Significance
About one-third of patients with type 1 diabetes mellitus develop nephropathy, which often progresses to end-stage renal diseases. The present study demonstrates that below-normal Elmo1 expression in mice ameliorates the albuminuria and glomerular histological changes resulting from long-standing type 1 diabetes, whereas above-normal Elmo1 expression makes both worse. Increasing Elmo1 expression leads to aggravation of oxidative stress markers and enhances the expression of fibrogenic genes. Suppressing Elmo1 action in human patients could be a promising option for treating/preventing the progressive deterioration of renal function in diabetes.
Keywords: reactive oxygen species, 3′-untranslated region, fibrosis
Abstract
Human genome-wide association studies have demonstrated that polymorphisms in the engulfment and cell motility protein 1 gene (ELMO1) are strongly associated with susceptibility to diabetic nephropathy. However, proof of causation is lacking. To test whether modest changes in its expression alter the severity of the renal phenotype in diabetic mice, we have generated mice that are type 1 diabetic because they have the Ins2Akita gene, and also have genetically graded expression of Elmo1 in all tissues ranging in five steps from ∼30% to ∼200% normal. We here show that the Elmo1 hypermorphs have albuminuria, glomerulosclerosis, and changes in the ultrastructure of the glomerular basement membrane that increase in severity in parallel with the expression of Elmo 1. Progressive changes in renal mRNA expression of transforming growth factor β1 (TGFβ1), endothelin-1, and NAD(P)H oxidase 4 also occur in parallel with Elmo1, as do the plasma levels of cystatin C, lipid peroxides, and TGFβ1, and erythrocyte levels of reduced glutathione. In contrast, Akita type 1 diabetic mice with below-normal Elmo1 expression have reduced expression of these various factors and less severe diabetic complications. Remarkably, the reduced Elmo1 expression in the 30% hypomorphs almost abolishes the pathological features of diabetic nephropathy, although it does not affect the hyperglycemia caused by the Akita mutation. Thus, ELMO1 plays an important role in the development of type 1 diabetic nephropathy, and its inhibition could be a promising option for slowing or preventing progression of the condition to end-stage renal disease.
Diabetic nephropathy is the leading cause of end-stage renal diseases in developed countries (1). Although the control of blood glucose levels remains the mainstay to prevent diabetic nephropathy, cumulative epidemiological studies have also provided evidence that genetic factors partly account for the severity of the disease (2, 3), and human genome-wide association studies have reported several candidate genes conferring susceptibility or resistance to diabetic nephropathy.
The gene coding for engulfment and cell motility protein 1 (ELMO1), first discovered as a gene required for phagocytosis of apoptotic cells and cell motility (4), is a strong candidate, but proof of causation is lacking. In a genome-wide case control association study in Japan, over 80,000 single-nucleotide polymorphism (SNP) loci were tested and one SNP locus in the 18th intron of the ELMO1 gene was found to be strongly associated with nephropathy due to type 2 diabetes (χ2 = 19.9; P = 0.000008; odds ratio, 2.7) (5). Later studies have demonstrated an association of SNPs in the ELMO1 gene and the susceptibility to type 1 diabetic nephropathy in Caucasians (6, 7), Pima Indians (8), African Americans (9), and Chinese population (10), although some other studies have reported that the association is not significant (11, 12).
To determine whether modest genetic changes in the levels of Elmo1 expression cause differences in the severity of diabetic nephropathy that occurs in type 1 diabetes, we have used our previously described method (13) to generate mice having five genetically graded levels of Elmo1 mRNA expression ranging from ∼30% to ∼200% normal, and have studied their phenotypes after making them type 1 diabetic by crossbreeding them with mice carrying the dominant Akita mutation in the insulin 2 gene (Ins2Akita).
Here, we show that the severity of renal fibrosis and the amount of urinary albumin excretion in the Akita diabetic mice parallels the genetic levels of Elmo1. The indices of reactive oxygen species (ROS) also increase as the expression of Elmo1 increases, although plasma glucose levels and blood pressure do not differ significantly among the mice with the five Elmo1 genetic levels. These results indicate that ELMO1 plays an important role in the development of diabetic nephropathy, probably by increasing oxidative stress.
