Abstract
Objective:
This study was designed to compare the efficacy and safety of enteral supplementation of a prebiotic mixture (SCGOS/LCFOS) on faecal microbiota in very premature infants who fed exclusively with human-milk.
Methods:
This double-center randomized control trial was conducted from December 2012 to November 2013 in the tertiary Neonatal Intensive Care Units of the Isfahan University of Medical Sciences. Fifty preterm infants (birth weight ≤1500 g who were not fed with formula) were randomly allocated to have enteral (tube feeding) supplementation with a prebiotic mixture (SCGOS/LCFOS; 9:1) or receive no prebiotics.
Findings:
The primary outcome (e.g., the effect of the prebiotic mixture on fecal microbiota pattern) was clearly different between the two groups. Despite greater coliforms colony counts in first stool cultures in the prebiotic group (Group P) (P = 0.67), coliforms were significantly lower in the third stool cultures in the Group P (P < 0.001). Furthermore, despite the much higher Lactobacillus colony counts, in the first stool cultures, in the control group (Group C) (P = 0.005); there was a trend toward significantly increased Lactobacillus colony counts in the Group P during the study, but the difference between Lactobacillus colony counts, in the third stool cultures, between two groups was no longer statistically significant (P = 0.11). Interestingly, the median length of hospital stay was significantly less in the Group P (16 [12.50–23.50] vs. 25 [19.50–33.00] days; P = 0.003).
Conclusion:
This suggests that it might have been “the complete removal of formula” which manifests a synergistic effect between nonhuman neutral oligosaccharides (prebiotics) and human oligosaccharides, which in turn, led to the rapid growth of beneficial Lactobacillus colonies in the gut of breast milk-fed preterm infants, while decreasing the number of pathogenic coliforms microorganisms. Therefore, further studies with larger sample sizes are recommended to investigate the issue.
Keywords: Fecal flora, oligosaccharides, prebiotic, preterm infant
INTRODUCTION
Colonization of the infant's sterile intestine starts immediately after birth by bifidobacterias and Lactobacillus.[1] In general, colonization of the neonatal intestine can be divided into two categories: Harmful and beneficial bacteria, with bifidobacteria and lactobacilli acting as beneficial bacteria for the infant's gastro-intestine (GI).[2] Colonization of the sterile intestine of infants starts during normal parturition and gets in touch with mother's vagina and intestinal flora microbe which in turn, results in the transfer of flora microbes such as bifidobacteria, chlisteridia, and Gram-positive cocci.[2] This flora microbe colonization changes rapidly under the effect of the infant's diet.[2] On the other hand, some researchers found that intestinal flora is different between term and preterm infants.[3] It has been found that the bifidobacteria and lactobacilli in preterm neonates are less compared with term neonates, while, the amount of potentially pathogenic bacteria in preterm neonates is more than that in term neonates.[3] Furthermore, prescription of antibiotics in preterm infants leads to a serious delay of GI colonization with bacteria.[4] In addition, in premature infants admitted to Neonatal Intensive Care Units (NICUs), the risk of neonatal infection is higher.[3] Although frequently serious infections in preterm infants are caused by coagulase-negative staphylococci that come from external sources of infants,[3] in many cases, serious infections in newborns can be the result of internal source bacteria (endogenous), particularly the colonized harmful bacteria in the infant's GI.[5] On the other hand, it has been shown that the existence of beneficial flora in the intestines has a positive impact on the development of the infant's immune system.[6,7] Also, researchers have found that breast milk has antibacterial adhesion and immunoregulatory effects on the infant's immune system.[3,8] These anti-adhesive effects are caused by the direct result of some oligosaccharides in the mother's milk, which sit on the receptor stations of microbes and prevent pathogens from binding to the epithelial cell walls of the infant's GI. It appears that the immuno-modulatory effects occur due to bifidogenic properties of human milk on the flora microbe of infant's GI.[8] High levels of oligosaccharides in human milk cause this bifidogenic property.[8] Also, it been seen that nonhuman milk oligosaccharides such as galacto-oligosaccharides (short chain) and fructooligosaccharides (long chain) have this effect.[9] In a series of studies on preterm and term infants, it has been found that the amount of beneficial flora can be increased by adding oligosaccharides, which also reduces the amount of pathogenic flora.[6,7,8] Furthermore, reduction in GI pH and an increment in the production of short-chain fatty acids (SCFAs) are caused by these oligosaccharides. Similarly, in one in vitro study, it was shown that SCFAs stimulate mucin-2 generation and increase intestinal integrity.[10] Some researchers have found that adding oligosaccharides to the infant's diet reduces the incidence of infections and atopy in them.[6,11] Furthermore, with a series of other studies, scholars observed that there was a trend toward decreased pathogenic bacteria counts in infants who were given prebiotic supplements.[12,13,14,15,16,17,18,19] Finally, in a recent study, with prebiotic enteral supplementation, Westerbeek et al. found a trend toward higher bifidobacteria counts.[20]
Based on the above and due to the importance of improving the health status of preterm infants also, since the existence of formula in previous studies may have had a negative effect, as a confounding factor, on intestinal flora; the present researchers decided to investigate their hypothesis; i.e., the complete removal of formula, in addition to eliminating the potentially harmful effects of formula, will cause a synergistic effect between nonhuman neutral oligosaccharides (prebiotic) and breast milk oligosaccharides; which has more favorable effects on fecal microbiota, particularly in very premature infants (infants with weight <1500 g [i.e., very low birth weight (VLBW)]).
