Significance
Despite the growing evidence that autoantibodies are team players in the pathogenesis of multiple sclerosis (MS), the target autoantigens are yet to be identified. In this work, we mined the autoantibody repertoire within MS by screening more than 2,000 plasma samples from patients with MS and controls and identified increased autoantibody reactivity against an ion-channel protein called “anoctamin 2” (ANO2). This finding points toward an ANO2 autoimmune sub-phenotype in MS and might contribute to the development of clinical algorithms to characterize a subgroup of MS patients.
Keywords: multiple sclerosis, autoimmunity, autoantibodies, protein microarrays, affinity proteomics
Abstract
Multiple sclerosis (MS) is the most common chronic inflammatory disease of the central nervous system and also is regarded as an autoimmune condition. However, the antigenic targets of the autoimmune response in MS have not yet been deciphered. In an effort to mine the autoantibody repertoire within MS, we profiled 2,169 plasma samples from MS cases and population-based controls using bead arrays built with 384 human protein fragments selected from an initial screening with 11,520 antigens. Our data revealed prominently increased autoantibody reactivity against the chloride-channel protein anoctamin 2 (ANO2) in MS cases compared with controls. This finding was corroborated in independent assays with alternative protein constructs and by epitope mapping with peptides covering the identified region of ANO2. Additionally, we found a strong interaction between the presence of ANO2 autoantibodies and the HLA complex MS-associated DRB1*15 allele, reinforcing a potential role for ANO2 autoreactivity in MS etiopathogenesis. Furthermore, immunofluorescence analysis in human MS brain tissue showed ANO2 expression as small cellular aggregates near and inside MS lesions. Thus this study represents one of the largest efforts to characterize the autoantibody repertoire within MS. The findings presented here demonstrate that an ANO2 autoimmune subphenotype may exist in MS and lay the groundwork for further studies focusing on the pathogenic role of ANO2 autoantibodies in MS.
Multiple sclerosis (MS), characterized by multifocal demyelination and axonal loss, is the most common progressive and disabling neurological disease among young adults. There are several indications that MS is an immune-mediated disease, most likely caused by autoimmune mechanisms (1). Sera of ∼30% of individuals with MS contain antibodies with affinity for myelin components, although with unknown specificity (2). Several autoimmune targets other than myelin antigens have been proposed, but the role of these autoantibodies and the interactions with their targets in the pathogenesis and progression of MS remains elusive (3), leaving ample room for further quests for autoimmune targets.
Multiplex proteomics approaches hold great potential for broad and unbiased exploration of the autoantibody repertoires in body fluids and for identifying novel antigenic targets in MS. Antigen microarrays, in particular, represent an appealing high-throughput platform to study antibody reactivity toward thousands of antigens in parallel. The Human Protein Atlas is an initiative systematically producing human protein fragments, where regions from protein-encoding genes are selected based on low similarity to other proteins in the proteome (4). Previously, using more than 11,000 protein fragments representing more than 7,500 unique human proteins in an MS-related sample set, we demonstrated the utility of such protein fragments on antigen arrays in determining plasma IgG reactivity profiles (5). In that pilot study we identified 51 antigens differentially recognized across various MS subtypes as compared with controls with neurological disorders other than MS (OND).
Here, using bead-based arrays, we validate our initial findings in a larger and independent set of plasma samples collected from incident MS cases and population-based controls (n = 2,169). The list of the selected antigens (n = 384) was based on our previous results and was complemented with proteins associated with MS risk, such as Epstein–Barr virus nuclear antigen-1 (EBNA-1), and protein fragments representing previously proposed autoimmune targets in MS, such as the potassium channel protein KIR4.1 (KCNJ10) (6). This extended analysis confirmed increased IgG reactivity in plasma samples of MS patients against a calcium-activated chloride-channel protein called “anoctamin 2” (ANO2), also denoted as “transmembrane protein 16B” (TMEM16B). ANO2 reactivity was confirmed by independent analyses in which ANO2 was either expressed as an alternative construct or was mapped on the peptide level using arrays of overlapping 15-mer and 20-mer peptides. Subsequently, the interaction between some established MS risk factors (such as HLA gene alleles), increased IgG levels against EBNA-1 antigen, and plasma IgG autoantibody reactivity against ANO2 were investigated. Additionally, immunofluorescence analysis in sections of human brain tissue was used to identify the distribution and immunogenicity patterns of ANO2 in normal brain tissue and in MS lesions.
Results
Here we analyzed IgG autoantibody reactivities in plasma samples from 1,063 MS patients and 1,106 population-based, non-MS controls (Fig. 1 and Table 1) using a total of 384 protein fragments representing 196 unique human proteins. This antigen set included 115 fragments representing the 51 protein targets identified in our initial discovery study (5) and protein fragments representing proteins reported in literature as potential autoimmune targets in the context of MS (SI Appendix, Fig. S1).
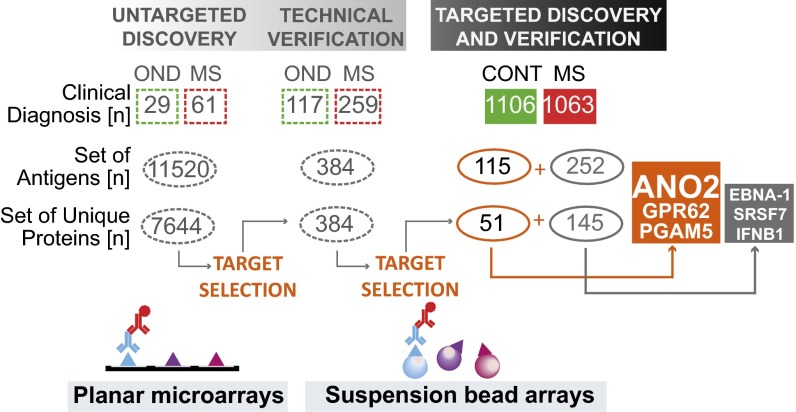
Fig. 1.
