Abstract
Background:
Vitamin D supplementation has been shown to decrease insulin resistance through which it might cause fatty liver. Fatty liver increasingly results in type 2 diabetes mellitus (T2DM). Insulin resistance and fatty liver are particularly closely related. The aim of present study is to examine the effect of vitamin D supplementation on blood sugar and different indices of insulin resistance in patients with non-alcoholic fatty liver disease (NAFLD).
Materials and Methods:
This randomized placebo-controlled clinical trial was conducted on 60 patients with NAFLD, who were divided equally into intervention and control groups. Patients in the intervention group received vitamin D3 (50,000 IU) and patients in the control group received placebo capsules every week for 10 weeks. Blood sugar, homeostatic model assessment-insulin resistance (HOMA-IR), and homeostatic model assessment-beta cell (HOMA-B) were checked at baseline and after 10 weeks of the intervention. Adjustment for variables was performed by analysis of covariance (ANCOVA).
Results:
Vitamin D supplementation resulted in increased serum 25-hydroxy vitamin D [25(OH) D] concentration in the intervention group compared to the control group [+68 (12) vs. −1.9 (2.44); P = 0.001]. Intake of vitamin D supplements led to a marginally significant decrease in fasting blood glucose [FBS: −12 (4) in the intervention group compared to − 3 (2) in the control group; P = 0.055]. Also, HOMA-IR decreased in the intervention group compared to the control group [−1.75 (0.23) vs. 0.12 (0.41); P = 0.066].
Conclusions:
Vitamin D supplementation resulted in decreased HOMA-IR and FBS concentration in patients with NAFLD; however, it did not affect the insulin level and HOMA-B significantly.
Keywords: Blood sugar, insulin resistance, non-alcoholic fatty liver, vitamin D
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a public health problem worldwide, with an incidence of 20-30% in European countries.[1] The spectrum of NAFLD ranges from usual steatosis to non-alcoholic steatohepatitis (NASH) and eventually carcinoma. Almost 5% of people with liver steatosis progress to NASH.[2] NAFLD is tightly correlated with overweight and is considered as a predictor of the metabolic syndrome. Incidence of NAFLD among patients with diabetes is considered to be 65%, which is much more than its incidence among healthy individuals.[3,4,5] NAFLD is a multi-factorial disease with intricate pathogenesis. Clinical features of NAFLD encompass overweight, insulin resistance (IR), and dyslipidemia.[6] Liver steatosis is associated with IR. IR increases the amount of fat tissue lipolysis and the flow of free fatty acids to the liver cell.[7] Hyperglycemia induces lipid reposition in the hepatocytes by increasing lipogenesis while blocking fatty acid oxidation (FAO) and lipid transfer in the liver.[8,9]
Several studies have shown a significant relationship between vitamin D level and chronic heart disease (CHD), diabetes, metabolic syndrome, and IR. Vitamin D plays a vital role in glucose intolerance and IR.[10,11,12,13] It has been shown that colon, pancreas, and also immune cells have vitamin D receptors.[14,15] Vitamin D has been suggested as having a main role in IR and pathology of type 2 diabetes mellitus or β-cell functioning.[16,17] Vitamin D deficiency increases serum parathyroid hormone (PTH) level and this, in turn, increases IR in the peripheral tissue. Several studies demonstrated that IR plays an important role in NAFLD.[10,13]
Therefore, the aim of this study was to determine the effect of vitamin D supplementation on IR in patients with NAFLD.
MATERIALS AND METHODS
A randomized controlled trial (IRCT registration code: IRCT2013060411763N8) was conducted on 60 patients with NAFLD. This study was conducted in Metabolic Liver Disease Research Center in Isfahan University of Medical Sciences. The study was performed with the approval of the local ethics committee of Isfahan University of Medical Sciences. Written informed consent was obtained from the participants. NAFDL was confirmed by ultrasound. Exclusion criteria of our study were: having acute illness, hepatitis C, B, or Wilson's disease; history of chronic liver disease or other conditions that affect the gallbladder and bile ducts; pregnancy; history of taking any drugs affecting the level of alanine aminotransferase (ALT), such as valproic acid, tamoxifen, 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA reductase) inhibitors, metformin, angiotensin-converting enzyme 1 (ACE1), and angiotensin-converting enzyme receptor 1 (ACER 1); and any kinds of medication addiction.