Generation of Akita Diabetic Mice Having Five Genetically Different Levels of Elmo1
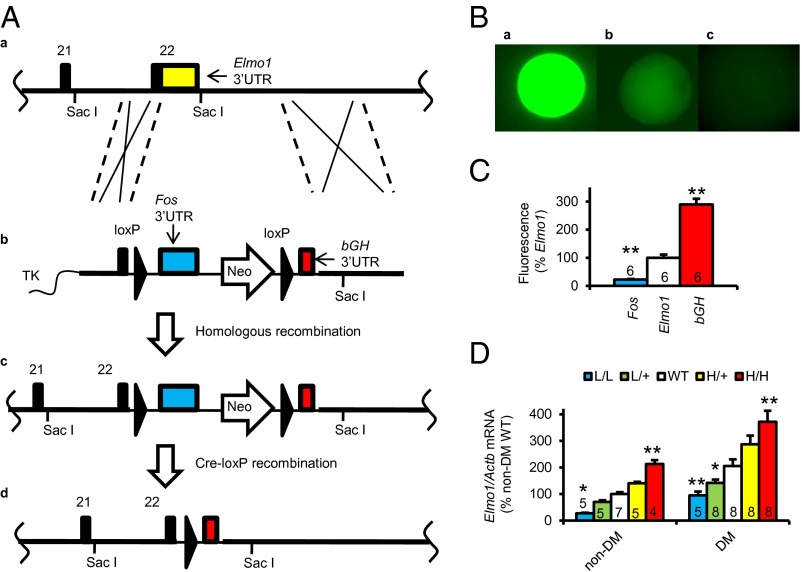
We first used our published method (13, 14) to generate mice having an Elmo1 allele in which the 3′-untranslated region (3′-UTR) of the gene is replaced with that of the cFos gene (to form a low-expressing L allele) or with that of bovine growth hormone gene, bGH (to form a high-expressing H allele) (Fig. 1A). Comparison of the effects of these 3′-UTRs on gene expression shows that the stability of an mRNA having the natural Elmo1 3′-UTR is intermediate between that of an mRNA having the Fos 3′-UTR and that of an mRNA having the bGH 3′-UTR (Fig. 1 B and C).
Fig. 1.
Generation of Akita diabetic mice having five graded expression levels of Elmo1. (A) The gene-targeting strategy. (a) The target locus, Elmo1, into which an exogenous 3′-untranslated region (3′-UTR) is introduced by homologous recombination. Coding sequences and the endogenous 3′-UTR of the Elmo1 gene are shown as black and yellow columns, respectively. (b) The targeting vector contains a loxP sequence, the c-Fos 3′-UTR (blue), a Neo gene with the MC1 promoter (pMC1), loxP, and the 3′-UTR of the bovine growth hormone gene (bGH 3′-UTR; red). TK, thymidine kinase gene. (c) The resulting locus after homologous recombination; the Elmo1 gene is now in its low-expression form (Elmo1L) because the stability of its mRNA is now controlled by the destabilizing 3′-UTR of Fos. (d) The resulting locus after Cre-lox P recombination; the Elmo1 gene is now in its high-expression form (Elmo1H) because the stability of its mRNA is now controlled by the stabilizing 3′-UTR of bGH. (B) In vitro comparison of the effects of 3′-UTRs and their G-rich elements (GREs) on expression of a green fluorescent protein gene. Fluorescence of colonies with the 3′-UTRs and GREs of bGH, Elmo1, and Fos is shown. (C) Mean fluorescence levels of the single cells with the 3′-UTR and GRE of bGH, Elmo1, and Fos from different colonies. *P < 0.05, **P < 0.01 vs. Elmo1. (D) The mRNA levels of Elmo1 in the kidney of the nondiabetic and Akita diabetic mice having five graded expression levels of Elmo1 at age 40 wk. *P < 0.05, **P < 0.01 vs. Elmo1 WT genotype.
