Abstract
Immunization programs are one of the most well-recognized and successful public health programs across the world. The immunization programs have achieved significant successes in a number of countries; however, the coverage with available vaccines remains sub-optimal in many low- and middle-income countries (LMICs). This article, based upon extensive review of literature and using universal immunization program (UIP) in India as a case study, summarizes the latest developments and initiatives in the area of vaccination and immunization in the last few years. The article analyzes initiatives under UIP in India from the “health system approach” and argues that it is possible to increase coverage with available vaccines and overall program performance by focused attention on various functions of health systems. It also discusses the emerging evidence that health systems could be strengthened prior to the introduction of new interventions (vaccines included) and the introduction of new interventions (including vaccines) could be planned in a way to strengthen the health systems. It concludes that immunization programs could be one of the entry points for strengthening health systems in the countries and lessons from vaccine introduction could pave pathway for scaling up other health interventions and therefore, could contribute to advancing Universal Health Coverage (UHC).
Keywords: Health system, India, universal health coverage, universal immunization program
Introduction
The first vaccine, targeting smallpox, was discovered and came into use in the late 1700s and early 1800; however, it was not until almost two centuries later that immunization services began to reach majority of the children in low- and middle-income countries (LMICs). World Health Organization (WHO) and UNICEF proposed the Expanded Program on Immunization (EPI) in 1974 with the goal of universal coverage by 1990.[1] By the year 2012, an estimated 2.5 million deaths were being averted each year by vaccination; however, nearly 1.5 million children still die from the diseases preventable by vaccines recommended by the WHO. About 29% of deaths in children 1–59 months were vaccine preventable in 2012.[2,3] As the world celebrated 40 years of EPI in the year 2014, this review article analyzes the immunization programs, with universal immunization program (UIP) in India—which completes 30 years in 2015—as a case study, to guide the policy makers in LMICs, to step back, deliberate and take appropriate actions to ensure that the benefits of available vaccines reach to all eligible children. This article also describes how the “health system approach” could be optimally used to improve immunization program performance.
Immunization programs–Brief global overview
Globally, overall vaccine coverage has increased in recent years and a total of 165 countries reported diphtheria, pertussis, and tetanus (DPT) vaccine coverage rates of 80% or greater in 2012. While all countries provide one dose of measles vaccine in their national program, 146 countries provide second dose of measles containing vaccine in their routine immunization program and remaining through supplementary immunization activities (SIAs).[2,3,4] A major challenge is that a considerable percentage of populations continue to suffer from limited access to immunization services, and the gap in coverage amongst well and poorly performing nations is widening annually. Twenty-three million children were not protected against diphtheria, pertussis, and tetanus diseases. Polio continued to be endemic in three countries (Afghanistan, Pakistan and Nigeria) and newer vaccines had low uptake in many regions. Poor access to health facilities and immunization campaigns, insufficient and inefficient use of resources, limited technical capacity of poorly empowered immunization decision-making bodies (often termed National Immunization Technical Advisory Group or NITAG), lack of political will, civil conflict and war, and natural disasters, all contribute to under-immunization.[1]
Universal Immunization Program in India
India adopted EPI in 1978 and then in 1985 renamed it as UIP. In the recent years, annually immunization program in India aims to reach out to approx. 27 million newborns through 9 million immunization sessions conducted by nearly 150,000 Auxiliary Nurse Midwives and supported by approx. 27,000 cold chain points. Currently vaccines are provided against nine VPDs (including JE and Haemophilus Influenzae type b (Hib) in selected district and states).[4,5] The major challenges in expanding the immunization program in the country are summarized in India's Comprehensive Multi Year strategic Plan (cMYP) for UIP (2013–17).[1,4]
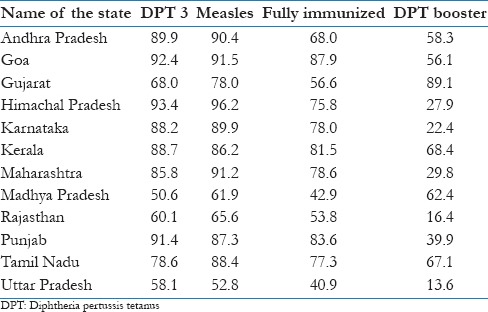
By Dec 2012, among 194 WHO member states, 131 (68%) achieved ≥90% DTP3 coverage and 59 (30%) achieved ≥80% DTP3 coverage in every district. In India, the nation-wide evaluated DPT3 coverage has been 72% indicating that India is far from achieving this target. What is worrisome is that in successive evaluation surveys in India, only a few states have achieved more than 80% coverage for three doses of DPT vaccine.[2] As per CES 2009, only three states of India Himachal Pradesh, Goa and Punjab had DPT3 coverage more than 90%. There were a few states such as Maharashtra, Kerala, Tamil Nadu and Andhra Pradesh where DPT3 coverage was between 85% and 89.9%. Only two states, Goa and Himachal Pradesh, had coverage for two antigens DPT3 and measles more than 90%. None of Indian state had proportion of fully immunized more than 90% [Table 1].[5]
Table 1.
