Significance
Release of the oocyte from the ovarian follicle at ovulation occurs with precise timing and spatial localization to the outer surface of the ovary to insure deposition of the oocyte in the oviduct for fertilization. The role that vasoconstriction of follicular vessels may play in follicle rupture was tested using multiphoton microscopy to measure repeatedly, at intervals, blood flow and the diameter of individual follicular vessels relative to follicle rupture in vivo. Blocking the acute vasoconstriction that occurred at the outer surface of the follicle before ovulation prevented follicle rupture, whereas restoring vasoconstriction induced rupture. These findings contribute to our understanding of the mechanics of ovulation, showing that vasoconstriction of follicular vessels is essential for follicle rupture.
Keywords: ovary, ovulation, endothelin, in vivo imaging, vasculature
Abstract
Rupture of the ovarian follicle releases the oocyte at ovulation, a timed event that is critical for fertilization. It is not understood how the protease activity required for rupture is directed with precise timing and localization to the outer surface, or apex, of the follicle. We hypothesized that vasoconstriction at the apex is essential for rupture. The diameter and blood flow of individual vessels and the thickness of the apical follicle wall were examined over time to expected ovulation using intravital multiphoton microscopy. Vasoconstriction of apical vessels occurred within hours preceding follicle rupture in wild-type mice, but vasoconstriction and rupture were absent in Amhr2cre/+SmoM2 mice in which follicle vessels lack the normal association with vascular smooth muscle. Vasoconstriction is not simply a response to reduced thickness of the follicle wall; vasoconstriction persisted in wild-type mice when thinning of the follicle wall was prevented by infusion of protease inhibitors into the ovarian bursa. Ovulation was inhibited by preventing the periovulatory rise in the expression of the vasoconstrictor endothelin 2 by follicle cells of wild-type mice. In these mice, infusion of vasoconstrictors (either endothelin 2 or angiotensin 2) into the bursa restored the vasoconstriction of apical vessels and ovulation. Additionally, infusion of endothelin receptor antagonists into the bursa of wild-type mice prevented vasoconstriction and follicle rupture. Processing tissue to allow imaging at increased depth through the follicle and transabdominal ultrasonography in vivo showed that decreased blood flow is restricted to the apex. These results demonstrate that vasoconstriction at the apex of the follicle is essential for ovulation.
During ovulation in typically mono-ovulatory species such as humans, as well as in poly-ovulatory species such as rodents, the oocyte is released from the preovulatory follicle by extrusion through a rupture site on the outer surface, or apex, of the follicle, which protrudes from the surface of the ovary (1). Precise timing and accurate spatial localization of rupture at the apex are essential to allow capture of the oocyte by a hormonally primed oviduct where fertilization occurs, but the mechanisms involved are not yet understood. The rupture site breaches multiple layers of cells and their associated extracellular matrix and basement membranes (2). These include the single layer of epithelial cells that covers the surface of the ovary, the basement membrane that supports it, and the multiple cell layers comprising the wall of the preovulatory follicle. The outer wall of the ovarian follicle contains androgen-secreting theca cells and extensive vasculature. This vasculature consists of an inner and an outer plexus of capillaries with associated arterioles and venules that supply nutrients to the entire follicle (3–5). Underlying the theca and separated from it by a basement membrane is the avascular granulosa cell layer that serves as the major source of estrogen. The oocyte resides in the center of the follicle surrounded by multiple layers of specialized granulosa cells known as “cumulus cells.” In a mature preovulatory follicle, formation of a fluid-filled antral cavity separates the oocyte–cumulus complex from the mural granulosa cells that form the wall of the follicle except at a region known as the “stalk,” which connects the oocyte–cumulus complex to the antral granulosa cells of the follicle wall. At ovulation the oocyte is released from the follicle in association with attached cumulus cells.
The preovulatory release of surge levels of luteinizing hormone (LH) from the anterior pituitary acts on receptors in the follicle to trigger events critical for the rupture and remodeling of the follicle and differentiation of granulosa and theca cells into progesterone-producing cells of the corpus luteum. The cumulus cells are induced to secrete a mucoelastic extracellular matrix which causes loosening of contacts between granulosa cells and between granulosa cells and the oocyte, a process known as “cumulus expansion,” which is essential for ovulation (1). Expression of proteases belonging to several major families, including the matrix metalloproteinase, plasminogen activator/plasmin, and ADAMTS (a disintegrin and metalloproteinase with thrombospondin-like motifs) families, increases. Simultaneously, follicle cells express protease inhibitors such as tissue inhibitors of metalloproteinases (TIMPs 1–4) and plasminogen activator inhibitors (PAI 1–3) (6, 7). The increase in protease activity is essential for rupture of the follicle and for the breakdown of the basement membrane separating theca and granulosa cells to allow the ingrowth of blood vessels to establish the corpus luteum. The mechanisms that regulate the balance of protease and protease inhibitor activity in the follicle to allow precise rupture at the apex while protecting most of the follicle structure from protease activity are not understood (1, 6, 7).
We postulated that vasoconstriction of vessels within the theca at the apex of the follicle is required to promote follicle rupture. Our first approach was to examine mice with conditional expression of a dominant active allele of smoothened (SMO), the transmembrane protein that relays signaling by the hedgehog (HH) pathway. In these Amhr2cre/+SmoM2 mice, preovulatory follicles develop normally in many respects, including changes in the expression of critical genes in response to the preovulatory LH surge (8, 9). However, follicles fail to rupture, and oocytes remain trapped as the follicles luteinize. The major ovarian phenotype in these mice is a pronounced deficiency of vascular smooth muscle (VSM) surrounding vessels in the theca cell layer, whereas other vessels that are present throughout the stroma of the ovary have normal maturation with VSM. Because VSM is required for vasoconstriction, the mice provided a model to test whether failure of vasoconstriction contributes to anovulation. In additional experiments with wild-type mice, we blocked the increase in the expression of endothelin 2 (Edn2) by granulosa cells that normally occurs within hours before follicle rupture (10, 11). Because EDN2 is a potent vasoconstrictor, this approach allowed us to test the effect on follicle rupture of inhibiting vasoconstriction versus treatment with exogenous compounds to restore vasoconstriction. In addition, treatment of wild-type mice with EDN2 receptor antagonists was used to test the role of EDN2 in vasoconstriction and rupture. Vasoconstriction and changes in the follicle wall were monitored repeatedly relative to the time of ovulation using intravital multiphoton microscopy.
Results
Transgenic Amhr2cre/+SmoM2 mice, in which thecal blood vessels are deficient in VSM (8, 9), and genotype-matched Amhr+/+SmoM2 control mice were used to test the requirement for vasoconstriction of thecal vessels in follicle rupture. Vessels of preovulatory follicles were imaged by multiphoton microscopy as described in detail in SI Materials and Methods and ref. 12. Prepubertal mice were treated with equine chorionic gonadotropin (eCG) to induce the growth of multiple preovulatory follicles followed 48 h later by injection with human CG (hCG), which causes ovulation within 12–13 h (8). Mice were anesthetized, and the ovary with attached bursa and reproductive tract were exteriorized and then were immobilized on a specialized dish for imaging and were perfused continuously with 37 °C lactated Ringer’s solution. The vasculature was labeled by retro-orbital injection of rhodamine-labeled dextran [molecular weight (MW) 2 × 106]. Multiple individual vessels within the theca layer at the outer surface of a selected preovulatory follicle were imaged by acquiring z-stacks every 60 min over 2-h time intervals between −2 h and 13 h after hCG injection (−2 to 0, 5–7, 8–10, and 11–13 h). Imaging protocols included repeated line scans to measure the velocity of red blood cells within a vessel and repeated z-series to measure the diameter and orientation of those vessels. These values were used to calculate blood flow (Fig. 1 A–E and Fig. S1). Leakage of some of the fluorescent dextran from vessels into the antral cavity provided sufficient contrast to identify the antrum and the granulosa and theca layers of the follicle, allowing the thickness of the apical follicle wall to be measured (Fig. 1F). The base of a preovulatory follicle could not be imaged because the typical diameter of a follicle (>300 μm) exceeds the ∼200 μm depth of imaging possible in the highly light-scattering ovarian tissue in vivo (12).
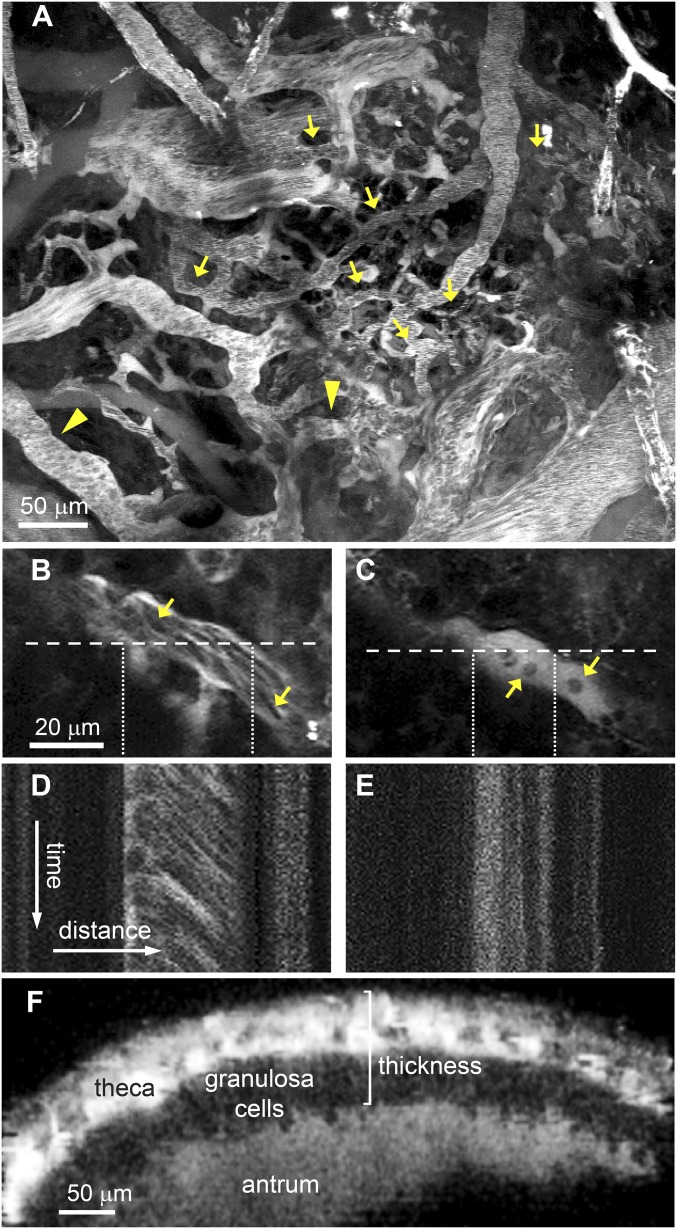
Fig. 1.