Mice having the five combinations of the low (L), WT (+), and high (H) alleles (Elmo1L/L, Elmo1L/+, Elmo1+/+, Elmo1H/+, and Elmo1H/H) were bred and at age 40 wk have Elmo1 mRNA expression graded in five steps from ∼30% to ∼200% of normal (Fig. 1D, Left).
We then crossbred these mice with mice having the Akita mutation in insulin 2 gene (Ins2Akita/+), to generate male Akita mice with the following genotypes—Elmo1L/L: Ins2Akita/+ (L/L Akita), Elmo1L/+:Ins2Akita/+ (L/+ Akita), Elmo1+/+:Ins2Akita/+ (Akita), Elmo1H/+:Ins2Akita/+ (H/+ Akita), and Elmo1H/H:Ins2Akita/+ (H/H Akita). Fig. 1D, Right, shows that these diabetic mice, like their nondiabetic counterparts, have Elmo1 mRNA expression graded in five steps, still ranging from genetically increased levels of Elmo1 to ∼200% relative to diabetic WT Elmo 1 mice but at an approximately two times higher level than nondiabetic WT Elmo1 mice.
Systemic Parameters Affecting Diabetic Nephropathy in Akita Diabetic Mice with Five Graded Expressions of Elmo1
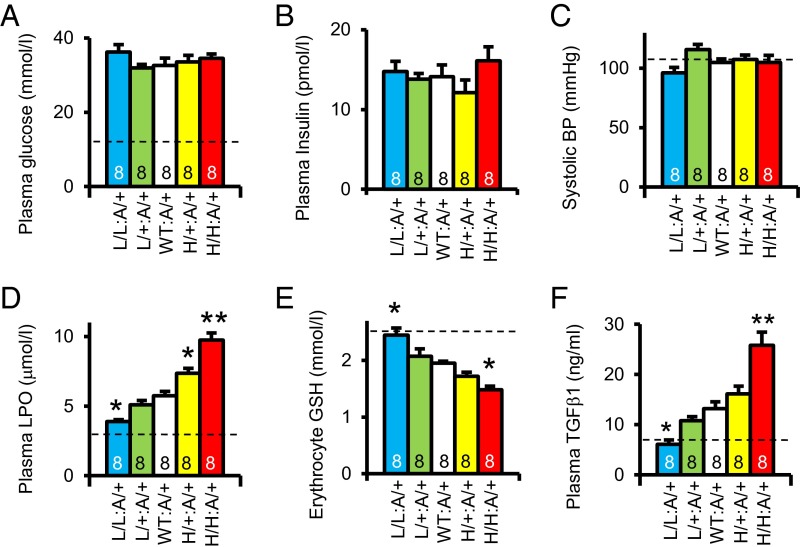
Plasma glucose and plasma insulin levels are not significantly different among the Akita diabetic mice with the five Elmo1 genotypes (Fig. 2 A and B), nor are their arterial pressures different (Fig. 2C). However, plasma lipid peroxides (a marker of oxidative stress) are progressively increased (Fig. 2D), and the levels of reduced form of glutathione in erythrocytes is reduced (Fig. 2E) as the expression of Elmo1 increases in the diabetic mice. Plasma levels of transforming growth factor β1 (TGFβ1) also increase as the expression of Elmo1 increases in the diabetic mice (Fig. 2F). These results demonstrate that increased expression of Elmo1 does not affect plasma glucose levels and blood pressure in the diabetic mice, but that it progressively enhances oxidative stress and increases the plasma levels of the fibrogenic cytokine TGFβ1.
Fig. 2.
Systemic parameters affecting diabetic nephropathy in Akita mice with five graded expressions of Elmo1 at age 40 wk. Dotted lines indicate nondiabetic WT levels. *P < 0.05, **P < 0.01 vs. Elmo1 WT. (A) Plasma glucose levels. (B) Plasma insulin levels. (C) Systolic blood pressure (BP) determined with a tail-cuff method. (D) Plasma levels of lipid peroxide (LPO). (E) Erythrocyte levels of reduced glutathione (GSH). (F) Plasma levels of transforming growth factor β1 (TGFβ1).
