Significance
Asthma exacerbations are frequently triggered by common cold infections. Whereas prior studies have swabbed individual patients to detect viruses, we take a population level approach to investigate common cold circulation and its consequences for asthmatics. We introduce a dynamic model of common cold transmission with different contact patterns for adults and children, which are modified by school vacations. By jointly fitting this and an asthma risk model to daily hospitalization rates in eight large cities in Texas, we found that the common cold is a primary driver of asthma exacerbations, and both are predictably influenced by the school calendar. This provides actionable insight for assessing asthma hospitalization risk and targeting preventive measures.
Keywords: asthma, common cold, mathematical model, transmission, Bayesian inference
Abstract
Asthma exacerbations exhibit a consistent annual pattern, closely mirroring the school calendar. Although respiratory viruses—the “common cold” viruses—are implicated as a principal cause, there is little evidence to link viral prevalence to seasonal differences in risk. We jointly fit a common cold transmission model and a model of biological and environmental exacerbation triggers to estimate effects on hospitalization risk. Asthma hospitalization rate, influenza prevalence, and air quality measures are available, but common cold circulation is not; therefore, we generate estimates of viral prevalence using a transmission model. Our deterministic multivirus transmission model includes transmission rates that vary when school is closed. We jointly fit the two models to 7 y of daily asthma hospitalizations in adults and children (66,000 events) in eight metropolitan areas. For children, we find that daily viral prevalence is the strongest predictor of asthma hospitalizations, with transmission reduced by 45% (95% credible interval =41–49%) during school closures. We detect a transient period of nonspecific immunity between infections lasting 19 (17–21) d. For adults, hospitalizations are more variable, with influenza driving wintertime peaks. Neither particulate matter nor ozone was an important predictor, perhaps because of the large geographic area of the populations. The school calendar clearly and predictably drives seasonal variation in common cold prevalence, which results in the “back-to-school” asthma exacerbation pattern seen in children and indirectly contributes to exacerbation risk in adults. This study provides a framework for anticipating the seasonal dynamics of common colds and the associated risks for asthmatics.
Asthma is a chronic airway condition with increasing prevalence in many countries (1, 2). Exacerbations, the worsening of asthma symptoms, are a growing public health concern, resulting in millions of missed work and school days and $50 billion in direct healthcare costs in the United States each year (3–5). Prior studies have examined environmental correlates of asthma exacerbations, including air quality measures (6–8), whereas others have considered the role of respiratory virus infections in triggering asthma exacerbation (9–13). However, none have simultaneously considered both infectious and noninfectious factors that potentially influence the large-scale spatiotemporal dynamics of asthma exacerbations.
Asthma-related hospitalizations exhibit an extraordinarily consistent seasonal pattern from year to year (14). In children, this pattern strongly reflects the school calendar (15–17). A wave of asthma exacerbations in children ensues shortly after the return to school after summer break (shown in Fig. 1 for Texas in mid-August). The return-to-school peak has been termed the “September epidemic of asthma” (14) and noted in the United Kingdom (18), Canada (17, 19), and elsewhere (20). Asthma hospitalizations also seem to rise after the 2-wk winter holiday (late December through early January) and 1-wk spring break (late March) (Fig. 1).
Fig. 1.
Daily number of asthma hospitalizations. Total hospitalizations in the eight largest metropolitan areas in Texas from August of 2004 to August of 2005, where markers indicate the first day of the month. Daily count values (light gray) and a spline-smoothed value (dark gray) in (A) children ages 5–18 y old and (B) adults ages 19–55 y old. In 2004, most Texas schools started in mid-August, took a 2-wk winter break in late December to early January, and took a 1-wk spring break in late March.
Respiratory virus infections, including those responsible for the common cold, are known to cause exacerbations in asthmatic children and to a lesser extent, adults suffering from respiratory diseases (11, 21, 22). In particular, rhinovirus has been widely implicated in asthma exacerbations and wheezing-related hospitalizations (13, 16, 23–29). Although asthma is not infectious, these aggravating viruses are infectious. Consequently, the dynamics of asthma hospitalizations can seem as if children are serving as transmission vectors for exacerbations (19). Data on the prevalence of these common viruses are infrequently available and are never available for large sample sizes.
