Abstract
Objective:
Opportunistic infections like cytomegalovirus (CMV) are among the primary causes of morbidity and mortality in patients undergoing hematipoetic stem cell transplantation (HSCT). This infection is frequently seen in early postengraftment period. So we determined to find the risk factors associated with CMV reactivation.
Methods:
We retrospectively evaluated the medical records of 126 consecutive patients who underwent allogenic-HSCT from peripheral blood stem cells from August 2011 to February 2013 in Shariati Hospital. We included HSCT patients with 15 years of age or older, who survived at least 100 days after transplantation. CMV reactivation was detected based on the weekly PP65 assessment. Patients with 10 or more positive cells per 50,000 cells were defined as having high-level antigenemia.
Findings:
From 126 patients which included in this study, 76 were male (60%). CMV antigenemia was documented in 43 patients (34%). The median time to CMV infection was 40 days (range: 3–77) after transplantation. The incidence of high-level antigenemia during the first 100 days following HSCT was 11%.
Conclusion:
We found that the significant risk factor for CMV antigenemia in multivariate analysis was prior graft-versus-host disease (GVHD) experience and higher donor age. For high-level antigenemia, GVHD or duration of its treatment was significant determinant.
Keywords: Allogeneic hematopoietic stem cell transplantation, cytomegalovirus infection, graft-versus-host disease
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a treatment option for a variety of hematologic and nonhematologic as well as the bone marrow failure disorders.[1]
Opportunistic infections are among the primary causes of morbidity and mortality in patients undergoing hematipoetic stem cell transplantation (HSCT).[2] Among them, infections caused by viruses are one of the important complications in these patients.[3] As an example, reactivation of human cytomegalovirus (CMV), one of the herpes virus family,[4] which occurs in patients with impaired cell-mediated immune response, is commonly seen following HSCT.[5] Especially, this infection is frequently seen in early post-engraftment period[2] and is still responsible for a majority of morbidities and mortalities in these patients.[6,7] However, it should be noted that late CMV infections has increased as the results of prophylaxis or preemptive therapy with ganciclovir.[8] When CMV is reactivated, the most severe complication is CMV pneumonitis,[9,10] while CMV gastrointestinal (GI) disease is the most common manifestation.[11]
Several risk factors have been determined for CMV. For example, the degree of immunosuppression and its duration were found to be associated with CMV[6] and increases the incidence of this infection.[12] CMV infection is also related to the serostatus of the patients and the donors,[12,13] conditioning regimen,[13] use of T-cell depletion[13,14] and the presence of acute graft-versus-host disease (GVHD).[15]
Currently, it is acceptable to monitor CMV pp65 antigen serially in peripheral blood and initiate preemptive therapy accordingly in positive cases.[13] This approach, places patients at risk under close monitoring for subclinical CMV infection while they are in critical period.[16] The aim of the present study was to assess the frequency of CMV reactivation in patients undergoing allo-HSCT. We also aimed to evaluate the role of different pretransplant and posttransplant factors on CMV infection in a series of allo-HSCT recipients.
METHODS
We retrospectively evaluated medical records of 126 consecutive patients that had received allo-HSCT from August 2011 to February 2013. Patients with 15 years of age or older, who received allo-HSCT from peripheral blood stem cells and survived at least 100 days after transplantation were included in this study. The donor and the recipients of the HSCT were matched related or unrelated donors; except for four patients who received haploidentical transplantation. In this study, all of the patients who survived at least 100 days following HSCT were included. Patients’ documents were evaluated until the 100th day after HSCT (inpatients’ charts and outpatients’ charts of follow-up visits in the clinic were reviewed). However, the duration of the hospitalization was different among patients.
Patients were hospitalized in single rooms and received the same care regarding nutritional support during hospitalization. Anti-infective prophylaxis generally consisted of acyclovir, cotrimoxazole and fluconazole for prevention of Herpes Zoster, Pneumocystis carinii and Candida infection respectively. Patients received blood and platelet transfusion based on their laboratory findings and their condition.
