteria of the Bacillus cereus group. Mol Microbiol. 2011;82(3):619–633. doi: 10.1111/j.1365-2958.2011.07839.x. [DOI] [PubMed] [Google Scholar]
- 57.Doan T, Marquis KA, Rudner DZ. Subcellular localization of a sporulation membrane protein is achieved through a network of interactions along and across the septum. Mol Microbiol. 2005;55(6):1767–1781. doi: 10.1111/j.1365-2958.2005.04501.x. [DOI] [PubMed] [Google Scholar]