Common cold viruses spread rampantly—typically causing two to four relatively mild infections in adults and three to eight infections in children annually (30, 31). Although asthmatics tend to experience more severe and prolonged illness on infection, studies suggest that the frequency of infection is similar for asthmatics and nonasthmatics (21). However, little is known about the transmission dynamics of these viruses or the extent to which they account for the complex annual cycles of asthma exacerbations. Mathematical models of viral transmission are widely used for estimating epidemiological parameters, such as transmission rates, from disease surveillance data (32–34). Such data are rare for common colds, because most infections are subclinical, never entering the healthcare system. Here, we exploit asthmatics as a “sentinel” population for the common cold to infer the transmission dynamics of these viruses.
Although viral infections are an important trigger for asthma exacerbations, they are not the only cause of asthma hospitalizations. In particular, poor air quality is thought to be a critical risk factor, and the link between pollution and asthma has been studied extensively (8, 35–39). Elevated particulate matter and ozone levels have both been associated with increased asthma exacerbations and hospitalization events.
By fitting a mathematical model of viral transmission jointly with a model of noninfectious drivers to asthma hospitalization data from eight metropolitan populations in Texas, we are able to both estimate epidemiological characteristics of common cold viruses and rigorously assess the relative contributions of proposed infectious and noninfectious drivers of asthma exacerbations. Our analysis provides insight into the dynamics of common cold viruses and a robust framework for predicting times of heightened risk and thus, key periods for clinical intervention in the growing population of asthmatic people.
Results
Predictors of Asthma Hospitalizations.
We tested models with different combinations of predictive variables and determined which explained the hospitalization data best. The variables tested are shown in Table 1, and the components of the best fitting model are indicated in column 4 of Table 1. The best fitting model from our study included common cold prevalence (Fig. 2), influenza prevalence, daily low temperature, a baseline hospitalization rate specific to each city, a term modifying the baseline rate on each day of the week, and a long-term temporal trend in hospitalization rates. Fitted values for this model are shown in Fig. 3 for children and adults, and coefficients for each parameter of the best fitting model are given in SI Appendix, Table S2. Bayesian model selection procedures excluded ozone and particulate matter variables as informative predictors of asthma hospitalization rate. A full description of each model compared is given in SI Appendix, section 10.
Table 1.
Name, source, and description of each explanatory variable considered
| Data | Source | Description | Included in best model |
| Common cold | SIRS transmission model | 4-d Aggregated common cold prevalence | * |
| Influenza prevalence | Hospitalization records | Daily state-level hospitalizations per million caused by influenza in adults and children (spline-smoothed) | * |
| Day of the week | Calendar | * | |
| Time trend | Daily index value | * | |
| Local intercept | Metropolitan boundary | Geographic variation in baseline hospitalization rate | * |
| Low temperature | CDC Wonder | Daily minimum temperature in counties in the metropolitan area (Celsius) | * |
| Ozone | AQS | Daily ozone (air quality index value) | |
| PM 2.5 | CDC Wonder | Maximum daily PM 2.5 for counties in the metropolitan area (micrograms per meter3) |
A complete description of each model and estimated coefficients of each variable is in SI Appendix. AQS, air quality system; CDC, Centers for Disease Control and Prevention; PM, particulate matter.
The variable was included in the best fitting model.
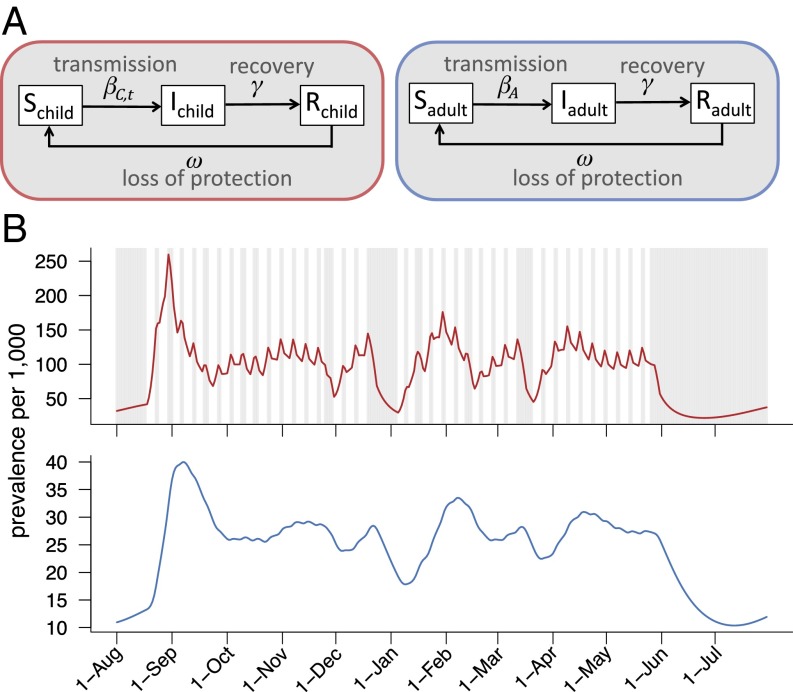
Fig. 2.