Study design. In a previous study (5), an untargeted discovery strategy was used in which randomly assembled collections of protein fragments in a planar microarray format were used for analysis of IgG reactivity in a representative plasma sample set of 61 MS cases and 29 controls with ONDs. After the analysis of autoantibody reactivity against a total of 11,520 protein fragments representing 7,644 unique proteins, 384 antigens were selected for further technical verification in an extended plasma sample set of 259 MS cases and 117 controls with ONDs using a suspension bead array format. Fifty-one of these antigens revealed differential autoantibody reactivity frequencies across various MS subtypes and controls with ONDs. Here, an even larger and independent sample set consisting of plasma samples from 1,106 MS cases and 1,063 population-based controls without MS was analyzed for reactivity against this set of 51 antigens, which were represented by 115 protein fragments (orange). The antigen bead array also was complemented with 252 protein fragments representing 145 targets (gray), which were suggested by previous studies. The most significant differences in plasma IgG reactivity were revealed for the protein fragment representing ANO2 belonging to the previously identified set of 51 antigens. Two other protein fragments from this set representing GPR62 and PGAM5 and the protein fragments representing literature-based targets EBNA-1, SRSF7, and IFNB1 revealed differential plasma IgG reactivity. Information on these targets can be found in the SI Appendix, SI Results.
Table 1.
Demographic data of the study set
| Sample group | No. of subjects | Female/male, % | Median age, y (range, y) |
| CIS-conv* | 37 | 76/24 | 40 (21–55) |
| RRMS* | 865 | 76/24 | 36 (16–66) |
| SPMS† | 128 | 68/32 | 46 (21–66) |
| PPMS† | 28 | 57/43 | 54 (35–76) |
| PRMS† | 5 | 60/40 | 48 (33–56) |
| MS | 1,063 | 74/26 | 38 (16–76) |
| Control | 1,106 | 77/24 | 40 (17–71) |
| Total | 2,169 |
CIS-conv, clinically isolated syndrome converters; PPMS. primary progressive MS; PRMS, primary relapsing MS; RRMS, relapsing remitting MS; SPMS, secondary progressive MS.
The CIS-conv and RRMS groups are categorized as “relapsing-remitting MS.”
The SPMS, PPMS, and PRMS groups are categorized as “progressive MS.”
Reactivity Profiles for ANO2 Fragments in Plasma.
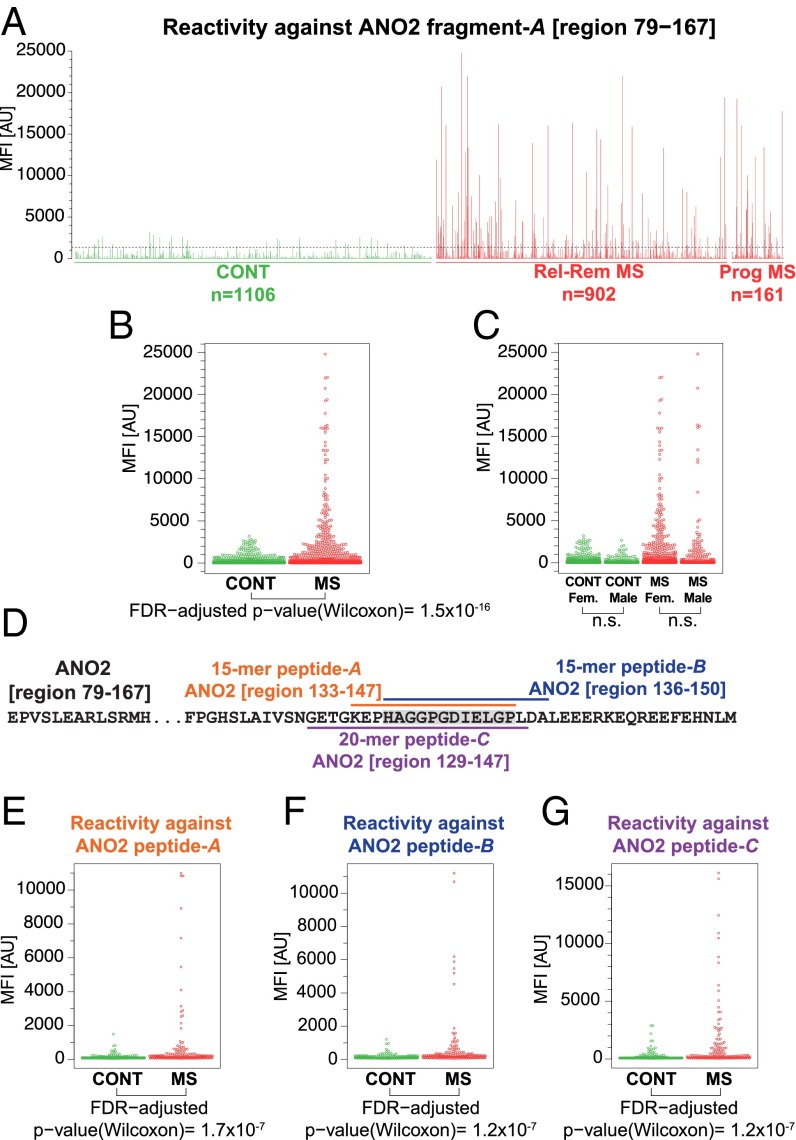
Across the entire set of 384 antigens, the statistically most significant differences between the MS cases and controls were revealed in IgG reactivity against the protein fragment representing the N-terminal region of ANO2 covering residues 79–167 (SI Appendix, Fig. S2 and Table S1), denoted here as “ANO2 fragment-A” (SI Appendix, Fig. S3). This antigen was part of the set of 51 follow-up targets that were identified in our previous discovery study comprising an unbiased selection of 11,520 antigens.
