Abstract
Background and Aim:
Polymorphisms in aryl hydrocarbon receptor nuclear translocator-like (ARNTL) gene, the key component of circadian clock manifests circadian rhythm abnormalities. As seasonal affective disorder (SAD) is associated with disrupted circadian rhythms, the main objective of this study was to screen an Indian family with SAD for ARNTL gene polymorphisms.
Materials and Methods:
In this study, 30 members of close-knit family with SAD, 30 age- and sex-matched controls of the same caste with no prior history of psychiatric illness and 30 age- and sex-matched controls belonging to 17 different castes with no prior history of psychiatric illness were genotyped for five different single nucleotide polymorphisms (SNPs) in ARNTL gene by TaqMan allele-specific genotyping assay.
Statistical Analysis:
Statistical significance was assessed by more powerful quasi-likelihood score test-XM.
Results:
Most of the family members carried the risk alleles and we observed a highly significant SNP rs2279287 (A/G) in ARNTL gene with an allelic frequency of 0.75.
Conclusions:
Polymorphisms in ARNTL gene disrupt circadian rhythms causing SAD and genetic predisposition becomes more deleterious in the presence of adverse environment.
Keywords: Aryl hydrocarbon receptor nuclear translocator-like, circadian rhythms, seasonal affective disorder, single nucleotide polymorphism
INTRODUCTION
Bipolar disorder (BD) associated seasonal pattern (mania during spring and summer together with depression during fall and winter) referred to as seasonal affective disorder (SAD) is associated with disrupted circadian rhythms.[1]
Aryl hydrocarbon receptor nuclear translocator-like (ARNTL) protein is a transcription factor and a core component of mammalian circadian rhythms regulatory network. Convergent functional genomics approach, integrating the genetics with functional genomics in human as well as animal models identified the ARNTL gene as the top candidate associated with BD.[2] The ARNTL gene knockout mice are reported to be complete arrhythmic in constant darkness.[3] Several polymorphisms in ARNTL gene are reported to be associated with BD and SAD in different ethnic groups.[4,5,6,7,8,9]
As the family members in the study were experiencing SAD, this study was undertaken to delineate the role of ARNTL gene in SAD. We attempted to decipher the five single nucleotide polymorphisms (SNPs) (rs2279287, rs1982350, rs7126303, rs969485, and rs2290035) in ARNTL gene [Figure 1] and correlated it to differential seasonal behavior. However, the interaction between photoperiodic mechanisms (light-dark cycle) and the circadian system in the onset of SAD is obscure.
Figure 1.
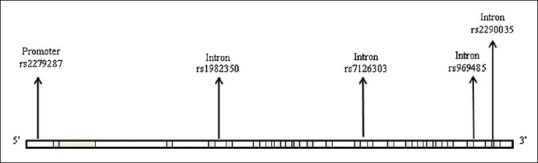
Distribution of the five single nucleotide polymorphisms in aryl hydrocarbon receptor nuclear translocator-like gene
MATERIALS AND METHODS
Study subjects
The subjects comprised 30 members of close-knit family with SAD (DSM-5 criteria was used in diagnosis) and age- and sex-matched 30 controls of the same caste with no prior history of psychiatric illness. In addition, 30 age- and sex-matched controls with no prior history of psychiatric illness belonging to 17 different castes formed as a negative control. All investigations were conducted in compliance of the principles of the declaration of Helsinki. Informed consents were obtained from all subjects. The Human Ethical Committee of Bharathiar University, Tamil Nadu, India approved the study. The clinical investigations of all the study participants were carried out in KG Hospital, Coimbatore, Tamil Nadu, India.
DNA isolation
DNA was isolated from the blood samples using Hi-PurA mini blood DNA isolation kit (HiMedia, India).
Selection of single nucleotide polymorphisms
Five SNPs across the ARNTL gene namely rs2279287 (A/G), rs1982350 (C/T), rs7126303 (C/T), rs969485 (A/G), and rs2290035 (A/T) were selected to test for their association as the risk factor in SAD.
TaqMan single nucleotide polymorphisms genotyping
SNPs were genotyped using TaqMan allele-specific genotyping assay (Applied Biosystems). Briefly, polymerase chain reaction (PCR) reactions were run in a total volume of 5 μl, containing 10 ng DNA, 2.5 μl 1 × PCR buffer, and 0.125 μl 40 × allelic discrimination primer-probe mix. Reactions were run in ABI 7500 real-time system (Applied Biosystems, USA) with the following cycle parameters: 95°C for 10 min; followed by 40 cycles at 95°C for 15 s and 60°C for 30 s. The post-PCR run was done at 60°C for 1 min. The assays were carried out in triplicate.