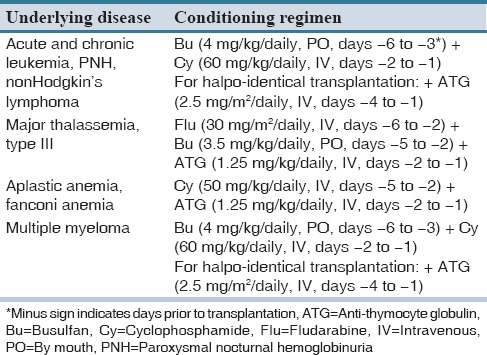
All of the patients received similar medications for the prophylaxis against GVHD, which consisted of low dose methotrexate and cyclosporine. In cases of GVHD development, the treatment included methylprednisolone 1 mg/kg intravenously. In steroid refractory cases, antithymocyte globulin (ATG) was administered. GVHD severity was also graded based on Glucksberg grading of acute GVHD.[17] Patients received different conditioning regimen based on the underlying disease as presented in Table 1.
Table 1.
Conditioning regimen based on underlying diseases
Donors and recipients CMV serostatus were documented before HSCT. Additionally, patients’ peripheral blood samples were tested for CMV antigen pp65 weekly until the 100 days after HSCT (+100). All the samples were assayed using electrochemiluminescence.
CMV antigenemia was defined as the presence of ≥1 positive cells per 50,000 leukocytes examined. Additionally, we categorized antigenemia as high-level with ≥10 positive cells per 50 000 cells and low-level with <10 positive cells.[18]
However, based on the institutional protocols the initiation of the treatment in this center was considered for patients with (1) ≥5 positive cells per 50,000 cells; (2) clinical presentation suggestive of CMV; (3) ≥1 positive cells per 50,000 cells with GVHD.
In these cases, patients received ganciclovir intravenously for the treatment. Patients’ demographic data, as well as medications and GVHD severity, were recorded.
Cox proportional hazard regression was used to identify the risk factors associated with CMV infection rate in the univariate and multivariate analyses. In the multivariate analysis, factors related to each other were not entered into the model simultaneously. Cumulative incidence of GVHD and CMV infection was estimated by Kaplan–Meier method. For the assessment of the incidence of GVHD on CMV infection, factors analyzed as the time-dependent covariates in the Cox model.
RESULTS
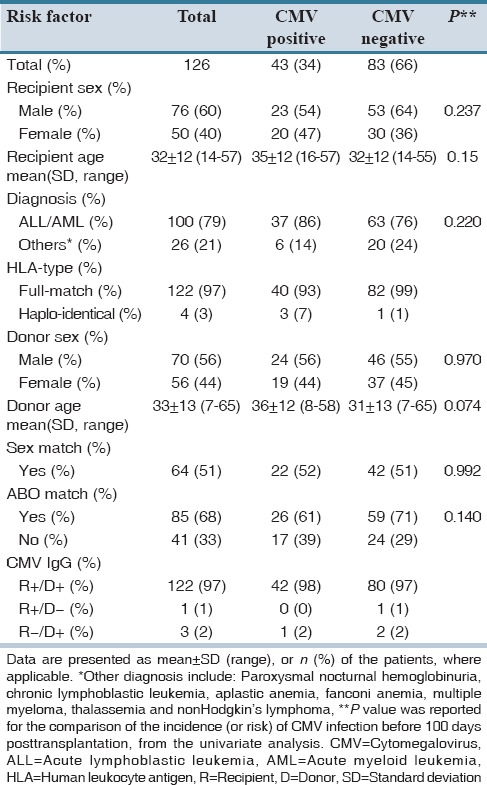
From the total of 126 patients included in this study, 76 (60%) were male. Patients, donors and transplantation characteristics are presented in Table 2. The majority of patients suffered from acute leukemia (80%). Other underlying diseases were as follows: Paroxysmal nocturnal hemoglobinuria (n = 2, 1.5%), chronic lymphoblastic leukemia (n = 1, 1%), aplastic anemia (n = 9, 7%), fanconi anemia (n = 3, 2%), multiple myeloma (n = 7, 5%), thalassemia (n = 2, 1.5%) and non-Hodgkin's lymphoma (n = 2, 1.5%). Most of the patients (97%) received full human leukocyte antigen-matched HSCT and only 4 (3%) patients received haplotype transplantation.