For ANO2 fragment-A covering region 79–167, we previously observed a significantly higher reactivity percentage in plasma of MS patients (32%), particularly in those with the relapsing-remitting subtype of MS (RRMS) (34%) compared with controls with OND (18%) (SI Appendix, Fig. S4). Analysis of a much larger plasma sample collection in the present follow-up study revealed a 5.3-fold change between the median fluorescent intensity (MFI) values for the group of MS cases and controls (Wilcoxon test; P = 1.5 × 10−16) (Fig. 2 A and B and SI Appendix, Fig. S5), thus reproducing and strengthening our initial observations. An MFI threshold set as the median plus 3 × SD of the MFI values obtained for the control samples disclosed positive reactivity percentages of 3.2% in controls, 15.4% in the relapsing MS group, 16.4% in the progressive MS group, and 15.5% in all MS cases, thus revealing a statistically significant difference in positive reactivity against this antigen in controls vs. all MS cases (Fisher’s exact test; P = 4.3 × 10−22).
Fig. 2.
Plasma autoantibody reactivity against ANO2. (A) The barplot represents the MFI values for plasma reactivity against ANO2 fragment-A (region 79–167) within 1,106 controls and 1,063 MS cases. The arbitrarily chosen MFI threshold, set as the median plus 3 × SD of MFI values obtained for the control samples, is shown by the dashed line. (B) The dotplot represents the MFI values and their spread for plasma reactivity against ANO2 fragment-A in 1,106 controls and 1,063 MS cases. (C) The dotplot represents the MFI values and their spread for plasma reactivity against ANO2 fragment-A in male and female nondiseased controls and MS cases; the differences were found to be statistically nonsignificant. (D) The position of the two overlapping 15-mer peptides and a 20-mer peptide residing within ANO2 fragment-A, revealing differences between the MS cases and controls on the peptide level. (E and F) The dotplots represent the MFI values and their spread for plasma reactivity against two overlapping 15-mer peptides representing ANO2-A (region 133–147) (E) and ANO2-B (region 136–150) (F) in 178 nondiseased controls and 185 MS cases. (G) The dotplot represents the MFI values and their spread for plasma reactivity against a 20-mer peptide representing ANO2 (region 129–148) in 178 nondiseased controls and 185 MS cases. Wilcoxon rank-sum test P values are reported below the plots. AU, arbitrary units.
Reactivity profiles across all antigens included in the bead array are shown in SI Appendix, Fig. S6 A and B for plasma samples from the two individual MS patients with the highest MFI values for ANO2 fragment-A. A further analysis of these two samples on an in-house–generated planar microarray containing 21,120 protein fragments and representing 12,412 unique human proteins confirmed the prominent antibody reactivity against ANO2 fragment-A among a much larger set of antigens and on a different array platform (SI Appendix, Fig. S6 C and D). In addition, the plasma reactivity against ANO2 fragment-A produced within the Human Protein Atlas has been replicated independently by another laboratory (German Cancer Research Center, Heidelberg) using a different expression system. In this independent analysis, both ANO2 region 79–167, i.e., fragment-A, and region 1–365, corresponding to the entire N-terminal portion of ANO2 predicted to reside in cytoplasmic space, were produced and used for profiling autoantibodies in a subset of plasma samples (SI Appendix, Fig. S7).
When the ANO2 fragment-A reactivity was dissected into gender-related profiles, males and females within the MS diagnosis group and controls did not differ (Fig. 2C). In addition, no correlations were found between age and reactivity against ANO2 fragment-A (SI Appendix, Fig. S8).
The bead-based array contained an antigen that represented the C terminus of ANO2, denoted here as ANO2 fragment-B (region 932–1003) (SI Appendix, Fig. S3). However, the reactivity against this region of ANO2 was not significantly different between the MS cases and controls (SI Appendix, Fig. S9).
Reactivity Mapping for ANO2 on the Peptide Level.
A bead-based peptide array consisting of 15-mer (n = 26) and 20-mer (n = 8) overlapping peptides representing ANO2 fragment-A (region 79–167) was generated and used for mapping the plasma IgG reactivity in a randomly selected subset of samples containing 185 MS cases and 178 controls. Reactivity against two overlapping 15-mer peptides representing region 133–147 and region 136–150 and a 20-mer peptide representing region 129–148 revealed statistically significant differences between the MS cases and controls (Fig. 2 E–G and SI Appendix, Fig. S10). This analysis showed that the sequence of amino acid residues HAGGPGDIELGP, which is shared by all three of these peptides, constituted the main region revealing the differences in plasma reactivity between MS cases and controls. This sequence in ANO2 showed no significant sequence homology to any viral, bacterial, or other human proteins (SI Appendix, Fig. S11). In addition, this sequence overlapped with the region of ANO2 fragment-A predicted to be a possible continuous B-cell epitope (SI Appendix, Fig. S12).
Immunohistochemical Findings for ANO2.
A rabbit polyclonal antibody raised against the N-terminal region of human ANO2 (SI Appendix, Fig. S13) combined with antibodies staining for astrocytes (GFAP) or macrophages/microglia (CD68) was applied to MS and normal human brain tissue using a multiplex fluorescent immunohistochemistry approach. Lesions were identified based on GFAP immunoreactivity and Sudan Black counterstaining, with the majority being localized at the gray–white matter border and characterized by large numbers of macrophages/microglia visualized mainly within and also surrounding the plaque(s).