Statistical analysis
The allelic frequency was calculated using Hardy–Weinberg law. Statistical significance was assessed by more powerful quasi-likelihood score test (MQLS)-XM,[10] a program written in C, that performs single-SNP, case-control association testing on the autosomal chromosomes in samples with related individuals. The program is applicable to association studies with completely general combinations of related and unrelated individuals, where the relationship among the sampled individuals is assumed to be known. For each SNP, the program computes three different test statistics for association. For autosomal SNPs, the three test statistics computed are MQLS,[11] WQLS and corrected χ2.[12] The link to the statistical software used is https://galton.uchicago.edu/~mcpeek/software/MQLS_XM/index.html.
RESULTS AND DISCUSSION
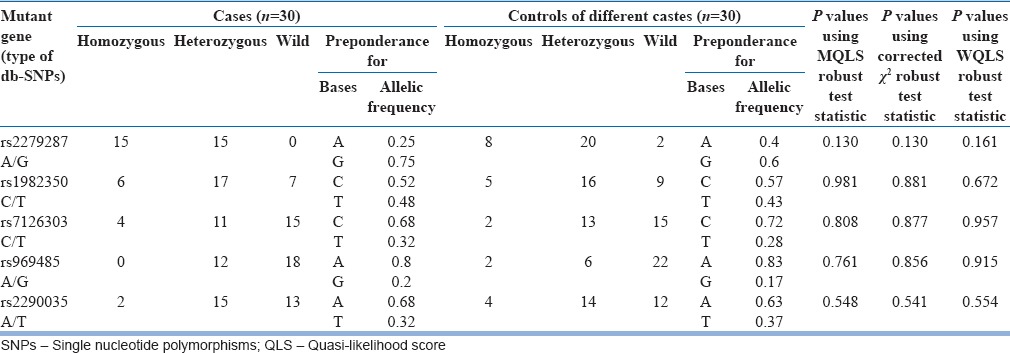
The polymorphic count and allele frequency distribution of different SNPs in ARNTL gene in cases and controls of the same caste are given in Table 1. The polymorphic count and allele frequency distribution of different SNPs in ARNTL gene in cases and controls of different castes are given in Table 2. Among the five SNPs of ARNTL gene, there was a significant variation in SNP rs2279287 between the cases and controls of same caste [Table 1]. The allelic frequency of the mutant allele (G) was found to be 0.75 in cases. Genotyping of the cases for this SNP showed that 50% of the cases are homozygous, and the remaining individuals are heterozygous for the mutant allele. None of the cases were found to be wild. In the control group belonging to the same caste, 20% were homozygous, 47% were heterozygous, and 33% were wild type. In the control group of different castes, 26% were homozygous, 66% were heterozygous, and 6% were wild type. The other 4 SNPs (rs1982350, rs7126303, rs969485, and rs2290035) were almost equally distributed both in the cases and controls [Tables 1 and 2]. Among the 90 subjects (30 cases and 30 controls of the same caste and 30 controls of different castes), only one individual is wild for all the five SNPs studied.
Table 1.
Polymorphic count and allele frequency distribution of different db-single nucleotide polymorphisms in ARNTL gene in cases and controls of same caste
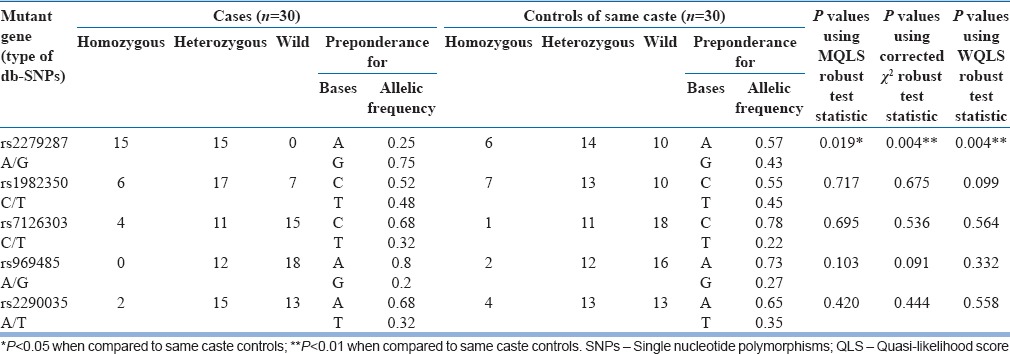
Table 2.
Polymorphic count and allele frequency distribution of different db-single nucleotide polymorphisms in ARNTL gene in cases and controls of different castes
SNP rs2279287, which is a significant marker in the family under study is located in the promoter region of ARNTL gene and hence the polymorphism in this region could affect the clock regulated processes.
The controls in this study carried the mutant alleles, and the same finding was reported by other studies.[2,7,13] The significant marker rs2279287 in the present study is a part of the most significant haplotype of ARNTL gene of Caucasians[4,5,6,8]
Similar to this study, association of ARNTL gene variation with a seasonal pattern in BD has been reported in different populations recently.[14,15] People with wild-type genotype of SNP rs2290035 in ARNTL gene are associated with less seasonal variation in energy level.[15] About 96% of patients with the mutant genotype of ARNTL gene have a routine seasonal variation of energy level.[16] The functionality of the ARNTL protein in terms of homozygous, heterozygous, and wild polymorphic nature is not elucidated still. A fully functional ARNTL protein is hypothesized to inhibit mania by inactivating dopamine through monoamine oxidase A and thus inhibiting the promanic effects of dopamine[17,18,19] and hence any defect in ARNTL gene (homozygous/heterozygous) is expected to promote mania.