Table 2.
Patients and donors characteristics
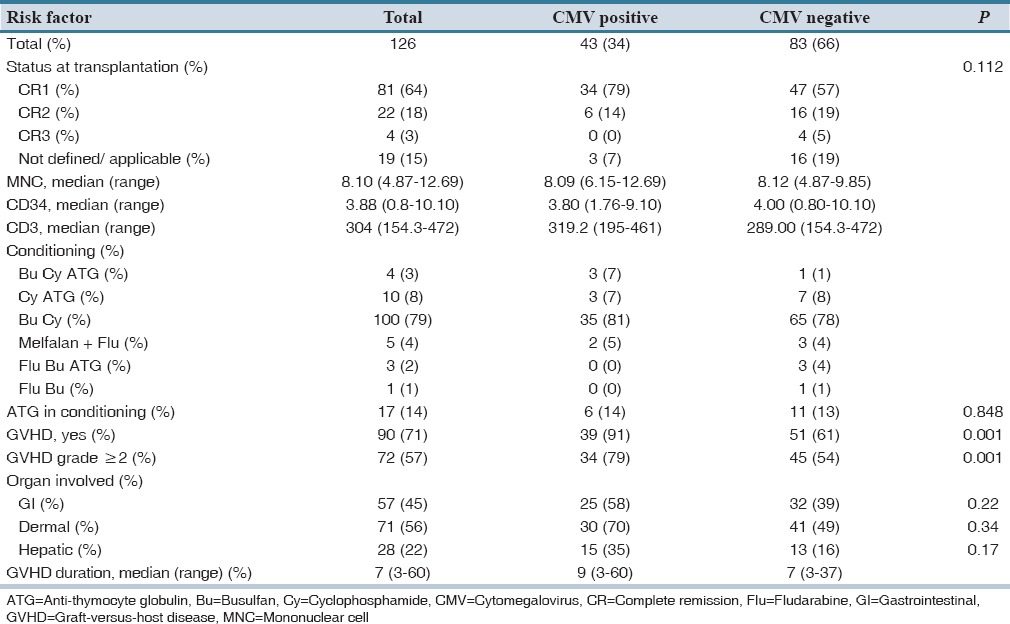
Ninety patients in our study experienced acute GVHD [Table 3]. The incidence of acute GVHD was 71% (95% confidence interval [CI]: 63–79). Moreover, the incidence of GVHD with grade II-IV was 57% (95% CI: 48–66). In patients who experienced GVHD, the median time to the GVHD initiation was 12 days (range: 7–93). Among patients with GVHD, 39 patients were CMV seropositive which consisted of 11 patients with high-level antigenemia. It should be noted that in 5 patients (two patients with high-level antigenemia) GVHD was diagnosed after detection of CMV antigenemia. However, due to the limited number of patients in this category the role of CMV infection in GVHD development could not be assessed.
Table 3.
Transplantation characteristics and intermediate outcomes before CMV infection
CMV antigenemia was documented in 43 patients (34%) at the end of the study follow-up. In these patients, the median time to CMV infection was 40 days (range: 3–77) after transplantation. By the days +30, +40, and +60 posttransplantation, 8% (standard error [SE] =0.02), 17% (SE = 0.03) and 31% (SE = 0.04) of patients were detected to be CMV positive respectively. The cumulative incidence of CMV infection is shown in Figure 1. All patients with high-level antigenemia received treatment with ganciclovir. However, ten patients with CMV positive state did not receive treatment based on institutionalized protocols. We evaluated the impact of different characteristics of patients, donors and transplantation on the incidence of CMV in a univariate analysis.