Patients were randomly assigned to consume vitamin D supplements (n = 30) or placebo (n = 30) for 6 weeks. Random assignment was performed using computer-generated random numbers. Placebo capsules were of the same appearance, color, odor, and taste as vitamin D3 capsules. Intervention period was 10 weeks, and patients received vitamin D supplements or placebo every week (1 vitamin D or placebo/week). The level of serum 25-hydroxy vitamin D [25(OH) D] was measured at the beginning and end of the study. Dietary records were collected every 2 weeks and intake was determined based on the estimated values in household measurements. To obtain the nutrient intake of the participants on the basis of these 5-d food diaries, we used NUTRITIONIST IV Software (Version 7.0; N-squared Computing, Salam, OR, USA), which was modified for Iranian food items. Five-day physical activity records were collected using 24-h physical activity record questionnaire (one per 2 weeks). Physical activity level was estimated as metabolic equivalent minutes per week (MET-hours/week). In order to calculate MET-hours/week, we used the following formula: days per week multiplied by hours of exercise each time × MET equivalent of exercise, and summed up all MET-hours/week values to estimate the total MET-hours/week for each person.[14]
Biochemical measurements
Fasting blood samples were taken and 25(OH) D was assessed using direct competitive immune assay kit (Diasercine Italian Company, Monza, Itsly) at the beginning and end of the study. Blood glucose level was measured using Hitachi auto-analyzer, and serum insulin levels were measured with radioimmunoassay kit Pars Azmoon, Tehran, Iran (Tehran Pars test; Tehran, Iran). IR was measured on the basis of the homeostasis model assessment of IR (HOMA-IR), and the percentage of β-cell function was assessed on the basis of the homeostasis model assessment of β (HOMA-β) using the following formulae:
HOMA-IR = [fasting insulin (mU/l) × fasting glucose (mg/l)/405]
%HOMA-β = [fasting insulin (mU/l) ×360/fasting glucose (mg/l) − 63]
Degree of fat accumulation in liver
Level of liver steatosis was measured using ultrasonography with Esaote medical ultrasound machine (convex 3.5 MHz) at the beginning and end of the study. Hepatic ultrasonography was done by someone who was blinded to the objectives of the study. For ultrasound, patients were required to fast for 8 h. Ultrasonography was performed in supine position. Right and left lobes of the upper and lower surfaces were studied. Echogenicity of the liver, presence or absence of bulky tumors, and cystic or solid calcification also were assessed. Intrahepatic bile ducts, portal vein, and hepatic artery were evaluated. Liver steatosis was scored semi-quantitatively on a scale of 0-3, where 0 denoted absent, 1 was given for mild, 2 for moderate, and 3 for severe steatosis. Steatosis was graded according to Saverymuttu et al.[18]
Statistical analysis
The normal distribution of variables was confirmed by the Kolmogorov-Smirnov test. Log transformation was used for non-normally distributed variables. Independent-sample Student's t-test was used to detect differences in general characteristics and dietary intake between the two groups, and paired t-test was used to assess differences within groups. These adjustments were performed using analysis of covariance (ANCOVA). Changes of NAFLD grades were analyzed by ordinal regression (adjustment for age and sex). Chi-square test was used to detect differences in fatty liver grades in the two groups at baseline. P < 0.05 was considered as the level of significance. All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS), version 16 (SPSS Inc.).
Ethical considerations
This study is approved by Ethical Committee of Isfahan University of Medical Sciences and Helsinki's guideline is followed, completely and addicts to any kinds of drug.
RESULTS
The study flowchart shows the screening, randomization, and follow-up of the participants [Figure 1]. In this study, 29 men and 31 women participated. Mean age of the participants was 48.5 years.
Figure 1.
