Significance
This report explores the biochemical and structural basis of the interactions of TAP binding protein, related (TAPBPR), a tapasin homolog, with MHC-I molecules. TAPBPR associates with MHC-I molecules early in their biosynthesis and folding but is not part of the peptide-loading complex (PLC). Here, by examining the interactions of recombinant TAPBPR with peptide-free and peptide-complexed MHC-I molecules, we show that TAPBPR serves as a peptide editor. Structural comparison of TAPBPR with tapasin indicates the similarities of the two molecules and provides a basis for evaluating the steps of peptide loading. Understanding the molecular underpinnings of peptide loading of MHC-I by TAPBPR and tapasin has wide-ranging influence on our ability to modulate peptide loading for vaccine design and T-cell recognition.
Keywords: antigen presentation, peptide loading, major histocompatibility complex, protein interactions, SAXS
Abstract
Peptide loading of major histocompatibility complex class I (MHC-I) molecules is central to antigen presentation, self-tolerance, and CD8+ T-cell activation. TAP binding protein, related (TAPBPR), a widely expressed tapasin homolog, is not part of the classical MHC-I peptide-loading complex (PLC). Using recombinant MHC-I molecules, we show that TAPBPR binds HLA-A*02:01 and several other MHC-I molecules that are either peptide-free or loaded with low-affinity peptides. Fluorescence polarization experiments establish that TAPBPR augments peptide binding by MHC-I. The TAPBPR/MHC-I interaction is reversed by specific peptides, related to their affinity. Mutational and small-angle X-ray scattering (SAXS) studies confirm the structural similarities of TAPBPR with tapasin. These results support a role of TAPBPR in stabilizing peptide-receptive conformation(s) of MHC-I, permitting peptide editing.
Adaptive T-cell responses to intracellular pathogens and tumor antigens are governed primarily by effector CD8+ T cells that recognize antigenic peptides. These peptides are generated by protein degradation or during translation and are presented at the cell surface by MHC-I molecules (1–7). The steps of antigen processing and presentation via the MHC-I pathway have been studied extensively, and the contributions of components of the peptide-loading complex (PLC), tapasin, endoplasmic reticulum protein 57 (ERp57), transporter associated with antigen processing (TAP), and calreticulin, as well as various endoplasmic reticulum-associated proteases (ERAAP, ERAP1/2, IRAP) have been elucidated by genetic and biochemical studies (8–12). The general features of peptide loading onto MHC-I depend upon proteasome processing in the cytoplasm (13), transport of peptides to the endoplasmic reticulum (ER) (14), amino-terminal trimming of peptides (15–17), and stabilization of peptide-receptive MHC-I by tapasin (9) and calreticulin (10). The role of tapasin as a chaperone mediating loading and editing of high-affinity peptides is clear, but the precise molecular mechanism by which tapasin performs these functions remains poorly understood (18–21). Amino acid residues of tapasin and MHC-I involved in the interaction have been identified by mutational studies (9, 20, 22–27), and the X-ray crystallographic structure of a complex of tapasin and ERp57 provides key information for understanding tapasin (27).
A human gene encoding a tapasin homolog, TAP binding protein, related (TAPBPR), was identified on chromosome 12 (12p13.3) (28, 29). The encoded type I membrane protein is expressed in the ER and Golgi and interacts with MHC-I during its maturation (30–33). TAPBPR is highly conserved among vertebrates (mouse and human proteins are 69% identical), is widely expressed, is inducible by IFN-γ, and coprecipitates with MHC-I molecules containing β2-microglobulin (β2m) (30), but not with components of the PLC. Results of site-directed mutagenesis of both HLA-A2 and TAPBPR are consistent with a view that tapasin and TAPBPR share a similar mode of MHC-I binding (31). The amino acid sequence and biochemical similarities to tapasin suggest that examination of the direct interactions of TAPBPR with MHC-I would not only reflect on the normal function of this molecule, but would also provide insight into the molecular mechanisms that govern tapasin. With these goals in mind, we have examined the binding interactions of recombinant human TAPBPR with human and murine MHC-I molecules, performed low-resolution structural studies of both tapasin and TAPBPR in solution, and exploited this information to illuminate the mechanism by which TAPBPR functions in peptide editing.
Results
Binding of TAPBPR to Peptide-Free MHC-I/β2m Complexes by Native Gel Shift and Size Exclusion Chromatography.
Because previous studies indicated that TAPBPR first interacts with MHC-I early in its biosynthesis (30, 31, 33), we investigated whether recombinant TAPBPR would bind peptide-free MHC-I with high affinity. Producing peptide-free recombinant MHC-I is difficult for most MHC-I alleles because such molecules are unstable and aggregate (34). We produced MHC-I complexes refolded with photosensitive peptides and generated peptide-free molecules by UV irradiation (35). The interaction of peptide-containing, or peptide-free MHC-I with either tapasin or TAPBPR was evaluated in a gel shift assay (Fig. 1A). For this experiment, HLA-A2 was complexed with human β2m (hβ2m) and a peptide, photo-FluM158–66 (35) (see Table S1 for list of peptides used; peptides containing a photosensitive amino acid are designated by the prefix “photo”). As shown in Fig. 1A, tapasin (lanes 1 and 2), TAPBPR (lanes 7 and 8), and HLA-A2 (lanes 5 and 6) either before or after UV irradiation have characteristic mobilities in this gel system. The mixture of tapasin and HLA-A2 showed no difference in mobility following UV exposure (lanes 3 and 4), but the mixture of TAPBPR and HLA-A2 showed evidence of their physical association (compare lanes 9 and 10).
Fig. 1.
TAPBPR binds HLA-A2 loaded with photosensitive FluM1 peptide (photo-FluM158–66) after UV irradiation. (A) Native gel shift analysis. Individual components: tapasin (lanes 1 and 2), HLA-A2 (lanes 5 and 6), TAPBPR (lanes 7 and 8), and mixtures of tapasin and HLA-A2 (lanes 3 and 4) or TAPBPR and HLA-A2 (lanes 9 and 10) were separated on native gel with (lanes 2, 4, 6, 8, and 10) or without (lanes 1, 3, 5, 7, and 9) UV irradiation as described in Materials and Methods (4 µg of each protein in 10 µL). The positions of migration of the individual components are indicated, and the shifted TAPBPR/HLA-A2 complex band is denoted with a line. (Lanes 1–4 and 5–10, respectively, were taken from different gels run in parallel.) (B) SEC analysis. Individual components, TAPBPR (green), HLA-A2/photo-FluM158–66 (magenta), and the mixture of TAPBPR and HLA-A2 after UV irradiation (blue) were analyzed by SEC (S200 HR10/300) as described in Materials and Methods. (Markers of 158, 44, and 17 kDa eluted at 16.2, 20.1, and 22.6 min, respectively). (C) SDS/PAGE analysis confirms the identity of the TAPBPR/HLA-A2 complex peak. The SEC peaks of TAPBPR/HLA-A2 complex (I) and HLA-A2 (II) were collected, concentrated, and analyzed by SDS/PAGE (5 µg loaded in 10 µL per lane). The bands of TAPBPR, HLA-A2 heavy chain (HLA-A2 HC), and β2m, as well as the position of comigrated molecular-weight standards are indicated. (D) SEC (S200 HR10/300) analysis of tapasin alone (green), HLA-A2/photo-FluM158–66 (magenta), and mixture of tapasin and HLA-A2 following irradiation (blue) shows no complex formation. (E) AUC analysis of TAPBPR (green), HLA-A2/photo-FluM158–66 (blue), and the purified TAPBPR/HLA-A2 complex after UV irradiation (red). Shown are representative normalized sedimentation coefficient distributions at 2 µM. (F) AUC analysis of tapasin (green), HLA-A2/photo-FluM158–66 (blue), and the mixture following irradiation (red). These analyses were performed with 20 µM of each component. (G) AUC analysis of TAPBPR association with HLA-A2/photo-FluM1 without UV irradiation at the representative concentrations indicated. The Inset shows the weighted-average (black) and reaction boundary sedimentation coefficients (gray) for all of the concentrations examined. (Representative sedimentation profiles are shown in Fig. S1.)
Table S1.
Peptides used in this study
| Peptide | Sequence | MHC |
| FluM158–66 | GILGFVGTL | HLA-A2 |
| HBV18–27 | FLPSDFFPSV | HLA-A2 |
| MCMVpp89168–176 | YPHFMPTNL | H2-Ld |
| FLUNP366–374 | ASNENMETM | H2-Db |
| Ldp29 | YPNVNIHNF | H2-Ld |
| P18-I10 | RGPGRAFVTI | H2-Dd |
| A1 motif | STAPGVLEY | HLA-A1 |
| Photo-FluM158–66 | GILGFVFJ*L | HLA-A2 |
| Photo-HBV18–27 | FLPSDFFPJ*V | HLA-A2 |
| Photo-FluNP366–374 | ASNENJ*ETM | H2-Db |
| Photo-Ldp29 | YPNVNIHJ*F | H2-Ld |
| Photo-P18-I10 | RGPGRAFJ*TI | H2-Dd |
| Photo-A1 motif | STAPGJ*LEY | HLA-A1 |
| FITC-HBV18–27 | FLPSDK(Fl)FPSV | HLA-A2 |
| FITC-PAP277–285 | IPSYK(Fl)RLIM | HLA-B7 |
| TDP43 | DLIIKGISV | HLA-A2 |
| RPL10 | DAKIRIFDL | HLA-A2 |
| YFVenv | SAGGFFTSV | HLA-A2 |
| HPVE7 | TLHEYMLDL | HLA-A2 |
| YFVNS5 | ATWASHIHL | HLA-A2 |
| FLUM1var | GILGFTFTL | HLA-A2 |
| Tcomp1 | LLDVVHPA | HLA-A2 |
The gel shift assay, although suggestive of an interaction between UV-irradiated HLA-A2/photo-FluM158–66 and TAPBPR, was confirmed and extended by size exclusion chromatography (SEC) analysis of a mixture of UV-irradiated HLA-A2/photo-FluM158–66 and TAPBPR (Fig. 1B). In this chromatographic system, TAPBPR and HLA-A2 elute at ∼19 and 20.5 min, respectively. The irradiated mixture shows a new peak eluting at 18 min, suggestive of an HLA-A2/TAPBPR complex, which was confirmed by SDS/PAGE (Fig. 1C). Tapasin and HLA-A2, treated in the same way, showed no evidence of association by the SEC assay (Fig. 1D). [TAPBPR/HLA-A2 complexes formed following irradiation contained no detectable photo-FluM158–66, and the 7-mer amino-terminal product of photolysis was only found at a level equivalent to 5% of the starting material (Materials and Methods and Table S2), indicating that the HLA-A2 in the observed complex with TAPBPR is largely devoid of bound peptide.]