Figure 1.
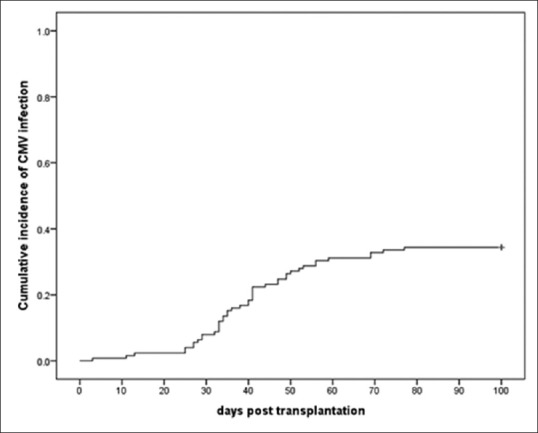
The cumulative incidence of cytomegalovirus infection. CMV: Cytomegalovirus
We categorized the underlying diseases which led to the transplantation as malignant (111 patients) or nonmalignant (15 patients). Incidence of CMV infection in both groups (36% in patients with malignant disease vs. 20% in patients with nonmalignant disease) was not significantly different (P = 0.22). Comparison of patients infected with CMV among those diagnosed as acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) (8/30 patients in ALL vs. 29/70 patients with AML) did not show a significant association (P = 0.17) for CMV reactivation.
Mean duration of hospital stay among patients with and without CMV infection was 17.3 ± 3.0 days and 17.7 ± 2.7 days, respectively.
In the univariate analysis, patients’ age was not associated with increased CMV reactivation (P = 0.150, relative risk [RR]: 1.018, 95% CI: 0.994–1.043). However, higher donor age was associated with an increase in CMV reactivation with a significant level of 0.1. (RR: 1.021, 95% CI: 0.998–1.044, P = 0.074) so, it was entered in the multivariate analyses.
Although the median age of donors in transplantations with positive and negative CMV infection was 36 versus 31 years respectively, multivariate analysis adjusted for incidence of GVHD (grade I-IV), showed that the risk of CMV infection was significantly higher for patients who received stem cells from donors with higher ages (P = 0.049, RR: 1.024, 95% CI: 1.00–1.048).
In the univariate analysis, we found that patients who experienced GVHD were at significantly higher risk for CMV infection (P = 0.002, RR: 3.402, 95% CI: 1.568–7.381). Among them, patients with grade II-IV GVHD were also at increased risk (P = 0.001, RR: 3.204, 95% CI: 1.638–6.270).
However, it should be noted that when patients with GVHD grade I were compared for the CMV infection susceptibility to patients with grade II-IV in univariate analysis, the results were not significantly different (P = 0.09, RR: 1.980, 95% CI: 0.906–4.326).
We found that patients age (RR: 1.018, 95% CI: 0.994–1.043, P = 0.15) as well as number of infused mononuclear cells (RR: 1.137, 95% CI: 0.859–1.505, P = 0.37), CD3 cells (RR: 1.003, 95% CI: 0.999–1.007, P = 0.18) and number of CD34 cells (RR: 1.059, 95% CI: 0.906–1.237, P = 0.47) were not significantly associated with CMV infection.