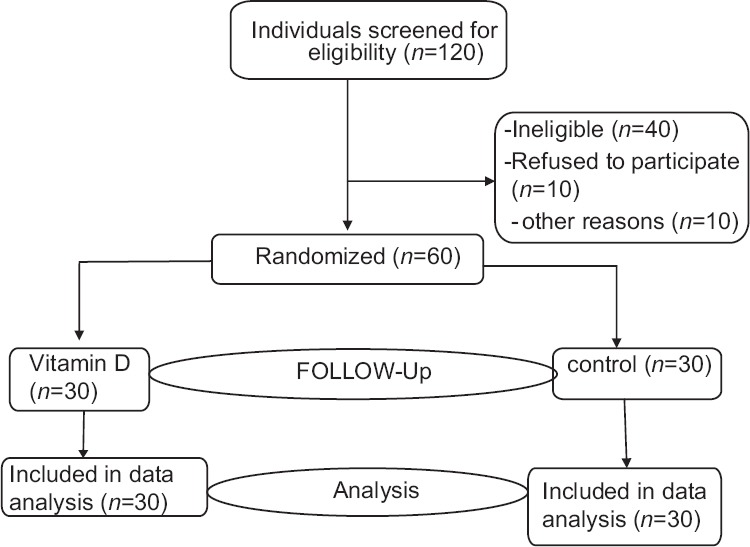
Study flow diagram
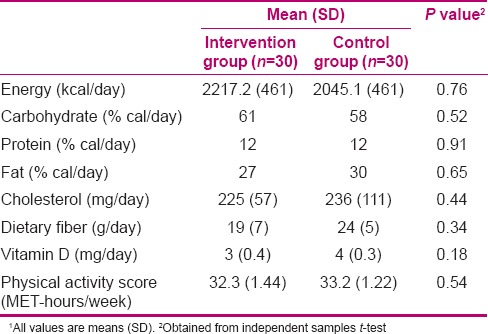
Glycemic indicator of 60 patients is given in Table 1. Compliance with the treatments was good in both groups and no side effects were reported. On the basis of 5-d dietary intake and physical activity records, no significant differences were seen between the two groups.
Table 1.
Laboratory characteristics in intervention and control groups1
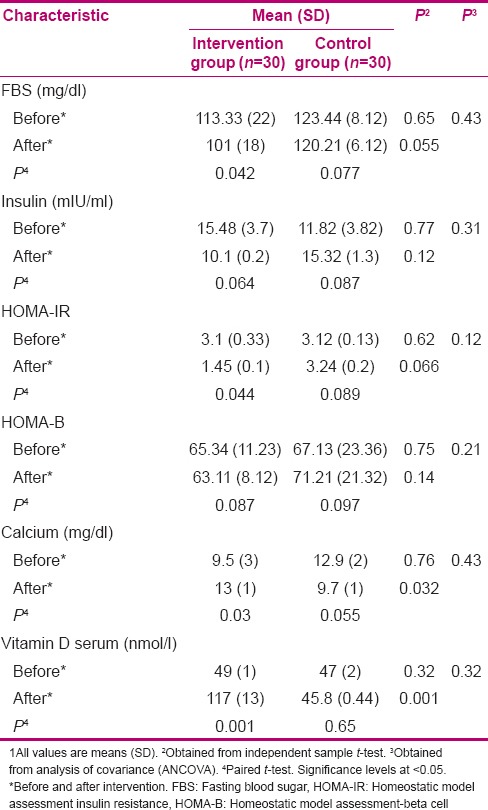
When the analyses were adjusted for baseline characteristics, vitamin D supplementation resulted in increase of serum 25(OH) D concentrations compared with placebo [+68 (12) compared to −1.9 (2.44) nmol/ml; P = 0.001] [Table 2]. Intake of vitamin D supplements led to a marginally significant reduction in fasting blood glucose (FBS) and HOMA-IR level [FBS: −12 (4) compared to − 3 (2) mg/dl in the intervention and control groups, respectively; P = 0.055 and HOMA-IR: −1.75 (0.23) compared to 0.12 (0.41) in the intervention and control groups, respectively; P = 0.066]. Moreover, serum calcium was increased in the intervention group compared to the control group [4 (0.4) compared vs. 3.2 (1) mg/dl; P = 0.032].
Table 2.
Dietary intake and physical activity of NAFLD of intervention and control groups1
DISCUSSION
The aim of this study was to assess the effect of vitamin D supplementation on blood sugar and different indices of IR in patients with NAFLD.
In this study, vitamin D supplementation caused a marginally significant decrease in FBS level and HOMA-IR, however, had no significant effect on insulin level and HOMA-B.
There are some evidences showing that vitamin D deficiency has a relationship with the risk factors of chronic diseases, including NAFLD and other metabolic risk factors.[17,18] It has been suggested that low serum levels of vitamin D may increase IR and, in turn, the risk of diabetes mellitus type 2.[19] NAFLD is associated with IR in both liver and muscle tissue. IR elevates the amount of fat tissue lipolysis and increases the flow of free fatty acids inside the liver cell.[19,20,21]
Effects of vitamin D supplementation on the metabolism of glucose have been demonstrated in several studies. Our findings are similar to the results of other studies, and IR was found to decrease after vitamin D intake. Inzucchi et al. demonstrated in their study a 60% reduction in IR by vitamin D supplementation, while the reduction observed on administration of metformin and troglitazone was 54% and 13%, respectively.[22] In another study, Von Hurst et al. demonstrated that vitamin D supplementation significantly increased insulin sensitivity and decreased IR.[23] Ken et al. found a negative association between 25(OH) D concentration and FBS.[24] However, Witham et al. showed that vitamin D (at several dosages) had no effects on IR or on FBS.[25] Nagpal et al. showed that vitamin D supplementation had no effect on insulin sensitivity, but supplementation with vitamin D for 2 years could reduce HOMA-IR.[26] In this study, they showed that long-term supplementation of vitamin D increased insulin sensitivity, while in our study vitamin D supplementation for a short term increased insulin sensitivity.