The SIRS dynamic transmission model of common cold circulation. (A) The child and adult populations are each divided into three infection classes: susceptible, infectious, and recovered. The recovered class is immune to infection. Transitions between compartments are governed by the rate parameters indicated. (B) Example model output for children (red) and adults (blue). Weekends and vacations for the Dallas–Fort Worth–Arlington metropolitan area in 2003–2004 are shown as gray areas. On those days, the transmission rate of children is decreased by σ. The prevalence of common cold infections in children more directly reflects the school calendar (i.e., weekends and holidays). Variation in adults is driven by changes in prevalence in children in the model. The estimated prevalence values are incorporated into an asthma hospitalization risk model to assess the relative impact of viral transmission on asthma exacerbation rates.
Fig. 3.
Fit of the best model in children and adults. Seven-day rolling means of observed hospitalizations in all cities (black) and simulations from the best fitting model (red). We sampled 20 parameter sets from the joint posterior distribution and generated five nonhomogeneous Poisson simulations for each set. Hospitalizations are shown for (A) children ages 5–18 y old and (B) adults ages 19–55 y old. Contribution of each factor to the predicted hospitalization rate in children for (C) Monday, August 11, 2003; (D) Monday, September 1, 2003; and (E) Monday, December 29, 2003 in the Dallas–Fort Worth–Arlington area (metropolitan code 19100). The heights of the bars in C–E sum to the fitted total asthma hospitalization rates on those days.
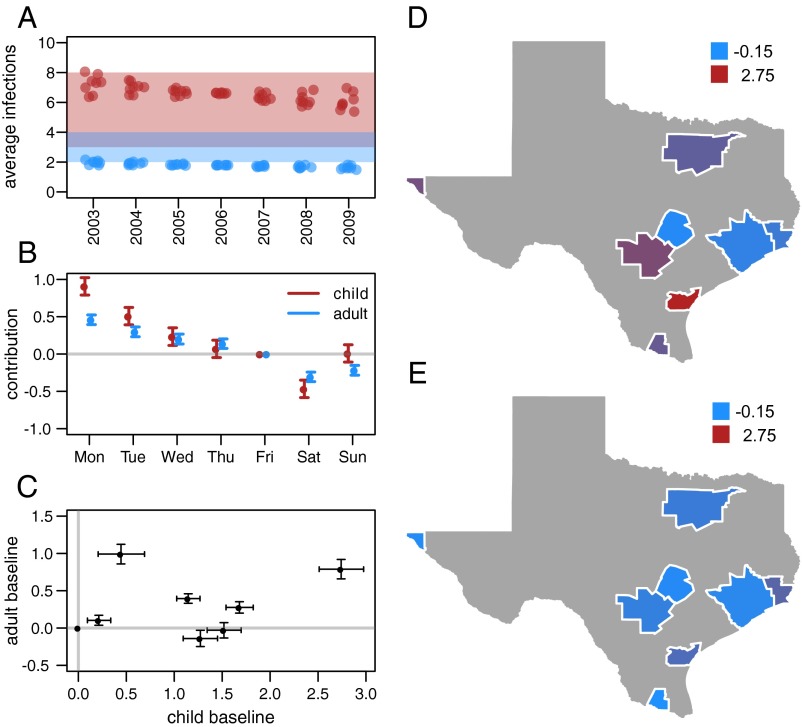
The coefficient of the day of the week variable has a pronounced pattern in both adults and children (Fig. 4B), where the contribution to the hospitalization rates steadily declines from Monday to Saturday. This pattern has been observed in asthma hospitalizations previously [for example, in Canada (40)]. Baseline hospitalization rates differ across metropolitan areas as shown by higher or lower addition to the baseline hospitalization rate (Fig. 4 D and E). The rate differences are not correlated in children and adults in the same metropolitan areas (Fig. 4C).
Fig. 4.