Intravital multiphoton microscopy of the preovulatory follicle was used to obtain repeated measurements of blood flow, diameters of thecal vessels, and the thickness of the apical follicle wall at various times relative to the expected time of ovulation. (A) A z-stack projection obtained at the surface of a representative follicle 48 h after eCG injection. Blood flow and vessel diameter were measured in thecal vessels (indicated by arrows) and vessels immediately external to the theca (arrowheads). (B and C) Representative images of a vessel at 11 (B) and 12 (C) h after hCG injection in an eCG-primed mouse. Arrows point to individual red blood cells. (D and E) Corresponding line scans of the vessel show moving blood cells at 11 h which leave a diagonal track (D) and stationary blood cells at 12 h which leave a vertical track (E). (F) A cross-section of a z-stack through a follicle at 48 h after eCG injection shows the theca and granulosa cell layers and the antrum, visible because of the leakage of rhodamine dextran out of vessels. This type of image was used to measure the thickness of the follicle wall, indicated by the bracket.
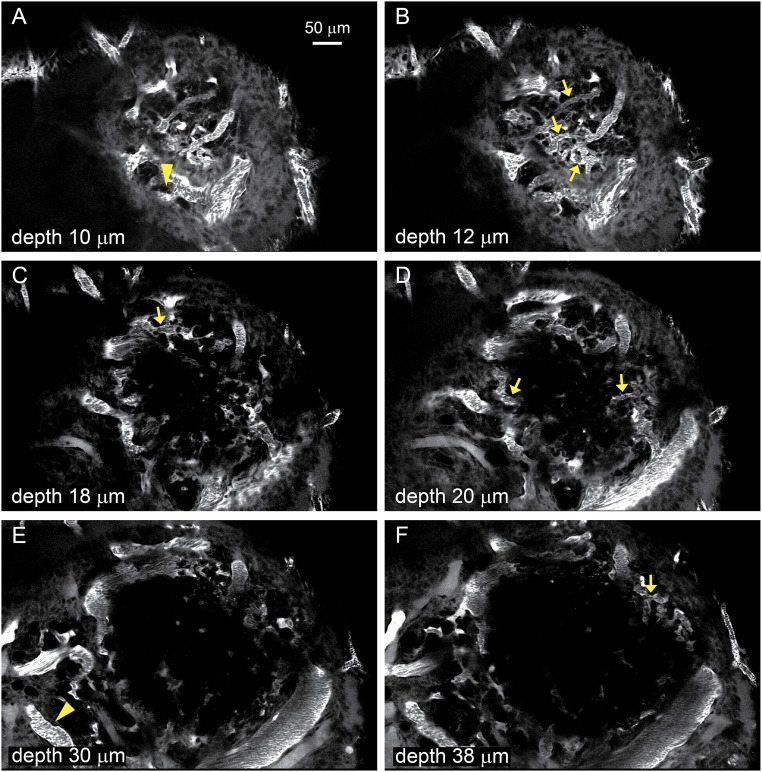
Fig. S1.
A–F show Individual images from a z-stack of the follicle shown as a projection in Fig. 1. The images were obtained at the indicated depths from the surface and show all the thecal vessels (arrows) and vessels external to the theca (arrowheads) analyzed for flow and diameter.
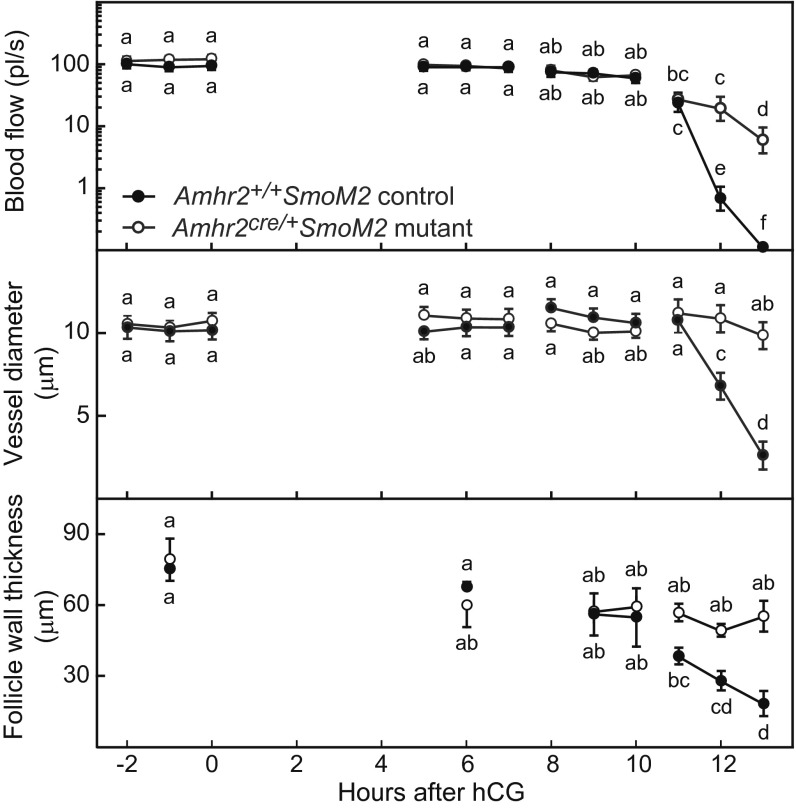
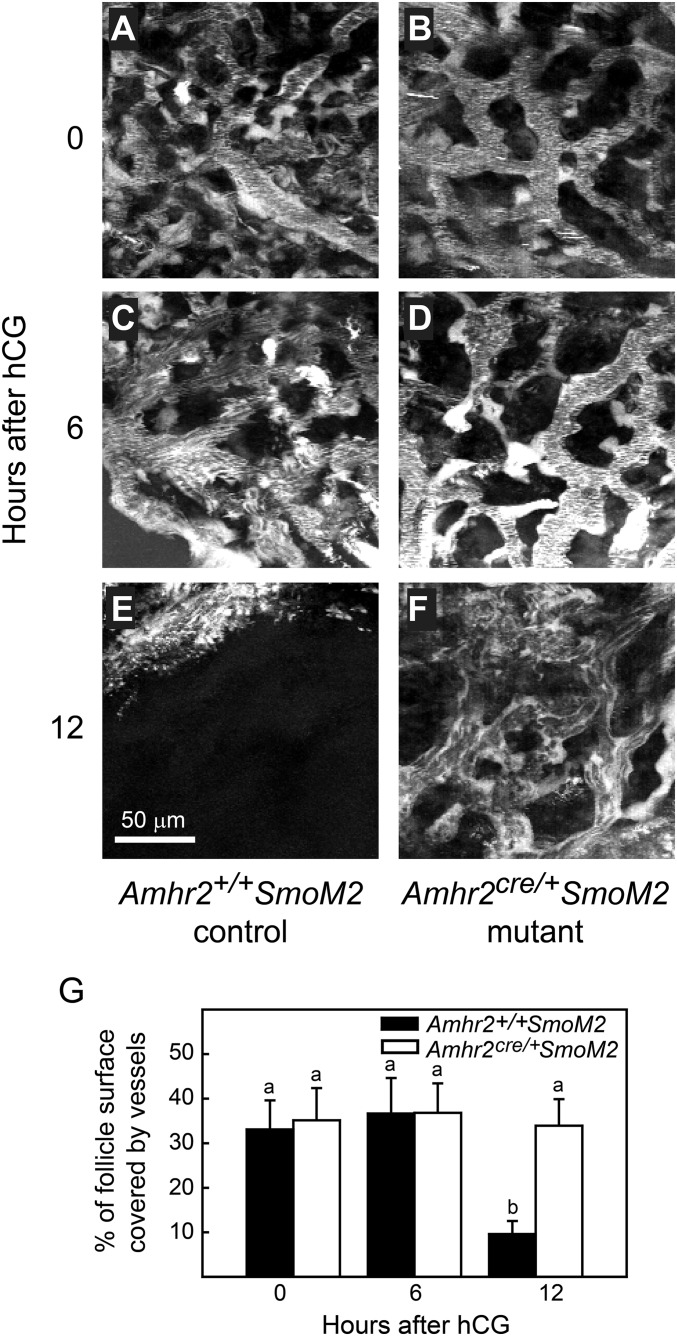
Blood flow and vessel diameter were constant in control and Amhr2cre/+SmoM2 mutant mice during time intervals from 2 h before to 10 h after hCG injection; these measures decreased acutely from 11–13 h after hCG injection in control mice but decreased only slightly in mutant mice (Fig. 2). A decrease in both blood flow and vessel diameter is indicative of vasoconstriction. A similar pattern was observed in larger vessels located in close proximity to the theca layer but just external to it, in the interstitium at the side of the follicle (Fig. S2). The thickness of the follicle wall remained relatively constant in both control and mutant mice from 2 h before to 11 h after hCG injection. In control mice the thickness of the follicle wall decreased acutely between 11 and 13 h postinjection, and ovulation occurred just after 13 h postinjection, but in mutant mice the thickness of the follicle wall did not change, and follicle rupture did not occur (Fig. 2). These data demonstrate that in control mice vasoconstriction of thecal vessels at the apex of the follicle occurs coincident with the acute thinning of the follicle wall before rupture. Acute thinning of the follicle wall and rupture fail to occur in Amhr2cre/+SmoM2 mice in which thecal vessels lack the normal association with VSM and do not constrict. Vasoconstriction before rupture was further supported by measurements of the area at the outer surface of follicles covered by rhodamine dextran-filled vessels. Vessel coverage declined between 6 h after hCG and prior to ovulation at 12 h after hCG in control mice but remained constant in mutants (Fig. S3).