Overexpression of Elmo1 Increases and Underexpression of Elmo1 Decreases Renal Histological Changes, Renal Excretory Function, Urinary Excretion of Albumin, and the Renal Expression of Fibrogenic Genes in Akita Diabetic Mice
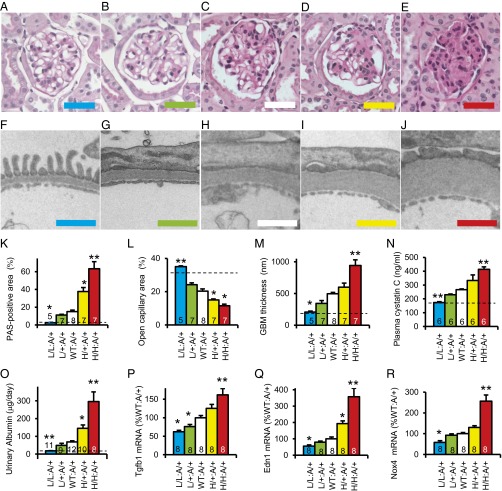
Microscopic studies of the glomeruli of the diabetic mice (Fig. 3 A–E) showed that the diabetic mice with wild-type Elmo1 alleles (WT:A/+) at age 40 wk had pathological changes in their glomeruli typical of diabetic nephropathy, including mesangial cell proliferation and accumulation of periodic acid–Schiff (PAS)-positive materials (Fig. 3C). These pathological changes were progressively exacerbated when expression of Elmo1 increased above normal in the H/+:A/+ and H/H:A/+ Akita diabetic mice (Fig. 3 D and E). In marked contrast, the pathological changes were completely absent in the Akita mice with the lowest expression of Elmo1 (L/L:A/+; Fig. 3A), and were still reduced in the L/+:A/+ mice (Fig. 3B) relative to those in the diabetic mice with wild-type Elmo1 (Fig. 3C). We quantitated the renal pathology in the five Elmo1 genotypes by measuring the fraction of PAS-positive area per glomerular tuft area, an indicator of mesangial expansion, and found that PAS-positive material increased more than 10-fold in the Akita diabetic animals as Elmo1 expression increased (Fig. 3K). The open capillary area in the L/L:A/+ hypomorphs decreased from almost twice that in the WT:A/+ diabetic mice to less than one-half in the H/H:A/+ diabetic mice (Fig. 3L).
Fig. 3.
Renal pathology in the Akita diabetic mice having five graded expression levels of Elmo1 at 40 wk of age. Dotted lines indicate nondiabetic WT levels. *P < 0.05, **P < 0.01 vs. Elmo1 WT. (A–E) Periodic acid–Schiff (PAS) staining with hematoxylin of the glomerulus in 40-wk-old male mice. (Scale bar: 50 μm.) (F–J) Transmission electron microscopy of the glomerular basement membrane in 40-wk-old male mice. (Scale bar: 1 μm.) (A and F) L/L Akita mouse. (B and G) L/+ Akita mouse. (C and H) WT Akita mice. (D and I) H/+ Akita mouse. (E and J) H/H Akita mouse. (K) Quantification of PAS-positive mesangial material per total glomerular tuft cross-sectional area. (L) Percentage of open capillary area per glomerular tuft. (M) Thickness of glomerular basement membrane (GBM). (N) Plasma levels of cystatin C. (O) Urinary albumin excretion. (P) Renal expression of Tgfb1. (Q) Renal expression of Edn1. (R) Renal expression of Nox4.