Results from the best fitting model. (A) Average number of colds in children (red points) and adults (blue points) for each of the eight metropolitan areas predicted by the model for each year of the study. The shaded windows indicate the public health estimate of three to eight colds per year for children and two to four colds per year for adults (30). (B) Estimated day of the week coefficients in the hospitalization model. Variation in these values captures, in part, variation in healthcare-seeking behavior on different days of the week. (C) Correlation of the baseline hospitalization rate in adults and children for each of the eight metropolitan areas in the study. (D and E) Baseline hospitalization rates in each metropolitan area for (D) children and (E) adults. Values represent the city-specific addition to the baseline asthma hospitalization rate. Positive values of this coefficient indicate higher baseline rate.
Temporal Variation in Exacerbation Triggers.
To investigate if there was a different dominant driver of asthma exacerbations at different times of year, we determined the contribution of each variable to the hospitalization rate on certain days. In Fig. 3 C–E, the total height of a bar is the fitted hospitalization rate on that day. We found that the key predictors of asthma exacerbations vary in importance through the year. For example, in 2003 in the Dallas–Fort Worth–Arlington metropolitan area, the common cold hardly contributes to late summer asthma activity (Fig. 3C), because prevalence is low in the summer when children are out of school and thus, have a lower transmission rate. Common cold prevalence substantially impacts the back-to-school wave of exacerbations (Fig. 3D). During winter break, low temperatures, common cold prevalence, and influenza all have moderate effects (Fig. 3E), because the temperature is low, and common cold and influenza prevalence is moderate. The other years of the study and metropolitan areas exhibit similar temporal patterns, and additional examination of the contribution of each variable to the fitted rate is given in SI Appendix, section 18.
Common Cold Transmission Rates.
The common cold susceptible–infectious–recovered–susceptible (SIRS) transmission model has six estimated parameters (Table 2). The posterior means and 95% credible intervals suggest that children infect each other much more than do other combinations of age groups. Specifically, the estimated adult-to-child and adult-to-adult transmission rates are 2.5% (1.4–4.2%) and 42% (35–48%) of the child-to-child rate, respectively. We estimate that, when schools close for weekends and holidays, transmission rates between children decrease by 45% (41–49%). Furthermore, we estimate that the common cold has an average infectious period of 3.0 d (2.6–3.5 d), and after recovery, that cross-protective immunity lasts an average of 19 d (18–21 d).
Table 2.
Parameter estimates for the common cold model
| Parameter | Symbol | Mean | 95% CI lower | 95% CI upper |
| Transmissibility | β0 | 0.74 | 0.68 | 0.79 |
| Adult–child scaling (%) | αAC | 2.54 | 1.39 | 4.24 |
| Adult–adult scaling (%) | αAA | 41.6 | 34.6 | 48.3 |
| Vacation effect (%) | σt | 45.1 | 41.0 | 49.0 |
| Duration of cross-immunity (d) | ω−1 | 19.3 | 17.7 | 21.1 |
| Duration of infection (d) | γ−1 | 3.01 | 2.64 | 3.45 |
Posterior means and 95% credible intervals (95% CIs) for the parameters of the SIRS common cold model.
As additional validation, we used the model to estimate the average number of common cold infections in each adult and child per year. Although the model was fitted to different data (daily asthma hospitalizations), the estimated numbers of colds per year were remarkably consistent with those reported in the literature and widely endorsed by public health agencies (Fig. 4A) (30, 31). In addition, when school start dates in Texas were delayed by 10 d in 2007 because of legislative change, the September asthma peak shifted accordingly. Our model provides a mechanistic link between the school calendar and asthma exacerbations and readily captures this epidemiological transition (SI Appendix, Fig. S16).
Robustness of Common Cold Model.
To further assess whether common cold prevalence is a critical predictor of asthma exacerbations, we performed likelihood ratio comparisons between the full model and two linear models that lacked the SIRS-driven common cold variable. One included only the other variables from the best fit model to test whether the common cold variable was necessary; the other also included a school closure indicator variable to test whether the school effect is linked to attendance at school rather than viral transmission at school. The likelihood ratio test indicated that the alternative models were significantly inferior (P < 0.01 and P < 0.01, respectively), further supporting the fundamental role of common colds in shaping large-scale spatiotemporal dynamics of asthma exacerbations (additional details are in SI Appendix, sections 11 and 12).
Discussion
Asthma hospitalization rates in children clearly reflect the school calendar. We hypothesized that this is mediated by viral transmission within schools rather than alternative triggers associated with the school environment. Through explicit modeling of respiratory virus circulation and comparison of model components, we found that the prevalence of respiratory infections explained asthma hospitalization patterns much better than the academic calendar alone. Our study combines both infectious and noninfectious drivers of asthma exacerbation; this two-tiered modeling strategy—coupling an asthma regression model with a respiratory virus transmission model—allowed us to simultaneously infer predictors of asthma hospitalization rates and epidemiological characteristics of the viruses that trigger asthma exacerbations.