Fig. 2.
Blood flow (Top) and the diameter of vessels within the theca at the apex of the preovulatory follicle (Center) decreased acutely within hours of follicle rupture in Amhr+/+SmoM2 control mice but remained relatively constant in anovulatory Amhr2cre/+SmoM2 mutant mice in which follicular vessels are deficient in VSM. (Bottom) The thickness of the apical follicle wall decreased simultaneously with decreased blood flow in control mice but not in mutant mice. Individual vessels and the thickness of the follicle wall were measured hourly in groups of anesthetized mice studied during 2-h intervals between −2 h to 13 h after injection of hCG. Ovulation occurred in control mice between 13 and 13.5 h after hCG injection. Data on blood flow and vessel diameter at each time point are from 22–36 vessels from individual follicles of five mice (mean ± SEM). Data on the thickness of the follicle wall are from single follicles measured at the times shown in three or four mice. Data points without a common super- or subscript are different (P < 0.05).
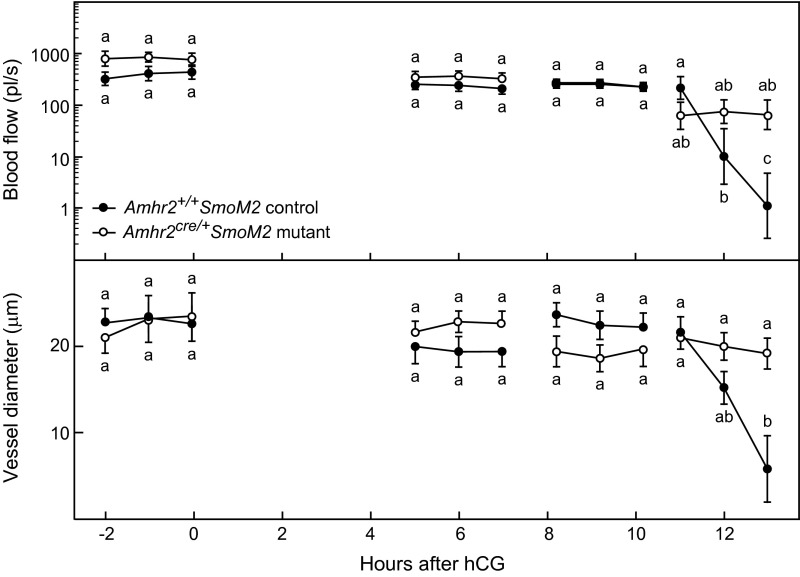
Fig. S2.
The patterns of changes in blood flow and in the diameter of vessels at the outer surface of the preovulatory follicle immediately external to the theca during the preovulatory period were similar to those observed for vessels within the theca (shown in Fig. 2). Blood flow (Upper) and vessel diameter (Lower) decreased acutely within hours of follicle rupture in Amhr+/+SmoM2 control mice but remained relatively constant in anovulatory Amhr2cre/+SmoM2 mutant mice in which follicular vessels are deficient in VSM. Individual vessels and the thickness of the follicle wall were measured hourly in groups of anesthetized mice studied during 2-h intervals between −2 h to 13 h after injection of hCG. Data on blood flow and vessel diameter are from 5–14 vessels from five mice (mean ± SEM) for each data point. Data points without a common super- or subscript are different (P < 0.05).
Fig. S3.
The percent of the surface of preovulatory follicles covered by rhodamine dextran-filled vessels decreased between 6 h and 12 h after hCG injection (just before ovulation) in Amhr+/+SmoM2 control mice but remained constant in anovulatory Amhr2cre/+SmoM2 mutant mice. A single image representing the surface density of vessels was created by projection of 20 sequential images at 2-μm intervals taken from the outer surface of the follicle toward the center. (A–F) Z-stack projections of representative follicles at various time points are shown. A single threshold level was applied to all images to determine the percent of the apical area that was rhodamine dextran-positive and therefore represented filled blood vessels. (G) Data (mean ± SEM) from single follicles of three mice of each genotype are shown. Data points without a common superscript are different (P < 0.05).
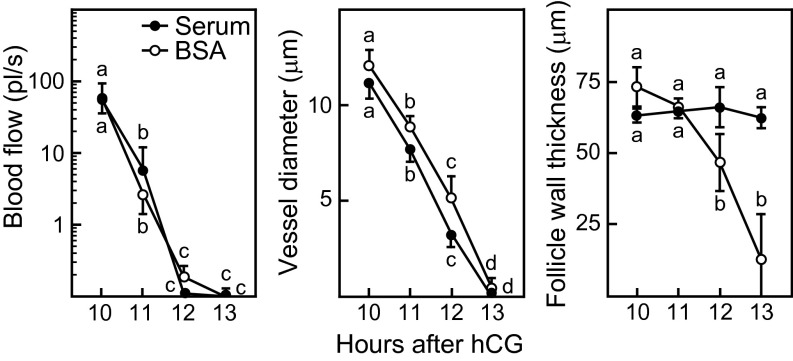
It was important to address the possibility that vasoconstriction at the apex of the follicle before rupture might simply be a response to the thinning of the follicle wall and a compensatory decrease in blood supply. If so, experimentally blocking thinning should prevent vasoconstriction. However, if vasoconstriction is independent of thinning, it should occur even when thinning is blocked. This question was tested by inhibiting the protease activity that leads to thinning of the follicle wall. Because serum is a rich source of protease inhibitors, a number of which are present at milligram-per-milliliter concentrations [e.g., alpha 2 macroglobulin and alpha 1 anti-trypsin (13)], we infused serum into the bursal cavity to inhibit protease activity and observed the effect on vasoconstriction. Thinning of the follicle wall was blocked in mice in which the bursa was infused with serum between 10 and 15 h after hCG injection; at 15 h only 1.3 ± 0.8 oocytes were recovered from the oviduct ipsilateral to infusion, and 16 ± 1.9 oocytes were recovered from the contralateral oviduct (Fig. 3). In contrast, in mice in which the bursa was infused with BSA as a control, the acute thinning of the follicle wall between 10 and 13 h after hCG injection occurred normally, and oocytes were recovered from oviducts ipsilateral and contralateral to infusion (15.7 ± 2.3 and 15.7 ± 1.1 oocytes, respectively). Despite this difference in the thickness of the follicle wall in response to BSA or serum infusion, the decrease in blood flow and vessel diameter occurred similarly (Fig. 3), indicating that vasoconstriction before follicle rupture is not initiated by or dependent on thinning of the follicle wall. This result allowed testing of the alternative possibility: that acute thinning of the follicle wall before rupture is dependent on vasoconstriction.
Fig. 3.
Infusion of serum into the bursal cavity, as a means to deliver protease inhibitors, blocked the thinning of the follicle wall that normally occurs before ovulation (Right) but did not prevent the decrease in blood flow (Left) or in the diameter of thecal blood vessels (Center). Serum (1:1 in saline) or 2% BSA in saline as control was infused into the bursa 10–15 h after hCG injection. Data are mean ± SEM from hourly repeated measurements of 14 or 15 vessels and of the wall thickness of single follicles from three mice at each time point. Data points without a common super- or subscript are different (P < 0.05).