Multivariate analysis, also confirmed that in the presence of donor age in the model, GVHD and GVHD grade II-IV were significant determinants of CMV infection. In addition, in the univariate analysis skin GVHD (hazard ratio [HR] =1.458, 95% CI: 1.029–2.066, P = 0.03], liver GVHD (HR = 1.710, 95% CI: 1.172–2.496, P = 0.005) and GI-GVHD (HR = 1.429, 95% CI: 1.03–1.982, P = 0.03) were significantly related to higher risk of CMV infection. However, in the multivariate analyses along with donor age, only liver (RR = 2.206, 95% CI: 1.177-4.137, P = 0.01) and GI (RR = 2.135, 95% CI: 1.160–3.930, P = 0.015) GVHD were remained significant in the models and skin GVHD (RR = 1.889, 95% CI: 0.984–3.628, P = 0.06) did not independently predict the CMV infection.
The median duration of hospital stay in patients with and without CMV infection was 18 days (range: 9–26) and 17 days (range: 12–28), respectively.
The incidence of detecting high-level antigenemia during the first 100 days following HSCT was 11% (95% CI: 5–17%). Additionally, we analyzed the risk factors associated with high-level antigenemia.
The incidence of high-level antigenemia in patients who experienced GVHD was 14%. Univariate analysis showed that patients with GVHD grade ≥2 were at a significantly higher risk for high-level CMV reactivation. Among 72 patients with GVHD grade ≥2, 10 patients were CMV positive (P = 0.035). When liver was the organ involved in GVHD, the association was not significant (P = 0.07).
We found that the only significant risk factors were grade II-IV of GVHD (RR: 3.753, 95% CI: 1.016–13.864, P = 0.05) and GVHD duration (RR: 1.042, 95% CI: 1.003–1.083, P = 0.035). However, other factors such as the affected organs did not have a significant effect.
DISCUSSION
In this study, we retrospectively evaluated the patients who had undergone HSCT to find the risk factors associated with CMV antigenemia. The incidence of CMV infection was 34% in this patient population. It should be noted that differences in the reported incidence of CMV infection among studies might be due to the differences in the definition of antigenemia and the method of diagnosis of CMV infection along with the characteristics of included patients and administration of prophylaxis.
For example, Peres et al. reported that 90% of patients included in their study experienced CMV infection.[5] They attributed this high incidence to the lack of prophylactic treatment and positive pretransplantation serostatus in all of the donors. However, these factors were similar to our center where patients did not receive prophylaxis against CMV infection, and 99% of donors were IgG positive for CMV. It is more probable that the multiple diagnostic approaches simultaneously might have increased the chance of detecting positive cases. They used the results of pp65 antigenemia assay and/or nested-polymerase chain reaction (PCR) and/or presence of specific viral load by real-time PCR for the diagnosis. It seems that the incidence of CMV infection in our center was approximately low with considering the fact that patients in this center did not receive ganciclovir for prophylaxis and majority were CMV seropositive. It has been reported that the incidence of CMV infection can be as high as 80% and 40% in CMV-seropositive patients without and with prophylaxis respectively after HSCT.[19] Based on our findings in univariate analysis, the presence of GVHD was a significant risk factor for CMV infection.
We found that there was not a significant difference between patients with grade I and grade II-IV GVHD in CMV reactivation in the present study. Additionally, in multivariate analysis, GVHD was an independent risk factor for CMV infection. These results can be interpreted as evidence for the fact that the presence of GVHD itself is associated with increased CMV infection regardless of the treatment with systemic corticosteroids. Since generally patients with GVHD grade I do not receive systemic corticosteroids.
The role of GVHD as a risk factor for CMV infection has been the focus of several studies. Similar to our findings, Miller et al. noted that the effect of immunosuppression caused by GVHD itself is more prominent than that of immunosuppressive agents used for the treatment of GVHD in CMV reactivation.[15] They found that CMV infection was not different among patients with GVHD, who were treated with high-dose corticosteroids or those treated topically.[15] Yanada et al. in a retrospective study on 241 HSCT patients found that patients who experienced acute GVHD grade II-IV were at a significantly higher risk for developing CMV antigenemia compared with those with Grade 0-I GVHD in multivariate analysis.[16]
Cantoni et al. found that in multivariate analysis, patients with GVHD grade I were at a greater risk for CMV replication compared with those without GVHD, but the difference was not significant. They also noted that patients with GVHD grade II-IV were at a significantly increased risk for CMV replication compared with both previously mentioned groups.[20]
All of these findings were consistent about the significance of GVHD especially Grade II-IV for CMV reactivation.