The pathology revealed by light microscopy was further evaluated by electron microscopy (Fig. 3 F–J). The results confirmed that, at age 40 wk, the Akita mice with wild-type Elmo1 alleles (WT:A/+) had ultrastructural changes typical of advanced diabetic nephropathy, including a severalfold increase in the thickness of the glomerular basement membrane (GBM) together with marked podocyte effacement (Fig. 3H). The GBM thickening (quantitated in Fig. 3M) was progressively exacerbated in the H/+:A/+ and H/H:A/+ mice with above-normal expression of Elmo1 (Fig. 3 I and J). The ultrastructure of the glomeruli in the L/L:A/+ mice was indistinguishable from that in wild-type nondiabetic C57BL/6 mouse (Fig. 3 F and M). Podocyte effacement was present in the glomeruli of all of the diabetic mice except the L/L:A/+ mice with one-third normal Elmo1 expression. We did not observe any obvious lesions in the renal vasculature other than in the glomeruli of the Akita mice. We conclude that the nephropathy observed in the mature Akita mice with type 1 diabetes is strongly affected by the expression of Elmo1, ranging from almost the same as in nondiabetic Elmo1 WT mice to severe diabetic nephropathy.
We studied kidney function by measuring plasma cystatin C and found that the cystatin C levels increase as the expression of Elmo1 is increased in Akita mice (Fig. 3N). Likewise, urinary excretion of albumin was progressively increased when expression of Elmo1 increased (Fig. 3O).
Renal expression of genes encoding TGFβ1 (Tgfb1; Fig. 3P), endothelin-1 (Edn1; Fig. 3Q), and NAD(P)H oxidase 4 (Nox4; Fig. 3R), which have been demonstrated to be involved in fibrogenesis and epithelial–mesenchymal transition, are progressively increased as the expression of Elmo1 is increased.
Thus, visual inspection of the light and electron microscope images of the glomeruli together with their quantitative evaluation show that below-normal Elmo1 expression ameliorates the nephropathy caused by diabetes whereas above-normal expression exacerbates it.
Discussion
In the current study, we have shown that genetically increased levels of Elmo1 in Akita diabetic mice lead to progressive increases in the concentrations of oxidative stress markers, in the severity of pathological features characteristic of diabetic nephropathy, including glomerular fibrotic changes and urinary excretion of albumin and the renal expression of fibrogenic genes. In contrast, genetically decreased levels of Elmo1 lead to less urinary output of albumin, less tissue fibrosis, and less expression of fibrogenic genes. Strikingly, the kidneys of the diabetic mice with ∼30% Elmo1 expression exhibited no pathological changes; their kidneys were the same as those of nondiabetic mice Elmo1 WT mice. Remarkably the plasma level of lipid peroxides (a measure of oxidative stress) and the erythrocyte level of reduced glutathione in the 30% Elmo1 diabetic mice are also indistinguishable from that in nondiabetic Elmo1 WT mice. Plasma glucose levels and blood pressures of the diabetic mice were unaffected by differences in Elmo1 expression. Thus, our results show that genetic increases in Elmo1 expression causally aggravate the severity of diabetic nephropathy, whereas reducing expression of Elmo1 to about one-third is sufficient to prevent any pathological effects of the diabetes.
Elmo1 was first discovered as a factor required for the phagocytosis of dying cells and cell migrations in Caenorhabditis elegans (4) that also acts as an upstream regulator of Rac1 in the Ras-related C3 botulinum toxin substrate (Rac) signaling pathway. Elmo1 is part of the Rac–guanine nucleotide exchange factor (Rac-GEF), which converts inactive Rac-GDP into active Rac-GTP (15). Earlier investigations have shown that expression of Elmo1 is increased in db/db type 2 diabetic mice relative to its expression in nondiabetic control mice (5). In our present study, we have found that Elmo1 expression is also elevated in Akita type 1 diabetic mice.
A possible chain of events leading from Elmo1 to an increase in ROS and to nephropathy is apparent. Thus, in KKA(y) mice, which develop obesity-related diabetic kidney disease, the activity of Rac1 is increased in mesangial cells, and treatment with a pan-Rac inhibitor (EHT1864) mitigates the renal pathology (16). Rac1, Rac2, and Rac3 increase the formation of superoxide anion via activation of NAD(P)H oxidases (17–20), which are encoded in Nox genes. Increased production of superoxide by the mitochondrial electron transport chain has been shown to be the causal link between elevated glucose and three major pathways of hyperglycemic damage (activation of protein kinase C, formation of advanced glycation end products, and activation of the polyol pathway) in cultured bovine endothelial cells (21), and there is growing evidence that NAD(P)H oxidase is a critical enzyme for producing ROS (22). Additionally, pharmacological inhibition of NAD(P)H oxidase with apocynin has been shown to reverse renal mesangial matrix expansion in streptozotocin-induced type 1 diabetic mice (23). Consequently, it is likely that increased expression of Elmo1 enhances the development of diabetic nephropathy by activating Rac and increasing NAD(P)H oxidase, which in turn increases ROS.