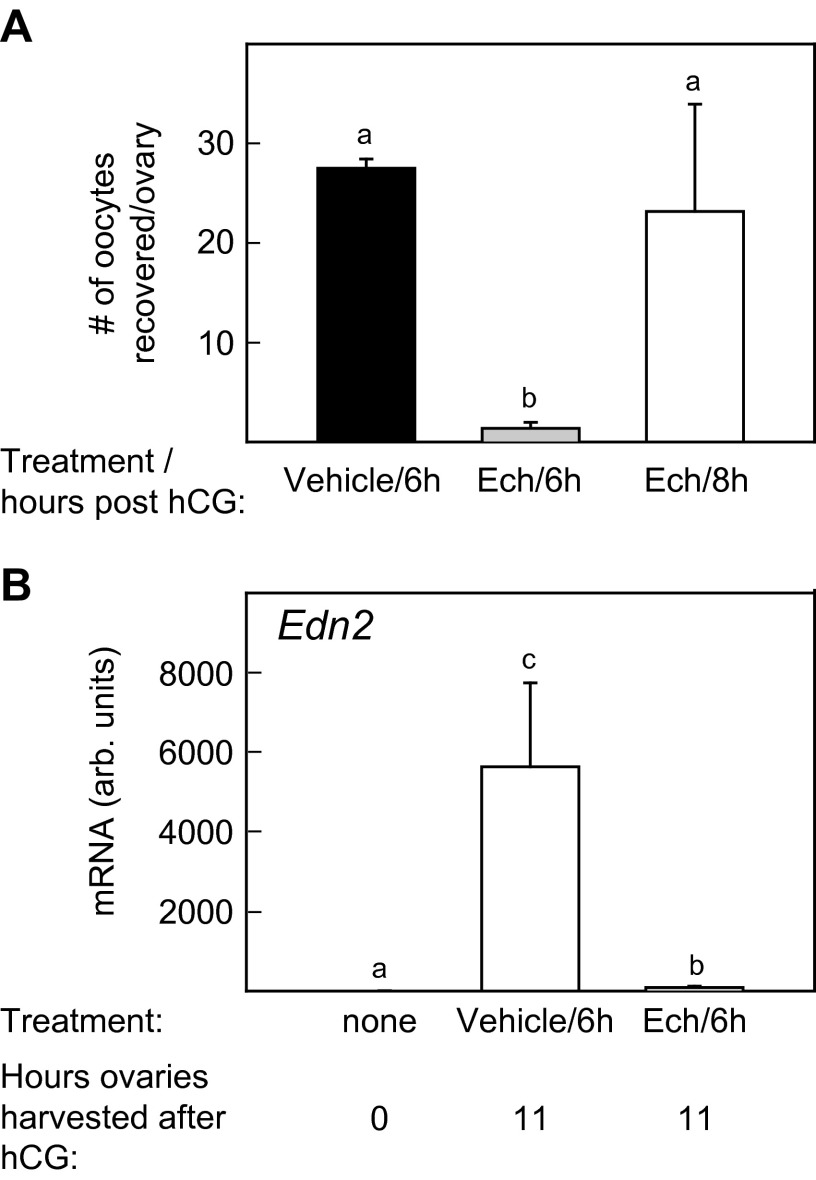
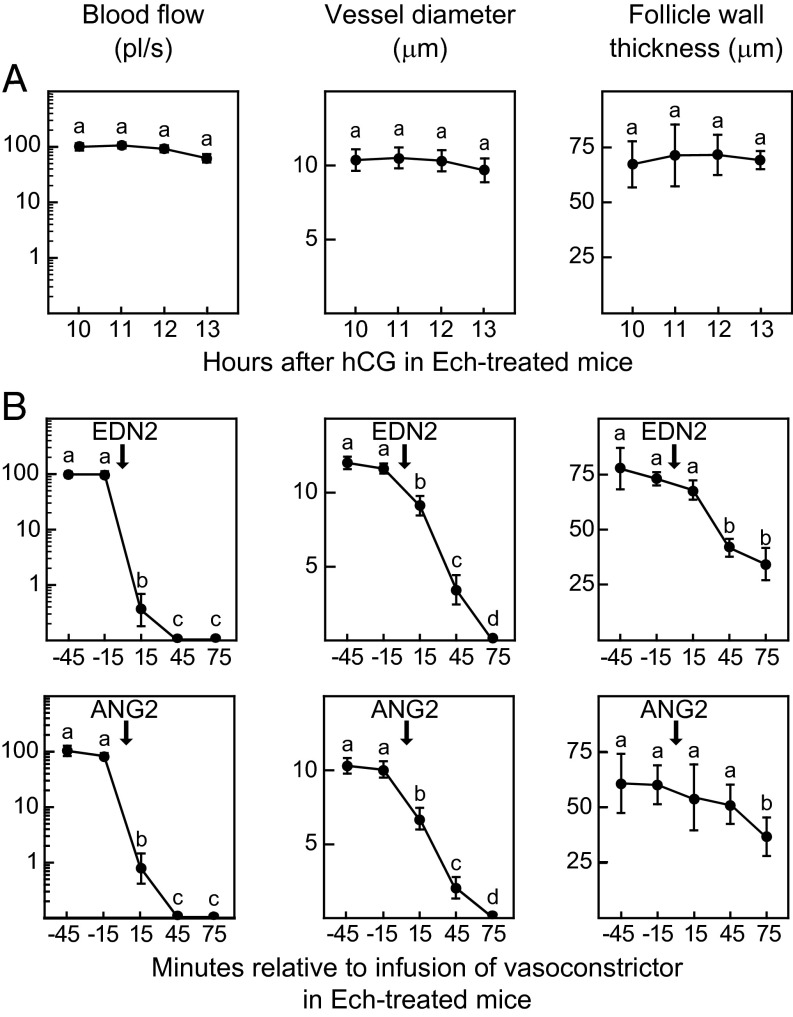
To test the requirement for vasoconstriction in follicle rupture, an anovulatory mouse model was used in which the rise in expression of the vasoconstrictor Edn2 by granulosa cells, which normally occurs several hours before rupture, was blocked. Previous studies showed that treatment of mice or rats with an inhibitor of the hypoxia-inducible factors (HIFs), echinomycin (Ech), reduced ovulation and suppressed the increase in Edn2 mRNA (14, 15). In our experiments, injection of Ech (1 mg/kg) at 6 h after hCG injection reduced the ovulation rate (Fig. S4A) and prevented the increase in Edn2 that normally occurred between 0 and 11 h after hCG injection (Fig. S4B). The follicles that failed to rupture in Ech-treated mice differed from normal follicles in that there was no reduction in blood flow or vessel diameter between 10 and 13 h after hCG injection, and the thickness of the follicle wall did not decrease (Fig. 4A). These results reinforced the positive association between vasoconstriction and follicle rupture. We then tested whether induction of vasoconstriction by bursal infusion of vasoconstrictors could overcome the block to ovulation in Ech-treated mice. A single bursal cavity of an Ech-treated mouse was first infused with 2% BSA-saline as a control beginning 11.25 h after hCG injection and continuing for 45 min; then the infusion was changed to 100 nM EDN2 until 15 h after hCG injection. During infusion with BSA-saline, the blood flow, vessel diameter, and thickness of the follicle wall were constant. Within 15 min after initiating infusion of EDN2, blood flow and vessel diameter were reduced; by 45 min the thickness of the follicle wall decreased (Fig. 4B); and follicles ovulated between 75 and 105 min after the initiation of perfusion. Collection of oocytes from the oviducts at 15 h after hCG injection resulted in recovery of 8.7 ± 1.1 oocytes from the oviduct ipsilateral to EDN2 infusion and 0.7 ± 0.7 oocytes from the contralateral oviduct (P < 0.001). A similar protocol in which the bursa of Ech-treated mice were infused with BSA-saline followed by the vasoconstrictor angiotensin 2 (ANG2) also resulted in vasoconstriction and thinning of the follicle wall (Fig. 4B) and induced ovulation from the infused ovary but not from the contralateral ovary (9.3 ± 0.4 and 0.7 ± 0.7 oocytes recovered, respectively). The fact that two different vasoconstrictors, EDN2 and ANG2, overcame the inhibitory effect of Ech on ovulation indicates that Ech-induced suppression of ovulation most likely was mediated by the inhibition of vasoconstriction, an effect that may have been caused by lack of Edn2 expression.
Fig. S4.
Treatment with the HIF inhibitor Ech suppressed ovulation and prevented the increase in expression of Edn2 by granulosa cells of preovulatory follicles. (A) Oocytes were recovered from the ampulla of each oviduct at 16 h after hCG injection in mice that were injected with Ech at 6 or 8 h after hCG injection or with vehicle at 8 h after hCG injection (n = 4 mice). (B) Levels of Edn2 mRNA were measured before hCG injection (0 h) and at 11 h after hCG injection in mice that were treated with Ech or vehicle at 6 h after hCG injection (n = 4 mice). Data points without a common superscript are different (P < 0.05).
Fig. 4.
Vascular changes within the theca at the apex of preovulatory follicles associated with the suppression of ovulation in Ech-treated wild-type mice and the restoration of ovulation by bursal infusion of vasoconstrictors in Ech-treated mice. (A) Blood flow (Left), the diameter of thecal vessels (Center), and the thickness of the apical follicle wall (Right) were constant from 10 to 13 h after hCG injection in Ech-treated mice in which ovulation was blocked. Data are mean ± SEM from hourly repeated measurements of 17 vessels and of the wall thickness of single follicles from three mice. (B) Infusion of EDN2 (Upper) or ANG2 (Lower) into the bursa of Ech-treated mice restored the changes in thecal vessels and the thinning of the follicle wall that normally occur before rupture. Treatment began at 11.25 h after hCG injection with continuous infusion of 2% BSA-saline for 45 m followed by continuous infusion of vasoconstrictors (arrows) until 15 h after hCG injection. Data are mean ± SEM from 13 EDN2-infused vessels or 15 ANG2-infused vessels and follicle walls imaged repeatedly at 30-min intervals (n = 3 mice for each vasoconstrictor). Data points without a common superscript are different (P < 0.05).