METHODS
This double-center randomized control trial (RCT) was conducted from December 2012 to November 2013 in the tertiary NICUs (Alzahra and Shahid Beheshti Hospital NICUs; general and maternity hospitals, respectively) of the Isfahan University of Medical Sciences. Preterm neonates were favorable for participation if BW ≤1500 g and if they were not fed with formula. To ensure tolerate milk initially, when the volume of breast milk reached 30 ml/kg/day, VLBW infants were enrolled in the RCT study. Exclusion criteria were asphyxia, major congenital anomalies, GI system anomalies, proven sepsis or infection immediately before the start of the study and feeding with formula.
The effects of enteral supplementation with galacto-oligosaccharides (short chain) and fructooligosaccharides (long chain) (SCGOS/LCFOS) mixture on fecal microbiota in premature infants were investigated in two groups of prebiotic (Group P) and control (Group C) groups. The neonates in the study were randomly assigned to have their feedings supplemented with either prebiotics (SCGOS/LCFOS mixture) Group P or placebo Group C. Randomization was performed 1:1 using a computer-generated randomization list prepared by an independent statistician not involved in the rest of the investigation. SCGOS/LCFOS mixture was prepared, sterilized and sent to us by nutricia MMP (Mashhad, Iran). Then several samples of SCGOS/LCFOS mixture were analyzed by a food processing industry specialist.
In both groups, after considering the inclusion and exclusion criteria, infants were entered into the study. In the Group P, 0.5 g/kg/day of SCGOS/LCFOS mixture was started, and maintained until the milk volume reached 70 ml/kg/day. When the milk volume reached 70–110 ml/kg/day, the dosage of SCGOS/LCFOS mixture was increased to 1 g/kg/day and at the milk volume of 110–150 ml/kg/day, the SCGOS/LCFOS mixture was increased to 1.5 g/kg/day, then the mixture was added to the infants’ diet for one-two additional days. Infants in Group C were received an equivalent volume of distilled water in their milk during the days of intervention. SCGOS/LCFOS and distilled water were prepared in similar syringes by a trained nurse who was blind to the group's assignments. Syringes were numbered for each infant entering the study by her then the investigator, who was blind to the numbering, allocated numbers to infants in the sequence of entry into the study. Three times; when the volume of milk reached approximately 30 (time 1), 70 (time 2) and 150 ml/kg/day (time 3), stool cultures were sent for an investigation of fecal microbiota (de Man Rogosa Sharpe [MRS] and Eosin Methylene Blue [EMB]). The infants were given parenteral nutritional support during the increase of milk volumes.
In both groups, infants were initially fed with 20 cc/kg/day on the day that the attending neonatologist decided to initiate the enteral feedings (tube feeding). Then on the next day, feeding volumes were increased to 40 cc/kg/day; on the 3rd day of the study, volumes were increased to 60 cc/kg/day, and so forth, until a volume of 150 cc/kg/day was achieved. To ensure tolerate milk initially, in both groups, the infants were entered in the study when the milk volume reached 30 cc/kg/day. Parenteral nutrition was gradually tapered as enteral feeding volumes were increased. Human milk fortifier was begun at volume milk of 150 cc/kg/day.
Stool samples were collected from the neonates in both Groups P and C at three sampling stages; on the 1st day of study and on the last day of each study interval in which increasing prebiotic concentration was included in the neonate's diet. The samples were kept refrigerated in thigh plastic containers for <6 h until they were transferred to the laboratory, where they were examined as soon as possible.
