Abstract
Aim:
The aim was to evaluate the role of high sensitivity troponin T and ischemia modified albumin (IMA) and in the early diagnosis of acute coronary syndrome (ACS).
Materials and Methods:
This was a cross-sectional study that comprised of 120 individuals of which 75 were cases and 45 healthy controls. On the basis of clinical history and 12-lead electrocardiogram, initial diagnosis of ACS was made in the cases. High sensitive cardiac troponin T (hs-cTnT) and IMA were measured in all the individuals.
Results:
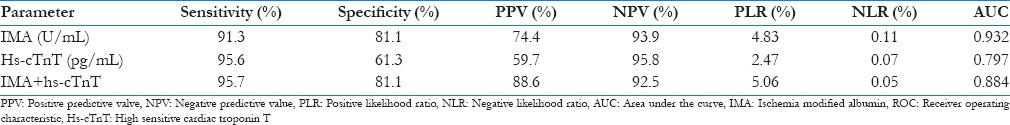
Levels of IMA were significantly higher in patients of ACS as compared to those in control group (means: 101.83 [95% confidence interval (CI): 91.96–111.70] vs. 41.11 [95% CI: 38.55–43.67]). By taking the cut-off as >65.23 U/mL for IMA, which was obtained from receiver operating characteristic (ROC) curve, the sensitivity was 91.3%, specificity was 81.1%, positive predictive value (PPV) was 74.4%, and negative predictive value (NPV) was 93.9%. Positive likelihood ratio was 4.83 while negative likelihood ratio was 0.11, whereas the corresponding values in case of hs-cTnT were 95.6% (95% CI: 85.2–99.5), 61.3% (95% CI: 49.5–72.6), 59.7%, 95.8%, 2.47 and 0.07 by taking cut-off as >14 pg/mL. The area under the ROC curves (AUC) of IMA and hs-cTnT at 0–6 h were 0.932 (95% CI: 0.87–0.97, P < 0.001) and 0.797 (95% CI: 0.71–0.86, P < 0.001), respectively. The logistic model combining the two markers yielded sensitivity, specificity, PPV, and NPV of 95.7%, 81.1%, 88.6%, and 92.5% respectively.
Conclusion:
hs-cTnT and IMA may be useful tools for risk stratification of ACS and can be used together with better accuracy in the early diagnosis of ACS.
Keywords: Acute coronary syndrome, high sensitivity troponin T, ischemia modified albumin
Introduction
Acute coronary syndrome (ACS) refers to a spectrum of clinical presentations caused by acute myocardial ischemia, ranging from those for ST-segment elevation myocardial infarction (STEMI) to presentations found in non-STEMI (NSTEMI) or in unstable angina (UA), which are categorized based on the 12-lead electrocardiogram at presentation. In the third universal definition of myocardial infarction, cardiac troponin T (cTnT) is defined as a standard for detection of myocardial ischemia.[1] The sensitivity of cTnT for the diagnosis of AMI was observed to be lower within 4 h of chest pain (55%) than at ≥12 h (97%) after presentation.[2] Hence, newer high-sensitivity cardiac assays like hs-troponin T (hs-cTnT) with increased analytical sensitivity and lower limits of detection are being used, which has improved the identification of patients with AMI presenting in the first 3 h following the onset of symptoms. A variety of clinical conditions other than myocardial infarction may be associated with elevated cardiac hsTnT levels because of its lower limits of detection.[3,4] Since the level of troponin rises only when there is an evidence of myocardial necrosis, biomarkers that are able to identify and differentiate the patients with chest pain of etiology other than myocardial necrosis, might serve an important role in a clinical setting. A novel biomarker for the detection of myocardial ischemia is ischemia modified albumin (IMA) which is produced because of alteration of human serum albumin by ischemia, and has recently been evaluated as a marker of ischemia.[5] The advantage of this assay over hs-cTnT is that the levels of IMA are positive within minutes of ischemia and remains elevated for up to several hours, allowing detection before the development of myocardial necrosis.[6] Hence, a negative IMA result may help in moving the patients into a low-risk category by initial evaluation based on the clinical presentation, thereby providing a major cost saving.[7] Since no study has been found in India showing the utility of IMA with hs-cTnT in ACS, the aim of this study was to evaluate the analytical performance of these markers together in patients of ACS.