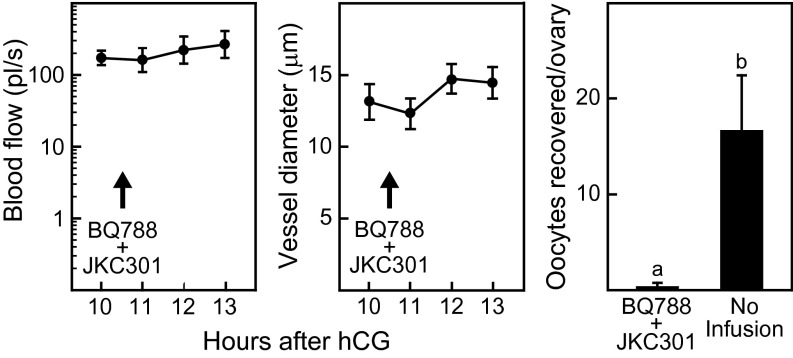
To test more directly the requirement for EDN2-induced vasoconstriction in follicle rupture, antagonists to the EDN2 receptors (EDNR) type A and B (JKC-301 for EDNRA and BQ-788 for EDNRB) were infused into the bursal cavity of wild-type mice. Beginning at 10 h after hCG injection, a single bursa was infused with 2% BSA-saline for 30 min; then the infusion was changed to 10 µm JKC-301 plus 10 µm BQ-788 in 2% BSA-saline, which was continued until 15 h after hCG injection. The decrease in blood flow and in the diameter of apical vessels that normally occur between 11 and 13 h after hCG injection were absent in follicles of the EDNR antagonist-infused ovaries, and ovulation was dramatically reduced in the infused ovary but not in the contralateral ovary (Fig. 5). These results show that inhibiting the action of endogenous EDN2 prevents vasoconstriction and suppresses ovulation.
Fig. 5.
Bursal infusion of EDN2 receptor antagonists in wild-type mice prevents vasoconstriction of vessels at the apex of the preovulatory follicle and suppresses ovulation. Beginning at 10 h after hCG, the bursa was infused with 2% BSA-saline for 30 min, followed by infusion of antagonists of EDN2 receptors type A and B (10 µm JKC-301 plus 10 µm BQ-788; arrows) until 15 h after hCG injection. Multiphoton microscopy imaging to determine blood flow (Left) and vessel diameter (Center) was performed on 15 vessels from three mice. (Right) The ovulation rate was determined by counting oocytes in the ampulla 15 h after hCG injection. Data points without a common superscript are different (P < 0.05).
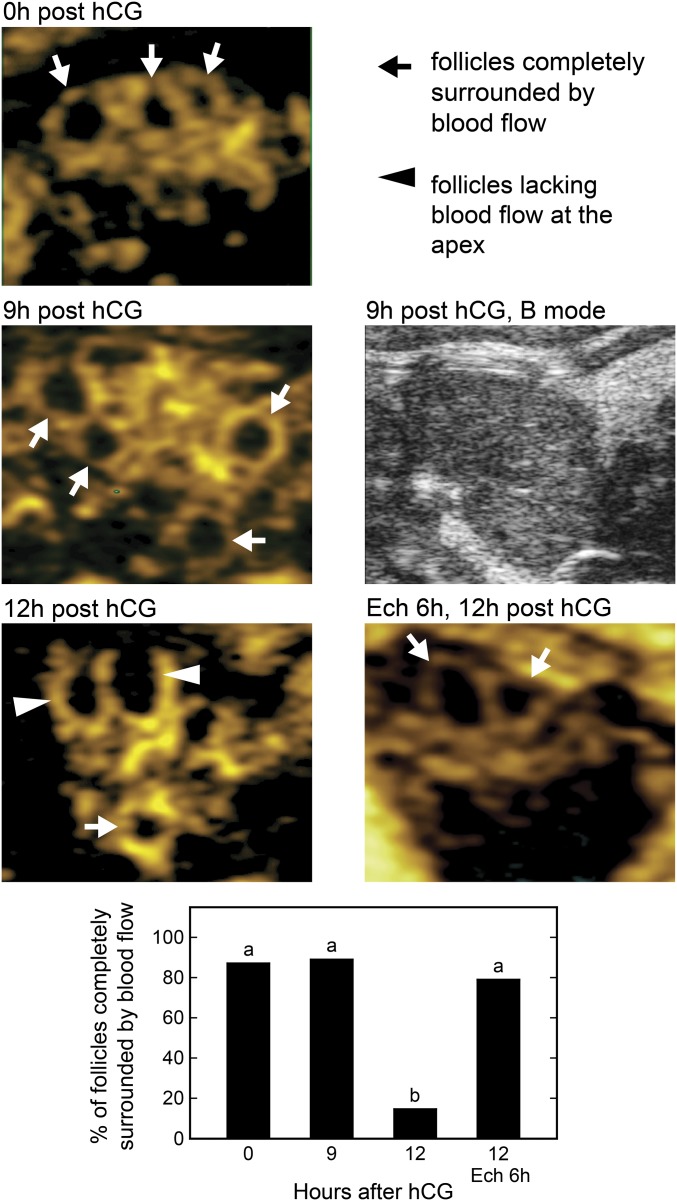
Although multiphoton microscopy was effective in imaging events at the follicle apex in vivo, light scattering prevented imaging deep enough into the follicle to analyze the base. Therefore additional methods were used to analyze vascular changes throughout the wall of the follicle. First, power Doppler ultrasonography was used to assess differences in blood flow between the apex and the base of preovulatory follicles as the time to ovulation approached. In the images generated, blood flow appears as a yellow signal surrounding dark circular areas which are the antral cavities of preovulatory follicles (Fig. 6). At 0 and 9 h after hCG injection in wild-type mice, most follicles had blood flow surrounding their circumference including the apex (89.2% and 88.9%, respectively), but at 12 h in mice that had not yet ovulated, few follicles (13.9%) had blood flow at the apex (Fig. 6). The results show that the decreased blood flow before follicle rupture in wild-type mice occurs at the apex of the follicle, as is consistent with the demonstration of vasoconstriction at the apex, and shows that blood flow to the base does not change. In mice that had been treated with Ech to inhibit ovulation (as described above), 80% of follicles had blood flow surrounding the circumference at 12 h after hCG injection, similar to the observations in wild-type mice at 0 h after hCG injection, indicating that the inhibition of ovulation was associated with a lack of vascular changes at the apex. As a second approach to assess vascular changes throughout the follicle, ovaries of anesthetized wild-type mice at 0 and 12 h after hCG injection (n = 3 mice per time period) were ligated and were removed 5 min after retro-orbital injection of rhodamine dextran. Ovaries were fixed and subjected to a clearing procedure to remove optically dense components that cause light scattering (16). This procedure allowed multiphoton imaging of rhodamine dextran-filled vessels throughout the wall of the follicle (Fig. S5). Projections of z-stacks (two follicles per ovary) were used to calculate the percent of the follicle area at the apex and the base covered by vessels (representative images are shown in Fig. S6). At 0 h after hCG injection, the percent of the area covered by vessels was similar at the apex and base of the follicle (26.7 ± 1.2% and 26.7 ± 4.9%, respectively). However, at 12 h after hCG injection, in mice that had not yet ovulated, the percent of the area covered by vessels was dramatically reduced at the apex (8.0 ± 2.9%, P < 0.05 vs. other values) compared with the base (20.0 ± 5.0%). The decrease in vessel coverage at the apex is consistent with the finding that vasoconstriction occurs at the apex before follicle rupture and indicates that similar vasoconstriction does not occur at the base.
Fig. 6.
Power Doppler ultrasound imaging of ovaries reveals changes in the pattern of blood flow around follicles at ovulation. Areas of blood flow appear yellow. Follicles are identified by a dark antral cavity associated with areas of blood flow and by their large size (>200 μm). There was continuous blood flow around the circumference of preovulatory follicles at 0 h and 9 h after hCG injection (arrows) and a lack of blood flow to the apex of follicles at 12 h after hCG injection (arrowheads), just before the expected time of follicle rupture. Ovaries from mice treated with Ech at 6 h and examined at 12 h after hCG injection also showed continuous blood flow around the circumference of follicles. A B-mode ultrasound image of a mouse ovary at 9 h after hCG injection, taken at the same depth as the power Doppler image, is shown. The percent of follicles completely surrounded by blood flow is shown in the bar graph. A total of 19–46 follicles from three to five mice were analyzed at each time point. Data points without a common superscript are different (P < 0.05).
Fig. S5.