Differences in age and metabolic risk factors of the subjects, as well as the doses of vitamin D supplementation can explain the differences observed in the results of our study and various other studies. Patients in our study were older than patients in other studies; therefore, synthesis of vitamin D was reduced in the patients.
Vitamin D regulates the gene transcription of anti-inflammatory marker and insulin. Moreover, vitamin D regulates calcium and phosphorous levels in plasma and cytoplasm.[13] It suggested that vitamin D increasing calcium in cells, in turns conduction to elevating glucose into the cells.[27] Vitamin D modulates nuclear peroxisome proliferative activated receptor (PPAR) which is a key factor in the IR.[28] Vitamin D serum reducing is in relationship with augments in inflammation. Vitamin D reduces the transcription of pro inflammatory cytokines that increase IR, such as interleukins like IL 1, IL 6, and tumor necrosis factor (TNF) α; also, vitamin D reduces the gene transcription of nuclear factor kappa light chain enhancer of activated B cells (NF κb).[29,30,31,32]
Increasing these inflammatory parameters can even lead to the lipid profile disorders which can put patients with non-alcoholic fatty liver disorder in metabolic syndrome and cardiovascular dysfunctions.[33,34]
The strength of the present study is that it was a double-blind RCT conducted in a country with a high prevalence of NAFLD and hyopovitaminosis vitamin D. In this study, we had several limitations. The first limitation was the use of ultrasound for the diagnosis of fatty liver disease, while for accurate diagnosis of fatty liver, liver biopsy is needed. The second limitation was the small sample size of participants, especially in grades one and two. A larger sample size with longer period of follow-up perhaps would have given more favorable results. More studies need to be conducted to demonstrate the effect of vitamin D supplementation on glycemic indicators.
CONCLUSION
Vitamin D supplementation was inversely associated with IR. Vitamin D supplementation may have beneficial effects on controlling the glycemic indicator.
ACKNOWLEDGMENTS
This study was extracted from an Msc dissertation which was approved by School of Nutrition and Food Sciences, Isfahan University of Medical Sciences (code 391214). We thank the College of Food Security Research Center and Department of Community Nutrition, School of Nutrition and Food Sciences, Isfahan University of Medical Sciences, Isfahan, Iran.
Footnotes
Source of Support: Food Security Research Center and Department of Community Nutrition, School of Nutrition and Food Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009;50:1282–93. doi: 10.1002/hep.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: A longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–8. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Molleston JP, White F, Teckman J, Fitzgerald JF. Obese children with steatohepatitis can develop cirrhosis in childhood. Am J Gastroenterol. 2002;97:2460–2. doi: 10.1111/j.1572-0241.2002.06003.x. [DOI] [PubMed] [Google Scholar]
- 5.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118:277–83. doi: 10.1161/CIRCULATIONAHA.107.739920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. [PubMed] [Google Scholar]
- 7.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 8.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–9. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 9.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinic pathological correlates of pediatric non-alcoholic fatty liver disease. J Pediatr. 2003;143:500–5. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 10.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose Homeostasis. Int J Endocrinol 2010. 2010:351–85. doi: 10.1155/2010/351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su Y, Ye L. Can vitamin D intake assist in improving the outcome of endodontic treatment for diabetic patients. Med Hypotheses. 2010;74:673–5. doi: 10.1016/j.mehy.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Foss YJ. Vitamin D deficiency is the cause of common obesity. Med Hypotheses. 2009;72:314–21. doi: 10.1016/j.mehy.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain consumption and the metabolic syndrome: A favorable association in Tehranian adults. Eur J Clin Nutr. 2005;59:353–62. doi: 10.1038/sj.ejcn.1602080. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Zhang Z. Comment on “Vitamin D deficiency is the cause of common obesity”. Med Hypotheses. 2009;73:123. doi: 10.1016/j.mehy.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Mattila C, Knekt P, Männistö S, Rissanen H, Laaksonen MA, Montonen J, et al. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007;30:2569–70. doi: 10.2337/dc07-0292. [DOI] [PubMed] [Google Scholar]