MRS agar (Merck, Germany, pH = 5.7) added with 0.5% cysteine hydrochloride (sigma, USA), and EMB agar were used for enumeration of Lactobacillus colonies and coliforms respectively.
Each fecal sample (about 0.5 g) was accurately weighed in a sterilized tube, thoroughly mixed with 5 ml sterile normal saline, and centrifuged for 5 min at 100 rpm. One milliliter of the upper phase was serially diluted 10-fold to 10−7 dilution.
One hundred microliters of the proper dilution were surface-cultured on the both types of plates. MRS plates were incubated anaerobically in an anaerobic jar using CO2 generating gas pack A (Merck, Germany) and were kept at 37–38°C for 48 h. EMB plates were incubated aerobically for 24 h in at the same temperature. Colony counting was done by expert eyes and expressed as a logarithm (log) of the colony-forming units (CFUs) per gram of fresh feces.
The primary outcome of the study in both groups was the effect of SCGOS/LCFOS mixture on fecal microbiota pattern. Secondary outcomes were the duration of dependency to oxygen, incidence of chronic lung disease (CLD) (defined as oxygen dependency at 28 days of life),[21] duration of hospitalization and death for each neonate, which were recorded daily. Gestational ages were assessed by prenatal ultrasonography.
This paper is derived from the residency thesis No. 392237 in Isfahan University of Medical Sciences. The study was approved by the Regional Ethical Review Board at the university. Written informed consents were obtained from the parents before the intervention. This trial was registered at www.irct.ir as IRCT2013090710026N2.
The sample size of 50 infants was based on the sample size design for the primary outcome of a previous study (the effect of SCGOS/LCFOS on stool bacterial flora in preterm infants).[15] The sample size was adjusted according to the opinion of a statistical consultant and based on this formula: 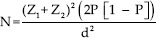 . “Z1” is confidence interval of 95% or 1.96. “Z2” is the power of the test that was 0/80 or 0/84. “P” is an estimate of the frequency of incidence of each the factors in both groups, in which 0.5 was considered regarding the variations in each group. “d” is the minimum difference of incidence of each variable between the two groups which shows statistically significant difference as 0.8P was considered. Normally distributed and nonparametric data were presented as means (± standard deviation [SD]) and median (range), respectively. The numeric variables were compared using the independent t-test (for parametric) and Mann–Whitney U-test (for nonparametric). To examine the effect of the intervention on quality variables in the areas of analysis, the researchers used the Kaplan–Meier with log-rank test and the calculation of hazard ratio (HR).
. “Z1” is confidence interval of 95% or 1.96. “Z2” is the power of the test that was 0/80 or 0/84. “P” is an estimate of the frequency of incidence of each the factors in both groups, in which 0.5 was considered regarding the variations in each group. “d” is the minimum difference of incidence of each variable between the two groups which shows statistically significant difference as 0.8P was considered. Normally distributed and nonparametric data were presented as means (± standard deviation [SD]) and median (range), respectively. The numeric variables were compared using the independent t-test (for parametric) and Mann–Whitney U-test (for nonparametric). To examine the effect of the intervention on quality variables in the areas of analysis, the researchers used the Kaplan–Meier with log-rank test and the calculation of hazard ratio (HR).
To study the changes of MRS and EMB in each group and compare the two groups, the repeated measures ANOVA test was used. To perform this procedure, the sphericity default was evaluated by Machel test. In order to rule out of this default, and to decide about the effect of time and group interaction, multivariate methods were used. P < 0.05 was considered statistically significant. The data were analyzed using the SPSS statistical software version 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
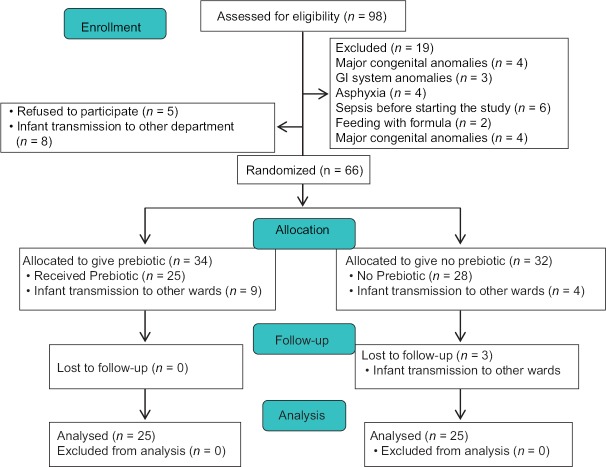
Throughout the trial, a total of 98 infants with a BW <1500 g were evaluated for eligibility to participate. Nineteen infants were excluded because of major congenital anomalies, GI system anomalies, asphyxia, and sepsis before starting the study [Figure 1]. There were a total of 79 infants who met the eligibility criteria, and 13 infants who refused to participate or were transmitted to other wards. Of the 66 enrolled neonates, 34 were allocated to the Group P and 32 to the Group C. Respectively, nine and seven neonates were transmitted to other wards from Groups P and C [Figure 1].