Evaluated immunization coverage in India
Analysis of District Level Household Surveys (DLHS) II (2002–04) and III (2007–08) reports from India[6,7] shows that evaluated immunization coverage demonstrates the marked variation in coverage rates both between and within states. It has been noted that between these two surveys, 379 (68%) districts surveyed (of total 555, for which data was available) showed an increase in the proportion of fully immunized children between two DLHS.[6,7] The average increase has been 19.0% (ranging from 0.1% to 74.6%). A total of 159 (29%) districts surveyed showed a decrease in the proportion of fully immunized between two surveys; the average decrease was 10.0% (ranging from 0.1% to 63.8%). Seventeen (03%) districts showed no change in full immunization rates. Clearly while a number of districts had shown improvement, the decline was noticeable in many districts. Further preliminary analysis of DLHS- 2 and 3 data, to have a look at the determinants of immunization coverage in India indicated an inverse relationship between urbanization and immunization coverage. It has plausible explanation as urban areas don’t have as well-organized immunization service delivery system in India and often slums and peri-urban areas are excluded from the service delivery. Immunization data reported in Health Management Information System (HMIS) web-portal of the Govt. of India noted that from Apr 2012 to Mar 2013, the national coverage with first dose of measles-containing vaccine (MCV1), given at 9 months of age, in India was 82.3% while the second dose of MCV2, given at 16–24 months, of age coverage was only 27.6%.[8]
Last few years for UIP in India- opportunities and hopes
In the last 3–4 years in India, since late 2010, there have been a number of new developments such as release of India's first national vaccine policy in 2011 and release of new comprehensive multi-year strategic plan (cMYP) for UIP (2013–17). A glimpse of the recent developments in vaccines in India, till March 2013, has been documented in a recently published article.[1] A closer look at the development since then (April 2013 to March 2014) has been provided in the following paragraphs, indicating how rapidly the immunization program landscape is evolving in the country.
One of the major developments was the announcement of the findings of phase III clinical trial of an indigenously researched and developed rotavirus vaccine, based on an indigenous strain called 116E, in May 2013. The vaccine, named ROTAVAC, trial covered nearly 6800 infants, the ratio of vaccine to placebo being 2:1 (4532 were administered the vaccine and 2267 placebo). The assessed efficacy and safety profile of the vaccine were reported to be comparable to those of other licensed and available rotavirus vaccines. The findings of this trial were subsequently published in the Lancet in 2014.[9] The development and successful completion of clinical trial of ROTAVAC is being considered a major milestone, and an example of successful “public–private partnership” and a “unique social innovation model.”[10] The indigenous production of a new vaccine in India has always had an effect on the availability of the vaccine, not only in the country but globally as well. It has always led to a reduction in the price of the vaccine which, in turn, contributes to an increase in the vaccine availability in LMICs. The Indian rotavirus vaccine is likely to be made available at cost of US$ 1 per dose (comparing to currently available Rotavirus vaccines which cost around US$ 4 to up to US$ 50 per dose) and can be stored at freezing temperature, a factor which is likely to contribute to further lowering the costs of the immunization program. This development underscores that the public–private partnership provides an opportunity for keeping the cost of the vaccines low, contrary to the scenario if vaccines are researched solely by private sector manufacturers. This success is likely to encourage more public–private partnerships for vaccine development in India.