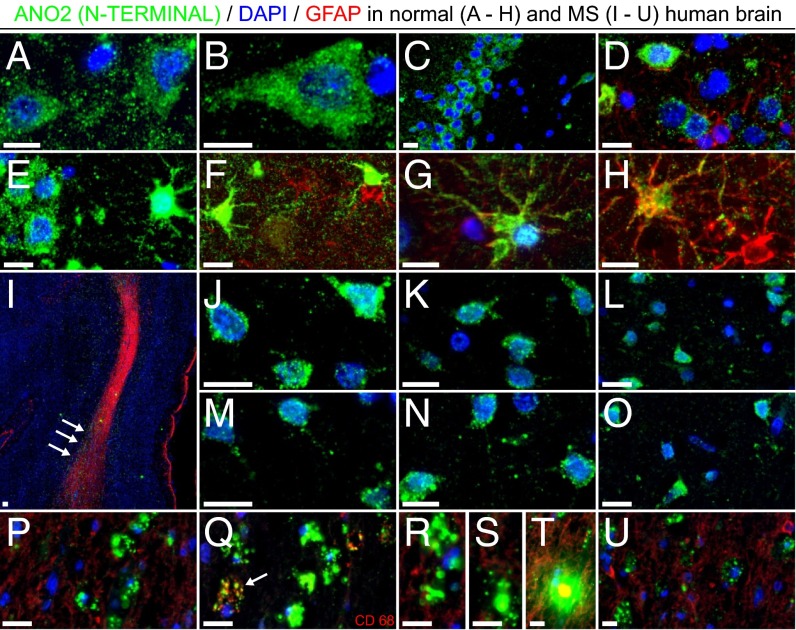
The antibody against ANO2 stained several neuronal and glial (some GFAP+) cells from normal hippocampal and cortical regions (Fig. 3 A–H). The same antibody also stained a number of cells of neuronal morphology from healthy-appearing areas of an MS brain section (Fig. 3 J–O), with ANO2 immunoreactivity localized mainly to the cytosolic compartment of cells and exhibiting a granular perinuclear moderate staining pattern. However, a visible increase in ANO2 staining intensity in the vicinity of and inside MS plaques (compared with more distal areas in the tissue) was observed, with the protein forming small cellular aggregates (Fig. 3 I and P–U) and occasionally colocalized with CD68+ macrophages (Fig. 3Q).
Fig. 3.
Annotated expression of ANO2 in human brain tissue. ANO2 was expressed in several neuronal and glial cells (GFAP+ cells are in red except in Q) from normal hippocampal and cortical regions (A–H) and in a number of cells of neuronal morphology from healthy-appearing areas of an MS brain section (J–O). A clear increase in ANO2 staining intensity near and inside MS plaques was observed (arrows in I), with the protein forming small cellular aggregates (I and P–U) and occasionally expressed in CD68+ macrophages (arrow in Q). (Scale bars, 10 μm.)
Interaction Between MS-Related HLA Risk Alleles and ANO2 Reactivity.
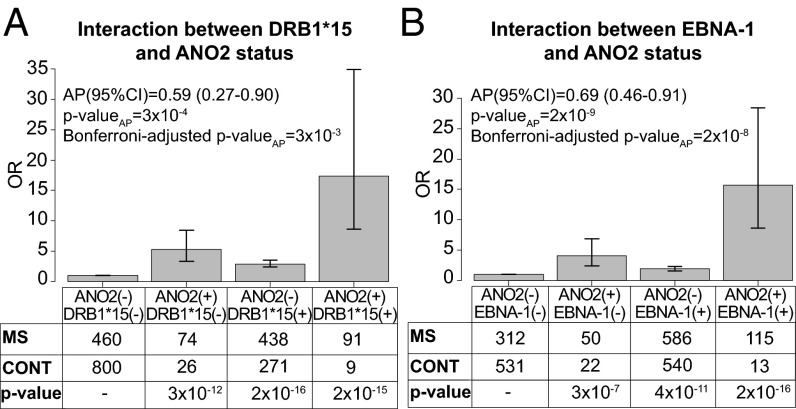
ANO2 IgG positivity in plasma was significantly associated with MS with an odds ratio (OR) of 5.78, 95% confidence interval (CI) of 4.00–8.57, and P value <2 × 10−16. We tested the interaction between ANO2 autoantibody reactivity and the strongest genetic risk factors for MS, namely the presence of HLA-DRB1*15, the absence of A*02, and increased levels of EBNA-1 IgG, all of which were found to be significantly associated with MS in this cohort. Interaction was estimated as the departure from additivity of the risk factors, indicating that the two risk factors are involved in the same sufficient cause for disease (7). We identified a significant departure from additivity for ANO2 positivity and DRB1*15, with an attributable proportion (AP) due to the interaction of 0.59 (95% CI 0.27–0.90) (Fig. 4A), and for increased EBNA-1 IgG levels, AP 0.69 (95% CI 0.46–0.91) (Fig. 4B). We did not find any interaction between ANO2 positivity and the absence of HLA-A*02.
Fig. 4.
Interaction analysis. Departure from additivity for pairs of risk factors was tested by calculating the proportion attributable to interaction (AP) using logistic regression analysis. The analysis was adjusted for age (12–24 y, 25–34 y, 35–44, and above 45 y of age; 25- to 34-y-olds were used as reference), gender, and ancestry (from Sweden, Norway, Denmark, Iceland, or elsewhere). Increased EBNA-1 IgG was defined as levels above the median among controls. (A) Interaction between ANO2 IgG positivity and the presence of HLA-DRB1*15. (B) Interaction between ANO2 IgG positivity and increased levels of EBNA-1. Adjusted ORs with 95% CIs for the risk of developing MS with different combinations of risk factors are shown.
We previously have identified an interaction between HLA-DRB1*15 and increased EBNA-1 IgG titers (8). We therefore tested the interaction between HLA-DRB1*15 and ANO2 both in individuals with increased EBNA-1 titers and in those without increased titers. We found a significant interaction only among those with EBNA-1 IgG titers below median (Table 2). Interactions between ANO2 IgG positivity and increased EBNA-1 IgG levels were found among both DRB1*15+ and DRB1*15− individuals but was stronger in the latter group (Table 3). The interactions among ANO2 positivity, carriage of the risk factor HLA-DRB1*15, and increased EBNA-1 IgG levels are illustrated in (SI Appendix, Fig. S14A).
Table 2.