Table S2.
Summary of LC/MS data of unirradiated and irradiated peptide alone, and in purified TABPR/HLA-A2 complex and TAPBPR + HLA/A2 mixture
| Condition | Amount, pmol | Peptide | Elemental composition | Calculated m/z | Elution time | EIC peak area | Observed m/z | Error, ppm | MS signal intensity |
| Peptides with and without irradiation | |||||||||
| Unirradiated | 12 | GILGFVFJL | C54H77N10O12 | 1,057.5717 | 47.4–47.8 | 4.55 e7 | 1,057.5759 | 3.97 | 3.16 e7 |
| Irradiated | 12 | GILGFVFJL | C54H77N10O12 | 1,057.5717 | 47.5–47.8 | 9.32 e5 | 1,057.5750 | 3.12 | 7.89 e5 |
| GILGFVF-NH2 | C39H59N8O7 | 751.4501 | 39.4–39.6 | 7.9 e7 | 751.4521 | 2.66 | 6.22 e7 | ||
| Unirradiated | 25 | GILGFVFJL | C54H77N10O12 | 1,057.5717 | 47.4–47.8 | 1.83 e8 | 1,057.5746 | 2.74 | 1.33 e8 |
| Irradiated | 25 | GILGFVFJL | C54H77N10O12 | 1,057.5717 | 47.5–47.8 | 2.15 e6 | 1,057.5769 | 4.9 | 1.62 e6 |
| GILGFVF-NH2 | C39H59N8O7 | 751.4501 | 39.4–39.6 | 2.24 e8 | 751.4536 | 4.6 | 1.86 e8 | ||
| TABPBPR/HLA-A2 complexes or TAPBPR + HLA-A2 mixture | |||||||||
| Unirradiated | 30 | GILGFVFJL | C54H77N10O12 | 1,057.5717 | 47.05–47.4 | 2.42 e8 | 1,057.5692 | −2.36 | 1.65 e8 |
| Irradiated | 30 | GILGFVFJL | C54H77N10O12 | 1,057.5717 | 47.5–47.8 | 2.59 e6 | 1,055.5526* | — | — |
| GILGFVF-NH2 | C39H59N8O7 | 751.4501 | 39.4–39.6 | 4.24 e7 | 751.4476 | −3.32 | 2.96 e7 | ||
| Unirradiated | 60 | GILGFVFJL | C54H77N10O12 | 1,057.5717 | 46.8–47.2 | 6.32 e8 | 1,057.5696 | −1.98 | 5.42 e8 |
| Irradiated | 60 | GILGFVFJL | C54H77N10O12 | 1,057.5717 | 47–47.4 | 6.74 e6 | 1,055.5536 | — | — |
| GILGFVF-NH2 | C39H59N8O7 | 751.4501 | 39.6–39.9 | 4.68 e7 | 751.4488 | −1.72 | 3.23 e7 | ||
We have noticed loss of two hydrogens in the full peptide after irradiation; this can be seen in the reduction by 2 Da in its molecular ion.
Binding of TAPBPR to HLA-A2 by Sedimentation Velocity–Analytical Ultracentrifugation.
The interaction of TAPBPR with HLA-A2 following photolysis of the photo-FluM158–66 peptide (Table S1) was analyzed by sedimentation velocity–analytical ultracentrifugation (SV-AUC). Analysis of a concentration series of the components with irradiation yielded a Kd value of 0.18 µM [95% confidence limits (Cl), 0.04, 0.49 µM]. An example of the AUC analysis comparing the behavior of the UV-irradiated purified complex of HLA-A2/TAPBPR with that of an irradiated mixture of HLA-A2 with tapasin is shown (Fig. 1 E and F). In the absence of irradiation, the HLA-A2/photo-FluM158–66/TAPBPR interaction was significantly weaker, with a Kd value of 1.11 µM (Cl, 0.86, 1.40) (Fig. 1G). The AUC results indicate that TAPBPR interacts strongly with HLA-A2 freed of peptide by photolysis. Furthermore, the weaker interaction with unirradiated HLA-A2/photo-FluM158–66 complexes may result from a population of molecules that have lost the unirradiated photo-FluM158–66 peptide. It remains possible that TAPBPR may bind alternative conformations of peptide-loaded molecules. No interaction of tapasin and UV-irradiated HLA-A2/photo-FluM158–66 was observed under these conditions (Fig. 1F).
Binding of TAPBPR to HLA-A2 by Surface Plasmon Resonance.
To explore further the interactions of various HLA-A2/peptide complexes to TAPBPR, we used surface plasmon resonance (SPR), which offers a sensitive method to examine molecular interactions in real time (Fig. 2A). Although HLA-A2 refolded with either HBV18–27 or with FluM158–66 showed very little binding, complexes prepared with photo-FluM158–66, even without irradiation, bound appreciably higher, with a dissociation rate (t1/2) of about 200 s (Fig. 2A, “wash” at t = 425–835 s, blue line). This prompted us to test whether the dissociation of HLA-A2 from TAPBPR would be accelerated by exposure to a high-affinity peptide, such as HBV18–27. Indeed, when HBV18–27 was included in the washout buffer, HLA-A2 dissociated much more rapidly and completely with a t1/2 that was too rapid for quantitative estimation (Fig. 2A, “peptide” at t = 825 s, blue line). To gain a quantitative estimate of the binding of HLA-A2/photo-FluM158–66 to TAPBPR, without irradiation, graded concentrations were offered to the TAPBPR-coupled surface (Fig. 2B). These kinetic binding curves could not be fit to a simple A + B ↔ AB model, suggesting a more complex association mechanism or structural heterogeneity of MHC molecules refolded with photolabile peptides. However, analysis of the equilibrium binding data with SEDPHAT (36) provided an estimate of the Kd value of 4.73 µM (Cl, 3.67, 6.23), similar to the value obtained with unirradiated components by SV-AUC (1.11 µM). Remarkably, HLA-A2 complexed with photo-FluM158–66, without irradiation showed rapid binding to the TAPBPR surface during the association phase, with relatively rapid dissociation during the buffer washout (Fig. 2 A and B, “wash”). To evaluate the role of specific peptide in the dissociation phase, HLA-A2/photo-FluM158–66 binding to TAPBPR was tested with sequential washouts (Fig. 2C). Following binding and a brief buffer wash (t = 400 s), exposure to the high-affinity HBV18–27 peptide (Fig. 2C, at t = 425 s, blue tracing), but not to a nonbinding control peptide (red), elicited a rapid dissociation from TAPBPR. Additional high-affinity peptide (Fig. 2C, at t = 835 s, “high dose”) caused rapid release of the residual bound HLA-A2 from TAPBPR. These binding experiments suggested that HLA-A2/photo-Flu-M158–66 can bind TAPBPR, but do not eliminate the possibility that a proportion of molecules in the HLA-A2/photo-Flu-M158–66 preparation are in a peptide-free state. The acceleration of HLA-A2 dissociation from the TABPBR surface by high-affinity peptide was consistent with the view that TAPBPR binds poorly or not at all to HLA-A2 complexed with high-affinity peptides.
Fig. 2.
Kinetics of interaction of MHC-I molecules with TAPBPR reveal allelic and peptide dependencies. (A) HLA-A2 refolded with different peptides binds differently to TAPBPR. HLA-A2 (2 µM) refolded with HBV18–27 (green), FluM158–66 (red), or with photo-FluM158–66 (blue) was injected at a flow rate of 20 µL/min over an SPR chip coupled with TAPBPR (2,000 RU) as described in Materials and Methods. Arrows indicate the initiation of the injection of analyte (45 s), buffer wash (345 s), and injection of HBV peptide (880 s). (B) Dose–response of HLA-A2 refolded with photo-FluM158–66 peptide binding to TAPBPR. Arrows indicate times of injection of analyte, buffer wash, and HBV peptide as shown in A. Graded concentrations offered were as follows: 0.15, 0.2, 0.26, 0.35, 0.47, 0.63, 0.84, 1.1, 1.5, and 2 µM, all at 20 µL/min. (C) Specific HLA-A2–binding peptide accelerates the dissociation of bound HLA-A2 from the TAPBPR-coupled surface. A TAPBPR surface (980 RU) was exposed to HLA-A2 refolded with photo-FluM158–66 (2 µM; flow rate, 20 µL/min), and then buffer wash (345 s) followed by HBV peptide (80 nM; blue), control peptide (80 nM; red), or buffer alone (blue) at 435 s (flow rate, 20 µL/min throughout). Additional HBV peptide wash (1 µM, 20 µL/min) was initiated at 825 s. (D) Different MHC-I molecules interact differently with TAPBPR. A TAPBPR-coupled surface (980 RU) was offered HLA-A1/photo-A1-motif (green), HLA-A2/photo-FluM158–66 (blue), H2-Ld /photo-Ldp29 (magenta), H2-Db/ photo-FluNP366–374 (cyan), or H2-Dd/photo-P18I10 (purple) at 2 µM (flow rate, 20 µL/min). In each case, a final washout with a mixture of A1 motif, FluM1, Ldp29, FluNP, and P18I10 (all at 1 µM) was used to regenerate the surface at 1,415 s. (E) HLA-A2 complexed with photo-FluM158–66 with or without irradiation binds TAPBPR better than HLA-A2/FluM158–66. (F) HLA-A2 complexed with photo-HBV18–27 binds TAPBPR better following irradiation. (G) H2-Dd complexes with or without irradiation bind to TAPBPR. For E–G, the TAPBPR-coupled surface was offered the indicated MHC complexes at 2 µM (flow rate, 20 µL/min).