We found that common cold infection is the primary determinant of asthma-related hospitalization patterns in children across eight major Texas metropolitan areas. Furthermore, the transmission of common colds is integrally linked to the school calendar, thus explaining the relationship between school vacation periods and asthma exacerbation. For adults, hospitalization rates have a different temporal signature dominated by a combination of common cold and influenza prevalence. In both age groups, low temperatures are a significant risk factor, and asthma hospitalization rates vary by day of the week.
It is critical to use a transmission model to generate the common cold prevalence input to our model, because actual viral prevalence data are not available for these study populations. Indeed, common cold prevalence is not known for any population on this scale or long time periods, such as in the 7 consecutive y of our study. Because common cold viruses cause mild, self-limited infections in healthy populations, there is little motivation for large studies to determine prevalence of these infections through time. By using very large-scale data, we are able to infer prevalence, which shows the power of transmission models to answer diverse public health questions.
Our viral transmission model captures the nonlinear interplay of waning immunity, cross-protection between different viruses, and contact patterns that both vary across age groups and change when schools are closed. The September epidemics noted in other asthma studies can be attributed to a resurgence of viral transmission at the beginning of the school year after an accumulation of susceptible children during summer vacation when transmission is lower. Later peaks occur after population-level waning of immunity during school vacation days, such as after Thanksgiving break.
Understanding the impact of school closures on the transmission of respiratory viral infections is valuable for not only asthma control but also, designing school closure strategies in planning for seasonal and pandemic influenza. We estimated transmission rates during school closures that are comparable with published estimates based on influenza surveillance data (41), sociological surveys (42), and measles outbreak dynamics (43). Unlike previous estimates, our analysis reflects contact patterns in normal vacation periods rather than during severe outbreaks, for which there may be additional changes in behavior that affect transmission rate.
Reducing severe asthma exacerbations remains a formidable challenge. Our analysis shows the critical influence of viral infections but does not explain the substantial variation in baseline asthma hospitalization rate observed between cities. We did not detect a significant effect of air pollutants, perhaps because measurements at the level of metropolitan areas are too coarse-grained. Our study is also limited to eight major cities in Texas and therefore, may not directly pertain to regions with different temperature and air quality values. We expect, however, that the common cold model may be generally applicable, with transmission reduced during school closures. In metropolitan areas with a high degree of heterogeneity in school calendar dates, common cold waves may be less pronounced. Furthermore, our model does not consider coinfection by multiple viruses, which could have a different probability of triggering asthma exacerbations than single infections. Nonspecific immunity may influence the frequency of coinfections by some viruses (44, 45), potentially leading to complex interactions between strains. Our model distills the multivirus transmission dynamics of the common cold into a parsimonious but biologically plausible system and could potentially be extended to consider additional complexity.
In Texas, asthmatic children tend to be at higher risk for exacerbations at the start of the school year and after other school breaks. Although reducing the burden of common cold viruses may not be feasible, asthma interventions that decrease the risk of exacerbation or hospitalization, including increased monitoring and preventive and therapeutic care, can be targeted at these high-risk periods. In general, future risk assessments and interventions for asthma, particularly in children, should explicitly consider both the school calendar and the seasonal dynamic of infectious triggers, either through spatiotemporal modeling or when possible, viral surveillance data.
Methods
We used asthma hospitalization data to jointly estimate the parameters of a population-level viral transmission model and coefficients of a multifactor linear model for asthma exacerbations in a Bayesian framework. We compared multiple models—including different combinations of predictors—using the deviance information criterion (DIC) (46).
Hospitalization Data.
To calculate the daily hospitalization rate per million, we use daily hospitalization records, which have a principal admission code indicating asthma (International Classification of Diseases version 9 code 493.XX) in each of the eight largest metropolitan areas of Texas from January 1, 2003 to December 30, 2009. There were 66,000 hospitalizations stratified into school-aged children (5–18 y old; 27,000 hospitalizations) and nonelderly adults (19–55 y old; 39,000 hospitalizations). We excluded age groups over 55 y old because of overlapping effects and diagnoses of chronic obstructive pulmonary disease. The eight focal populations totaled 14.8 million people in 2009, which are ∼59% of the state population. Additional details of the data are provided in SI Appendix, sections 1–5. This study was approved by Texas Department of State Health Services Institutional Review Board #1. Informed consent was not required from patients because data were extracted from administrative records. The data used is aggregated and nonidentifiable.