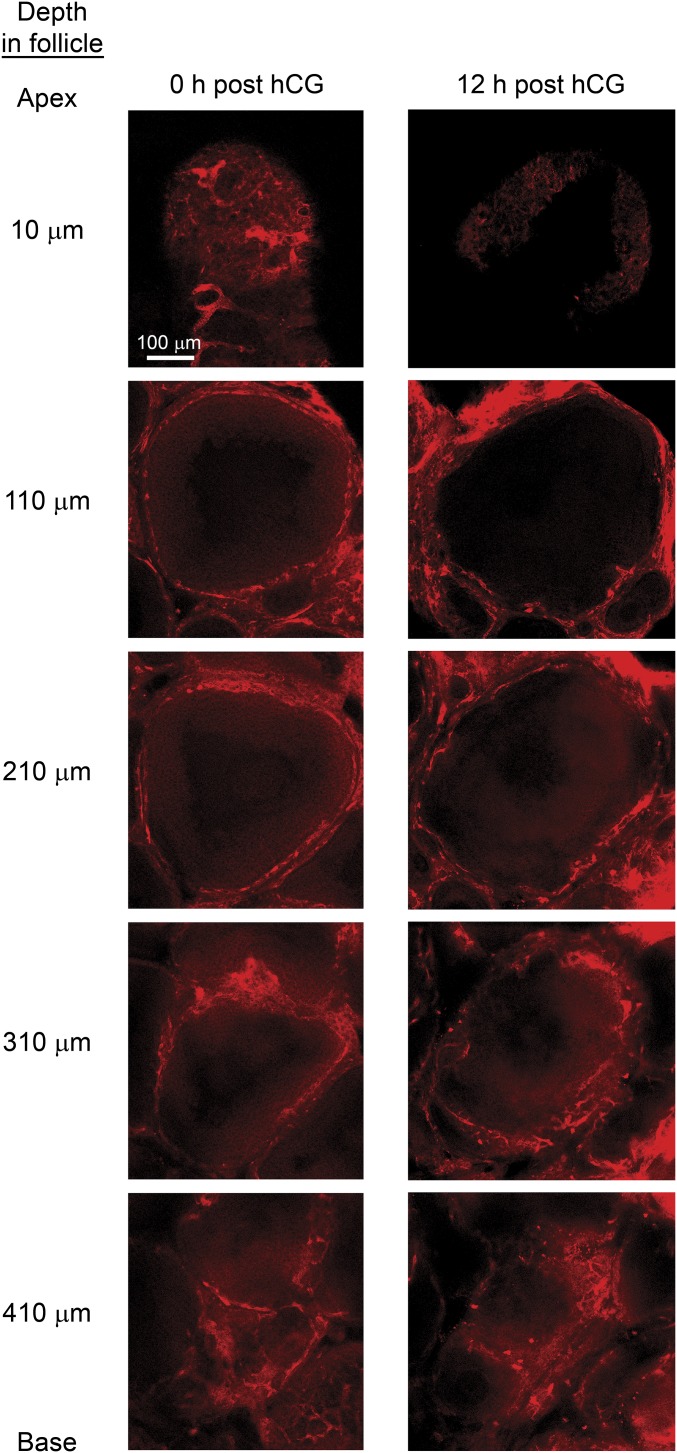
Images of the vasculature throughout representative preovulatory follicles at 0 (Left) and 12 (Right) h after hCG injection. Ovaries were harvested from mice 5 min after retro-orbital injection of rhodamine dextran and were processed to reduce light scattering and to enable deep imaging through the follicle according to the SeeDB optical clearing procedure as described in SI Materials and Methods. The topmost images, at a depth of 10 µm, are within the apical regions of the follicles and show the presence of filled vessels at 0 h after hCG injection and the absence of filled vessels at 12 h after hCG injection. Images from lateral and basal areas shown at progressively deeper positions within the follicles (110–410 µm) indicate that filling of vessels, representing blood flow, is present in these areas at both 0 h and 12 h after hCG injection. Two follicles from each of three mice were analyzed at each time point. Representative z-stack projections of apical and basal regions of follicles are shown in Fig. S6.
Fig. S6.
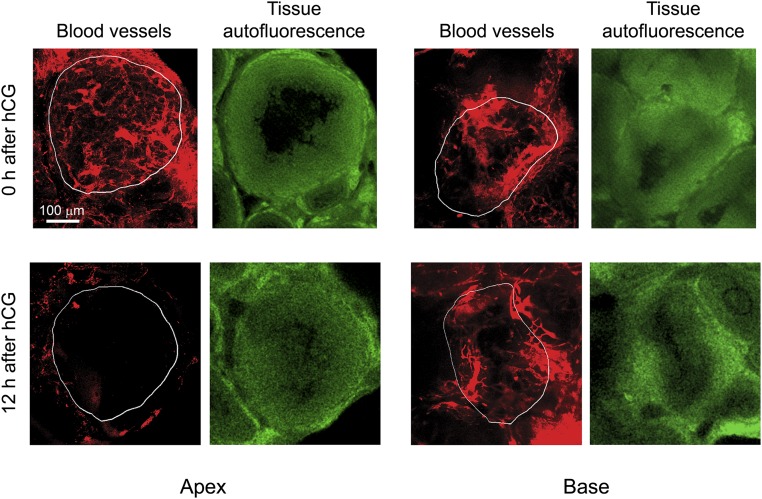
Representative z-stack projections of rhodamine dextran-filled vessels at the apex and base of preovulatory follicles in optically cleared ovaries processed as described in the legend to Fig. S5. Z-stack projections were prepared from 29 sequential multiphoton microscopy images at 3.52-μm intervals at both the apex and the base. These projections represent 100-μm-thick sections from the apex and base of the follicle. The red images show the projection of rhodamine dextran-filled vessels. The green images are single frames from the z-stack 100 μm from the base or apex showing autofluorescence of the tissue which was used to identify follicle boundaries (outlined in white on the red projections). At 0 h after hCG injection, filling of vessels was similar at the apex and base, but at 12 h after hCG injection filling at the apex was decreased relative to the base. These images were used to quantify the percent of rhodamine dextran-filled vessels at the apex and base and are discussed in Results in the main text.
SI Materials and Methods
Multiphoton Microscopy of Preovulatory Follicles in Vivo.
Details of the methodology used to image individual blood vessels at the surface of preovulatory follicles repeatedly over time in vivo are described in our previous publication (12). The major steps are described here: Mice were maintained under isoflurane-induced anesthesia. The right ovary was exteriorized and then immobilized on a raised Sylgard 184 (Dow Corning) shelf within a custom-made dish using insect pins placed through the fat pad, oviduct, and uterus. The ovary and lower abdomen of the mouse were submerged in circulating 37 °C lactated Ringer’s solution (a picture of the preparation is provided in ref. 12). The vasculature was labeled by retro-orbital injection of tetramethylrhodamine-labeled dextran (2 × 106 MW) (Life Technologies). The ovary was imaged using a custom-made multiphoton microscope with an Olympus 20×/0.95W XLUMPlanFL water immersion lens (details are given in ref. 12). Two-photon excitation was at 880 nm (∼150-fs pulse width at the sample). One follicle per ovary in an optimal position was selected for repeated imaging. Images were taken at 2-μm intervals in z starting from the outer surface of the follicle to generate a 160- to 200-μm-thick z-stack. Immediately after the z-stack was obtained, blood vessels were selected for measurement of blood flow. These vessels were categorized according to the location within the follicle where measurements were made, as described below. A vessel measured at the apex of the follicle was considered to be within the theca layer, and a vessel measured toward the side of the follicle within the interstitial tissue was considered to be immediately external to the theca layer. Blood flow was measured by taking line scans (2 ms per line) across each selected vessel (Fig. 1 B–E). In this technique, individual red blood cells, which do not take up the rhodamine dye, move through vessels along the scanned line leaving a shadow at an angle corresponding to their velocity (in micrometers per second). The measured velocity was corrected for the divergence of the vessel angle from the scanned line. The diameter of the vessel at the site of the line scan was measured in the z-stack, and the cross-sectional area (in square micrometers) was calculated and multiplied by the velocity of red blood cells (in micrometers per second) to determine blood flow (in cubic micrometers per second). Values were converted to picoliters per second for presentation in figures. Z-stacks and line scans of the previously scanned vessels were obtained at 30-m to 1-h intervals over a 2- to 3-h period relative to the expected time of ovulation. Data were analyzed using ImageJ software (NIH).
To determine the thickness of the wall at the apex of the follicle, the center of the apex was determined from z-stacks, and cross-sections through the center of the x–z and y–z planes were constructed. The thickness at the center of the apex and at four additional points 50 μm in each direction on the x and y axes were measured using ImageJ software, and the average thickness was calculated. A representative cross-section is shown in Fig. 1F.
The density of blood vessels at the apex was calculated using the z-stacks from each follicle, collected as described above. A single image representing the surface density of vessels was created by projection of 20 sequential images taken at 2-μm intervals from the outer surface of the follicle toward the center. A single threshold level was applied to all images to determine the percent of the apical area that was rhodamine dextran-positive and therefore represented blood vessels. Representative images are shown in Fig. S3.
Multiphoton Microscopy of Preovulatory Follicles ex Vivo.
The vasculature surrounding the entire follicle was imaged in fixed ovaries using the SeeDB method, a procedure developed for imaging deep within brain tissue (16). This method uses a saturated fructose solution to clarify tissues optically without causing excessive swelling or shrinking, disruption of fine morphology, or loss of fluorescence. Wild-type mice were maintained under isoflurane-induced anesthesia. A loop of braided suture was placed over the ovary. Rhodamine-labeled dextran was injected retro-orbitally as described above. Five minutes after injection the ovary was tied off with the suture and excised, and the bursa was removed. The ovary was fixed in 4% paraformaldehyde overnight and then was incubated with increasing concentrations of fructose in PBS as described (16). Cleared ovaries were imaged using a Zeiss 880 confocal/multiphoton microscope on an Axio Examiner Z1 stand with a Zeiss 10×/0.45 C-Apochromat water immersion lens. Two-photon excitation was at 880 nm. Fluorescent images were collected to visualize vasculature (red) and to identify follicle boundaries by tissue autofluorescence (green). Images were obtained at 3.52-μm intervals in z starting from the outer surface of the follicle and continuing through the base, generating 450- to 600-μm-thick z-stacks. Images at intervals through representative follicles are shown in Fig. S5. Data were analyzed using ImageJ software. Z-projections were created from 29 sequential images at 3.52-μm intervals at the apex and base (100-μm thickness) and were used to calculate the percent of these regions covered by filled vessels. Follicle boundaries were determined from the green autofluorescence frame obtained 100 μm from the apex or base of the follicle. Representative images are shown in Fig. S6. A single threshold level was applied to all images to determine the percent of the region that was rhodamine dextran-positive and therefore represented filled blood vessels.
Power Doppler Ultrasonography.