Figure 1.
Flowchart of the participants
In our study, 50 neonates were randomized and completed the study [Figure 1]. Differences in demographic characteristics were not statistically important [Table 1].
Table 1.
Basic and clinical characteristics of study infants*
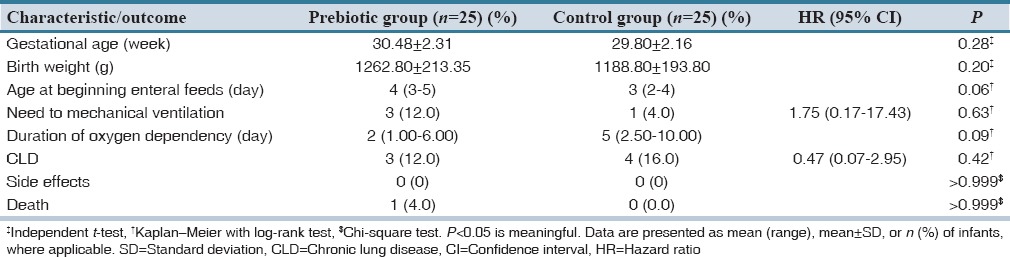
Average gestational ages in Groups P and C were 30.48 ± 2.31 and 29.80 ± 2.16, respectively [P = 0.28, Table 1]. Average BW in Group P was 1262.80 ± 213.35 g. and in Group C, 1188.80 ± 193.80 [P = 0.20, Table 1]. The median age at the start of feeding was 4 (3–5) and 3 (2–4) days in Groups P and C respectively [P = 0.06; Table 1]. The requirement for MV, usually in the 1st days after birth, in the Group P was slightly lower, compared to the Group C although this was not statistically meaningful; (1 [4%] vs. 3 [12%]; HR: 1.75, 95% confidence interval [95% CI]: 0.17–17.43; P = 0.63; Table 1).
Adverse events such as diarrhea, constipation, or weight loss were not observed (P > 0.999). Prebiotic oligosaccharides were safe and well tolerated by VLBW infants.
The primary outcome was clearly different between the two groups. Prebiotic oligosaccharides led to rapid growth of beneficial Lactobacillus colonies in the gut of breast milk-fed preterm infants while decreasing the numbers of pathogenic coliforms microorganisms. Average log of the CFUs per gram for coliforms on EMB agar in the Group P were 7.24 ± 2.87, 8.55 ± 1.07 and 3.09 ± 0.89 in the first, second and third stool cultures, respectively [Table 2]. Also, figures in the Group C were 6.84 ± 3.29, 8.99 ± 1.30 and 9.29 ± 1.08, respectively [Table 2].
Table 2.
Primary outcomes*
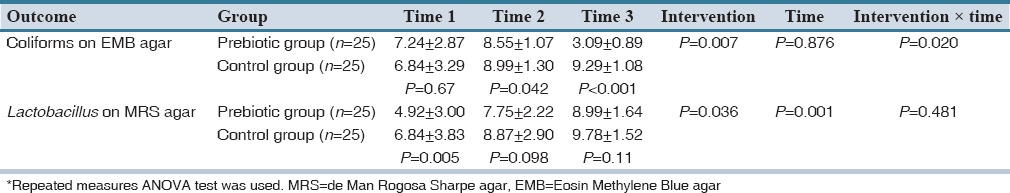
Therefore, we observed that, despite greater mean ± SD coliforms colony counts in first stool cultures in Group P [P = 0.67, Table 2 and Figure 2], mean ± SD coliforms colony counts were greater in second stool cultures in the Group C [P = 0.042, Table 2 and Figure 2]. Then, interestingly; mean ± SD coliforms colony counts were significantly lower in the third stool cultures in the Group P [P < 0.001, Table 2 and Figure 2].