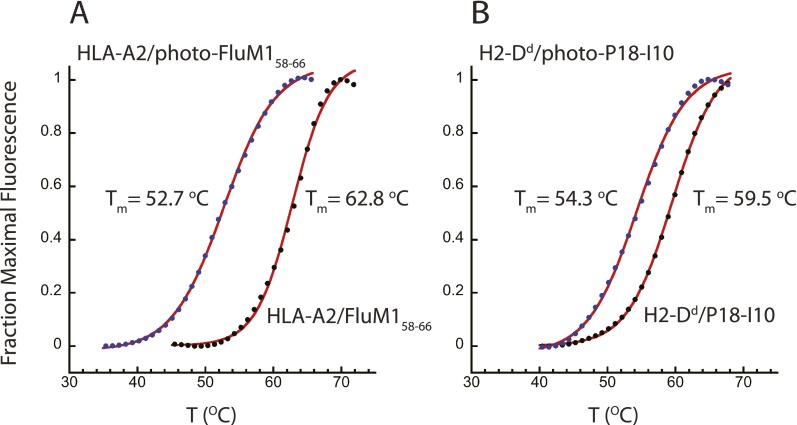
We then compared several different MHC-I complexes prepared with photosensitive peptides without irradiation for binding to the TAPBPR biosensor surface (Fig. 2D). Although HLA-A1/photo-A1 motif failed to bind at all, several other molecules, including the mouse MHC molecules H2-Ld, H2-Dd, and H2-Db, bound to different levels and dissociated at different apparent rates (Fig. 2D, “wash”). [A mixture of peptides that bind the five MHC-I molecules elicited rapid dissociation of the complexes from the TAPBPR surface (Fig. 2D, “peptide”).] We then compared the binding of HLA-A2 loaded with different photosensitive peptides with their nonphotosensitive counterparts with or without prior UV irradiation (Fig. 2 E–G). As noted in Fig. 2A, the HLA-A2/FluM158–66 complex barely binds to TAPBPR (Fig. 2E, red) and HLA-A2/HBV18–27 fails to bind (Fig. 2F, red). However, even without irradiation, HLA-A2 complexes made with the photosensitive variants of the FluM158–66 and HBV18–27 peptides bind appreciably (Fig. 2 E and F, green) and, following irradiation, also bind well (Fig. 2 E and F, blue). The increase in binding due to irradiation is greater for the photo-HBV18–27 peptide than for photo-FluM158–66. The mouse MHC-I molecule, H2-Dd, complexed with a high-affinity peptide P18-I10 or its photosensitive analog, photo-P18-I10, with or without irradiation, revealed interaction with TAPBPR (Fig. 2G, all tracings). The apparent kinetic dissociation rate of irradiated H2-Dd/photo-P18-I10 from TAPBPR (Fig. 2G, blue, “wash”) was slower than that of the unirradiated H2-Dd/photo-P18-I10 (Fig. 2G, green, “wash”), which in turn was slower than that of the H2-Dd/P18-I10 complex (Fig. 2G, red). Thus, depending on the particular MHC-I molecule and the particular peptide, interaction with TAPBPR can occur even without photolysis of the bound peptide. One possible explanation for the increased ability of HLA-A2 or H2-Dd complexed with photosensitive compared with the classical epitopic peptides to bind better to TAPBPR is that these complexes might be more unstable, generating a population of empty or peptide-receptive molecules available for TAPBPR binding. We tested this possibility by examining the thermal stability of HLA-A2 and H2-Dd complexed with either the antigenic viral peptides or the photosensitive variants (Fig. S2). For both pairs, the complexes prepared with the photosensitive peptides revealed a lower Tm value. HLA-A2/FluM158–66 melted at 62.8 °C, and complexes prepared with the photosensitive variant, HLA-A2/photo-FluM158–66, without irradiation melted at 52.7 °C. Similarly, H2-Dd/P18-I10 revealed a Tm value of 59.5 °C and H2-Dd/photo-P18-I10, a Tm value of 54.3 °C. Although the complexes containing the photosensitive peptides were less thermostable, they were not detectably denatured at temperatures below 40 °C. These data are consistent with a view that TAPBPR can interact with peptide-complexed, folded molecules, but do not formally rule out the possibility that the molecules that interact with TAPBPR represent a population of peptide-free molecules in each of these preparations. In some cases (e.g., HLA-A2/FluM158–66, Fig. 2E, and HLA-A2/HBV18–27, Fig. 2F), irradiation of an MHC complex containing a photolabile peptide allowed for greater interaction with TAPBPR, but in others (H2-Dd/P18-I10, Fig. 2G), the difference was not observed.
Fig. S2.
HLA-A2 and H2-Dd are less thermostable complexed with photosensitive peptides compared with antigenic peptides. Five micromolar concentration of each purified complex consisting of (A) HLA-A2/FluM158–66 (black circles) or HLA-A2/photo-FluM158–66 (blue circles) (without irradiation); or (B) H2-Dd/P18-I10 (black circles) or H2-Dd/photo-P18-I10 (blue circles) in 20 µL of Tris-buffered saline were incubated with 1× SyPro Orange (Thermo Fisher), and subjected to thermal denaturation in a 96-well PCR tray, in an Applied Biosystems Quant Studio 7-Flex Real Time PCR system, held for 2 min at 25 °C, and then heated to 99 °C at a rate of 0.05 °C/s, and fluorescence emission was recorded. Triplicate runs were averaged, normalized, and fit to a Boltzmann sigmoid function to obtain the curve fits (red) and the indicated Tm values. For clarity, only 1/10 data points are shown.
TAPBPR Augments Peptide Exchange.
To evaluate the role of TAPBPR in peptide exchange, we compared the ability of free and TAPBPR-bound HLA-A2 to bind a FITC-labeled high-affinity peptide using a fluorescence polarization assay, in which graded concentrations of protein were offered a fixed concentration (10 nM) of fluorescent indicator peptide. As shown in Fig. 3A, the HLA-A2/FluM158–66 complex bound the fluorescent indicator peptide FITC-HBV18–27 (Table S1), at a half-maximal protein concentration of about 300 nM. We then purified the TAPBPR/HLA-A2 complex generated by irradiation of a mixture of TAPBPR and HLA-A2/photo-FluM158–66 by SEC and tested this for peptide binding. Half-maximal binding was achieved by the purified complex at a 100-fold lower concentration of about 3 nM. A control HLA-B7 peptide, FITC-PAP277–285, was not bound by either of the protein preparations. HLA-A2/FluM158–66 complexes were exposed to the higher affinity FITC-HBV18–27 along with graded concentrations of TAPBPR (Fig. 3B). As TAPBPR concentration increased from 0 to 500 nM, the binding curve shifted to the left, indicating that TAPBPR facilitated the replacement of FluM158–66 by FITC-HBV18–27. In a more stringent test of the influence of TAPBPR on peptide exchange, HLA-A2 refolded with the higher affinity HBV18–27 peptide was tested with graded doses of TAPBPR included in the binding reaction. Increasing TAPBPR from 0 to 500 nM augmented the peptide exchange (Fig. 3C). The control HLA-B7 binding fluorescent peptide, FITC-PAP277–285, failed to exchange onto HLA-A2 even in the presence of TAPBPR (Fig. 3D). Thus, the TAPBPR augmentation of peptide exchange may be observed either when present as TAPBPR/HLA-A2 complexes, or when provided free to HLA-A2/peptide complexes.
Fig. 3.
TAPBPR augments peptide binding by HLA-A2. (A) TAPBPR/HLA-A2 complexes (red), HLA-A2/FluM158–66 complexes (black), or TAPBPR alone (blue) at the indicated protein concentrations were offered either a high-affinity, fluorescent peptide [FITC-HBV18–27 (circles)], or a control fluorescent peptide [FITC-PAP277–285 (squares)] at 10 nM. (B) The HLA-A2/FluM158–66 exchange of peptide with fluorescent peptide is augmented by TAPBPR. Purified HLA-A2/FluM158–66 complexes were mixed in the indicated amounts with 10 nM FITC-HBV18–27 with or without additional purified recombinant TAPBPR, and FP was measured as described in Materials and Methods. Circles represent data points, and curves represent best fits. Graded concentrations of TAPBPR ranged from 0 (black), 3.9 (red), 7.8 (light blue), 15.6 (green), 31.2 (magenta), 62.5 (orange), 125 (brown), 250 (red), and 500 nM (blue). (C) HLA-A2/HBV18–27 complexes were similarly prepared and purified and analyzed with the same FITC-HBV18–27 peptide as in C. The family of curves is coded for the same concentrations of TAPBPR as in B. (D) No exchange of fluorescent peptide is observed when the probe peptide does not bind HLA-A2. Peptide FITC-PAP277–285 at 10 nM was used to test for exchange with HLA-A2/FluM158–66 complexes in the absence or presence of graded concentrations of TAPBPR. All experiments shown are representative of at least three similar ones.
HLA-A2–Binding Peptides Release TAPBPR and HLA-A2 from the Complex in an Affinity-Dependent Manner.