The development of two more vaccines was announced in 2013. In August, a leading Indian vaccine manufacturer announced the development of the typhoid conjugate vaccine, Typbar-TCV (a fourth-generation vaccine against typhoid disease), proven to provide protection to adults and infants of the age of 9 months and above in India.[11] In September 2013, the indigenous Japanese encephalitis (JE) vaccine, JENVAC, was licensed in India. This new JE vaccine was jointly developed by scientists from the National Institute of Virology, Indian Council of Medical Research and Bharat Biotech Ltd. The candidate strain (821564 XZ) for this vaccine had been isolated from the blood sample of an encephalitic patient in the Government Hospital in the district of Kolar in Karnataka in November—December 1981.[12,13]
In late 2012, an Indian vaccine manufacturer had acquired Bilthoven Biologicals of the Netherlands and was able to access the technology and expertise required to produce inactivated polio vaccine (IPV). Subsequently, the Indian media reported the possibility of IPV being made available at half of the prevalent market rate at the cost of approx. US$ 1 per dose.[14] This is a significant development, considering that the South-east Asia region of the WHO has been certified polio-free and there are plans to make a switch to IPV in the immunization programs. With this development, sustainable availability of IPV is likely to increase in India and other countries.
India achieved one of its biggest public health successes since the eradication of smallpox in the late 1970s when it completed 3 years of a polio-free status on 11 January 2014. Thereafter, on 27 March 2014, the entire WHO South-East Asia region, consisting of 11 countries, became the fourth WHO region in the world to have been certified as polio-free.[15]
The other major milestone includes the completion of the first round of the measles catch-up campaigns in all targeted 14 states of India by mid-2013. The mortality from measles has come down to the lowest ever level and the government of India has targeted for “measles elimination” by 2020.[16] The outbreak-based measles surveillance is being extended to additional states of India.
The other developments during this period were conducting special immunization weeks from April to July 2014 to increase coverage in those districts of the country that are performing poorly in immunization, and completion of second post-introduction evaluation (PIE) of Hib-containing pentavalent vaccine in February March 2014, in six states where vaccine was introduced in late 2012 and early 2013.[1]
On policy and program front, the second comprehensive Multi-Year Strategic Plan (cMYP) for the UIP, for the period of 2013–17 was approved. This was the first time that detailed costing and financing for the immunization program was undertaken and included in the cMYP of India.[15] During the year, the Government of India received a US$ 107 million (or INR 642 crores) grant from the Gavi: The vaccine alliance for immunization-related health system strengthening. On behalf of the Government of India, this 3 year grant is being administered by three development partners.[17]
These events in the sphere of vaccines and immunization over the last few years indicate a dynamic and rapidly evolving scenario, where it is likely that the development and introduction of new vaccines in India could shape the global vaccine market in the years to come. These developments also corroborate and strengthen the case for greater investment in research and development of vaccines, strengthening of vaccine-preventable disease surveillance and improvement in the coverage of vaccines being offered as part of UIP in the country.
Discussion
Much of the progress in UIP of India is appreciated; however, this is less than what one would expect considering socio-economic development in the country in the recent years and what has been achieved during the similar period by comparator countries such as Brazil, Russia, China and South Africa etc. A “World Bank” report in the year 2000 noted that the immunization program coverage is a very good indicator of health system performance. It noted that the usefulness of immunization coverage is not simply a measure of the implementation of a health intervention, but a proxy for the overall performance of the “health system” to support priority health interventions.[18]
The health system comprises of “all the organizations, institutions and resources that are devoted to producing health actions”. The “health actions” are defined as “any effort, whether in personal health care, public health services or through inter-sectoral initiatives, whose primary purpose is to improve health.”[19] In a widely accepted view, health system includes all public and private organizations, institutions and resources mandated to improve, maintain or restore health within the political and institutional framework of each country. The “health system” approach provides an opportunity to look at the systems comprehensively.