Figure 2.
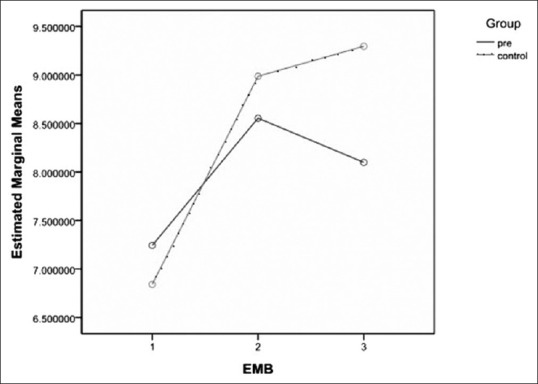
Estimated marginal means logarithm of the colony-forming unit per gram of coliforms colony counts on Eosin Methylene Blue agar
Average log of the CFU per gram for Lactobacillus on MRS agar in the Group P were 4.92 ± 3.00, 7.75 ± 2.22 and 8.99 ± 1.64 in the first, second and third stool cultures, respectively [Table 2]. In the Group C, the figures were 6.84 ± 3.83, 8.87 ± 2.90, and 9.78 ± 1.52, respectively [Table 2]. Apparently, notwithstanding the much higher mean ± SD Lactobacillus colony counts, in the first stool cultures, in the Group C [P = 0.005; Table 2, Figures 3 and 4]; there was a trend toward significant increased mean ± SD Lactobacillus colony counts in the Group P during the study, so that the difference between mean ± SD Lactobacillus colony counts, in the third stool cultures, in the two groups was no longer statistically significant [P = 0.11, Table 2, Figures 3 and 4].
Figure 3.
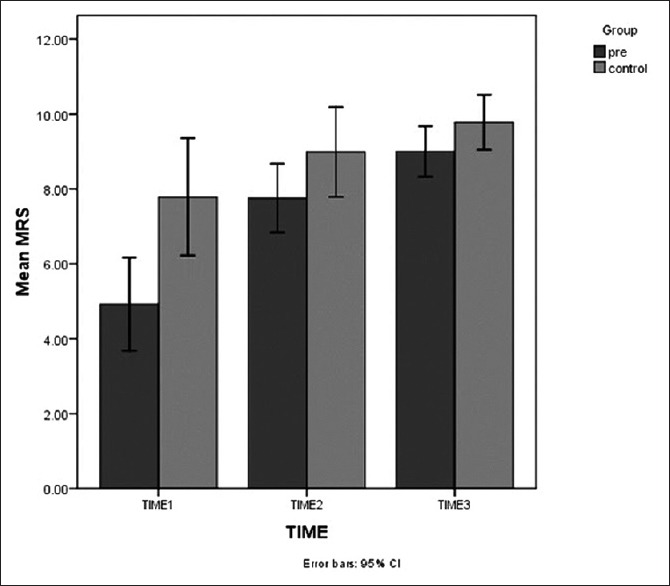
Means of Lactobacillus colony counts on de Man Rogosa Sharpe agar during the study
Figure 4.
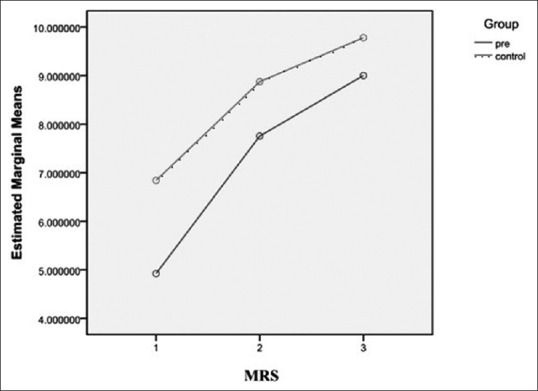
Estimated marginal means logarithm of the colony-forming unit per gram of Lactobacillus colony counts on de Man Rogosa Sharpe agar
Secondary outcomes were also investigated in our study. The median length of hospital stay was significantly less in the Group P versus Group C [16 (12.50–23.50) vs. 25 (19.50–33.00) days; P = 0.003; Table 1]. We observed that the median duration of oxygen dependency was meaninglessly shorter in Group P versus Group C (2 [1.00–6.00] vs. 5 [2.50–10.00] days, respectively) [P = 0.09; Table 1]. Also, the incidence of CLD was 3 (12.0%) versus 4 (16.0%) [HR: 0.47, 95% CI: 0.07–2.95; P = 0.42; Table 1]. The mortality rate was very low, with only one neonate dead in Group P [P > 0.999; Table 1].