Common Cold Transmission Model.
We developed a dynamic SIRS transmission model for common cold viruses (Fig. 2). The population (N) is stratified into adults and children who may be susceptible (S), infected (I), or recovered (R). Recovered individuals are protected against infection. The governing equations are
where i represents age group [adults (A) or children (C)], γ is the recovery rate, and ω is the rate at which cross-protective immunity wanes. The age-specific transmission rates (βi,t) are given by
where β0 is the baseline child-to-child transmission rate; the αij terms are scaling factors for transmission rates between age groups, where αAC and αCA are assumed to be equal, σt is time-dependent and represents the decrease in child-to-child transmission rates during school closures on weekends and school holidays, and σt is one when school is in session and estimated during weekends and vacation periods. Therefore, the transmission rate of children, βC,t, is time-dependent. We assume that transmission rates involving adults are not affected by school closures.
Multiple cocirculating viruses cause common colds, and recovery from one virus does not provide lasting immunity against other viruses. Thus, the recovered class models short-term broad-spectrum immunity against all common cold viruses. Although not fully understood, broad cross-protection after infection has been noted for other respiratory viruses (47, 48) and may be mediated by innate immune mechanisms (49, 50). Individuals return to the susceptible class after a period of protection, which has duration ω−1.
Holiday periods were collated for each metropolitan area for each year of the study from the largest (or second largest) school district in the metropolitan area (additional details are in SI Appendix, section 4). Temporal changes in population size and age composition were calibrated to the 2000 Census and 2010 Census in the two age groups. We assume that there is a maximum delay of 4 d between initial infection and hospitalization for asthma exacerbation (39, 51–53). We solve the ordinary differential equation model using a fourth-order Runge–Kutta method with a fifth-order error term.
We use this age-stratified SIRS model to generate daily common cold prevalence in adults and children for each metropolitan area (Fig. 2B). The parameters that govern transitions between compartments are estimated. The time series of prevalence values serves as input into our asthma hospitalization model, which is described below.
Hospitalization Model.
We developed a linear regression model to fit the daily hospitalization rate per million adults and children in each metropolitan area using potential predictors of variation in asthma hospitalization rate (SI Appendix, section 7). The variables included in model selection were common cold prevalence, influenza prevalence, particulate matter (2.5 μm), ozone, low temperature, city-specific difference in hospitalization rate, day of the week variation, and secular trend in hospitalization rate (Table 1). For the common cold, we used the SIRS model to generate daily prevalence. For influenza, we estimated daily prevalence directly from hospitalization records and did not explicitly model transmission dynamics. For all other variables, daily measurements were obtained from publicly available sources (SI Appendix, sections 2 and 3). Model components were compared extensively using the DIC, where DIC = D̄ + pv, and pv = 0.5var(D̄) (46, 54). Lower values indicate a better fit of the model to data, and a difference of 5 units is the customary threshold for distinguishing model variants.
We jointly fitted the transmission and hospitalization models using Markov Chain Monte Carlo. To sample the transmission model parameters more efficiently, we explicitly marginalized over the other parameters at each step by a Laplace approximation. Additional details on fitting methods and model comparison are given in SI Appendix, sections 6–10.
Supplementary Material
Acknowledgments
We thank Simon Cauchemez for helpful discussion, Thomas Hladish for technical assistance, and Karen Wylie for school calendar collation. This work was funded by National Institute of General Medical Sciences Models of Infectious Disease Agent Study Grant U01GM087719.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518677113/-/DCSupplemental.
References
- 1.Moorman JE, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012;35(2012):1–58. [PubMed] [Google Scholar]
- 2.de Marco R, et al. GEIRD Study Group Trends in the prevalence of asthma and allergic rhinitis in Italy between 1991 and 2010. Eur Respir J. 2012;39(4):883–892. doi: 10.1183/09031936.00061611. [DOI] [PubMed] [Google Scholar]
- 3.Barnett SBL, Nurmagambetov TA. Costs of asthma in the United States: 2002-2007. J Allergy Clin Immunol. 2011;127(1):145–152. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Bahadori K, et al. Economic burden of asthma: A systematic review. BMC Pulm Med. 2009;9(1):24. doi: 10.1186/1471-2466-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC 2011 Asthma in the US: Vital Signs. Available at www.cdc.gov/vitalsigns/asthma/. Accessed April 25, 2015.