The importance of recipient pretransplant serology for CMV infection following transplantation is reported in several studies.[15,21] Even it has been proposed that the serostatus of donors and recipients significantly influence the viral load of CMV infection.[22] Additionally, in their study, George et al. categorized patients based on the CMV serostatus and found that the incidence of CMV reactivation was significantly higher in patients in high-risk group consisting of seropositive recipients with either negative or positive donors (D−/R+ or D+/R+). They also noted that none of the D−/R− cases developed CMV reactivation.[13] However, in our study we had 123 seropositive patients (out of 126) which made the assessment of this issue as a risk factor for developing CMV infection posttransplantation impossible.
In the multivariate analysis, we found that along with the incidence of GVHD, higher donor age remained in the model as a borderline significant risk factor. However, recipients’ age was not found to be a significant risk factor for CMV reactivation in our study. It should be noted that results of the studies vary in terms of determining the role of age as a risk factor for CMV infection. For example, Miller et al. reported that older patients had a nonsignificant tendency towards higher rate of CMV infection. They attributed this finding to the increased possibility of seropositive status in older individuals.[15] Schetelig et al. in their study showed that despite detecting a significant association between recipient age and incidence of CMV-antigenemia in univariate analysis, this factor was no more significant in the multivariate analysis.[21] Additionally, in the study by George et al. patients’ age was not found to be a significant risk factor for CMV infection.[13]
Most of our patients (79%) were conditioned with the combination of busulfan and cyclophosphamide for transplantation; so we could not assess the impact of different conditioning regimens as a risk factor for CMV reactivation. However, we evaluated the role of presence of ATG in conditioning regimen, and it was found that it did not significantly affect the development of CMV antigenemia (P = 0.85). This finding was comparable to the study by George et al.[13] In another study, the comparison of the patients who received reduced-intensity conditioning regimen (fludarabine, busulfan and ATG) with patients who had undergone HSCT with myeloablative conditioning regimen did not show a significant difference in CMV infection.[21]
In the present study, the high-level antigenemia was detected in 11% of patients. We noted that GVHD grade II-IV (RR: 3.753, 95% CI: 1.016–13.864, P = 0.05) or duration of GVHD (RR: 1.042, 95% CI: 1.003–1.083, P: 0.035) were the significant risk factors in HSCT patients for developing high-level CMV infection in multivariate analysis.
For the assessment of high-level antigenemia Kanda et al. categorized patients to high-risk and low risk groups. The high-risk group consisted of patients who received HSCT from alternative donors, conditioned with a regimen containing ATG, those experiencing grade II-IV acute GVHD, and patients treated with more than 0.5 mg/kg of methylprednisolone. They found that high-level antigenemia was significantly higher in high-risk group patients.[18]
There were several limitations in the present study. This study was performed retrospectively which have some limitations regarding the availability of accurate documentation about CMV disease or the involved organs. It should be noted that the method of detecting CMV in the center where the study was conducted was using pp65 antigenemia, which has limitations in patients with neutropenia.[23] Additionally, other risk factors such as the presence of fungal and bacterial infections should have been addressed. There is also the possibility of some changes in the results if we had not excluded the patients who died before 100 days after transplantation.
The present study showed that patients who experienced GVHD and those received stem cells form donors with higher age are at significantly increased risk of CMV infection in multivariate analysis.