Interaction between ANO2 positivity and HLA-DRB1*15 positivity
| Group of individuals | HLA-DRB1*15− | P value | HLA-DRB1*15+ | P value | AP (95% CI) | P valueAP | ||||
| No. of cases | No. of controls | OR (95% CI) | No. of cases | No. of controls | OR (95% CI) | |||||
| All cases and controls | ||||||||||
| ANO2− | 460 | 800 | 1.0 (–) | — | 438 | 271 | 2.9 (2.4–3.5) | 2 × 10−16 | 0.6 (0.3–0.9) | 3 × 10−4 |
| ANO2+ | 74 | 26 | 5.3 (3.3–8.5) | 3 × 10−12 | 91 | 9 | 17.3 (8.6–34.9) | 2 × 10−15 | ||
| Individuals with EBNA1 IgG below median among controls | ||||||||||
| ANO2− | 180 | 419 | 1.0 (–) | — | 138 | 112 | 2.7 (2.0–3.7) | 5 × 10−10 | 0.6 (0.2–1.0) | 7 × 10−3 |
| ANO2+ | 24 | 17 | 3.4 (1.7–6.5) | 4 × 10−4 | 26 | 5 | 12.5 (4.7–33.2) | 5 × 10−7 | ||
| Individuals with EBNA1 IgG above median among controls | ||||||||||
| ANO2− | 280 | 381 | 1.0 (–) | — | 306 | 159 | 2.7 (2.1–3.5) | 2 × 10−14 | 0.5 (0.0×1.1) | n.s. |
| ANO2+ | 50 | 9 | 8.4 (4.0–17.5) | 2 × 10−8 | 65 | 4 | 22.0 (7.9–61.4) | 4 × 10−9 | ||
Adjusted ORs with 95% CIs of developing MS for ANO2 positivity and different combinations HLA-DRB1*15 status and increased EBNA-1 IgG levels.
Table 3.
Interaction between ANO2 positivity and EBNA-1 IgG levels
| Group of individuals | EBNA-1 IgG below median among controls | EBNA-1 IgG above median among controls | AP (95% CI) | P valueAP | ||||||
| No. of cases | No. of controls | OR (95% CI) | P value | No. of cases | No. of controls | OR (95% CI) | P value | |||
| All cases and controls | ||||||||||
| ANO2− | 312 | 531 | 1.0 (–) | — | 586 | 540 | 1.9 (1.6–2.3) | 4 × 10−11 | 0.7 (0.5–0.9) | 2 × 10−9 |
| ANO2+ | 50 | 22 | 4.0 (2.4–6.8) | 3 × 10−7 | 115 | 13 | 15.7 (8.6–28.4) | 2 × 10−16 | ||
| Individuals who are HLA-DRB1*15− | ||||||||||
| ANO2− | 188 | 419 | 1.0 (–) | — | 280 | 381 | 1.7 (1.4–2.2) | 2 × 10−5 | 0.7 (0.5–0.9) | 5 × 10−8 |
| ANO2+ | 24 | 17 | 3.4 (1.7–6.5) | 4 × 10−4 | 50 | 9 | 13.9 (6.8–29.5) | 2 × 10−12 | ||
| Individuals who are HLA-DRB1*15+ | ||||||||||
| ANO2− | 132 | 112 | 1.0 (–) | — | 306 | 159 | 1.7 (1.2–2.4) | 2 × 10−3 | 0.6 (0.1–1.1) | 2 × 10−2 |
| ANO2+ | 26 | 5 | 4.6 (1.7–12.4) | 3 × 10−3 | 65 | 4 | 14.0 (4.9–40.1) | 9 × 10−7 | ||
Adjusted ORs with 95% CIs of developing MS for ANO2 positivity and different combinations HLA-DRB1*15 status and EBNA-1 IgG levels.
Identification of Additional Potential Autoimmune Targets in MS.
In addition to ANO2, we found differential autoantibody reactivity between MS cases and controls for the protein fragments representing phosphoglycerate mutase family member 5 (PGAM5) (SI Appendix, Fig. S15) and G protein-coupled receptor 62 (GPR62) (SI Appendix, Fig. S16). As was ANO2, these antigens were proposed by our previous discovery study. Further results regarding these and other literature-based targets such as KIR4.1 are available in the SI Appendix, SI Results.
Discussion
We used bead-based arrays of 384 antigens representing 196 unique proteins for profiling plasma IgG reactivity in a very large case-control cohort. The antigen array included human protein fragments suggested by our previous efforts to be potential autoimmune targets in MS (5) and other human protein fragments representing MS-related targets, such as KIR4.1, proposed in the literature. Analysis of a total of 2,169 plasma samples revealed an increased autoimmune reactivity against ANO2 and highlighted this protein as a previously unreported autoimmune target candidate within MS. Our previous discovery study included controls with other diseases (SI Appendix, Fig. S4), including neurological and inflammatory conditions apart from MS; those findings might indicate that the autoantibodies against ANO2 are specific for MS. In addition, access to MS risk genotypes in this large sample enabled us to demonstrate interactions with ANO2 reactivity (Fig. 4 and SI Appendix, Fig. S14A), reinforcing its potential role in MS etiopathogenesis.
One of the main focus areas in MS research is the identification of molecular biomarker candidates, which would facilitate diagnosis and prognostic assessments in MS (9). CNS tissue, and in particular the MS lesion tissue forming the actual sites of disease, is an outstanding source to search for specific markers of disease-related mechanisms and pathophysiology in MS (10, 11). However, assembling and accessing large collections of samples of brain tissue for such discovery-driven approaches is challenging. Among the body fluids, cerebrospinal fluid (CSF) provides an ideal matrix for the detection of disease-related molecular changes in MS. In recent years, several potential CSF biomarkers have been proposed (12, 13). However, the invasive nature of the CSF collection procedures makes blood plasma or serum an alternate body fluid with unique potential clinical utility and value because of its minimally invasive collection (14).
Antigen arrays have been used in the context of MS to identify new biomarker candidates in body fluids (15), but such approaches mainly have adopted a targeted approach and have used lipid (16) or myelin compound arrays (17, 18); these arrays also have been used to profile serum from mice with experimental autoimmune encephalomyelitis (19). Only a few studies have taken an entirely unbiased and systematic approach and have used commercially available antigen arrays (20, 21), but these studies focused on the analysis of the autoantibody repertoire in CSF. Thus, in the context of MS, our previous study (5), in which we profiled plasma IgG reactivity against 11,520 antigens, can be regarded as the first (to our knowledge) large-scale and unbiased plasma autoantibody profiling study in MS. The implications of our current findings will require further investigations, but the follow-up study we present here clearly demonstrates that our initial discovery approach is an efficient strategy for the exploration of autoantibody repertoires in MS.