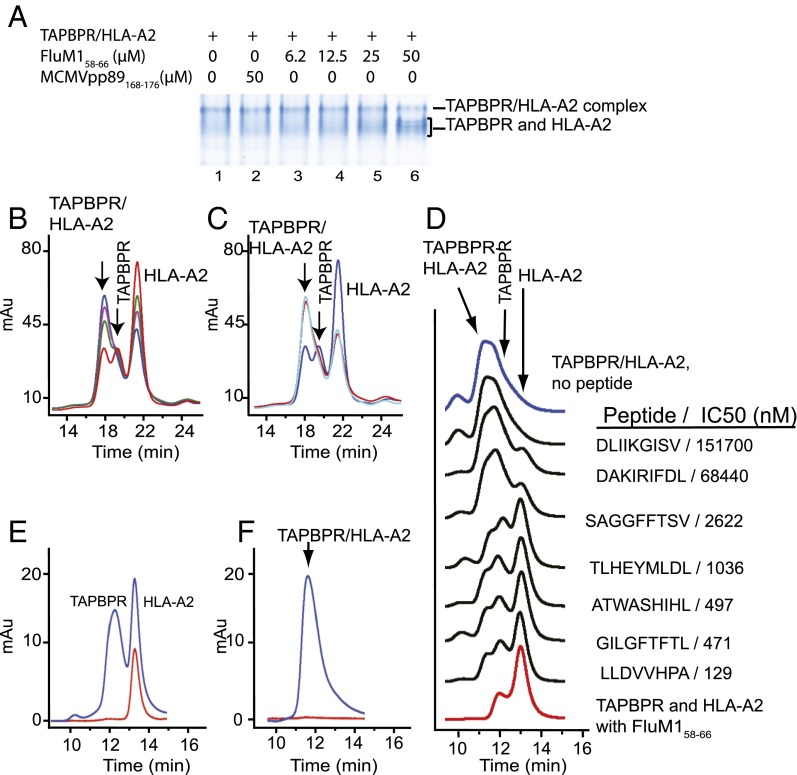
The observation that TAPBPR interacts strongly with HLA-A2 after photolysis of bound peptide (Fig. 1B) suggested that TAPBPR might function in peptide loading in a manner similar to tapasin and the PLC. In addition, SPR experiments indicated that cognate peptides could accelerate the dissociation of MHC-I from TAPBPR (Fig. 2). Addition of free FluM158–66 in graded doses to preformed TAPBPR/HLA-A2 complexes resulted in the release of free TAPBPR and HLA-A2 as shown in the gel shift assay (Fig. 4A) and by SEC (Fig. 4B). In contrast, a peptide that does not bind HLA-A2 (MCMVpp89168–176) failed to release the components of the complex (Fig. 4 A and C). To confirm that the TAPBPR/HLA-A2 complex was released by exposure to HLA-A2–binding peptides, and to address the hypothesis that the dissociation is related to the intrinsic affinity of a peptide for HLA-A2, we screened a set of peptides of known affinity for HLA-A2, using each peptide at a fixed concentration. As shown in Fig. 4D, exposure of the TAPBPR/HLA-A2 complex to free peptide resulted in the dissociation of TAPBPR and HLA-A2 in direct proportion to the known affinity of the peptide for HLA-A2. Using a fluoresceinated version of an HLA-A2–binding peptide as a probe, we established that, on dissociation of TAPBPR from HLA-A2 by high-affinity peptide, the peptide associates with the HLA-A2 but not the TAPBPR component as indicated by the coelution of HLA-A2 (the 280-nm absorbance peak) with the 500-nm FITC-HBV18–27 absorbance peak (Fig. 4E). On exposure to a control peptide, no dissociation of the complex or binding to HLA-A2 was observed (Fig. 4F). Taken together with the SPR and fluorescence polarization results, these experiments are consistent with the view that the TAPBPR/MHC complex, whether in solution or immobilized on a biosensor chip, dissociates as peptide binds to the MHC-I molecule.
Fig. 4.
Specific peptides release TAPBPR and HLA-A2 from the TAPBPR/HLA-A2 complex. (A) Native gel shift assay. A mixture of TAPBPR (10 µM) and HLA-A2/photo-FluM158–66 (10 µM) was UV irradiated as described in Materials and Methods and then incubated without (lane 1), with control peptide (lane 2), or with graded concentrations of FluM158–66 peptide (lanes 3–6). (B) SEC assay of a similar experiment in which TAPBPR (10 µM) and HLA-A2/photo-FluM158–66 (10 µM) were mixed, irradiated, and then treated without (blue) or with graded concentrations of FluM158–66 (25 µM, purple; 50 µM, green; 100 µM, red) before analytical chromatography on Superdex S200 column. (C) Similar experiment as in B but including incubation with 100 µM pMCMVpp89 (light blue). (D) Peptides release TAPBPR and HLA-A2 from the TAPBPR/HLA-A2 complex proportionate to the affinity of the peptide for HLA-A2. Mixtures of TAPBPR (10 µM) and HLA-A2/photo-FluM1 (2 µM) were UV irradiated as described in Materials and Methods, and then incubated with 20 µM of the indicated peptides before SEC on Shodex column as described in Materials and Methods. (Molecular weight standards of 660, 158, 44, and 17 kDa eluted at 9.7, 11.2, 12.3, and 14.2 min, respectively.) IC50 as determined by fluorescence polarization assay (37) for each peptide is indicated. (E) SEC analysis of an irradiated TAPBPR/HLA-2/photo-FluM1 mixture after incubation with a fluoresceinated HLA-A2–binding peptide [FITC-HBV18–27, FLPSDK(Fl)FPSV], and (F) shows the same experiment using a control peptide [FITC-PAP277–285, IPSYK(Fl)RLIM] that does not bind HLA-A2. For E and F, the blue and red curves describe protein absorbance at 280 nm and FITC absorbance at 500 nm, respectively.
Site-Directed TAPBPR Mutant Clusters Map Amino Acid Residues Conserved Between Tapasin and TAPBPR to an MHC-I Interaction Site.
Previous analysis of site-directed mutants of both tapasin (27) and TAPBPR (31), using transfection and antibody pull-down experiments, mapped the binding site(s) for MHC-I to several patches of the first, second, and third domains, based on amino acid sequence alignment (29). We have extended these observations by examining recombinant site-directed mutants of TAPBPR in each of its three major domains for their ability to bind HLA-A2/photo-FluM158–66 following UV irradiation by the SEC assay. Using mutant assignments originally made for tapasin (27), we examined several TAPBPR mutants (Fig. 5). As shown in Fig. 5B, we found that, whereas TAPBPR readily forms a complex with irradiated HLA-A2/photo-FluM158–66 (blue), the TN1 mutant (D17K, F19A, magenta) binds detectably but forms less of the high–molecular-weight complex than does parental TAPBPR. Neither TN6 (E205K, R207E, Q209S, Q272S; green) nor TC3 (Q336D, S337D; brown) binds at all. These results indicate that the direct interaction of TAPBPR with peptide-free HLA-A2 is dependent on contributions from the second and third domains and is influenced by residues in the N domain as well. Tapasin and TAPBPR thus share similar modes of binding to MHC-I.
Fig. 5.
TAPBPR mutants TN6 and TC3 fail to interact with HLA-A2 following peptide photolysis. (A) Site-directed mutants of TAPBPR in each of the three domains are illustrated on a TAPBPR homology model. TAPBPR model based on the tapasin moiety of the tapasin/ERp57 structure was generated with MMM (47) as described in Materials and Methods. Location of TN1, TN6, and TC3 mutants is indicated by spheres superposed on ribbon illustration. (B) SEC analysis of binding of parental (blue), TN1 (magenta), TN6 (green), and TC3 (brown) TAPBPR mutants. TAPBPR or the indicated TAPBPR mutant proteins were mixed with HLA-A2/photo-FluM158–66 at concentrations of 15 µM, UV irradiated, and analyzed by SEC on a Superdex HR 200 column as described in Materials and Methods.
Structure Determination of TAPBPR and Tapasin by Small-Angle X-Ray Scattering.