Female mice were anesthetized with 3.5% (vol/vol) isoflurane and then placed on their left side and maintained at 1.5% (vol/vol) isoflurane on a Vevo integrated rail stage adjusted to 37 °C (VisualSonics Inc.). Ovaries were imaged with a VisualSonics Vevo 2100 μLtrasound system equipped with a MS700 (30–79 MHz) transducer (VisualSonics Inc.). The ovary was located using B-mode, and power Doppler mode was used to study blood flow. Power Doppler ultrasound detects movement with greater sensitivity than color Doppler but does not provide information on the direction of flow. It is designed to determine the extent of vascularity in tissues. The system was set to detect very low flow using a preset option; critical settings for imaging blood flow included Doppler gain (55–60 dB), sensitivity (5), dynamic range (15–25 dB), and velocity (1 kHz). Images were captured while moving progressively through the entire ovary (350–400 images per ovary) and were saved as cineloops. The cineloops were analyzed by two independent observers as follows: A structure was identified as a follicle if it had a rounded shape and a dark cavity representing the antrum and if the antrum combined with the tissue immediately surrounding it had a diameter greater than 250 µm. Follicles were scored and divided into two categories: follicles in which blood flow was continuous around the circumference and follicles in which flow was absent at a region within the circumference. There was 68% agreement between the two observers in the structures identified as follicles and 5% disagreement in the categorization of the follicles based on the pattern of blood flow. Only follicles identified and categorized similarly by both observers were included in the analysis. Image analysis was performed using ImageJ software (NIH) and Photoshop (Adobe Systems). After imaging, each mouse was immediately killed, and the imaged ovary with the attached bursa and oviduct was examined to confirm that the ovary was properly stimulated by gonadotropin treatment and that, in the case of ovaries examined at 12 h after hCG injection, ovulation had not yet occurred.
Mouse Treatments.
In all experiments, ovulation was synchronized by hormonal stimulation. Prepubertal mice at 21–23 d of age were given an i.p. injection of 5 IU eCG (Sigma-Aldrich) followed 48 h later by an i.p. injection of 5 IU hCG (Sigma-Aldrich). This treatment results in ovulation 12–14 h after hCG injection; in our mouse colony ovulation occurs most frequently at 13 h after hCG injection. In some experiments, ovulation was inhibited in wild-type mice by i.p. injection of Ech (Cayman).
In some experiments the ovarian bursa was cannulated for infusions before imaging. The cannula was created by attaching each end of a length of polyethylene tubing (PE 10; Intramedic) to 30-G needles; the needle at one end was attached to a valve which allowed connections to two syringes containing different treatment solutions. The hub was removed from the needle at the other end of the cannula, and the bevel of the needle was dulled and smoothed by filing. The bursa was punctured with a 30-G needle, and the smoothed point of the cannula was advanced into the bursa and secured with a loop of surgical thread that was passed through connective tissue surrounding the oviduct. The ovary and cannula were immobilized using pins, as in other multiphoton imaging experiments. The dead space of the cannula was 80 µL. Solutions were infused into the bursa at a rate of 200 μL/h via a syringe pump (Harvard Apparatus). All cannulation experiments began with a period of infusion of 2% BSA in saline as control. The infusion then was switched to a test solution by operating the valve attached to syringes. Test solutions included serum diluted 1:2 in saline (vol:vol), 100 nM EDN2 or 10 µM ANG2 (both from Sigma-Aldrich) diluted in 2% (wt/vol) BSA-saline, and 10 µM BQ-788 plus 10 µM JKC-301 (Enzo Life Sciences) diluted in 2% (wt/vol) BSA-saline.
In some experiments, the number of ovulating follicles was determined by killing mice at 15 h after hCG injection, removing the ovary with attached bursa and oviducts, and opening the ampulla and bursa in dishes containing saline to release oocytes. Oocytes were counted after treatment with 300 µg/mL hyaluronidase in saline (Sigma-Aldrich).
Real-Time RT-PCR.
Total RNA was extracted from whole ovaries using an RNeasy Micro Kit (Qiagen). Reverse transcription was performed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time RT-PCR was performed on a StepOnePlus thermocycler (Applied Biosystems) using a mouse-specific assay for Edn2 (Mm00432983_m1; Applied Biosystems). A standard curve was constructed by serial dilution of cDNA prepared from an RNA pool of ovaries of immature, gonadotropin-stimulated mice. Values of samples and standards were normalized to 18S rRNA (4319413E; Applied Biosystems), and results are presented as arbitrary units by comparison with the standard curve.
Discussion
Intravital multiphoton microscopy was used to demonstrate, for the first time to our knowledge, that vasoconstriction of thecal vessels at the apex of the preovulatory follicle is necessary for follicle rupture at ovulation. The acute decrease in the thickness of the apical follicle wall within hours of rupture occurred simultaneously with a decrease in vessel diameter and blood flow indicative of vasoconstriction. The fact that vasoconstriction, acute thinning of the follicle wall, and rupture failed to occur in Amhr2cre/+SmoM2 mice, in which thecal vessels are deficient in VSM, supports a positive association between vasoconstriction and rupture. Previous studies using corrosion casts of the vasculature in a number of species reported a clearing of vessels at the apex of the preovulatory follicle before rupture (5, 17, 18), and light microscopy visualization in vivo revealed decreased blood flow to the apex before ovulation in rat and rabbit (19, 20). Multiphoton microscopy of fluorescently labeled vessels in the current study allowed direct testing of the relationship between vasoconstriction and follicle rupture in vivo. An initial step was to demonstrate that vasoconstriction is not simply a compensatory response to the thinning of the follicle wall; when thinning of the follicle wall and ovulation were blocked experimentally by bursal infusion of serum as a source of protease inhibitors, vasoconstriction occurred with normal timing after hCG injection. This result allowed further testing of the alternative possibility that vasoconstriction contributes to follicle rupture.
A dramatic increase in the expression of Edn2 by granulosa cells occurs within several hours before ovulation, and ovulation was suppressed by treatment of mice with EDNR antagonists (10, 11) and by global knockout of Edn2 (21). These reports suggested an important role for EDN2 in ovulation, but the mechanism had not been determined. Treatment of mice with the HIF inhibitor Ech was previously shown to block the increase in the expression of Edn2 by the preovulatory follicle and to suppress ovulation (14). Here we showed that both vasoconstriction and follicle rupture were inhibited in mice in which the preovulatory rise in Edn2 was suppressed by treatment with Ech. Our finding that bursal infusion of a vasoconstrictor, either EDN2 or ANG2, restored vasoconstriction and ovulation in Ech-treated mice indicates that vasoconstriction is critical for follicle rupture. Furthermore, inhibiting the action of endogenous EDN2 by infusing EDN2 receptor antagonists into the bursa prevented vasoconstriction and ovulation. The results suggest that the major role of EDN2 in ovulation is likely to be the induction of vasoconstriction to allow follicle rupture.
The light-scattering properties of ovarian tissue in vivo prevented the use of multiphoton microscopy to image deeply enough into the preovulatory follicle to determine whether vasoconstriction before ovulation is restricted to the apex. However, optical clearing of ovarian tissue ex vivo allowed multiphoton imaging of rhodamine dextran-filled vessels throughout the follicle. This procedure revealed decreased coverage by rhodamine dextran-filled vessels at the apex of the follicle close to the time of rupture but no change in vessel coverage at the base. Additional analysis of ovaries in vivo using Doppler ultrasound showed that blood flow changed as ovulation approached, from completely surrounding the follicle to being absent at the apex immediately before follicle rupture, a pattern that is consistent with vasoconstriction occurring only at the apex. Doppler ultrasound of ovaries in women and cattle also showed that blood flow to the apex of the follicle decreases around the time of ovulation, but blood flow to the base of the follicle remains strong (22, 23).
The mechanism whereby vasoconstriction promotes follicle rupture at ovulation must be determined. We postulate that vasoconstriction at the apex causes the localized depletion of components derived from serum that prevent the breakdown of the follicle wall; their absence thus promotes rupture. Protease inhibitors are present in serum at high concentrations (13), and their role in contributing to homeostasis in a number of tissues has been recognized. The potency of these inhibitors in the ovary is supported by our finding that thinning of the follicle wall and rupture were blocked by bursal infusion of diluted serum. The preovulatory LH surge causes increased expression of proteases in cells throughout the follicle, and these proteases are believed to contribute to the tissue remodeling critical for follicle rupture and the formation of the corpus luteum. The expression of tissue inhibitors of proteases by follicular cells also increases in response to the LH surge (1, 6, 7). Furthermore, the LH surge increases the permeability of follicular vessels (24, 25), which would promote the influx of serum protease inhibitors, some of which have relatively high MW but are present in follicular fluid (26–30). The presence of protease inhibitors may balance the activity of proteases to protect follicular tissue while allowing remodeling to form the corpus luteum. Localized, acute vasoconstriction at the apex of the preovulatory follicle may prevent access of protease inhibitors in serum, shifting the balance toward destruction of a small region at the apex and rupture to release the oocyte. Although the cause of the selective vasoconstriction at the apex of the follicle is not understood, it might be generated by the actions of vasoconstrictors on the unique vascular structure of the follicle (4, 5). Precise timing of vasoconstriction within the preovulatory follicle may be essential to release the oocyte from the apex before it becomes trapped by the luteinization process.
Knowing that vasoconstriction is essential for follicle rupture contributes to a more complete understanding of the mechanics of ovulation. This information has the potential to contribute to future developments of methods for regulating fertility. For example, the causes of a condition in women in which follicles fail to rupture and the oocyte becomes trapped in the luteinized follicle (31) could be investigated with an appreciation for the importance of vasoconstriction.