DISCUSSION
Among VLBW infants, we observed that prebiotic neutral oligosaccharides (SCGOS/LCFOS mixture) led to rapid growth of beneficial Lactobacillus colonies in the gut of breast milk-fed preterm infants while decreasing the number of pathogenic coliforms microorganisms. Our findings in these aspects were similar to those described by previous researchers.[15,16,17] Interestingly, despite the presence of only breast milk and its oligosaccharides; and complete removal of formula, in our study, still the beneficial effects of nonhuman milk oligosaccharides in other previous studies were found on increasing the growth of beneficial bacteria and reducing that of harmful bacteria.
Boehm et al. observed that infants who received oligosaccharide supplemented formula had significantly high colony counts of bifidobacteria (P = 0.0008). However, colony counts of pathogenic microorganism were similar in the two groups.[15] Kapiki et al. reported that the number of colony counts of fecal beneficial bacteria was higher in the group fed fructooligosaccharides than the control bottle-fed infants (P = 0.032). There was also a meaningful lower number of enterococci and Escherichia coli in the Group P (P = 0.025 and P = 0.029, respectively)[16] which our results are in line with. Also by Costalos et al. noted a significant reduction of the percentage of clostridia in bottle-fed infants who received formula supplemented with prebiotic (P = 0.42).[17] Modi et al.[18] and Riskin et al.[19] observed lower colony counts of pathogenic bacteria in infants who received prebiotic supplementation. However, no statistical significance was observed in either of the studies.
Westerbeek et al. reported that, in both groups they studied, the fecal bacterial colonization increased in the study (Exp. 35.41; 95% CI: 15.82–79.24, P < 0.001). Furthermore, they observed that intestinal colonization of specific bacterial groups was not affected by prebiotic supplementation; although there was meaninglessness increasing trend of bifidobacteria counts.[20]
The differentiations in the growth of fecal bacteria in previous studies could be described by different combinations and doses of prebiotics used. Riskin et al.[19] used 1% lactulose and observed a preferential intestinal proliferation of lactobacilli, and Kapiki et al.[16] used a fructooligosaccharide-supplemented formula and reported increase in the growth of fecal beneficial bacteria, while other researchers, such as Knol et al.,[22] used a combination of short chain GOS and long chain FOS and demonstrated an increase in the growth of bifidobacteria and Lactobacillus. On the other hand, Moro et al. observed that growth of fecal beneficial bacteria followed by prebiotic supplementation was dose-dependent.[23]
Interestingly, in other studies, the presence of formula was serious or semi-serious and so, as a confounding factor may have had a negative effect on the results. Fortunately, we were able to convince the majority of mothers to feed their babies with their own milk. Therefore, due to formula feeding, few infants were excluded from our study.
The present study showed that despite higher levels of pathogenic bacteria in the first stool cultures in Group P, the growth of these pathogenic bacteria in the last stool cultures in the group were significantly lower, and despite the significantly higher level of beneficial bacteria in first stool cultures in the Group C, after several days of receiving SCGOS/LCFOS mixture, proliferation of beneficial bacteria increased significantly in the Group P, so that the growth of these beneficial bacteria in the last stool cultures was not statistically significant in the two groups.
We think that perhaps the complete removal of formula, and consequently, elimination of its inherent adverse characteristics, caused a synergistic effect between nonhuman neutral oligosaccharides (prebiotic) and human oligosaccharides and clear effectiveness in the results.
Moro et al. found that “the degree of prebiotic response is also dependent on baseline colony counts of bifidobacteria,”[23,24] while in our study, despite the unexpected colony counts of beneficial/pathogenic bacteria in the first stool cultures, favorable results of growth of beneficial/pathogenic bacteria in the last stool cultures was obtained.
Interestingly in contrast to other studies, and probably because of the complete removal of formula in this present study, the duration of hospitalization was more favorable in the Group P [P = 0.003; Table 1]. However Riskin et al.[19] observed that the mean ± SD age at discharge from hospital for the prebiotic versus the Group C was of 52.5 ± 41.8 versus 71.6 ± 52.4 days, (P > 0.05), and also Westerbeek et al.[3] reported that the median length of hospitalization was 52 (30–111) and 54 (30–181) (P = 0.69), where, in this respect, no difference was found between the two groups in their studies.