Materials and Methods
The study having 1-year duration was carried out at Shree Krishna Hospital and H M Patel Centre for Medical Care and Education, a 550 bedded tertiary care rural based, teaching hospital attached to Pramukh Swami Medical College, Karamsad, from August 2013 to August 2014. A study protocol was set before undertaking this study, and it was approved by Institutional Human Research Ethical Committee. The present study comprised of 120 individuals between the age of 30 and 80 years which included 75 patients admitted in the emergency department and intensive cardiac care unit within 6 h of clinical signs and symptoms of ACS. 45 out of 75 patients who had acute chest pain and evidence of myocardial ischemia (their hs-cTnT being <100 pg/mL but had more than 14 pg/mL in initial 6 h of presentation and further rose by 100% after 6–12 h of first estimation) were included in group 1 (ischemic chest pain), while 30 out of 75 patients who had acute chest pain, but no subsequent evidence of cardiac involvement (their hs-cTnT was <100 pg/mL but more than 14 pg/mL and did not have significant rise after 6–12 h of first estimation) was included in group 2 (nonischemic chest pain). As a control (group 3) 45 normal individuals coming for routine checkup were taken. Diagnosis of myocardial infarction was done on the basis of the third universal definition of myocardial infarction. All the patients with sepsis, pulmonary embolism, peripheral vascular disease, stroke, liver cirrhosis, and renal failure were excluded. Blood was collected from all the enrolled patients within 1 h of admission before any treatment was started, in red top vacutainer tubes, containing no anticoagulant. Specimens were routinely centrifuged within 1 h of collection for 15 min at 2000 rpm, and serum was separated. hs-cTnT were estimated immediately after the separation of serum. After estimation of the tests, sera were frozen at −20°C for IMA estimation at a later stage. hs-cTnT was measured quantitatively using electrochemiluminescence (ECL immune assay) based on ECL technology (high sensitivity troponin T, Cobas e411, Roche, Mannheim, Germany). Measuring range: 3.0–10,000 pg/mL. IMA was estimated using albumin cobalt binding (ACB) test.[5] IMA assay was standardized in the Department of Biochemistry using different concentrations of CoCl2 ranging from 10.0 to 40.0 μg CoCl2/mL. Standard curve was prepared in the range 10.0–40.0 μg CoCl2 /mL. One IMA unit was defined as “μg of free Co+2 in the reaction mixture per milliliter of serum sample.” Calibration was done before running each batch of samples using freshly prepared CoCl2 and dithiothreitol reagents. Before running each batch control was done using known patient sample from a previous batch which had been frozen after the test.
Statistical analysis
Statistical analysis was performed using the commercially available statistical software SPSS for Windows, Version 14.0.SPSS Inc., Chicago), MedCalc for Windows, version 12.5 (MedCalc Software, Ostend, Belgium) and Microsoft Excel. The P < 0.05 was considered statistically significant. The unpaired t-test was applied for the statistical analysis and the results were expressed in mean ± standard deviation. Receiver operating characteristic (ROC) curve analysis and calculation of the area under the curve (AUC) were done for all parameters included in the study population according to the method of Hanley and McNeil. The optimum cut-off was used to dichotomously classify the positive or negative serum IMA and hs-cTnT levels, and it was also used for calculating the diagnostic sensitivity and the specificity.
Results
The common age group of 120 individuals enrolled was between 30 and 80 years (divided into 45 patients of ischemic chest pain, 30 patients of nonischemic chest pain and 45 healthy individuals as controls) with a mean age of 58.08 ± 12.93. Among those 120 individuals, 77 were males and 43 were females [Table 1 and Figure 1].
Table 1.
Demographic data of all individuals included in study groups (n=120)
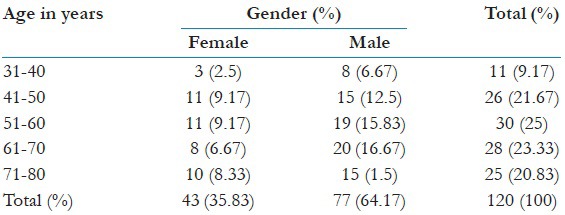
Figure 1.