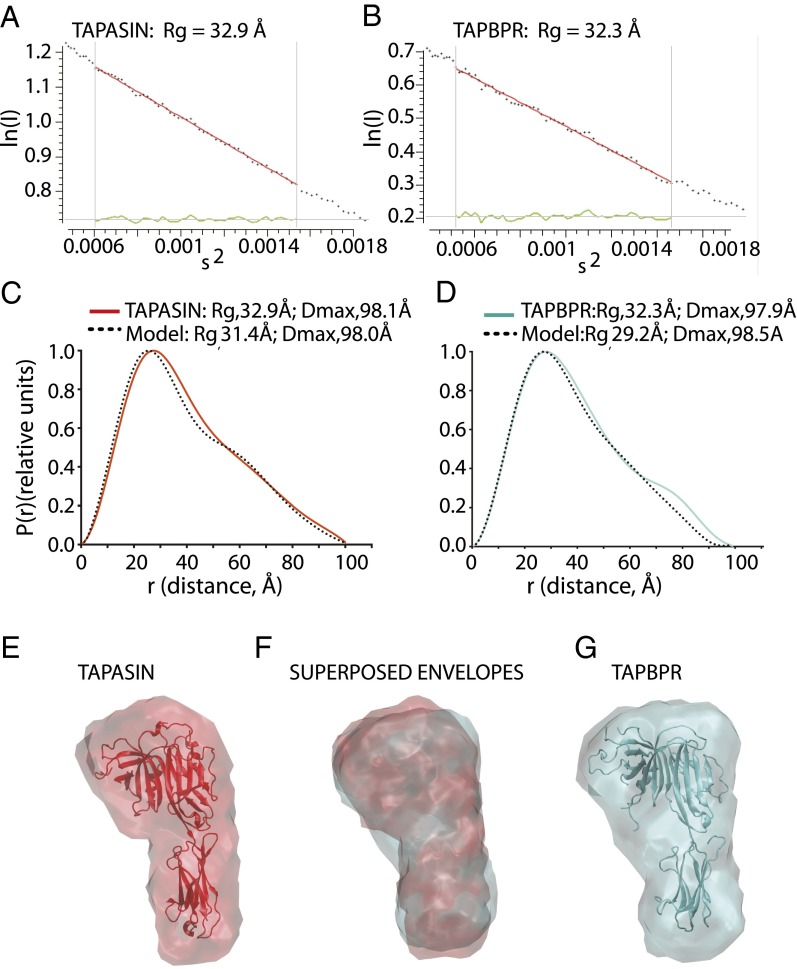
The amino acid sequence similarities of tapasin and TAPBPR, the characteristics of their interactions with MHC-I, and the mutational analyses all support the prediction that they share 3D structural features. Although the structure of tapasin has been determined crystallographically in complex with the oxidoreductase ERp57 (27), no structural information is available for TAPBPR. To explore further the similarities of tapasin and TAPBPR, we determined both structures at low resolution by small-angle X-ray scattering (SAXS). Data were collected and processed (Table S3) as described in Materials and Methods, giving Guinier fits as shown in Fig. 6 A and B. The radii of gyration Rg for tapasin and TAPBPR were 32.9 and 32.3 Å, respectively. The maximum particle sizes, Dmax, were 98.1 and 97.9 Å, respectively. The pairwise distance distribution function [P(r)] from the observed tapasin data (Fig. 6C) (red) compared favorably with that calculated from the tapasin model derived from the X-ray structure (3F8U) (dotted black). The P(r) of TAPBPR (Fig. 6D) (cyan) also compared well with the structural homology model of TAPBPR (dotted black) based on the tapasin structure. From the SAXS data, envelopes were calculated and were docked with the X-ray structure for tapasin (Fig. 6E) or with the tapasin-based TAPBPR model (Fig. 6G). The similarity of the two structures determined by SAXS is supported by the closeness of the Rg and Dmax values, and is readily illustrated by the superposition of the two experimentally determined envelopes (Fig. 6F). TAPBPR and tapasin are thus clearly related not only in amino acid sequence and in conservation of some interacting residues, but also by their low-resolution 3D structures. The SAXS structure of tapasin indicates that the envelope of the isolated luminal domain in solution closely approximates the X-ray structure of tapasin bound with ERp57, and establishes that the TAPBPR luminal domain maintains a similar solution structure. Thus, TAPBPR likely exploits an MHC recognition surface similar to that used by tapasin and may sterically compete for tapasin binding when MHC-I is accessible to both molecules.
Table S3.
SAXS data collection, structural parameters and model statistics
| Parameter | TAPASIN | hTAPBPR |
| Data collection parameters | ||
| Beamline/instrument | 18ID at APS | 18ID at APS |
| Detector | PILATUS 1M | PILATUS 1M |
| Distance to sample, mm | 3,500 | 3,500 |
| Wavelength, Å | 1.033 | 1.033 |
| q range recorded, Å−1 | 0.004–0.330 | 0.004–0.330 |
| Exposure time, s* | 1.0 | 1.0 |
| Concentration range, mg/mL* | 0.0–1.0 | 0.0–1.0 |
| Temperature, K | 293 | 293 |
| Structural parameters | ||
| Redundancy (average over frames)* | 74 | 40 |
| I(0), cm−1 (from Guinier) | 3.96 ± 0.01 | 2.31 ± 0.01 |
| Rg, Å (from Guinier) | 32.97 ± 1.38 | 32.88 ± 3.23 |
| s Rg limits | 0.81–1.29 | 0.75–1.26 |
| I(0), cm−1 [from P(r)] | 3.88 | 2.24 |
| Rg, Å [from P(r)] | 32.87 | 32.29 |
| Dmax, Å | 98.13 | 97.86 |
| Porod volume estimate, Å3 | 73,925 | 79,967 |
| q range used, Å−1 | 0.020–0.213 | 0.020–0.160 |
| Molecular-mass determination | ||
| Molecular mass, Mr [from I(0)] | 42,900 | 44,400 |
| Calculated monomeric Mr from sequence | 43,144 | 42,979 |
| Molecular mass (from fitting PDB model) | 38,980 | 39,250 |
| Model results and validation | ||
| NSD† (SD) | 0.584 (0.014) | 0.602 (0.071) |
| No. runs (models for averaging) | 10 | 10 |
| Symmetry enforced | P1 | P1 |
| χ2 (q max) in fitting experimental data | 1.19 (0.20) | 0.97 (0.16) |
| Rg, Å (from PDB model) | 31.43 | 28.28 |
| Dmax, Å (from PDB model) | 98.00 | 98.50 |
| Software used | ||
| Primary data reduction | 18ID at APS | 18ID at APS |
| Data processing and analysis | PRIMUS | PRIMUS |
| Ab initio modeling and analysis | DAMMIF | DAMMIF |
| Validation and averaging | DAMAVER | DAMAVER |
| Computation of model intensities | CRYSOL | CRYSOL |
| Envelopes | SITUS/VMD | SITUS/VMD |
| SAXS data deposition ID‡ | TAPSNP | TAPBPP |
Size exclusion chromatography: flow rate, 0.75 mL/min; 1 s per frame; loading sample concentration, 5 mg/mL.
Normalized spatial discrepancy.
SAXS database: www.bioisis.net.
Fig. 6.
SAXS structure determination of tapasin and TAPBPR. Purified recombinant tapasin or TAPBPR were subjected to X-ray scattering data collection upon synchrotron irradiation as described in Materials and Methods. (A) Guinier plot of tapasin scattering data, s Rg limits (0.81–1.29) and (B) Guinier plot of TAPBPR scattering data, s Rg limits (0.75–1.26). (C) Pairwise distance distribution P(r) as a function of r for the experimental data (red) and for a theoretical model for tapasin calculated from the X-ray coordinates (dotted black) (PDB ID code 3f8U). (D) P(r) as a function of r for TAPBPR experimental data (cyan) is compared with a homology model for TAPBPR based on the tapasin structure. (E) Tapasin structure was docked to the SAXS envelope (red), and (G) TAPBPR homology model was docked to its SAXS envelope (cyan). (F) Superposed envelopes of tapasin (red) and TAPBPR (cyan).
Discussion
TAPBPR is an MHC-I chaperone structurally related to tapasin that coprecipitates with MHC-I molecules but whose function in antigen presentation is unclear. The experiments we report here detailing the molecular interactions between purified soluble TAPBPR and MHC-I molecules demonstrate that (i) the human recombinant TAPBPR luminal domain interacts directly with purified soluble human as well as murine MHC-I molecules; (ii) the TAPBPR-bound MHC-I molecule is in a peptide-receptive state; (iii) the binding of high-affinity peptide by MHC-I dissociates the TAPBPR/MHC-I complex; and (iv) TAPBPR and tapasin are structurally similar and also share similar modes of binding to their MHC-I ligands.
We observed peptide preferences and allele dependencies in the interaction of TAPBPR with MHC-I. HLA-A2, when peptide-free following photolysis of a bound photolabile peptide or when complexed with moderate-affinity peptides (such as photo-FluM158–66 and photo-HBV18–27 with or without irradiation), binds to TAPBPR. However, HLA-A2 complexed with the high-affinity HBV18–27 peptide fails to bind TAPBPR. The preference of TAPBPR for “empty” MHC-I molecules or those loaded with intermediate- or weak-affinity peptides may be related to its peptide-editing function. TAPBPR, like its homolog tapasin in the PLC, can permit higher affinity peptides to exchange onto molecules that were previously loaded with weaker binding peptides, or onto molecules that were released from bound photosensitive peptides by photolysis.
As shown for tapasin (37), TAPBPR also demonstrates allelic preferences, as HLA-A1 even when released from a photolysable peptide fails to bind. Human TAPBPR interacts not only with HLA-A2 but also shows broad cross-reactivity with the murine molecules, H2-Dd, H2-Ld, and H2-Db. This reflects conservation of crucial structural features of MHC-I molecules necessary for TAPBPR interaction and also the similarities in MHC-I antigen processing and presentation pathways. The variation in the extent of TAPBPR binding to different MHC-I/peptide complexes may reflect differences in the dynamic structure or stability of different MHC-I molecules.
We provide evidence for an editing function of TAPBPR by demonstrating the ability of added TAPBPR to augment peptide exchange in a fluorescence polarization assay and the ability of TAPBPR to stabilize MHC-I molecules in a peptide-receptive form. In addition, the determination of the structural similarity of TAPBPR to tapasin by SAXS and the behavior of mutants in predicted homologous positions to those observed for tapasin suggest that the mechanism of binding and stabilization and the site of binding of MHC-I by TAPBPR is similar to that of tapasin. The chaperone/editing function of TAPBPR, like tapasin, also shows important similarities to the functions of the MHC-II chaperone, HLA-DM, which stabilizes MHC-II molecules by binding to a region of MHC-II on the α1-helix of MHC-II, a position symmetrically related to the site mapped by mutagenesis to MHC-I on the α2-helix (22, 31). A role of the MHC-I light-chain β2m in facilitating peptide exchange has been recognized for many years (37, 38), likely reflecting the dynamic and cooperative nature of MHC/β2m/peptide interactions.