In our study, the duration of oxygen dependency, during the study period, was similar in both groups (P = 0.09). This is in line with the result of a study by Riskin et al., who observed that the duration of oxygen dependency was 25.8 ± 36.4 and 29.5 ± 36.6 days in the Groups P and C, respectively (P > 0.05).[19]
In one study,[3] use of prebiotics has been associated with a reduction in the incidence of CLD while we did not find a significant difference between the two study groups.
The present study suffers from a number of limitations. The major limitation of the study could be the rather small number of the infants included (50 premature neonates), even though the results clearly indicated a significant difference between the Groups P and C. Furthermore, the timely submission of stool samples was difficult because infants did not pass stool when we had planned. The strengths of the study include the RCT design in high-risk neonates (i.e., VLBW infants), the complete removal of formula and very good cooperation with professionals from other disciplines involved in the study.
The present study suggests that it might have been the complete removal of formula which manifests a synergistic effect between nonhuman neutral oligosaccharides (prebiotics) and human-milk-oligosaccharides, which, in turn, led to rapid growth of beneficial Lactobacillus colonies in the gut of breast milk-fed preterm infants, while decreasing the number of pathogenic coliforms microorganisms. Therefore, further studies with larger sample sizes are recommended to investigate the issue.
AUTHORS’ CONTRIBUTION
Amir-Mohammad Armanian: Dr. Armanian conceptualized, designed and supervised the study, drafted the initial manuscript, and approved the final manuscript as submitted.
Ali-Reza Sadeghnia: Dr. Sadeghnia designed and supervised the study and reviewed and revised the manuscript, and approved the final manuscript as submitted.
Maryam Hoseinzadeh: Samples were collected by Dr. Hoseinzadeh and she reviewed the manuscript, and approved the final manuscript as submitted.
Maryam Mirlohi: Dr. Mirlohi designed and supervised the study and reviewed and revised the manuscript, and approved the final manuscript as submitted.
Awat Feizi: Dr. Feizi received the results of the study and provided statistical analysis of our results with appropriate statistical methods, and approved the final manuscript as submitted.
Nima Salehimehr: Dr. Salehimehr helped in study design, initial manuscript draft, and approved the final manuscript as submitted.
Moloud Torkan: Ms. Torkan designed the data collection instruments, and coordinated and supervised data collection at one of the four sites, critically reviewed the manuscript, and approved the final manuscript as submitted.
Zahra Shirani: Ms. Shirani designed the data collection instruments, and coordinated and supervised data collection at one of the four sites, critically reviewed the manuscript, and approved the final manuscript as submitted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rao S, Srinivasjois R, Patole S. Prebiotic supplementation in full-term neonates: a systematic review of randomized controlled trials. Arch Pediatr Adolesc Med. 2009;163:755–64. doi: 10.1001/archpediatrics.2009.94. [DOI] [PubMed] [Google Scholar]
- 2.Thomas DW, Greer FR. Probiotics and prebiotics in pediatrics. Pediatrics. 2010;126:1217–31. doi: 10.1542/peds.2010-2548. [DOI] [PubMed] [Google Scholar]
- 3.Westerbeek EA, van den Berg JP, Lafeber HN, Fetter WP, Boehm G, Twisk JW, et al. Neutral and acidic oligosaccharides in preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2010;91:679–86. doi: 10.3945/ajcn.2009.28625. [DOI] [PubMed] [Google Scholar]
- 4.Westerbeek EA, van den Berg A, Lafeber HN, Knol J, Fetter WP, van Elburg RM. The intestinal bacterial colonisation in preterm infants: a review of the literature. Clin Nutr. 2006;25:361–8. doi: 10.1016/j.clnu.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Van der Zwet WC, Kaiser AM, van Elburg RM, Berkhof J, Fetter WP, Parlevliet GA, et al. Nosocomial infections in a Dutch neonatal intensive care unit: Surveillance study with definitions for infection specifically adapted for neonates. J Hosp Infect. 2005;61:300–11. doi: 10.1016/j.jhin.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Field CJ. The immunological components of human milk and their effect on immune development in infants. J Nutr. 2005;135:1–4. doi: 10.1093/jn/135.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Vos AP, M’Rabet L, Stahl B, Boehm G, Garssen J. Immune-modulatory effects and potential working mechanisms of orally applied nondigestible carbohydrates. Crit Rev Immunol. 2007;27:97–140. doi: 10.1615/critrevimmunol.v27.i2.10. [DOI] [PubMed] [Google Scholar]