ARNTL protein has period-Arnt-single-minded domains which regulate biological responses to light[20] and hence mutations in the ARNTL gene could alter the sensitivity to light which is observed in SAD subjects. Thus, one cannot rule out the essential nature of fully functional ARNTL gene.
CONCLUSION
SAD is a highly heritable psychiatric disorder. Several studies have reported the association of ARNTL gene polymorphisms with BD and SAD. Similar to previous reports, the present study also identified a potentially functional polymorphism, rs2279287 in ARNTL gene in an Indian family diagnosed with SAD. Conclusively, we propose that polymorphisms in ARNTL gene disrupt the circadian rhythms causing SAD, and genetic predisposition becomes more deleterious in the presence of adverse environment. This is the first report on ARNTL gene mutations aggravated by environment associated with SAD in Indian population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 2.Le-Niculescu H, Patel SD, Bhat M, Kuczenski R, Faraone SV, Tsuang MT, et al. Convergent functional genomics of genome-wide association data for bipolar disorder: Comprehensive identification of candidate genes, pathways and mechanisms. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:155–81. doi: 10.1002/ajmg.b.30887. [DOI] [PubMed] [Google Scholar]
- 3.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–17. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansour HA, Wood J, Logue T, Chowdari KV, Dayal M, Kupfer DJ, et al. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav. 2006;5:150–7. doi: 10.1111/j.1601-183X.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- 5.Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, et al. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:234–41. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Partonen T, Treutlein J, Alpman A, Frank J, Johansson C, Depner M, et al. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann Med. 2007;39:229–38. doi: 10.1080/07853890701278795. [DOI] [PubMed] [Google Scholar]
- 7.Patel SD, Le-Niculescu H, Koller DL, Green SD, Lahiri DK, McMahon FJ, et al. Coming to grips with complex disorders: Genetic risk prediction in bipolar disorder using panels of genes identified through convergent functional genomics. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:850–77. doi: 10.1002/ajmg.b.31087. [DOI] [PubMed] [Google Scholar]
- 8.Soria V, Martínez-Amorós E, Escaramís G, Valero J, Pérez-Egea R, García C, et al. Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacology. 2010;35:1279–89. doi: 10.1038/npp.2009.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy MJ, Nievergelt CM, Kelsoe JR, Welsh DK. A survey of genomic studies supports association of circadian clock genes with bipolar disorder spectrum illnesses and lithium response. PLoS One. 2012;7:e32091. doi: 10.1371/journal.pone.0032091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornton T, Zhang Q, Cai X, Ober C, McPeek MS. XM: Association testing on the X-chromosome in case-control samples with related individuals. Genet Epidemiol. 2012;36:438–50. doi: 10.1002/gepi.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornton T, McPeek MS. Case-control association testing with related individuals: A more powerful quasi-likelihood score test. Am J Hum Genet. 2007;81:321–37. doi: 10.1086/519497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourgain C, Hoffjan S, Nicolae R, Newman D, Steiner L, Walker K, et al. Novel case-control test in a founder population identifies P-selectin as an atopy-susceptibility locus. Am J Hum Genet. 2003;73:612–26. doi: 10.1086/378208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geoffroy PA, Lajnef M, Bellivier F, Jamain S, Gard S, Kahn JP, et al. Genetic association study of circadian genes with seasonal pattern in bipolar disorders. Sci Rep. 2015;5:10232. doi: 10.1038/srep10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovanen L, Saarikoski ST, Aromaa A, Lönnqvist J, Partonen T. ARNTL (BMAL1) and NPAS2 gene variants contribute to fertility and seasonality. PLoS One. 2010;5:e10007. doi: 10.1371/journal.pone.0010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnusson A, Partonen T. The diagnosis, symptomatology, and epidemiology of seasonal affective disorder. CNS Spectr. 2005;10:625–34. doi: 10.1017/s1092852900019593. [DOI] [PubMed] [Google Scholar]
- 17.Greenwood TA, Schork NJ, Eskin E, Kelsoe JR. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Mol Psychiatry. 2006;11:125–33. doi: 10.1038/sj.mp.4001764. 115. [DOI] [PubMed] [Google Scholar]
- 18.Berk M, Dodd S, Kauer-Sant’anna M, Malhi GS, Bourin M, Kapczinski F, et al. Dopamine dysregulation syndrome: Implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand Suppl. 2007;434:41–9. doi: 10.1111/j.1600-0447.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 19.Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, et al. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol. 2008;18:678–83. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Huang ZJ, Edery I, Rosbash M. PAS is a dimerization domain common to Drosophila period and several transcription factors. Nature. 1993;364:259–62. doi: 10.1038/364259a0. [DOI] [PubMed] [Google Scholar]