There are a number of frameworks evolved in the recent years to explain the health system approach. Two such frameworks are shown in Figures 1 and 2.[20,21] One that by the WHO consist of six building blocks and the other describes four functions.
Figure 1.
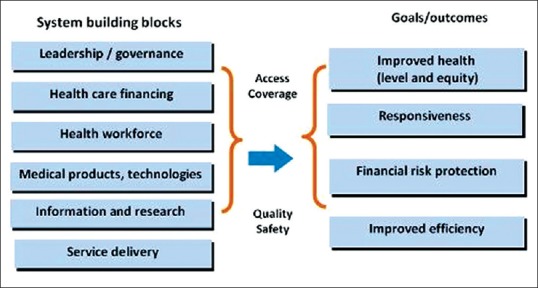
WHO Health system framework
Figure 2.
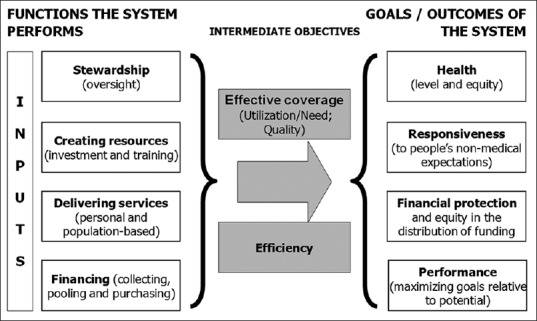
Health system framework
It is generally agreed that health systems strive for four final outcomes/goals, which includes improving health levels and equity. The other goals of health systems are to meet the non-medical needs and expectations of the people (responsiveness); protect individuals from the financial consequences of disease (Financial protection or affordability), and ensure minimal cost and maximum output (Efficiency). These final outcomes/goals are achieved by means of a few intermediate objectives such as increasing access, improving utilization, and assuring the quality of care, etc.
The relationship between resources used and results achieved unfolds through the inter-play of four sets of repeated activities or often termed functions of health systems:
Health services provision, refers to the actors and institutions actually providing the care to the population, i.e. mainly health professionals and hospitals. The health providers may include public, private for profit, not for profit institutions, employed or self-employed actors. Improving health requires preventive, promotive, therapeutic, diagnostic (laboratory and radiological), rehabilitative and palliative services targeted at individuals (–personal health services), and/or groups and society at large (–population health services)
Health financing, refers to how health systems are funded, that how people pay for the care they need or receive and how healthcare providers are paid for the services and products they deliver. These include collection, pooling of the funds, strategic purchase, etc., The point is that the out of pocket payment should be minimum and the mechanisms such as prepayment through insurances, which can be through either private institutions or public healthcare institutions, should be encouraged
Resource-generating component refers to education, training, recruitment, distribution and retention of health workforce as well as development and availability of medical products (drugs and diagnostics) and institutions
Stewardship function refers to overall supervision and coherence of health systems and setting rules as well as providing strategic direction for all the actors involved. The function is mainly related to the establishing and managing appropriate health information system, steering role of policy makers and program managers, leadership and governance, amongst others.
These functions are achieved through a series of inputs: Human resources, materials, supplies, equipments, infrastructure, information system, etc.
Thus, a health system approach could provide an opportunity to look holistically at all components (contrary to if this approach is not followed, there is risk of some component being missed and undue attention on select aspects) of the immunization programs. Thus any shortcomings in health systems reflect on the immunization outcomes of the country, and any improvements is likely to contribute to strengthening not only the immunization services, but may have a tonic effect on the provision of other aspects such as maternal and child health services.
The effect of routine immunization program implementation on health systems has not been documented in detail for multiple reasons. However, the impact of new vaccine introduction on improving immunization coverage and strengthening health system has been reviewed in the recent years. An analysis from recent evidence suggest that introduction of new vaccine may have variable effect on immunization coverage; it contributes to improving health systems, provided a focused attention is paid to the key health system functions.