A possible chain of events between increases in Elmo1 expression and glomerulosclerosis is also apparent. Thus, pharmacological inhibition of different ROS sources, including NAD(P)H oxidase and the mitochondrial respiratory chain, decreases the transcription of Tgfb1 via reduced activity of activated protein 1 (24), indicating that ROS-induced enhancement of the transcription of Tgfb1 may at least in part account for the increase in Tgfb1 expression in diabetes. In vitro overexpression of Elmo1 in cultured COS cells increases the expression of Tgfb1 and its downstream extracellular matrix genes, including fibronectin, collagen 1A1 (5), and integrin-linked kinase (5). Consequently, it is likely that the overexpression of Elmo1 in diabetes increases Tgfb1 expression and enhances the development of nephropathy via its effects on ROS formation.
In summary, we have studied the renal phenotypes of Akita type 1 diabetic male mice having five different genetically determined levels of Elmo1 expression and find that Elmo1 expression levels positively correlate with glomerular fibrosis and urinary excretion of albumin, although no significant differences in plasma glucose or blood pressure are seen among the five genotypes. Most notable is our finding that the nephropathic effects of diabetes are completely prevented if Elmo1 expression is genetically decreased to one-third, even though the diabetic hyperglycemia persists. Thus, Elmo1 plays an important pathophysiological role in the development of albuminuria and the tissue fibrotic changes characteristic of diabetic nephropathy. The remarkable effects of modestly decreasing its levels genetically in mice suggest that pharmacologically suppressing Elmo1 action in the kidney could be a promising option for preventing the progression of early stages of nephropathy to end-stage renal disease in patients with type 1 diabetes.
Materials and Methods
Animals.
Mice having five graded expression levels of Elmo1 were generated by targeted replacement of the 3′-UTR of Elmo1 with unstable Fos 3′-UTR or with stable bGH 3′-UTR, as previously described (13).
To study the effects of Elmo1 on the phenotype in diabetes, heterozygous and homozygous mice having hypomorphic (L) or hypermorphic (H) alleles for Elmo1 generated on a C57BL/6 genetic background (Ingenious Targeting Laboratory) were crossbred with mice heterozygous for the Akita mutation in the insulin 2 gene on a C57BL/6 genetic background (The Jackson Laboratory).
All mice were kept under husbandry conditions conforming to the guidelines of the University of North Carolina (UNC) Institutional Animal Care and Use Committee.
Measurement of Biological Parameters.
Plasma glucose levels were determined with the glucose oxidase method (Wako Chemical). Plasma insulin levels were determined with ELISA (Crystal Chem). Plasma TGFβ1 levels were determined with ELISA (Quantikine Mouse/Rat/Porcine/Canine TGFβ1 Immunoassay; R&D Systems). Plasma cystatin C levels were determined with ELISA (Quantikine Mouse/Rat Cystatin C Immunoassay; R&D Systems). Plasma lipid peroxide was quantified as thiobarbiturate reactive substances (Cayman Chemical). The reduced form of glutathione (GSH) in erythrocytes was determined with a Glutathione assay kit (EMD Millipore). Metabolic balance studies were performed using metabolic cages (Solo Mouse Metabolic Cage; Tecniplast).
Histology.