AUTHORS’ CONTRIBUTION
Bahareh Valadkhani; Data Acquisition, Literature Search, Mona Kargar; Design, Manuscript Preparation, Asieh Ashouri; Data Analysis, Statistical Analysis, Molouk Hadjibabaie; Concept, Manuscript Editing, Design, Kheirollah Gholami; Definition of Intellectual Content, Manuscript Review, Ardeshir Ghavamzadeh; Concept, Manuscript Review.
Financial support and sponsorship
This manuscript is derived from the Pharm.D. thesis of Dr. Bahareh Valadkhani.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Costanzo ES, Juckett MB, Coe CL. Biobehavioral influences on recovery following hematopoietic stem cell transplantation. Brain Behav Immun. 2013;30 Suppl:S68–74. doi: 10.1016/j.bbi.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wingard JR. Opportunistic infections after blood and marrow transplantation. Transpl Infect Dis. 1999;1:3–20. doi: 10.1034/j.1399-3062.1999.10102.x. [DOI] [PubMed] [Google Scholar]
- 3.Watcharananan SP, Kiertiburanakul S, Piyatuctsanawong W, Anurathapan U, Sungkanuparph S, Pakakasama S, et al. Cytomegalovirus, adenovirus, and polyomavirus co-infection among pediatric recipients of allogeneic stem cell transplantation: Characteristics and outcome. Pediatr Transplant. 2010;14:675–81. doi: 10.1111/j.1399-3046.2010.01325.x. [DOI] [PubMed] [Google Scholar]
- 4.Reinke P, Prösch S, Kern F, Volk HD. Mechanisms of human cytomegalovirus (HCMV) (re) activation and its impact on organ transplant patients. Transpl Infect Dis. 1999;1:157–64. doi: 10.1034/j.1399-3062.1999.010304.x. [DOI] [PubMed] [Google Scholar]
- 5.Peres RM, Costa CR, Andrade PD, Bonon SH, Albuquerque DM, de Oliveira C, et al. Surveillance of active human cytomegalovirus infection in hematopoietic stem cell transplantation (HLA sibling identical donor): Search for optimal cutoff value by real-time PCR. BMC Infect Dis. 2010;10:147. doi: 10.1186/1471-2334-10-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ksouri H, Eljed H, Greco A, Lakhal A, Torjman L, Abdelkefi A, et al. Analysis of cytomegalovirus (CMV) viremia using the pp65 antigenemia assay, the amplicor CMV test, and a semi-quantitative polymerase chain reaction test after allogeneic marrow transplantation. Transpl Infect Dis. 2007;9:16–21. doi: 10.1111/j.1399-3062.2006.00171.x. [DOI] [PubMed] [Google Scholar]
- 7.Borchers S, Luther S, Lips U, Hahn N, Kontsendorn J, Stadler M, et al. Tetramer monitoring to assess risk factors for recurrent cytomegalovirus reactivation and reconstitution of antiviral immunity post allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2011;13:222–36. doi: 10.1111/j.1399-3062.2011.00626.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Kim JG, Lee NY, Sung WJ, Sohn SK, Suh JS, et al. Risk factors for late cytomegalovirus infection after allogeneic stem cell transplantation using HLA-matched sibling donor: Donor lymphocyte infusion and previous history of early CMV infection. Bone Marrow Transplant. 2004;34:21–7. doi: 10.1038/sj.bmt.1704528. [DOI] [PubMed] [Google Scholar]
- 9.Barkholt L, Lewensohn-Fuchs I, Ericzon BG, Tydén G, Andersson J. High-dose acyclovir prophylaxis reduces cytomegalovirus disease in liver transplant patients. Transpl Infect Dis. 1999;1:89–97. doi: 10.1034/j.1399-3062.1999.010202.x. [DOI] [PubMed] [Google Scholar]