An inference could be drawn from Table 1, which shows that the states such as Goa, Punjab and Southern Indian states which have high immunization coverage also have better functioning health systems. If focus is restricted to a few components of immunization program delivery only, it may improve the delivery of a few antigens in a short period but sustainability would be difficult. Another example is that the states with reportedly not so well-functioning health system have lower coverage with DPT booster dose than states which have stronger health systems. In India, one of the reasons attributable to sub-optimal and erratic immunization coverage could be limited attention on systematic approaches and the health systems continue to remain weak. In the following sections, this article analyzes UIP in India, as an example, with an underlying hypothesis that with minor variations the challenges remain similar in LMICs, to suggest how a health system approach could be adopted to achieve desired final goals in immunization programs.
Governance and stewardship
The governance or stewardship includes strengthening policy decision-making process, regulation and improving health intelligence. In the last few years, there have been stronger institutional mechanisms for policy decision making in India such as setting up the National Technical Advisory Group on Immunization (NTAGI) and a few state level technical advisory groups as well. These have guided the decision making and implementation process in the country and a large part of the progress could be attributed to these mechanisms. However, there are existing challenges such as weak disease surveillance system. The Acute Flaccid Paralysis (AFP) surveillance system for polio eradication has demonstrated that with right kind of political will an efficient and effective surveillance systems can be established even in resource limited settings and at a relatively low cost. Another existing challenge is slow decision making on inclusion (or not) of new vaccines in national schedule. It is expected that a mechanism such as National Technical Advisory Group on Immunization will be further empowered in coming years to expedite the decision making. The process would be followed as outlined in the National Vaccine Policy of India and these policy documents would be considered live documents, with expectation of regular update.
Immunization information systems help staff plan and manage immunization activities and resources while ensuring that adequate quantities of vaccines are always available to meet the demand. A well-functioning health information system is one that ensures the production, analysis, dissemination and use of reliable and timely information on health determinants, health systems performance and health status. Various studies suggest that in order to have accurate immunization coverage reports in a country there is need to develop efficient monitoring information systems.[18] Accurate monitoring and evaluation of immunization coverage is necessary to inform decision making, to measure success in delivering vaccines, and to provide knowledge of health system bottlenecks. Developing ways of measuring change in key dimensions of the health system can guide resource allocation to where it is needed most and will improve accountability.
The weak surveillance systems in India, at times, hinder the availability of accurate information on the disease burden of VPDs. There is a need for more studies to quantify disease burden to assist the policy makers to informed decisions about the introduction of new vaccines, and more country-specific cost effectiveness analysis of new vaccines in India. There is lack of institutional mechanisms to coordinate appropriate research into disease burden studies and cost-effectiveness analysis. However, things appear to be improving and one example of higher stewardship for the program is that on 3 July 2014 Prime Minister's Office (PMO) of India announced the introduction of four new vaccines as part of India's UIP: IPV (inactivated polio vaccine), rubella vaccine; rotavirus vaccine and JE vaccine for adults in 179 endemic districts.[22] Which indicates highest level of political leadership and commitment.
Service delivery
Many of the infants and children in India are not vaccinated because immunization services are not accessible to the families of these children. Therefore, good micro-planning and close monitoring of the program are the possible way out to increase accessibility and availability of services. This has been achieved in polio eradication in the country and could be hoped that the lessons be learnt and closely replicated in the routine immunization program as well. The introduction of a new vaccine should be viewed as an opportunity to strengthen immunization systems, increase vaccine coverage and reduce inequities of access to health services. This has successfully been done and learnt from the introduction of Haemophilus influenzae type b (Hib) containing pentavalent (DPT+HepB+Hib) vaccine in selected states of India. There is need for innovative methods to increase service utilization and need of operational and implementation research in the field. The time has come that novel approaches of social marketing and community mobilization are adopted and extensively used in immunization programs in LMICs.