The main finding in this study was the confirmation of increased autoantibody reactivity against ANO2 in the plasma of MS patients as compared with the plasma of randomly selected, population-based controls without MS. ANO2, also known as TMEM16B, is one of 10 members of the recently discovered anoctamin family (22, 23). Anoctamins are predicted to have eight transmembrane regions with intracellular N and C termini. The first two members, ANO1 and ANO2, have been shown to belong to the family of calcium-activated chloride channels, which are involved in several physiological processes including, but not limited to, sensory transduction and neuronal excitation (24). Similarly, anoctamins recently have been implicated in a wide range of physiological processes (25, 26). ANO2 was reported to be expressed in photoreceptor synaptic terminals and in the cilia of mature olfactory neurons, thus possibly having a role in ion transport and in the cell physiology involved in olfaction (27, 28) and photoreception (29). Because little is known about the expression of ANO2 in human brain tissue and MS lesions, we performed immunohistochemistry analysis. This analysis showed moderate ANO2 staining in neuronal cell bodies from healthy-appearing tissue, but a clear increase in ANO2 staining intensity was observed in the proximity of and inside MS lesions, with the protein forming cellular aggregates (Fig. 3). This finding is in accordance with the autoantibody reactivity findings in plasma and further supports the notion that reactivity toward ANO2 might represent an autoimmune component of MS.
Here, the differences identified between MS cases and controls were affirmed for a protein fragment representing 89 residues of the N-terminal region of ANO2. These findings were corroborated further by analysis of a selected subset of the same sample collection in assays performed by another laboratory, in which both the 89-aa and an extended 365-aa N-terminal region of ANO2 were expressed independently and used for plasma analysis (SI Appendix, Fig. S7).
The selective reactivity against the N-terminal region fragment suggested that this fragment is the binding region of the ANO2 autoantibodies because the other fragment representing the C terminus revealed no prominent autoantibody reactivity (SI Appendix, Fig. S9). Further analysis of reactivity toward the N-terminal region on peptide level indeed narrowed down the binding to region 136–147 of ANO2 (Fig. 2 E–G). Although this region has been predicted to reside at the intracellular N-terminal domain of ANO2, superposition of this epitope on the very recently reported experimental structure of ANO1 (30) reveals that it is equally likely to reside on either the intracellular or the extracellular surface (SI Appendix, Fig. S17). Although the latter possibility makes the extracellular surface-exposed and thus potentially pathogenic autoantibody binding plausible, autoantibody reactivity against intracellular epitopes has been reported previously, e.g., for serum autoantibodies against aquaporin 4 (31), a water channel protein known to be an autoimmune target in neuromyelitis optica. Nevertheless, dedicated epitope-mapping studies representing the entire ANO2 protein and further investigations using a full-length version of ANO2 and its isoforms will certainly provide a better understanding of the exact binding site of ANO2 autoantibodies in MS.
The analysis we present of interactions with genetic and environmental risk factors of MS reinforces the notion that autoimmune reactivity against ANO2 might have a central role. The presence of an interaction between causal factors indicates that they might take part in the same pathogenic pathway but not that there is necessarily a direct physicochemical interaction between the interacting components. The findings of our interaction analysis thus strengthen a potential central role of ANO2 antibody reactivity in MS and strongly encourage more dedicated studies, e.g., studies of T-cell reactivities, pathogenicity of the antibodies in vitro, and the development of suitable rodent model systems. Besides, considering the implicated role for ANO2 in olfactory signal transduction and the number of studies describing olfactory dysfunction within MS and its correlation with the number of MS lesions (32, 33), further studies investigating the correlation between ANO2 reactivity data and standardized tests of odor identification ability might be potentially interesting.
In conclusion, profiling IgG-derived autoimmune reactivity in plasma highlighted ANO2 as an important candidate for future autoimmunity-profiling studies within MS, which will reveal whether ANO2 reactivity is indicative of a primary immune response as part of MS pathophysiology or is a secondary and rather more long-term effect following the neuroinflammation initiated by reactivity against other self-antigens. The data we present point toward an ANO2 autoimmune subphenotype in MS and pave the way for further studies investigating the role of ANO2 in the development and progression of MS.
Materials and Methods
Detailed information on materials and experimental procedures is available in SI Appendix, SI Materials and Methods.
Plasma Samples.
The EDTA plasma samples were collected within the Epidemiological Investigation of MS (EIMS) study, which is a population-based, case-control study comprising the population aged 16–70 y in geographically defined areas of Sweden. The study participants were recruited between April 2005 and June 2011 and comprised 1,063 MS cases and 1,106 controls (Table 1). Incident cases were recruited at 40 clinical centers, including all university hospitals in Sweden. All patients were examined and diagnosed by a neurologist and fulfilled the McDonald criteria. Controls were randomly selected from the national population registry, matched with cases by residential area, sex, and age (within predetermined 5-y age groups).
HLA Genotyping.
Allelic dosage of HLA-DRB1*15 and HLA-A*02 were obtained by imputation of HLA types from SNP genotypes in the MHC region using the HLA*IMP:02 program (34). The genotypes were generated using the Illumina Human Quad 660 chip, the Immunochip custom array, or both. Genotyping was carried out in previous studies analyzing the genetic risk of MS for the individuals included in this study (35, 36). Only HLA genotypes with a quality score above 0.7 were included.
Antigens and Assays on Bead-Based Antigen Arrays.