- 6.Schildcrout JS, et al. Ambient air pollution and asthma exacerbations in children: An eight-city analysis. Am J Epidemiol. 2006;164(6):505–517. doi: 10.1093/aje/kwj225. [DOI] [PubMed] [Google Scholar]
- 7.Le TG, et al. HEI Collaborative Working Group on Air Pollution, Poverty, and Health in Ho Chi Minh City Effects of short-term exposure to air pollution on hospital admissions of young children for acute lower respiratory infections in Ho Chi Minh City, Vietnam. Res Rep Health Eff Inst. 2012;169(2012):5–72. [PubMed] [Google Scholar]
- 8.Halonen JI, et al. Urban air pollution, and asthma and COPD hospital emergency room visits. Thorax. 2008;63(7):635–641. doi: 10.1136/thx.2007.091371. [DOI] [PubMed] [Google Scholar]
- 9.Custovic A, Simpson A, Bardin PG, Le Souëf P. Allergy is an important factor in asthma exacerbation: A pro/con debate. Respirology. 2010;15(7):1021–1027. doi: 10.1111/j.1440-1843.2010.01826.x. [DOI] [PubMed] [Google Scholar]
- 10.Green RM, et al. Synergism between allergens and viruses and risk of hospital admission with asthma: Case-control study. BMJ. 2002;324(7340):763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray CS, et al. Study of modifiable risk factors for asthma exacerbations: Virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax. 2006;61(5):376–382. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rakes GP, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159(3):785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 13.Johnston SL, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310(6989):1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston NW, et al. The September epidemic of asthma exacerbations in children: A search for etiology. J Allergy Clin Immunol. 2005;115(1):132–138. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendley JO, Gwaltney JM, Jr, Jordan WS., Jr Rhinovirus infections in an industrial population. IV. Infections within families of employees during two fall peaks of respiratory illness. Am J Epidemiol. 1969;89(2):184–196. doi: 10.1093/oxfordjournals.aje.a120928. [DOI] [PubMed] [Google Scholar]
- 16.Johnston SL, et al. The relationship between upper respiratory infections and hospital admissions for asthma: A time-trend analysis. Am J Respir Crit Care Med. 1996;154(3 Pt 1):654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 17.Sears MR, Johnston NW. Understanding the September asthma epidemic. J Allergy Clin Immunol. 2007;120(3):526–529. doi: 10.1016/j.jaci.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston NW. The similarities and differences of epidemic cycles of chronic obstructive pulmonary disease and asthma exacerbations. Proc Am Thorac Soc. 2007;4(8):591–596. doi: 10.1513/pats.200706-064TH. [DOI] [PubMed] [Google Scholar]
- 19.Johnston NW, Johnston SL, Norman GR, Dai J, Sears MR. The September epidemic of asthma hospitalization: School children as disease vectors. J Allergy Clin Immunol. 2006;117(3):557–562. doi: 10.1016/j.jaci.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Scheuerman O, et al. The September epidemic of asthma in Israel. J Asthma. 2009;46(7):652–655. doi: 10.1080/02770900902963102. [DOI] [PubMed] [Google Scholar]
- 21.Corne JM, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: A longitudinal cohort study. Lancet. 2002;359(9309):831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 22.Seemungal T, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 23.Bizzintino J, et al. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J. 2011;37(5):1037–1042. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khetsuriani N, et al. Novel human rhinoviruses and exacerbation of asthma in children. Emerg Infect Dis. 2008;14(11):1793–1796. doi: 10.3201/eid1411.080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller EK, et al. New Vaccine Surveillance Network A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123(1):98–104.e1. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olenec JP, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125(5):1001–1006.e1. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heymann PW, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114(2):239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller EK, et al. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195(6):773–781. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smuts HE, Workman LJ, Zar HJ. Human rhinovirus infection in young African children with acute wheezing. BMC Infect Dis. 2011;11:65. doi: 10.1186/1471-2334-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NHS 2013 NHS Common Cold. Available at www.nhs.uk/Conditions/Cold-common/Pages/Introduction.aspx. Accessed August 20, 2013.