In the coming months and year, there would be need for increasing involvement of private practitioners in immunization service delivery. There is a vast network of pediatricians and general practitioners in India (and also in other LMICs settings), who administer vaccines. Their role needs to be optimally utilized for increasing awareness and vaccine coverage.[23] Their knowledge and expertise in vaccine preventable disease and adverse events following immunization (AEFI) surveillance could be vital and instrumental in improving vaccine coverage.
Creating resources
Immunization program requires sufficient resources in terms of vaccines, supply chain (cold chain equipment's and storage points), vaccinators and other aspects. Availability of sufficient resources for the immunization program in the coming years would need that training schools and training facilities are well equipped and are in functioning status to produce sufficient human-resource and facilities to deliver vaccines in a safe and effective manner. Strengthening of recording and reporting system, especially through use of information and communication technology (ICT), is needed. Setting up new vaccine manufacturing units has to be an integral part of assuring vaccine security in the countries.
Financing immunization program
UIP in India is a 100% centrally sponsored program, where central government supplies the vaccines to the states and also pay the salary of various category of staff. Insufficient financial investment on vaccine research and not setting up new vaccine manufacturing units often delay the availability of new vaccines in the immunization programs in LMICs. The additional and dedicated public sector funding is required for conducting vaccine research and making vaccines available and to increase uptake of the vaccines in LMICs. The new vaccines are relatively costlier (often referred “Dollar vaccines”) than traditional vaccines (“Penny vaccines”). Therefore, any decision to introduce new vaccine is likely to increase the cost of the program and would require sustained funding even if initial funding support is provided through external funding agency. There is felt need for use of various funding sources, including those of health system strengthening efforts, and the opportunities which come with the introduction of new vaccines, polio eradication and measles elimination-related efforts. The countries have to utilize these opportunities to increase immunization program performance and to take measures to prevent that undue delay in providing benefits of new vaccines to the children most in the need.
The goal of financing for immunization is to ensure adequate spending on immunization (relative to income at national, local government and household levels) and effective allocation of financial resources to different components of immunization services. The government also needs to invest more on vaccine research. Indian vaccine manufacturers provide nearly half to two-third of all vaccine supply to the developing world through UNICEF or Gavi: The vaccine alliance and the industry needs to be more innovative and should increase and sustain funding for vaccine research and manufacturing.[24] These innovations have to come from public sector as well. There is need for closer coordination and linkage between Department of Biotechnology (In Ministry of Science and Technology) and Department of Health (In the Ministry of Health and Family Welfare, Government of India) to support these mechanisms.
In the future, more funding for UIP will be needed, e.g. The Government of India currently spends over Indian Rupees 200 crores (US$ 30 Million approx.) annually on the procurement of the six UIP vaccines (excluding the Pulse Polio Immunization Program)[25]. Inclusion of vaccines against infections such as rotavirus, mumps, measles, rubella (MMR), human papilloma virus (HPV), pneumococcal, meningococcal and others in any national program, whether universal or selective, will push the total Government procurement budget and this should be factored in for costing and financing of immunization program in India.
Intermediate objectives of health system
There is need for making targeted efforts and activities directed toward increased utilization (effective coverage) of the immunization services at various health facilities, by the people. Similarly, the combination vaccine has improved the efficiency of the service delivery and health system. On the “outcomes” of the health systems, there are evidence that program reviews and new vaccine introduction are opportunities to improve equity (earlier, if the vaccines was available in private sector and only those who could afford to pay were able to get this vaccine).[26,27,28] With introduction of new vaccines in public program, these become universally available for all children. The free of cost availability of the vaccines in public program ensures the financial protection.
A specific case study of health system approach contributing in the introduction of new interventions such as pentavalent vaccine in two states of India in 2011–12 has been documented. The opportunity to introduce Hib as pentavalent (DPT+HepB+Hib) vaccine was used to prepare health system to introduce this vaccine [Box 1].[26,27] This experience from India suggests that the health systems could be strengthened prior to the introduction of new health interventions (including vaccines) and the introduction of new interventions (including vaccines) could be planned in a way to strengthen the health system in the due course.
Box 1.