- 17.Nam GE, Kim do H, Cho KH, Park YG, Han KD, Kim SM, et al. 25-Hydroxyvitamin D insufficiency is associated with cardiometabolic risk in Korean adolescents: The 2008-2009 Korea National Health and Nutrition Examination Survey (KNHANES) Public Health Nutr. 2014;17:186–94. doi: 10.1017/S1368980012004855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292:13–5. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacifico L, Anania C, Osborn JF, Ferraro F, Bonci E, Olivero E, et al. Low 25(OH) D3 levels are associated with total adiposity, metabolic syndrome, and hypertension in Caucasian children and adolescents. Eur J Endocrinol. 2011;165:603–11. doi: 10.1530/EJE-11-0545. [DOI] [PubMed] [Google Scholar]
- 20.Muldowney S, Lucey AJ, Paschos G, Martinez JA, Bandarra N, Thorsdottir I, et al. Relationships between vitamin D status and cardio-metabolic risk factors in young European adults. Ann Nutr Metab. 2011;58:85–93. doi: 10.1159/000324600. [DOI] [PubMed] [Google Scholar]
- 21.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–5. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 22.Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J, Sovijärvi A, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–8. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 23.Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, et al. Efficacy and metabolic effect of metformin and troglitazone in type 2 Diabetes mellitus. N Engl J Med. 1998;338:867–72. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- 24.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in non-diabetic adult. Diabetes Care. 2007;30:980–6. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 25.Lind L, Pollare T, Hvarfner A, Lithell H, Sorensen OH, Ljunghall S. Long-term treatment with active vitamin D (alphacalcidol) in middle-aged men with impaired glucose tolerance. Effects on insulin secretion and sensitivity, glucose tolerance and blood pressure. Diabetes Res. 1989;11:141–7. [PubMed] [Google Scholar]
- 26.Witham MD, Dove FJ, Druburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D (3) on markers of vascular health in patients with type 2 diabetes: A randomised controlled trial. Diabetologia. 2010;53:2112–9. doi: 10.1007/s00125-010-1838-1. [DOI] [PubMed] [Google Scholar]
- 27.Bland R, Markovic D, Hills CE, Hughes SV, Chan SL, Squires PE, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol. 2004;89-90:121–5. doi: 10.1016/j.jsbmb.2004.03.115. [DOI] [PubMed] [Google Scholar]
- 28.Ojuka EO. Role of calcium AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc Nutr Soc. 2004;63:275–8. doi: 10.1079/PNS2004339. [DOI] [PubMed] [Google Scholar]
- 29.Dunlop TW, Vaisanen S, Frank C, Molnar F, Sinkkonen L, Carlberg C. The human peroxisome proliferator-activated receptor δ gene is a primary target of 1α, 25-dihydroxyvitamin D3 and its nuclear receptor. J Mol Biol. 2005;349:248–60. doi: 10.1016/j.jmb.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 30.Cohen-Lahav M, Douvdevani A, Chaimovitz C, Shany S. The anti-inflammatory activity of 1,25 dihydroxy vitamin D3 in macrophages. J Steroid Biochem Mol Biol. 2007;103:558–62. doi: 10.1016/j.jsbmb.2006.12.093. [DOI] [PubMed] [Google Scholar]
- 31.Foroughi M, Maghsoudi Z, Ghiasvand R, Iraj B, Askari G. Effect of Vitamin D Supplementation on C reactive Protein in Patients with Nonalcoholic Fatty Liver. Int J Prev Med. 2014;5:969–75. [PMC free article] [PubMed] [Google Scholar]
- 32.Mehdi Foroughi, Zahra Maghsoudi, Gholamreza Askari. Relationship between non-alcoholic fatty liver disease and inflammation in patients with non-alcoholic fatty liver. ABR. 2015 doi: 10.4103/2277-9175.176368. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehdi Foroughi, Zahra Maghsoudi, Gholamreza Askari. The effect of vitamin D supplementation on lipid profile in patients with non-alcoholic fatty liver (NAFLD) IJNMR. 2015 In press. [Google Scholar]
- 34.Mehdi Foroughi, Zahra Maghsoudi, Gholamreza Askari. The effect of vitamin D supplementation on blood sugar and different indices of insulin resistance in patients with non-alcoholic fatty liver disease (NAFLD) IJNMR. 2015 doi: 10.4103/1735-9066.174759. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