- 31.Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiol Infect. 1993;110(1):145–160. doi: 10.1017/s0950268800050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cauchemez S, et al. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med. 2009;361(27):2619–2627. doi: 10.1056/NEJMoa0905498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao DL, Halloran ME, Longini IM., Jr School opening dates predict pandemic influenza A(H1N1) outbreaks in the United States. J Infect Dis. 2010;202(6):877–880. doi: 10.1086/655810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber A, Weber M, Milligan P. Modeling epidemics caused by respiratory syncytial virus (RSV) Math Biosci. 2001;172(2):95–113. doi: 10.1016/s0025-5564(01)00066-9. [DOI] [PubMed] [Google Scholar]
- 35.Rage E, Siroux V, Künzli N, Pin I, Kauffmann F. Epidemiological Study on the Genetics and Environment of Asthma Air pollution and asthma severity in adults. Occup Environ Med. 2009;66(3):182–188. doi: 10.1136/oem.2007.038349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh N-H, Liao C-M. Fluctuations in air pollution give risk warning signals of asthma hospitalization. Atmos Environ. 2013;75(2013):206–216. [Google Scholar]
- 37.Tolbert PE, et al. Air quality and pediatric emergency room visits for asthma in Atlanta, Georgia, USA. Am J Epidemiol. 2000;151(8):798–810. doi: 10.1093/oxfordjournals.aje.a010280. [DOI] [PubMed] [Google Scholar]
- 38.Lee JT, et al. Air pollution and asthma among children in Seoul, Korea. Epidemiology. 2002;13(4):481–484. doi: 10.1097/00001648-200207000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Nastos PT, Paliatsos AG, Anthracopoulos MB, Roma ES, Priftis KN. Outdoor particulate matter and childhood asthma admissions in Athens, Greece: A time-series study. Environ Health. 2010;9(1):45. doi: 10.1186/1476-069X-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cakmak S, Dales RE, Coates F. Does air pollution increase the effect of aeroallergens on hospitalization for asthma? J Allergy Clin Immunol. 2012;129(1):228–231. doi: 10.1016/j.jaci.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 41.Cauchemez S, Valleron A-J, Boëlle P-Y, Flahault A, Ferguson NM. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature. 2008;452(7188):750–754. doi: 10.1038/nature06732. [DOI] [PubMed] [Google Scholar]
- 42.Eames KT, Tilston NL, Brooks-Pollock E, Edmunds WJ. Measured dynamic social contact patterns explain the spread of H1N1v influenza. PLoS Comput Biol. 2012;8(3):e1002425. doi: 10.1371/journal.pcbi.1002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fine PE, Clarkson JA. Measles in England and Wales—I: An analysis of factors underlying seasonal patterns. Int J Epidemiol. 1982;11(1):5–14. doi: 10.1093/ije/11.1.5. [DOI] [PubMed] [Google Scholar]
- 44.Greer RM, et al. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J Clin Virol. 2009;45(1):10–15. doi: 10.1016/j.jcv.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karppinen S, Toivonen L, Schuez-Havupalo L, Waris M, Peltola V. Interference between respiratory syncytial virus and rhinovirus in respiratory tract infections in children. Clin Microbiol Infect. October 16, 2015 doi: 10.1016/j.cmi.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Spiegelhalter DJ, Best NG, Carlin BP, van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B Stat Methodol. 2002;64(4):583–639. [Google Scholar]
- 47.Cowling BJ, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis. 2012;54(12):1778–1783. doi: 10.1093/cid/cis307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly H, Barry S, Laurie K, Mercer G. Seasonal influenza vaccination and the risk of infection with pandemic influenza: A possible illustration of non-specific temporary immunity following infection. Euro Surveill. 2010;15(47):19722. doi: 10.2807/ese.15.47.19722-en. [DOI] [PubMed] [Google Scholar]
- 49.Hayden FG, et al. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101(3):643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGill J, Heusel JW, Legge KL. Innate immune control and regulation of influenza virus infections. J Leukoc Biol. 2009;86(4):803–812. doi: 10.1189/jlb.0509368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Restrepo CE, Simonoff JS, Thurston GD, Zimmerman R. Asthma hospital admissions and ambient air pollutant concentrations in New York City. J Environ Prot. 2012;3:1102–1116. [Google Scholar]
- 52.Storr J, Lenney W. School holidays and admissions with asthma. Arch Dis Child. 1989;64(1):103–107. doi: 10.1136/adc.64.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silverman RA, Ito K, Stevenson L, Hastings HM. The relationship of fall school opening and emergency department asthma visits in a large metropolitan area. Arch Pediatr Adolesc Med. 2005;159(9):818–823. doi: 10.1001/archpedi.159.9.818. [DOI] [PubMed] [Google Scholar]
- 54.Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian Data Analysis. 2nd Ed Chapman & Hall/CRC; London: 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.