- 8.Bode L. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J Nutr. 2006;136:2127–30. doi: 10.1093/jn/136.8.2127. [DOI] [PubMed] [Google Scholar]
- 9.Boehm G, Stahl B, Mattila-Sandholm T, Saarela M. Oligosaccharides. Functional Dairy Products. 2003:203–43. ISBN 1-85573-584-9. [Google Scholar]
- 10.Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52:1442–7. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arslanoglu S, Moro G, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138:1091. doi: 10.1093/jn/138.6.1091. [DOI] [PubMed] [Google Scholar]
- 12.Fanaro S, Boehm G, Garssen J, Knol J, Mosca F, Stahl B, et al. Galacto-oligosaccharides and long-chain fructo-oligosaccharides as prebiotics in infant formulas: a review. Acta Paediatr Suppl. 2005;94:22–6. doi: 10.1111/j.1651-2227.2005.tb02150.x. [DOI] [PubMed] [Google Scholar]
- 13.Boehm G, Jelinek J, Stahl B, van Laere K, Knol J, Fanaro S, et al. Prebiotics in infant formulas. J Clin Gastroenterol. 2004;38(6 Suppl):S76–9. doi: 10.1097/01.mcg.0000128927.91414.93. [DOI] [PubMed] [Google Scholar]
- 14.Bruzzese E, Volpicelli M, Squeglia V, Bruzzese D, Salvini F, Bisceglia M, et al. A formula containing galacto-and fructo-oligosaccharides prevents intestinal and extra-intestinal infections: An observational study. Clin Nutr (Edinb, Scotl) 2009;28:156. doi: 10.1016/j.clnu.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Boehm G, Lidestri M, Casetta P, Jelinek J, Negretti F, Stahl B, et al. Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2002;86:F178–81. doi: 10.1136/fn.86.3.F178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapiki A, Costalos C, Oikonomidou C, Triantafyllidou A, Loukatou E, Pertrohilou V. The effect of a fructo-oligosaccharide supplemented formula on gut flora of preterm infants. Early Hum Dev. 2007;83:335–9. doi: 10.1016/j.earlhumdev.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Costalos C, Kapiki A, Apostolou M, Papathoma E. The effect of a prebiotic supplemented formula on growth and stool microbiology of term infants. Early Hum Dev. 2008;84:45–9. doi: 10.1016/j.earlhumdev.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Modi N, Uthaya S, Fell J, Kulinskaya E. A randomized, double-blind, controlled trial of the effect of prebiotic oligosaccharides on enteral tolerance in preterm infants (ISRCTN77444690) Pediatr Res. 2010;68:440–5. doi: 10.1203/PDR.0b013e3181f1cd59. [DOI] [PubMed] [Google Scholar]
- 19.Riskin A, Hochwald O, Bader D, Srugo I, Naftali G, Kugelman A, et al. The effects of lactulose supplementation to enteral feedings in premature infants: a pilot study. J Pediatr. 2010;156:209–14. doi: 10.1016/j.jpeds.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Westerbeek EA, Slump RA, Lafeber HN, Knol J, Georgi G, Fetter WP, et al. The effect of enteral supplementation of specific neutral and acidic oligosaccharides on the faecal microbiota and intestinal microenvironment in preterm infants. Eur J Clin Microbiol Infect Dis. 2013;32:269–76. doi: 10.1007/s10096-012-1739-y. [DOI] [PubMed] [Google Scholar]
- 21.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 22.Knol J, Boehm G, Lidestri M, Negretti F, Jelinek J, Agosti M, et al. Increase of faecal bifidobacteria due to dietary oligosaccharides induces a reduction of clinically relevant pathogen germs in the faeces of formula-fed preterm infants. Acta Paediatr Suppl. 2005;94:31–3. doi: 10.1111/j.1651-2227.2005.tb02152.x. [DOI] [PubMed] [Google Scholar]
- 23.Moro G, Minoli I, Mosca M, Fanaro S, Jelinek J, Stahl B, et al. Dosage-related bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants. J Pediatr Gastroenterol Nutr. 2002;34:291–5. doi: 10.1097/00005176-200203000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasjois R, Rao S, Patole S. Prebiotic supplementation in preterm neonates: Updated systematic review and meta-analysis of randomised controlled trials. Clin Nutr. 2013;32:958–65. doi: 10.1016/j.clnu.2013.05.009. [DOI] [PubMed] [Google Scholar]