Indeed, the peptide-editing function of tapasin in the PLC is clear (3, 21), and our results are consistent with a similar function for TAPBPR. However, the biological need for what may appear to be redundant peptide loading/exchange systems (i.e., PLC vs. TAPBPR) remains perplexing. Several differences in the two systems suggest plausible reasons for their evolutionary preservation. Major biochemical differences between the PLC and the TAPBPR system include the following: (i) the PLC is localized to the ER while TAPBPR (which is not glycosylated and lacks any ER retention signal) is also found beyond the medial Golgi (30); (ii) TAPBPR is a single polypeptide chain, whereas the PLC consists of multiple chains ensuring additional chaperone function (by calreticulin), proximity to peptide delivery via TAP, and stabilization by ERp57; (iii) the luminal domain of TAPBPR seems to interact more tightly with susceptible MHC-I molecules than does the luminal domain of tapasin.
Although the studies of TAPBPR interactions with MHC-I to date are limited, taken together they permit us to formulate a mechanistic model of TAPBPR binding and function, which is illustrated schematically in Fig. S3. This model posits the interaction of TAPBPR (i) with peptide-free MHC/β2m complexes early in biosynthesis in the ER; (ii) with low-affinity peptide-containing MHC/β2m complexes either in the ER or a post-Golgi compartment; (iii) with peptide-free MHC/β2m complexes in equilibrium with low-affinity peptide-containing complexes; and (iv) conceivably with alternate conformations of high-affinity peptide-containing MHC/β2m complexes, which may lead to the rapid dissociation of TAPBPR from the TAPBPR/MHC/β2m/peptide complex.
Fig. S3.
Models for the interaction of TAPBPR with MHC-I molecules during peptide loading. Pathways for assembly of MHC-I heavy chain with β2m, TAPBPR, and either high-affinity (PHA) or low-affinity (PLA) peptides are illustrated. MHC-I conformations: MHC-INC (nascent chain), MHC-IPR (peptide-receptive), MHC-IF (folded) are indicated schematically and are labeled. The nascent MHC-I heavy chain (MHC-INC) first folds partially and interacts with β2m, early in the biosynthetic pathway (1). This peptide-receptive MHC/β2m complex, MHC-IPR/β2m (2) assembles with TAPBPR (3) to form the stabilized TAPBPR/MHC-IPR/β2m (4). Encounter complexes, formed with either a high-affinity peptide TAPBPR/MHC-IPR/PHA (5) or with a low-affinity peptide TAPBPR/MHC-IPR/PLA (7), then serve as precursors either to the fully folded (and stable) MHC-IF/PHA complex (6), which can be transported to the cell surface, or to a TAPBPR-free MHC-IPR/PLA complex (8), which is unstable but can be stabilized by TAPBPR. The biologically important editing role of TAPBPR is evident in the transition from conformation 7 to conformation 5, which occurs with the exchange of low- for high-affinity peptides.
The particular MHC-I/peptide complexes that interact with TAPBPR may be viewed as a continuum, but three general kinds have been observed: those MHC or MHC/peptide complexes with which TAPBPR fails to interact, irrespective of the assay used (e.g., HLA-A1); those MHC/peptide complexes that contain weakly binding or photosensitive peptides, with or without irradiation (e.g., HLA-A2 with a variety of peptides; H2-Dd, H2-Ld, and H2-Db complexed with photosensitive peptides); or those MHC alleles that bind particularly well even when complexed with high-affinity peptides (e.g., H2-Dd). It is worth noting that the photosensitive peptides that we have used in these experiments contain 3-amino-3-(2-nitro)phenyl-propionic acid, a β-amino acid, that may contribute to a distortion of the MHC/peptide complex that reads out in greater interaction with TAPBPR. This distortion is reflected in the decreased thermal stability of HLA-A2 and H2-Dd when complexed with photolabile peptides. Whether initial interactions of TAPBPR at a molecular level are only with peptide-free MHC-I or with MHC-I/peptide complexes in particular conformations is a subtle distinction that may require high-resolution binding assays in solution, such as NMR methods, to discriminate.
Following submission of our manuscript, Hermann et al. (39) provided similar findings to our own, offering data indicative of the peptide-editing function of TAPBPR. Extensive data on the ability of TAPBPR to interact with MHC/peptide complexes and to accelerate dissociation of weakly binding peptides, consistent with our own findings, were reported.
Despite our progress in understanding binding aspects of TAPBPR and establishing a low-resolution structural similarity to tapasin, many questions concerning the biochemistry and biology of TAPBPR remain to be explored, including understanding the details of the role that TAPBPR plays in intracellular peptide loading, the dynamics of TAPBPR/MHC association, and the high-resolution structural basis for its peptide-editing functions.
Materials and Methods
Expression Constructs.
Plasmid pMT-V5-His (Invitrogen) directed expression in Drosophila S2 cells of the luminal domain of human TAPBPR (residues 1–405, including the 18-aa signal sequence) linked to a C-terminal His6 tag. Plasmid pRMHa3 (40) was used for Drosophila S2 cell expression of the luminal domain of human tapasin (residues 1–412, including the 20-aa signal sequence) linked to a C-terminal thrombin cleavage site and His6 tag. Constructs were cotransfected with plasmids for resistance to puromycin and blasticidin S. Mutant TAPBPR proteins were generated by site-directed mutagenesis, and transfection of S2 cells. pHN1 expressing the HLA-A*02:01 heavy chain (41) was the kind gift of Dr. David Garboczi, National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH), Rockville, MD. cDNA encoding luminal domains of heavy chains of H2-Db, H2-Dd, and H2-Ld molecules were cloned into pET21 (Novagen). pET24 (Novagen) was used for expression of HLA-A*01:01. HLA-A*01:01 and HLA-A*02:01 as constructs encoding BirA C-terminal tags, were provided by Dr. R. Willis of the NIAID tetramer facility. (HLA proteins are referred to as HLA-A1, HLA-A2, etc.) cDNA encoding human β2m (hβ2m) was expressed in pET21d (42). Proteins expressed in Drosophila cells were cultured in Insect-Xpress (Lanza) medium at 27 °C and induced with 1 mM CuSO4 for 4 d. The filtered supernatant was subjected to chelate agarose chromatography (43), and bound protein was eluted with 250 mM imidazole followed by SEC on Superdex 200 16/60 (GE Healthcare Life Science) in PBS. MHC-I heavy chains were expressed as inclusion bodies in Escherichia coli BL21(DE3). The preparation of inclusion bodies, denaturation in 6 M guanidine hydrochloride, reduction of disulfides with DTT, refolding by dilution into arginine, reoxidation in the presence of glutathione, and assembly of heavy chain and β2m with the appropriate synthetic peptides were performed essentially as described previously (41). All MHC-I molecules were refolded with human β2m. Refolded heavy-chain/hβ2m/peptide complexes were concentrated in Amicon stirred cells (Millipore), filtered to remove aggregates, and subjected to SEC on Superdex 200 16/60 (GE Healthcare Life Science) in PBS. The peak corresponding to the assembled complex was recovered, concentrated by ultrafiltration, and subjected to ion exchange chromatography on monoQ 5/50 GL (GE Healthcare Life Science). Protein purity and identity were confirmed by SDS/PAGE and by N-terminal sequencing.
Peptides.
Synthetic peptides as listed in Table S1 from Biopeptek or CPC Scientific, were provided as >90% pure, and identity was confirmed by mass spectrometry. Conditional photosensitive peptides were synthesized with one residue replaced by 3-amino-3-(2-nitro)phenyl-propionic acid) designated J* as reported by Toebes et al. (35). Throughout the text, we refer to peptides containing the J* amino acid with a prefix of “photo” and specifically indicate when these have been irradiated.
UV-Mediated Cleavage of Photosensitive Peptides and Mass Spectrometry Analysis.
Purified proteins refolded with photolabile peptides were irradiated (366 nm) in a CAMAG UV-illuminator box for 40 min. After UV irradiation, the samples were equilibrated at room temperature for 30 min before further analysis. In some cases, photolysis of peptides was confirmed by reverse-phase HPLC of the MHC molecules refolded with photolysed peptides. For mass spectrometry analysis, size exclusion-purified HLA-A2/TAPBPR complexes were diluted with buffer A (0.1% formic acid in milli-Q water), followed by heating at 95 °C for 10 min to dissociate the complex. Samples were loaded onto a Trap-elute jumper chip using an Eksigent Nanoflex cHiPLC system. Each sample was switched onto the microfluidic trap column (ChromXP C18-CL 3 µm, 120 Å, 200 µm × 0.5 mm) to remove the salts and buffer from the sample using an isocratic gradient of 50% B (0.1% formic acid in acetonitrile) for 5 min, at 4 µL⋅min−1 flow rate. Analytical (ChromXP C18-CL 3 µm, 120 Å, 75 µm × 15 cm) microfluidic column was then switched in line and peptides were separated on a linear gradient eluting with 70% B (0.1 % formic acid in acetonitrile) over 75 min (300 nL⋅min−1). Eluted peptides were directly introduced onto the electrospray ionization source using Nanospray of the Thermo Q-Exactive mass spectrometer (MS). Full MS spectra were recorded at 70,000 resolution, MS/MS spectra were acquired using high-collision energy dissociation (HCD MS/MS) at 17,500 resolution and 25 eV of normalized collision energy. The intact peptide of the nanomer photo-FluM158–66 (GILGFVFJ*L), cleaved 7-mer GILGFVF-NH2, and J*L were eluted at 47–48 min, 39–40 min, and 34–35 min with corresponding masses at m/z 1,057.5711/1+, m/z 751.4549/1+, and m/z 324.1573/1+. The relative quantitation of the irradiated peptides was determined by extracting the ion chromatograms of respective ions from the base peak chromatogram. Tabulation of the quantitation is shown in Table S2.
Native Gel Shift Assay.