Conclusions
The immunization programs in all low- and middle-income countries (LMICs) are maturing at a rapid speed. In India, noticeable developments have taken place which have been documented in this article. The analysis indicates that adoption of health system approach by the countries has potential to accelerate the performance of immunization programs. The immunization programs could be one of the entry points for strengthening health systems in the countries and lessons from vaccine introduction could pave pathway for scaling up of other health interventions, as well. As the countries are embarking upon journey towards Universal Health Coverage (UHC), the learning and initiatives for scaling up coverage in immunization programs and experiences from new vaccines introduction, combined with health system approach, should be optimally utilized for expansion of other health interventions. The policy makers and program managers in LMICs may take note of these observations for better health outcomes in their settings.
Acknowledgement
The contribution of Dr. Neelesh Kapoor and Dr. Priyanka Singh in the review of literature for this article is highly appreciated.
Footnotes
Disclaimer: The opinions expressed in this review article are solely of the author and should not be attributed to any institution/organization; he has been affiliated in the past or at present.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Lahariya C. A brief history of vaccines and vaccination in India. Indian J Med Res. 2014;139:491–511. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global routine Vaccination Coverage, 2012. Weekly Epidemiological Rec. 2013;88:482–5. [Google Scholar]
- 3.Geneva: World Health Organization; 2013. WHO and UNICEF. Global Immunization Data-2012; pp. 1–4. [Google Scholar]
- 4.New Delhi: Ministry of Health and Family Welfare; 2005. Government of India. Multi Year strategic Plan for Universal Immunization Program in India (2005-2010) [Google Scholar]
- 5.UNICEF Coverage Evaluation survey, 2009 National Fact Sheet. [Last accessed on 2009]. Available from: http://www.unicef.org/india/National_Fact_Sheet_CES_2009.pdf .
- 6.International Institute of Population Sciences. District Level Household Survey -2 (DLHS 2): IIPS Mumbai and ORC Macro, Maryland. [Last accessed on 2014 Jul 14]. Available from: http://www.rchiips.org/pdf/rch2/National_Report_RCH.II.pdf .
- 7.International Institute of Population Sciences. District Level Household Survey -3 (DLHS 3): IIPS Mumbai and ORC Macro, Maryland. [Last accessed on 2014 Jul 14]. Available from: http://www.rchiips.org/pdf/INDIA_REPORT_DLHS-3.pdf .
- 8.New Delhi: Ministry of Health and Family Welfare, Nirman Bhawan; 2013. Govt. of India. Health management Information System (HMIS data, MoHFW, Govt. of India, as on Oct 2013, as presented in SEPIO Training workshop. [Google Scholar]
- 9.Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, et al. for the India Rotavirus Vaccine Group. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: A randomised, double-blind, placebo-controlled trial. Lancet. 2014;383:2136–43. doi: 10.1016/S0140-6736(13)62630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhan MK, Glass RI, Ella KM, Bhandari N, Boslego J, Greenberg HB, et al. Team science and the creation of a novel rotavirus vaccine in India: A new framework for vaccine development. Lancet. 2014;383:2180–3. doi: 10.1016/S0140-6736(14)60191-4. [DOI] [PubMed] [Google Scholar]
- 11.Pilla V. Mint, Delhi: 2013. Typhoid vaccine with longer immunity launched; p. 11. [Google Scholar]
- 12.Govt. of India. Press release from Press Information Bureau: Shri Ghulam Nabi Azad launches JE Vaccine (JENVAC) produced by NIV, ICMR and Bharat Biotech. Govt of India; MoHFW, New Delhi. 04 Oct 2014. [Last accessed on 2014 April 10 2014;21:30 IST]. Available from: http://pib.nic.in/newsite/erelease.aspx?relid=99873 .
- 13.Dhar A. India launches vaccine to prevent Japanese encephalitis: JENVAC is the first vaccine to be manufactured in the public-private partnership mode. Hindu, New Delhi. 2013. [Last accessed on 2014 April 10 2014;21:30 IST]. Available from: http://www.thehindu.com/news/national/india-launches-vaccine-to-prevent-japanese-encephalitis/article5201813.ece .