- 10.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin North Am. 2010;24:319–37. doi: 10.1016/j.idc.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Cho BS, Yahng SA, Kim JH, Yoon JH, Shin SH, Lee SE, et al. Impact of cytomegalovirus gastrointestinal disease on the clinical outcomes in patients with gastrointestinal graft-versus-host disease in the era of preemptive therapy. Ann Hematol. 2013;92:497–504. doi: 10.1007/s00277-012-1632-x. [DOI] [PubMed] [Google Scholar]
- 12.El-Cheikh J, Devillier R, Crocchiolo R, Fürst S, Calmels B, Faucher C, et al. Impact of pretransplant donor and recipient cytomegalovirus serostatus on outcome for multiple myeloma patients undergoing reduced intensity conditioning allogeneic stem cell transplantation. Mediterr J Hematol Infect Dis. 2013;5:e2013026. doi: 10.4084/MJHID.2013.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George B, Pati N, Gilroy N, Ratnamohan M, Huang G, Kerridge I, et al. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl Infect Dis. 2010;12:322–9. doi: 10.1111/j.1399-3062.2010.00504.x. [DOI] [PubMed] [Google Scholar]
- 14.George B, Kerridge IH, Gilroy N, Huang G, Hertzberg MS, Bradstock KF, et al. A risk score for early cytomegalovirus reactivation after allogeneic stem cell transplantation identifies low-, intermediate-, and high-risk groups: Reactivation risk is increased by graft-versus-host disease only in the intermediate-risk group. Transpl Infect Dis. 2012;14:141–8. doi: 10.1111/j.1399-3062.2011.00706.x. [DOI] [PubMed] [Google Scholar]
- 15.Miller W, Flynn P, McCullough J, Balfour HH, Jr, Goldman A, Haake R, et al. Cytomegalovirus infection after bone marrow transplantation: An association with acute graft-v-host disease. Blood. 1986;67:1162–7. [PubMed] [Google Scholar]
- 16.Yanada M, Yamamoto K, Emi N, Naoe T, Suzuki R, Taji H, et al. Cytomegalovirus antigenemia and outcome of patients treated with pre-emptive ganciclovir: Retrospective analysis of 241 consecutive patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;32:801–7. doi: 10.1038/sj.bmt.1704232. [DOI] [PubMed] [Google Scholar]
- 17.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Kanda Y, Mineishi S, Saito T, Seo S, Saito A, Suenaga K, et al. Pre-emptive therapy against cytomegalovirus (CMV) disease guided by CMV antigenemia assay after allogeneic hematopoietic stem cell transplantation: A single-center experience in Japan. Bone Marrow Transplant. 2001;27:437–44. doi: 10.1038/sj.bmt.1702805. [DOI] [PubMed] [Google Scholar]
- 19.Serio B, Rosamilio R, Giudice V, Pepe S, Zeppa P, Esposiito S, et al. Low-dose valgancyclovir as cytomegalovirus reactivation prophylaxis in allogeneic haematopoietic stem cell transplantation. Infez Med. 2012;20 Suppl 2:26–34. [PubMed] [Google Scholar]
- 20.Cantoni N, Hirsch HH, Khanna N, Gerull S, Buser A, Bucher C, et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:1309–14. doi: 10.1016/j.bbmt.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Schetelig J, Oswald O, Steuer N, Radonic A, Thulke S, Held TK, et al. Cytomegalovirus infections in allogeneic stem cell recipients after reduced-intensity or myeloablative conditioning assessed by quantitative PCR and pp65-antigenemia. Bone Marrow Transplant. 2003;32:695–701. doi: 10.1038/sj.bmt.1704164. [DOI] [PubMed] [Google Scholar]
- 22.Ljungman P, Perez-Bercoff L, Jonsson J, Avetisyan G, Sparrelid E, Aschan J, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 2006;91:78–83. [PubMed] [Google Scholar]
- 23.Ross SA, Novak Z, Pati S, Boppana SB. Overview of the diagnosis of cytomegalovirus infection. Infect Disord Drug Targets. 2011;11:466–74. doi: 10.2174/187152611797636703. [DOI] [PMC free article] [PubMed] [Google Scholar]