Equal amounts of MHC-I/β2m complexed with photosensitive peptide were mixed with TAPBPR or tapasin (4 µg of each in 10 µL), UV-irradiated for 40 min at room temperature, and rested for 30 min, and were then analyzed in acrylamide gels devoid of SDS using the Mini-Protean system (Bio-Rad), with the Tris-glycine buffer system. The samples were mixed with native gel loading buffer [250 mM Tris (pH 8.8), 10% glycerol], loaded on discontinuous polyacrylamide gels (4% stacking, 13% resolving), and run at 100 V at room temperature for 3.5 h in 25 mM Tris, 190 mM glycine running buffer. Following electrophoresis, proteins were visualized with RAPIDstain (G-Biosciences) or PageBlue Protein staining solution (Thermo Scientific).
SEC.
Analytical SEC was performed on either a Superdex S200 HR10/300 or on a Shodex KW802.5 column at a flow rate of 0.75 mL/min, developed under native conditions (PBS, pH 7.3), calibrated with standard molecular-weight markers (Bio-Rad; 1511901).
AUC.
Analytical ultracentrifugation experiments were conducted in an XL-I analytical ultracentrifuge (Beckman Coulter) with an An50Ti rotor, following previously described protocols (44). Protein samples were prepared by dilution of concentrated stocks with the working buffer (PBS). Individual protein molecules were characterized in the primary AUC experiments with TAPBPR at 2 µM and HLA-A2 refolded with photo-FluM158–66 peptide in a concentration series from 0.1 to 10 µM. In an independent experiment, purified TAPBPR and HLA-A2/photo-FluM158–66 were irradiated and examined over a range of concentrations. The samples were loaded into standard double-sector charcoal-filled Epon centerpieces with 12- or 3-mm path length and sapphire windows. The sedimentation process of the protein molecules was monitored using both Rayleigh interference and UV absorbance at 280-nm detection at 20 °C and 195,000 × g at a radius of 7 cm. The acquired sedimentation velocity data were analyzed with SEDFIT (https://sedfitsedphat.nibib.nih.gov/software) using the c(s) sedimentation coefficient distribution approach (45), from which the signal weighted-average sedimentation coefficient (sw) was obtained by integration. To determine the binding affinity, the isotherm of sw as a function of macromolecular concentrations was loaded into SEDPHAT (https://sedfitsedphat.nibib.nih.gov/software) and fitted with the 1:1 heterodimerization model as follows:
where cA and cB denote the molar concentration of the free species of A and B, respectively, which relate with mass conservation to the total component loading concentrations, s denotes the sedimentation coefficient, and KAB (KD = 1/KAB) is the equilibrium association binding constant. In the analysis, sA and sB were fixed at the experimentally determined values, whereas KAB and sAB were subject to optimization through nonlinear regression. The error surface projection analysis was exploited to determine the error intervals of the best-fit KD values at a 95% confidence level. The AUC graphs were prepared in GUSSI (biophysics.swmed.edu/MBR/software.html) (46). Representative sedimentation profiles of TAPBPR, HLA-A2/photo-FluM158–66, and of the mixture following irradiation are given in Fig. S1.
Fig. S1.
Representative sedimentation profiles of TAPBPR, HLA-A2/photo-FluM158–66, and an irradiated mixture of TAPBPR and HLA/A2/photo-FluM158–66. SV-AUC analyses were carried out and analyzed as described in detail in Materials and Methods, and profiles were acquired at 280 nm with absorbance optical detection system. Sedimentation profiles of the SV data, curve fits, and residuals for runs of (A) TAPBPR at 2 µM, of (B) HLA-A2/photo-FluM158–66 at 2 µM, and of (C) the mixture (not irradiated) of TAPBPR (2 µM) and HLA-A2/photo-FluM158–66 (10 µM).
Fluorescence Polarization.
Binding of HLA-A2 (i.e., HLA-A2/peptide complexes) alone or in the presence of graded amounts of TAPBPR, or of HLA-A2/TAPBPR complexes (previously purified by SEC following UV irradiation and complex formation) to fluorescent peptides was examined by fluorescence polarization using a modification of previously published methods (37). Individual wells of a black polystyrene 384-well Corning plate were loaded with indicated proteins at concentrations as indicated in the figure legends, to a final volume of 70 µL. Peptide exchange was initiated by addition of 10 µL of 40 nM FITC-labeled peptide. The plates were incubated at 18 °C for 12–24 h including appropriate controls for buffer, protein alone, and peptide alone. FP values given as milli-Polarization (mP) are calculated by the following equation: Polarization (mP) = 1,000 (S – GP)/(S + GP), where S and P are background-subtracted intensities of the fluorescence measured in the parallel (S) and perpendicular (P) directions, respectively, and G (grating) is an instrument and assay-dependent correction factor.
SPR Binding Assays and Data Analysis.
All SPR experiments were performed on a BIAcore T100 Biosensor (BIAcore) at 25 °C in 10 mM Hepes, pH 7.4, 150 mM NaCl, and 3 mM EDTA. About 1,000 response units (RU) of purified recombinant TAPBPR were immobilized on a research-grade CM5 chip (BIAcore) with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)/N-hydroxysuccinimide (NHS) coupling chemistry. After buffer exchange to HBS-EP, HLA-A1, HLA-A2, H2-Db, H2-Dd, and H2-Ld refolded with hβ2m and the indicated conventional or photosensitive peptides were offered to the immobilized protein surface at 20 µL/min. After binding and washout, residual bound MHC-I was further dissociated from the immobilized TAPBPR with specific peptides, and sensorgrams were monitored for return to baseline. All measurements were made in at least two independent experiments. Data were analyzed by BIAevaluation 3.0 software (GE Healthcare Life Sciences) or SEDPHAT (36) as indicated.
Molecular Modeling.
A homology model of hTAPBPR was made based on the structure of the tapasin chain in the tapasin/ERp57 complex reported by Dong et al. (PDB code 3F8U) (27) using the homology modeling MMM server (47). The TAPBPR model was used in comparison with the SAXS dummy atom model described below.
SAXS.
Models of the structural shapes of unliganded TAPBPR and tapasin were determined by SAXS data analysis. SAXS data were collected at beamline BioCAT (18ID-D) at the Advanced Photon Source at the Argonne National Laboratory. Purified TAPBPR or tapasin was run in-line on a Superdex 200 Increase 10/300 column at a flow rate of 0.75 mL/min directly connected to the sample chamber, and images were collected during 1-s exposures. Two-dimensional diffraction images were reduced to one-dimension scattering data, and buffer scattering was subtracted. Data analysis and radius of gyration (Rg) and pairwise distance distribution function P(r) calculations were performed using PRIMUS (48), and dummy residue models were produced using DAMMIN/DAMMIF (49, 50). SAXS envelopes were produced by pdb2vol of SITUS (situs.biomachina.org), and rendered by VMD (www.ks.uiuc.edu/Research/vmd/). A comparison of Rg and fitness between PDB structures and derived models and the SAXS experimental data were made with CRYSOL (51). SAXS data and models have been deposited in the BioIsis database (www.bioisis.net) under ID codes TAPSNP and TAPBPP. Data collection and scattering-derived parameters are shown in Table S3.
Acknowledgments
We thank Drs. David Garboczi [National Institute of Allergy and Infectious Diseases (NIAID)] and Rick Willis and John Altman of the NIAID tetramer facility for plasmids used for generating some MHC constructs; Drs. Ted Hansen, Rose Mage, Nathan May, and Rajat Varma for comments on the manuscript; and Robert Tampé for discussion. We appreciate the help of Dr. Tengchuan Jin with fluorescence polarization. Drs. Srinivas Chakravarthy and Weifeng Shang at Bio-CAT/APS (18IDD) assisted with small-angle X-ray scattering (SAXS) data collection. SAXS research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357, a project supported by Grant 9 P41 GM103622 from the National Institute of General Medical Sciences of the National Institutes of Health. Our research was supported by the intramural research programs of the National Institute of Allergy and Infectious Diseases (Project AI0000394) and National Institute of Biomedical Imaging and Bioengineering (Project EB000008)/National Institutes of Health and the Center for Drug Evaluation and Review/Federal Drug Administration (Project 1617).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper has been deposited in the BioIsis database, www.bioisis.net (ID codes TAPSNP and TAPBPP).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519894113/-/DCSupplemental.