A total of 367 protein fragments were used in this study, representing 196 unique Ensembl Gene IDs. The protocols for the design and expression of these antigens within the Human Protein Atlas framework were applied as previously described (37). In brief, using a whole-genome bioinformatics approach, antigens of 80- to 150-aa acid residues were designed in silico based on the principle of lowest sequence similarity to other human proteins and were expressed in Escherichia coli. In addition to the protein fragments, the antigen set included a full-length protein for the viral protein EBNA-1 (Tebu-Bio) and other control analytes, building a final set of 384 analytes. All antigens were coupled to carboxylated magnetic beads (MagPlex-C; Luminex Corp.) as recently described (5).
Assay conditions for the bead-based antigen arrays were applied as recently described (5). Briefly, plasma samples were diluted 1:150 in an assay buffer, and an R-phycoerythrin–conjugated Fcγ-specific goat anti-human IgG F(ab')2 fragment (Jackson ImmunoResearch) was used to detect bound human IgG in plasma. All measurements were done on the same FlexMap3D instrument (Luminex Corp.), and binding events were displayed as MFI values.
Reactivity Mapping for ANO2 on Bead-Based Peptide Arrays.
With the use of the PEPscreen Library Design Tool (Sigma-Aldrich), a total of 34 N-terminally biotinylated peptides derived from ANO2 fragment-A (region 79–167) were designed to be 15- or 20-aa long with a 12- or 10-aa overlap, respectively. In accordance with a recently described protocol (38), each biotinylated peptide (50 µM) was coupled on magnetic beads coated with NeutrAvidin (Thermo Scientific) (250 µg/mL). Following the previously described assay steps, including a preadsorption against NeutrAvidin-specific plasma antibodies (38), the bead-based peptide array was used for multiplex mapping of the IgG reactivity against ANO2 in a randomly chosen subset of plasma samples consisting of 185 MS cases and 178 controls.
Data Analysis and Statistics.
All data analysis and statistics were performed using R and various R packages (39). The nonparametric Wilcoxon rank-sum or Kruskal–Wallis test was applied for statistical analysis of differences in MFI values obtained for two- or multigroup comparisons, respectively. Reactivity of a given plasma sample against an antigen was considered positive if the MFI value exceeded an MFI threshold set as the median plus 3 × SD of the MFI values obtained for the control samples. Fisher’s exact test was used for the statistical evaluation of differences in fractions of positive antibody reactivity within the sample groups. All P values were adjusted for multiple testing using the false-discovery rate (FDR) correction. Correlation coefficients were calculated using nonparametric Spearman’s correlation.
For HLA interaction analysis, interactions between risk factors were examined by calculating the departure from additivity of effects, the AP due to interaction with 95% confidence intervals, and the corresponding P value (APp) (40, 41). The AP was estimated with logistic regression adjusted for age, gender, and ancestry. When the interaction with increased EBNA1 IgG levels was analyzed, increased EBNA1 IgG levels were defined as being those above the median EBNA-1 IgG titer among controls, as previously suggested (8). Both nominal P values and P values corrected for multiple comparisons [i.e., multiplied by number of tests performed (n = 9)] are presented.
Immunofluorescence Histochemistry on Brain Sections.
Paraffin-embedded human brain sections (7 µm thick) were deparaffinized, rehydrated, and treated in an EDTA-based solution to unmask the antigens. Slides subsequently were incubated in normal donkey serum, followed by the addition of the primary antibody mixture containing antibodies against CD68 (Abcam), GFAP (Millipore), and ANO2 (Origene). Sections were washed and incubated with the secondary antibody mixture, washed again, and, after incubation in Sudan Black to quench autofluorescence, slides were mounted in DAPI-containing mounting medium (Life Technologies). All tissue-covered areas were scanned using a 20× primary objective, and individual field-of-view images were stitched to generate a large, four-channel fluorescence image of the entire specimen with microscopic resolution.
Study Approval and Ethical Statement.
Study enrollment followed the recommendations of the Declaration of Helsinki, and the study was approved by the Regional Ethics Committee of the Karolinska Institute, Stockholm. Sample donors received verbal and written information and gave consent in writing before inclusion in the study.
Supplementary Material
Acknowledgments
We thank the entire staff of the Human Protein Atlas for their efforts in producing the antigens used in this study. This study was supported by the Knut and Alice Wallenberg Foundation; the ProNova VINN Excellence Centre for Protein Technology (VINNOVA, Swedish Governmental Agency for Innovation Systems); the AFA Foundation; the Söderberg Foundation; the Swedish Research Council; the Swedish Brain Foundation; the Swedish Research Council for Health, Working Life and Welfare; The Science for Life Laboratory Stockholm; and the KTH Center for Applied Proteomics funded by the Erling–Persson Family Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.H.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518553113/-/DCSupplemental.