After cutting the inferior vena cava, the left ventricle was punctured with a 23-gauge needle and perfused with PBS for 3 min and with 4% (wt/vol) paraformaldehyde for 5 min. Thereafter, the tissues were dissected out and put in 4% (wt/vol) paraformaldehyde for at least 3 d. They were then paraffin embedded and sectioned. Stained sections were prepared by the Center for Gastrointestinal Biology and Diseases Histology Core at UNC and imaged on an Olympus BX61 microscope. For electron microscopy, grids were prepared by the Microscopy Services Laboratory at UNC and imaged on a Zeiss TEM 910 transmission electron microscope.
Blood Pressure and Pulse Rate Measurement.
Blood pressure and pulse rate were measured with a tail-cuff method (25).
Quantitative Reverse Transcription–PCR.
Total RNA was extracted from different tissues, and the mRNAs were assayed by quantitative reverse transcription–PCR as previously described (26). The primers and probes used to measure the mRNAs are shown in Table S1.
Table S1.
Primers and probes for quantification of mRNA with real-time quantitative reverse transcription–PCR
| Gene symbol | Primers and probes | Sequences |
| Actb | Forward primer | 5′-AAG AGC TAT GAG CTG CCT GA-3′ |
| Reverse primer | 5′-ACG GAT GTC AAC GTC ACA CT-3′ | |
| Probe | 5′-FAM-CAC TAT TGG CAA CGA GCG GTT CCG-Tamra-3′ | |
| Edn1 | Forward primer | 5′-TGC CAC CTG GAC ATC ATC TG-3′ |
| Reverse primer | 5′-ACG CTT GGA CCT GGA AGA AC-3′ | |
| Probe | 5′-FAM-TCC CGA GCG CGT CGT ACC GTA TG-Tamra-3′ | |
| Elmo1 | Forward primer | 5′-ATC CTC AAA GTG GGC GAG CT-3′ |
| Reverse primer | 5′-CCT CAA AAG ATC TGT CAT GGG T-3′ | |
| Probe | 5′-FAM-AGT GAG ACC TGC AAC GAC TTT CAC CCG-Tamra-3′ | |
| Nox4 | Forward primer | 5′-AGT AGT AGG AGA CTG GAC AG-3′ |
| Reverse primer | 5′-AAT GAA GGG CAG AAT CTC AGA-3′ | |
| Probe | 5′-FAM-TCC GGG ATT TGC TAC TGC CTC CAT CAA G-Tamra-3′ | |
| Tgfb1 | Forward primer | 5′-TGACGTCACTGGAGTTGTACGG-3′ |
| Reverse primer | 5′-GGTTCATGTCATGGATGGATGGTGC-3′ | |
| Probe | 5′-FAM-TTCAGCGCTCACGTCTCTTGTGACAG-Tamra-3′ |
Statistical Analysis.
Data are expressed as means ± SEs. To compare groups, we used one-factor ANOVA. Post hoc pairwise comparisons were performed by Tukey–Kramer honestly significant difference test (JMP 8.0; SAS Institute).
Acknowledgments
This work was supported by NIH Grants HL49277, HL70523, and HL71266, and by Career Development Award 2006-102 from Juvenile Diabetes Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600511113/-/DCSupplemental.