- 14.Isalkar U. New Delhi: [Last accessed on 2013 Mar 8]. Serum Polio vaccine likely in National Plan. Times of India. Available from: http://timesofindia.indiatimes.com/city/pune/Serums-pol i o-vaccine-likely-in-national-plan/articleshow/18856737.cms . [Google Scholar]
- 15.World Health Organization. WHO South-East Asia Region is officially certified polio-free. WHO South-east Asia regional office, New Delhi. 2014. [Last accessed 2014 April 10 21:30 IST]. Available from: http://www.searo.who.int/entity/campaigns/polio-certification/en/
- 16.Vaidya SR. Commitment of measles elimination by 2020: Challenges in India. Indian Pediatr. 2015;52:103–6. doi: 10.1007/s13312-015-0580-7. [DOI] [PubMed] [Google Scholar]
- 17.GAVI Alliance. Health Systems Stregthening proposal India. [Last accessed on 2014 Mar 28 at 19:30 IST]. Available from: http://www.gavi.org/country-documents/IND_201304_Proposal_HSS.pdf .
- 18.Bos E, Batson A. Washington: The World Bank; 2000. Using Immunization Coverage for monitoring health sector performance: Measurement and interpretation issues. Health and Nutrition Discussion Paper. [Google Scholar]
- 19.Geneva: World Health Organization; 2000. World Health Organization. World Health report 2000: Health Systems: Improving performance. [Google Scholar]
- 20.Geneva: World Health Organization; 2007. World Health Organization. Everybody's business: Strengthening health system to improve health outcomes – WHO's framework for action. [Google Scholar]
- 21.Duran A, Kutzin J, Martin JM, Travis P. Understanding health systems: Scope, functions and objectives. In: McKee M, Figueras J, editors. Health Systems: Health, Wealth, Society and well-being. New york: Open University Press, McGraw Hill; 2011. pp. 19–37. [Google Scholar]
- 22.New Delhi: Ministry of Health and Family Welfare, Government of India; 2011. [Last accessed 2014 Jul 14]. PMO Press release 03 July 2014. Government of India. National vaccine policy. Available from: http://www.mohfw.nic.in/WriteReadData/l892s/1084811197NATIONAL%20VACCINE%20POLICY%20BOOK.pdf . [Google Scholar]
- 23.Vashishtha VM, Choudhury P, Kalra A, Bose A, Thacker N, Yewale VN, et al. Indian Academy of Pediatrics (IAP) recommended immunization schedule for children aged 0 through 18 years--India, 2014 and updates on immunization. Indian Pediatr. 2014;51:785–800. doi: 10.1007/s13312-014-0504-y. [DOI] [PubMed] [Google Scholar]
- 24.Gupta SS, Nair GB, Arora NK, Ganguly NK. Vaccine development and deployment: Opportunities and challenges in India. Vaccine. 2013;31(Suppl 2):B43–53. doi: 10.1016/j.vaccine.2012.11.079. [DOI] [PubMed] [Google Scholar]
- 25.GAVI Alliance. GAVI Annual Progress Report 2012. [last accessed on 2014 Mar 28]. Available from: http://www.gavi.org/country/india/documents/
- 26.Gupta SK, Sosler S, Lahariya C. Introduction of Haemophilus Influenzae type b as liquid pentavalent (DPT+HepB+Hib) vaccine in 2 states of India. Indian Pediatr. 2012;49:707–9. doi: 10.1007/s13312-012-0151-0. [DOI] [PubMed] [Google Scholar]
- 27.New Delhi, India: WHO; 2013. World Health Organization Country Office for India. Post Introduction Evaluation of Pentavalent (DPT+HepB+Hib) vaccine in Tamil Nadu and Kerala, India 2012; pp. 11–48. [Google Scholar]
- 28.Lahariya C, Subramanya BP, Sosler S. An assessment of hepatitis B vaccine introduction in India: Lessons for roll out and scale up of new vaccines in immunization programs. Indian J Public Health. 2013;57:8–14. doi: 10.4103/0019-557X.111357. [DOI] [PubMed] [Google Scholar]