References
- 1.Saunders PM, van Endert P. Running the gauntlet: From peptide generation to antigen presentation by MHC class I. Tissue Antigens. 2011;78(3):161–170. doi: 10.1111/j.1399-0039.2011.01735.x. [DOI] [PubMed] [Google Scholar]
- 2.Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 3.Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yewdell JW, Antón LC, Bennink JR. Defective ribosomal products (DRiPs): A major source of antigenic peptides for MHC class I molecules? J Immunol. 1996;157(5):1823–1826. [PubMed] [Google Scholar]
- 5.Apcher S, Manoury B, Fåhraeus R. The role of mRNA translation in direct MHC class I antigen presentation. Curr Opin Immunol. 2012;24(1):71–76. doi: 10.1016/j.coi.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Eisenlohr LC, Huang L, Golovina TN. Rethinking peptide supply to MHC class I molecules. Nat Rev Immunol. 2007;7(5):403–410. doi: 10.1038/nri2077. [DOI] [PubMed] [Google Scholar]
- 7.Shastri N, Nagarajan N, Lind KC, Kanaseki T. Monitoring peptide processing for MHC class I molecules in the endoplasmic reticulum. Curr Opin Immunol. 2014;26:123–127. doi: 10.1016/j.coi.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulsson K, Wang P. Chaperones and folding of MHC class I molecules in the endoplasmic reticulum. Biochim Biophys Acta. 2003;1641(1):1–12. doi: 10.1016/s0167-4889(03)00048-x. [DOI] [PubMed] [Google Scholar]
- 9.Solheim JC, Harris MR, Kindle CS, Hansen TH. Prominence of beta 2-microglobulin, class I heavy chain conformation, and tapasin in the interactions of class I heavy chain with calreticulin and the transporter associated with antigen processing. J Immunol. 1997;158(5):2236–2241. [PubMed] [Google Scholar]
- 10.Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5(2):103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 11.Abele R, Tampé R. The ABCs of immunology: Structure and function of TAP, the transporter associated with antigen processing. Physiology (Bethesda) 2004;19:216–224. doi: 10.1152/physiol.00002.2004. [DOI] [PubMed] [Google Scholar]
- 12.Hansen TH, Bouvier M. MHC class I antigen presentation: Learning from viral evasion strategies. Nat Rev Immunol. 2009;9(7):503–513. doi: 10.1038/nri2575. [DOI] [PubMed] [Google Scholar]
- 13.Song R, Harding CV. Roles of proteasomes, transporter for antigen presentation (TAP), and beta 2-microglobulin in the processing of bacterial or particulate antigens via an alternate class I MHC processing pathway. J Immunol. 1996;156(11):4182–4190. [PubMed] [Google Scholar]
- 14.Spies T, DeMars R. Restored expression of major histocompatibility class I molecules by gene transfer of a putative peptide transporter. Nature. 1991;351(6324):323–324. doi: 10.1038/351323a0. [DOI] [PubMed] [Google Scholar]
- 15.Kanaseki T, et al. ERAAP and tapasin independently edit the amino and carboxyl termini of MHC class I peptides. J Immunol. 2013;191(4):1547–1555. doi: 10.4049/jimmunol.1301043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serwold T, Gonzalez F, Kim J, Jacob R, Shastri N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature. 2002;419(6906):480–483. doi: 10.1038/nature01074. [DOI] [PubMed] [Google Scholar]
- 17.Hammer GE, Gonzalez F, James E, Nolla H, Shastri N. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nat Immunol. 2007;8(1):101–108. doi: 10.1038/ni1409. [DOI] [PubMed] [Google Scholar]
- 18.Santos SG, et al. Major histocompatibility complex class I-ERp57-tapasin interactions within the peptide-loading complex. J Biol Chem. 2007;282(24):17587–17593. doi: 10.1074/jbc.M702212200. [DOI] [PubMed] [Google Scholar]
- 19.Tan P, et al. Recruitment of MHC class I molecules by tapasin into the transporter associated with antigen processing-associated complex is essential for optimal peptide loading. J Immunol. 2002;168(4):1950–1960. doi: 10.4049/jimmunol.168.4.1950. [DOI] [PubMed] [Google Scholar]
- 20.Suh WK, et al. Interaction of murine MHC class I molecules with tapasin and TAP enhances peptide loading and involves the heavy chain alpha3 domain. J Immunol. 1999;162(3):1530–1540. [PubMed] [Google Scholar]
- 21.Wearsch PA, Cresswell P. Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat Immunol. 2007;8(8):873–881. doi: 10.1038/ni1485. [DOI] [PubMed] [Google Scholar]
- 22.Yu YY, et al. An extensive region of an MHC class I alpha 2 domain loop influences interaction with the assembly complex. J Immunol. 1999;163(8):4427–4433. [PubMed] [Google Scholar]
- 23.Carreno BM, et al. TAP associates with a unique class I conformation, whereas calnexin associates with multiple class I forms in mouse and man. J Immunol. 1995;155(10):4726–4733. [PubMed] [Google Scholar]
- 24.Peace-Brewer AL, et al. A point mutation in HLA-A*0201 results in failure to bind the TAP complex and to present virus-derived peptides to CTL. Immunity. 1996;4(5):505–514. doi: 10.1016/s1074-7613(00)80416-1. [DOI] [PubMed] [Google Scholar]
- 25.Lewis JW, Sewell A, Price D, Elliott T. HLA-A*0201 presents TAP-dependent peptide epitopes to cytotoxic T lymphocytes in the absence of tapasin. Eur J Immunol. 1998;28(10):3214–3220. doi: 10.1002/(SICI)1521-4141(199810)28:10<3214::AID-IMMU3214>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.Harris MR, Lybarger L, Yu YY, Myers NB, Hansen TH. Association of ERp57 with mouse MHC class I molecules is tapasin dependent and mimics that of calreticulin and not calnexin. J Immunol. 2001;166(11):6686–6692. doi: 10.4049/jimmunol.166.11.6686. [DOI] [PubMed] [Google Scholar]
- 27.Dong G, Wearsch PA, Peaper DR, Cresswell P, Reinisch KM. Insights into MHC class I peptide loading from the structure of the tapasin-ERp57 thiol oxidoreductase heterodimer. Immunity. 2009;30(1):21–32. doi: 10.1016/j.immuni.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Pasquier L. The phylogenetic origin of antigen-specific receptors. Curr Top Microbiol Immunol. 2000;248:160–185. [PubMed] [Google Scholar]
- 29.Teng MS, et al. A human TAPBP (TAPASIN)-related gene, TAPBP-R. Eur J Immunol. 2002;32(4):1059–1068. doi: 10.1002/1521-4141(200204)32:4<1059::AID-IMMU1059>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 30.Boyle LH, et al. Tapasin-related protein TAPBPR is an additional component of the MHC class I presentation pathway. Proc Natl Acad Sci USA. 2013;110(9):3465–3470. doi: 10.1073/pnas.1222342110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermann C, Strittmatter LM, Deane JE, Boyle LH. The binding of TAPBPR and Tapasin to MHC class I is mutually exclusive. J Immunol. 2013;191(11):5743–5750. doi: 10.4049/jimmunol.1300929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porter KM, Hermann C, Traherne JA, Boyle LH. TAPBPR isoforms exhibit altered association with MHC class I. Immunology. 2014;142(2):289–299. doi: 10.1111/imm.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermann C, Trowsdale J, Boyle LH. TAPBPR: A new player in the MHC class I presentation pathway. Tissue Antigens. 2015;85(3):155–166. doi: 10.1111/tan.12538. [DOI] [PubMed] [Google Scholar]
- 34.Bouvier M, Wiley DC. Structural characterization of a soluble and partially folded class I major histocompatibility heavy chain/beta 2m heterodimer. Nat Struct Biol. 1998;5(5):377–384. doi: 10.1038/nsb0598-377. [DOI] [PubMed] [Google Scholar]
- 35.Toebes M, et al. Design and use of conditional MHC class I ligands. Nat Med. 2006;12(2):246–251. doi: 10.1038/nm1360. [DOI] [PubMed] [Google Scholar]
- 36.Zhao H, Piszczek G, Schuck P. SEDPHAT—a platform for global ITC analysis and global multi-method analysis of molecular interactions. Methods. 2015;76:137–148. doi: 10.1016/j.ymeth.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchli R, Vangundy RS, Giberson CF, Hildebrand WH. Critical factors in the development of fluorescence polarization-based peptide binding assays: An equilibrium study monitoring specific peptide binding to soluble HLA-A*0201. J Immunol Methods. 2006;314(1-2):38–53. doi: 10.1016/j.jim.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Kozlowski S, et al. Excess beta 2 microglobulin promoting functional peptide association with purified soluble class I MHC molecules. Nature. 1991;349(6304):74–77. doi: 10.1038/349074a0. [DOI] [PubMed] [Google Scholar]
- 39.Hermann C, et al. TAPBPR alters MHC class I peptide presentation by functioning as a peptide exchange catalyst. eLife. 2015;4:4. doi: 10.7554/eLife.09617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bunch TA, Grinblat Y, Goldstein LS. Characterization and use of the Drosophila metallothionein promoter in cultured Drosophila melanogaster cells. Nucleic Acids Res. 1988;16(3):1043–1061. doi: 10.1093/nar/16.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garboczi DN, Hung DT, Wiley DC. HLA-A2-peptide complexes: Refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci USA. 1992;89(8):3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shields MJ, Assefi N, Hodgson W, Kim EJ, Ribaudo RK. Characterization of the interactions between MHC class I subunits: A systematic approach for the engineering of higher affinity variants of beta 2-microglobulin. J Immunol. 1998;160(5):2297–2307. [PubMed] [Google Scholar]
- 43.Waeber U, Buhr A, Schunk T, Erni B. The glucose transporter of Escherichia coli. Purification and characterization by Ni+ chelate affinity chromatography of the IIBCGlc subunit. FEBS Lett. 1993;324(1):109–112. doi: 10.1016/0014-5793(93)81542-8. [DOI] [PubMed] [Google Scholar]
- 44.Zhao H, Brautigam CA, Ghirlando R, Schuck P. 2013. Overview of current methods in sedimentation velocity and sedimentation equilibrium analytical ultracentrifugation. Curr Protoc Protein Sci Chapter 20:Unit 20.12.
- 45.Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys J. 2000;78(3):1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balbo A, Brown PH, Braswell EH, Schuck P. 2007. Measuring protein-protein interactions by equilibrium sedimentation. Curr Protoc Immunol Chapter 18:Unit 18.18.
- 47.Rai BK, Fiser A. Multiple mapping method: A novel approach to the sequence-to-structure alignment problem in comparative protein structure modeling. Proteins. 2006;63(3):644–661. doi: 10.1002/prot.20835. [DOI] [PubMed] [Google Scholar]
- 48.Konarev PV, Volkov VV, Sokolova AV, Koch MHJ, Svergun DI. PRIMUS: A Windows PC-based system for small-angle scattering data analysis. J Appl Crystallogr. 2003;36(5):1277–1282. [Google Scholar]
- 49.Svergun DI. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys J. 1999;76(6):2879–2886. doi: 10.1016/S0006-3495(99)77443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franke D, Svergun DI. DAMMIF, a program for rapid ab-initio shape determination in small-angle scattering. J Appl Crystallogr. 2009;42(2):342–346. doi: 10.1107/S0021889809000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Svergun D, Barberato C, Koch MHJ. CRYSOL—a program to evaluate x-ray solution scattering of biological macromolecules from atomic coordinates. J Appl Crystallogr. 1995;28(6):768–773. [Google Scholar]