Materials and Methods
Mouse strains, surgical preparation of mice, and the multiphoton microscopy and Doppler ultrasonography methods used to image preovulatory follicles are detailed in SI Materials and Methods. Protocols for superovulation, cannulation, infusions into the ovarian bursa, collection of oocytes from the oviduct, and RT-PCR are described in SI Materials and Methods.
Mice.
Amhr2cre/+ mice, provided by Richard Behringer, M.D. Anderson Cancer Center, University of Texas, Houston (32), and GT(ROSA)26Sortm1(smo/YFP)Amc/J mice (33), purchased from The Jackson Laboratory, were mated to obtain Amhr2cre/+SmoM2 mice (mutants) and Amhr+/+SmoM2 controls. Mice were genotyped from tail DNA using protocols from The Jackson Laboratory. B6/129 mice were obtained from The Jackson Laboratory. Mice were maintained according to the NIH Guide for the Care and Use of Laboratory Animals (34), and studies were approved by the Cornell University Institutional Animal Care and Use Committee.
Statistical Analyses.
General linear mixed models were used to analyze blood flow (log 10 scale) and vessel diameter and thickness of the follicle wall (arithmetic scale) with JMP Pro 10 software. The models included fixed effects for type (mouse genotype or treatment), time of sampling, and their interaction and random effects contributed by repeated measurements of multiple vessels. Tukey’s honest significant difference test was used for pairwise post hoc tests. Gene-expression data were log transformed and analyzed by one-way ANOVA followed by post hoc comparisons with the Student–Newman–Keuls procedure. Ovulation rates were compared by Student’s t test or ANOVA. Ultrasound data were analyzed by the χ2 test.
Acknowledgments
This work was supported by NIH Grant R03 HD082536 (to S.M.Q.), by Cornell Center for Vertebrate Genomics funding (S.M.Q. and R.M.W.), and by NIH Grant P41 RR04224 (to W.R.Z.). Imaging data were acquired through the Cornell University Biotechnology Resource Center, with NIH Grants S10OD018516 and NIH S10OD016191 for the shared Zeiss LSM 880 multiphoton microscope and VisualSonics Vevo-2100 ultrasound, respectively.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512304113/-/DCSupplemental.
References
- 1.Russell DL, Robker RL. Molecular mechanisms of ovulation: Co-ordination through the cumulus complex. Hum Reprod Update. 2007;13(3):289–312. doi: 10.1093/humupd/dml062. [DOI] [PubMed] [Google Scholar]
- 2.Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod. 1994;50(2):233–238. doi: 10.1095/biolreprod50.2.233. [DOI] [PubMed] [Google Scholar]
- 3.Bassett DL. The changes in the vascular pattern of the ovary of the albino rat during the estrous cycle. Am J Anat. 1943;73(2):251–291. [Google Scholar]
- 4.Macchiarelli G, Jiang JY, Nottola SA, Sato E. Morphological patterns of angiogenesis in ovarian follicle capillary networks. A scanning electron microscopy study of corrosion cast. Microsc Res Tech. 2006;69(6):459–468. doi: 10.1002/jemt.20305. [DOI] [PubMed] [Google Scholar]
- 5.Kanzaki H, et al. Scanning electron microscopic study of rabbit ovarian follicle microvasculature using resin injection-corrosion casts. J Anat. 1982;134(Pt 4):697–704. [PMC free article] [PubMed] [Google Scholar]
- 6.Curry TE, Jr, Smith MF. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med. 2006;24(4):228–241. doi: 10.1055/s-2006-948552. [DOI] [PubMed] [Google Scholar]
- 7.Ohnishi J, Ohnishi E, Shibuya H, Takahashi T. Functions for proteinases in the ovulatory process. Biochim Biophys Acta. 2005;1751(1):95–109. doi: 10.1016/j.bbapap.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Ren Y, Cowan RG, Harman RM, Quirk SM. Dominant activation of the hedgehog signaling pathway in the ovary alters theca development and prevents ovulation. Mol Endocrinol. 2009;23(5):711–723. doi: 10.1210/me.2008-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren Y, Cowan RG, Migone FF, Quirk SM. Overactivation of hedgehog signaling alters development of the ovarian vasculature in mice. Biol Reprod. 2012;86(6):174. doi: 10.1095/biolreprod.112.099176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko C, et al. Endothelin-2 in ovarian follicle rupture. Endocrinology. 2006;147(4):1770–1779. doi: 10.1210/en.2005-1228. [DOI] [PubMed] [Google Scholar]
- 11.Palanisamy GS, et al. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol Endocrinol. 2006;20(11):2784–2795. doi: 10.1210/me.2006-0093. [DOI] [PubMed] [Google Scholar]
- 12.Migone FF, Cowan RG, Williams RM, Zipfel WR, Quirk SM. Multiphoton microscopy as a tool to study ovarian vasculature in vivo. Intravital. 2013;2(1):e24334. [Google Scholar]
- 13.Travis J, Salvesen GS. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Bagchi IC, Bagchi MK. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology. 2009;150(7):3392–3400. doi: 10.1210/en.2008-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, et al. Effects of echinomycin on endothelin-2 expression and ovulation in immature rats primed with gonadotropins. Exp Mol Med. 2012;44(10):615–621. doi: 10.3858/emm.2012.44.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ke MT, Fujimoto S, Imai T. SeeDB: A simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat Neurosci. 2013;16(8):1154–1161. doi: 10.1038/nn.3447. [DOI] [PubMed] [Google Scholar]
- 17.Shimoda K, Sato E, Tanaka T, Takeya T, Toyoda Y. Morphological differentiation of the microvasculature during follicular development, ovulation and luteinization of mouse ovaries. Dev Growth Differ. 1933;35:431–437. doi: 10.1111/j.1440-169X.1993.00431.x. [DOI] [PubMed] [Google Scholar]
- 18.Martelli A, et al. Blood vessel remodeling in pig ovarian follicles during the periovulatory period: An immunohistochemistry and SEM-corrosion casting study. Reprod Biol Endocrinol. 2009;7:72. doi: 10.1186/1477-7827-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zackrisson U, et al. Alterations of follicular microcirculation and apex structure during ovulation in the rat. Eur J Obstet Gynecol Reprod Biol. 2011;157(2):169–174. doi: 10.1016/j.ejogrb.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Dahm-Kähler P, et al. An intravital microscopy method permitting continuous long-term observations of ovulation in vivo in the rabbit. Hum Reprod. 2006;21(3):624–631. doi: 10.1093/humrep/dei394. [DOI] [PubMed] [Google Scholar]
- 21.Cacioppo JA, et al. Loss of function of endothelin-2 leads to reduced ovulation and CL formation. PLoS One. 2014;9(4):e96115. doi: 10.1371/journal.pone.0096115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brännström M, et al. Preovulatory changes of blood flow in different regions of the human follicle. Fertil Steril. 1998;69(3):435–442. doi: 10.1016/s0015-0282(97)00544-x. [DOI] [PubMed] [Google Scholar]
- 23.Acosta TJ, Hayashi KG, Ohtani M, Miyamoto A. Local changes in blood flow within the preovulatory follicle wall and early corpus luteum in cows. Reproduction. 2003;125(5):759–767. [PubMed] [Google Scholar]
- 24.Gerdes U, Gåfvels M, Bergh A, Cajander S. Localized increases in ovarian vascular permeability and leucocyte accumulation after induced ovulation in rabbits. J Reprod Fertil. 1992;95(2):539–550. doi: 10.1530/jrf.0.0950539. [DOI] [PubMed] [Google Scholar]
- 25.Mitsube K, Brännström M, Haraldsson B. Modulation of microvascular permeability in the preovulatory rat ovary by an ovulatory gonadotropin stimulus. Fertil Steril. 2013;99(3):903–909. doi: 10.1016/j.fertnstert.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Gulamali-Majid F, Ackerman S, Veeck L, Acosta A, Pleban P. Kinetic immunonephelometric determination of protein concentrations in follicular fluid. Clin Chem. 1987;33(7):1185–1189. [PubMed] [Google Scholar]
- 27.Gentry PA, et al. Human ovarian follicular fluid has functional systems for the generation and modulation of thrombin. Fertil Steril. 2000;73(4):848–854. doi: 10.1016/s0015-0282(99)00635-4. [DOI] [PubMed] [Google Scholar]
- 28.Curry TE, Jr, et al. Identification and characterization of metalloproteinase inhibitor activity in human ovarian follicular fluid. Endocrinology. 1988;123(3):1611–1618. doi: 10.1210/endo-123-3-1611. [DOI] [PubMed] [Google Scholar]
- 29.Zhu C, Woessner JF., Jr A tissue inhibitor of metalloproteinases and alpha-macroglobulins in the ovulating rat ovary: Possible regulators of collagen matrix breakdown. Biol Reprod. 1991;45(2):334–342. doi: 10.1095/biolreprod45.2.334. [DOI] [PubMed] [Google Scholar]
- 30.Curry TE, Jr, Mann JS, Estes RS, Jones PB. Alpha 2-macroglobulin and tissue inhibitor of metalloproteinases: Collagenase inhibitors in human preovulatory ovaries. Endocrinology. 1990;127(1):63–68. doi: 10.1210/endo-127-1-63. [DOI] [PubMed] [Google Scholar]
- 31.Qublan H, et al. Luteinized unruptured follicle syndrome: Incidence and recurrence rate in infertile women with unexplained infertility undergoing intrauterine insemination. Hum Reprod. 2006;21(8):2110–2113. doi: 10.1093/humrep/del113. [DOI] [PubMed] [Google Scholar]
- 32.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet. 2002;32(3):408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 33.Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18(8):937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.