References
- 1.Collins AJ, Chen SC, Gilbertson DT, Foley RN. CKD surveillance using administrative data: Impact on the health care system. Am J Kidney Dis. 2009;53(3) Suppl 3:S27–S36. doi: 10.1053/j.ajkd.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 2.Krolewski AS, Warram JH, Rand LI, Kahn CR. Epidemiologic approach to the etiology of type I diabetes mellitus and its complications. N Engl J Med. 1987;317(22):1390–1398. doi: 10.1056/NEJM198711263172206. [DOI] [PubMed] [Google Scholar]
- 3.Quinn M, Angelico MC, Warram JH, Krolewski AS. Familial factors determine the development of diabetic nephropathy in patients with IDDM. Diabetologia. 1996;39(8):940–945. doi: 10.1007/BF00403913. [DOI] [PubMed] [Google Scholar]
- 4.Gumienny TL, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107(1):27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 5.Shimazaki A, et al. Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes. 2005;54(4):1171–1178. doi: 10.2337/diabetes.54.4.1171. [DOI] [PubMed] [Google Scholar]
- 6.Craig DW, Millis MP, DiStefano JK. Genome-wide SNP genotyping study using pooled DNA to identify candidate markers mediating susceptibility to end-stage renal disease attributed to type 1 diabetes. Diabet Med. 2009;26(11):1090–1098. doi: 10.1111/j.1464-5491.2009.02846.x. [DOI] [PubMed] [Google Scholar]
- 7.Pezzolesi MG, et al. Confirmation of genetic associations at ELMO1 in the GoKinD collection supports its role as a susceptibility gene in diabetic nephropathy. Diabetes. 2009;58(11):2698–2702. doi: 10.2337/db09-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson RL, et al. ELMO1 variants and susceptibility to diabetic nephropathy in American Indians. Mol Genet Metab. 2010;101(4):383–390. doi: 10.1016/j.ymgme.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leak TS, et al. Variants in intron 13 of the ELMO1 gene are associated with diabetic nephropathy in African Americans. Ann Hum Genet. 2009;73(2):152–159. doi: 10.1111/j.1469-1809.2008.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu HY, et al. Association of ELMO1 gene polymorphisms with diabetic nephropathy in Chinese population. J Endocrinol Invest. 2013;36(5):298–302. doi: 10.3275/8525. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, et al. Examination of association with candidate genes for diabetic nephropathy in a Mexican American population. Clin J Am Soc Nephrol. 2010;5(6):1072–1078. doi: 10.2215/CJN.06550909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams WW, et al. GENIE Consortium Association testing of previously reported variants in a large case-control meta-analysis of diabetic nephropathy. Diabetes. 2012;61(8):2187–2194. doi: 10.2337/db11-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakoki M, et al. Altering the expression in mice of genes by modifying their 3′ regions. Dev Cell. 2004;6(4):597–606. doi: 10.1016/s1534-5807(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 14.Kakoki M, et al. Primary aldosteronism and impaired natriuresis in mice underexpressing TGFβ1. Proc Natl Acad Sci USA. 2013;110(14):5600–5605. doi: 10.1073/pnas.1302641110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brugnera E, et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol. 2002;4(8):574–582. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida S, et al. Local mineralocorticoid receptor activation and the role of Rac1 in obesity-related diabetic kidney disease. Nephron Exp Nephrol. 2014;126(1):16–24. doi: 10.1159/000358758. [DOI] [PubMed] [Google Scholar]
- 17.Abo A, et al. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353(6345):668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 18.Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254(5037):1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- 19.Ando S, et al. Post-translational processing of rac p21s is important both for their interaction with the GDP/GTP exchange proteins and for their activation of NADPH oxidase. J Biol Chem. 1992;267(36):25709–25713. [PubMed] [Google Scholar]
- 20.Miyano K, Koga H, Minakami R, Sumimoto H. The insert region of the Rac GTPases is dispensable for activation of superoxide-producing NADPH oxidases. Biochem J. 2009;422(2):373–382. doi: 10.1042/BJ20082182. [DOI] [PubMed] [Google Scholar]
- 21.Nishikawa T, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 22.Guzik TJ, et al. Vascular superoxide production by NAD(P)H oxidase: Association with endothelial dysfunction and clinical risk factors. Circ Res. 2000;86(9):E85–E90. doi: 10.1161/01.res.86.9.e85. [DOI] [PubMed] [Google Scholar]
- 23.Asaba K, et al. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67(5):1890–1898. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 24.González-Ramos M, et al. Intracellular redox equilibrium is essential for the constitutive expression of AP-1 dependent genes in resting cells: Studies on TGF-β1 regulation. Int J Biochem Cell Biol. 2012;44(6):963–971. doi: 10.1016/j.biocel.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25(5):1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 26.Kim HS, Lee G, John SW, Maeda N, Smithies O. Molecular phenotyping for analyzing subtle genetic effects in mice: Application to an angiotensinogen gene titration. Proc Natl Acad Sci USA. 2002;99(7):4602–4607. doi: 10.1073/pnas.072083799. [DOI] [PMC free article] [PubMed] [Google Scholar]