References
- 1.McFarland HF, Martin R. Multiple sclerosis: A complicated picture of autoimmunity. Nat Immunol. 2007;8(9):913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 2.Elliott C, et al. Functional identification of pathogenic autoantibody responses in patients with multiple sclerosis. Brain. 2012;135(Pt 6):1819–1833. doi: 10.1093/brain/aws105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schirmer L, Srivastava R, Hemmer B. To look for a needle in a haystack: The search for autoantibodies in multiple sclerosis. Mult Scler. 2014;20(3):271–279. doi: 10.1177/1352458514522104. [DOI] [PubMed] [Google Scholar]
- 4.Uhlén M, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 5.Ayoglu B, et al. Autoantibody profiling in multiple sclerosis using arrays of human protein fragments. Mol Cell Proteomics. 2013;12(9):2657–2672. doi: 10.1074/mcp.M112.026757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava R, et al. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med. 2012;367(2):115–123. doi: 10.1056/NEJMoa1110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd Ed Lippincott Williams & Wilkins; Philadelphia: 2008. [Google Scholar]
- 8.Sundqvist E, et al. Epstein-Barr virus and multiple sclerosis: Interaction with HLA. Genes Immun. 2012;13(1):14–20. doi: 10.1038/gene.2011.42. [DOI] [PubMed] [Google Scholar]
- 9.Farias AS, Pradella F, Schmitt A, Santos LM, Martins-de-Souza D. Ten years of proteomics in multiple sclerosis. Proteomics. 2014;14(4-5):467–480. doi: 10.1002/pmic.201300268. [DOI] [PubMed] [Google Scholar]
- 10.Lock C, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8(5):500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 11.Han MH, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451(7182):1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- 12.Tumani H, et al. BioMS Study Group Cerebrospinal fluid biomarkers in multiple sclerosis. Neurobiol Dis. 2009;35(2):117–127. doi: 10.1016/j.nbd.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Fitzner B, Hecker M, Zettl UK. Molecular biomarkers in cerebrospinal fluid of multiple sclerosis patients. Autoimmun Rev. 2015;14(10):903–913. doi: 10.1016/j.autrev.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 14.D’Ambrosio A, et al. Peripheral blood biomarkers in multiple sclerosis. Autoimmun Rev. 2015;14(12):1097–1110. doi: 10.1016/j.autrev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Fraussen J, Claes N, de Bock L, Somers V. Targets of the humoral autoimmune response in multiple sclerosis. Autoimmun Rev. 2014;13(11):1126–1137. doi: 10.1016/j.autrev.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Kanter JL, et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med. 2006;12(1):138–143. doi: 10.1038/nm1344. [DOI] [PubMed] [Google Scholar]
- 17.Quintana FJ, et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci USA. 2008;105(48):18889–18894. doi: 10.1073/pnas.0806310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintana FJ, et al. Antigen microarrays identify CNS-produced autoantibodies in RRMS. Neurology. 2012;78(8):532–539. doi: 10.1212/WNL.0b013e318247f9f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson WH, et al. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat Biotechnol. 2003;21(9):1033–1039. doi: 10.1038/nbt859. [DOI] [PubMed] [Google Scholar]
- 20.Beyer NH, Lueking A, Kowald A, Frederiksen JL, Heegaard NH. Investigation of autoantibody profiles for cerebrospinal fluid biomarker discovery in patients with relapsing-remitting multiple sclerosis. J Neuroimmunol. 2012;242(1-2):26–32. doi: 10.1016/j.jneuroim.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Querol L, et al. Protein array-based profiling of CSF identifies RBPJ as an autoantigen in multiple sclerosis. Neurology. 2013;81(11):956–963. doi: 10.1212/WNL.0b013e3182a43b48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang YD, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455(7217):1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 23.Caputo A, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 24.Duran C, Thompson CH, Xiao Q, Hartzell HC. Chloride channels: Often enigmatic, rarely predictable. Annu Rev Physiol. 2010;72:95–121. doi: 10.1146/annurev-physiol-021909-135811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreiber R, et al. Expression and function of epithelial anoctamins. J Biol Chem. 2010;285(10):7838–7845. doi: 10.1074/jbc.M109.065367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian Y, Schreiber R, Kunzelmann K. Anoctamins are a family of Ca2+-activated Cl- channels. J Cell Sci. 2012;125(Pt 21):4991–4998. doi: 10.1242/jcs.109553. [DOI] [PubMed] [Google Scholar]
- 27.Stephan AB, et al. ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc Natl Acad Sci USA. 2009;106(28):11776–11781. doi: 10.1073/pnas.0903304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurya DK, Menini A. Developmental expression of the calcium-activated chloride channels TMEM16A and TMEM16B in the mouse olfactory epithelium. Dev Neurobiol. 2014;74(7):657–675. doi: 10.1002/dneu.22159. [DOI] [PubMed] [Google Scholar]
- 29.Stöhr H, et al. TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J Neurosci. 2009;29(21):6809–6818. doi: 10.1523/JNEUROSCI.5546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunner JD, Lim NK, Schenck S, Duerst A, Dutzler R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature. 2014;516(7530):207–212. doi: 10.1038/nature13984. [DOI] [PubMed] [Google Scholar]
- 31.Kampylafka EI, et al. Fine specificity of antibodies against AQP4: Epitope mapping reveals intracellular epitopes. J Autoimmun. 2011;36(3-4):221–227. doi: 10.1016/j.jaut.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Doty RL, Li C, Mannon LJ, Yousem DM. Olfactory dysfunction in multiple sclerosis. N Engl J Med. 1997;336(26):1918–1919. doi: 10.1056/NEJM199706263362617. [DOI] [PubMed] [Google Scholar]
- 33.Doty RL, Li C, Mannon LJ, Yousem DM. Olfactory dysfunction in multiple sclerosis. Relation to plaque load in inferior frontal and temporal lobes. Ann N Y Acad Sci. 1998;855:781–786. doi: 10.1111/j.1749-6632.1998.tb10658.x. [DOI] [PubMed] [Google Scholar]
- 34.Dilthey A, et al. Multi-population classical HLA type imputation. PLOS Comput Biol. 2013;9(2):e1002877. doi: 10.1371/journal.pcbi.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawcer S, et al. International Multiple Sclerosis Genetics Consortium Wellcome Trust Case Control Consortium 2 Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476(7359):214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beecham AH, et al. International Multiple Sclerosis Genetics Consortium (IMSGC) Wellcome Trust Case Control Consortium 2 (WTCCC2) International IBD Genetics Consortium (IIBDGC) Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45(11):1353–1360. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berglund L, et al. A whole-genome bioinformatics approach to selection of antigens for systematic antibody generation. Proteomics. 2008;8(14):2832–2839. doi: 10.1002/pmic.200800203. [DOI] [PubMed] [Google Scholar]
- 38.Ayoglu B, et al. Bead arrays for antibody and complement profiling reveal joint contribution of antibody isotypes to C3 deposition. PLoS One. 2014;9(5):e96403. doi: 10.1371/journal.pone.0096403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Team RC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2013. [Google Scholar]
- 40.Källberg H, Ahlbom A, Alfredsson L. Calculating measures of biological interaction using R. Eur J Epidemiol. 2006;21(8):571–573. doi: 10.1007/s10654-006-9037-6. [DOI] [PubMed] [Google Scholar]
- 41.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112(4):467–470. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.