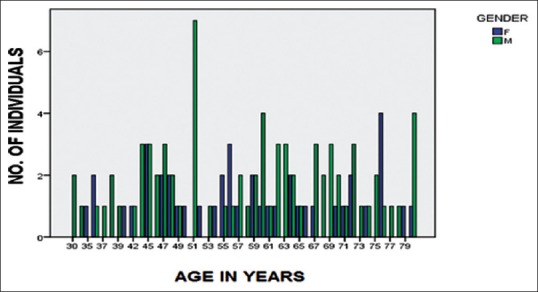
Age and gender wise distribution of all individuals included in study groups
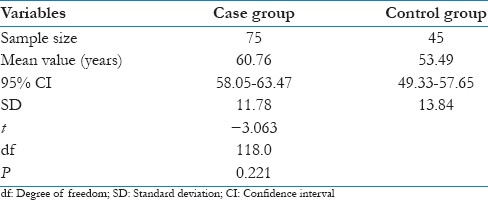
Among 45 patients from ischemic chest pain group (26 patients with ST segment elevation, 19 patients without ST segment elevation which included patients with ST depression and left bundle branch block) had a mean age of 61.29 ± 10.76 and among them 31 individuals were males. The 30 patients from the nonischemic chest pain group had a mean age of 60.07 ± 12.89 and among them 19 were males. The control group consisting 45 individuals had a mean age group of 53.49 ± 13.84 and among them 27 individuals were males. The unpaired t-test showed t = −3.063, degree of freedom = 118 and P = 0.221. This showed that there was no significant difference between these age groups [Table 2].
Table 2.
Comparison of age between case and control groups
The IMA values for the control reference population are normally distributed. Values for the control reference population [Table 3 and Figure 2] are 17.81–54.03 U/mL (mean, 41.11 U/mL; median, 40.27 U/mL). The upper 95th percentile was 44.81 U/mL.
Table 3.
Summary of distribution of serum IMA levels amongst different study groups
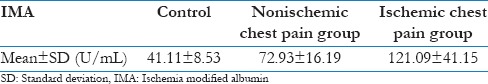
Figure 2.
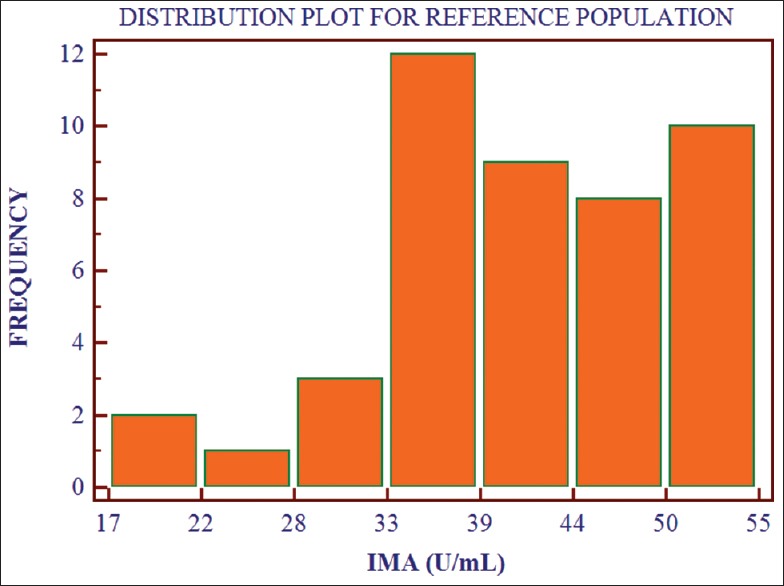
Distribution of ischemia modified albumin values for the control reference population (n = 45)
To find out whether there was any correlation of serum IMA levels in between case and control group, unpaired t-test was performed, and P value was derived. The value P ≤ 0.001 suggested a significant difference in values of IMA between these two groups. Similarly, when unpaired t-test was performed between ischemic and nonischemic chest pain group, the value P = 0.012 suggested that levels of IMA in patients of chest pain with ischemia were statistically significantly higher compared with patients of chest pain without ischemia [Tables 4 and 5, Figures 3 and 4].
Table 4.
Comparison of serum IMA levels between case and control group
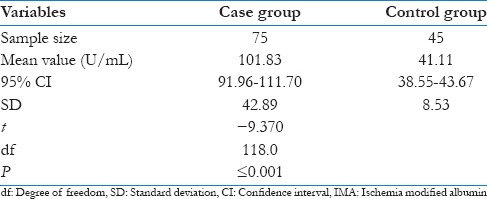
Table 5.
Comparison of serum IMA levels between ischemic chest pain and nonischemic chest pain groups
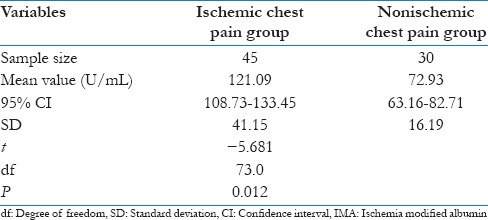
Figure 3.
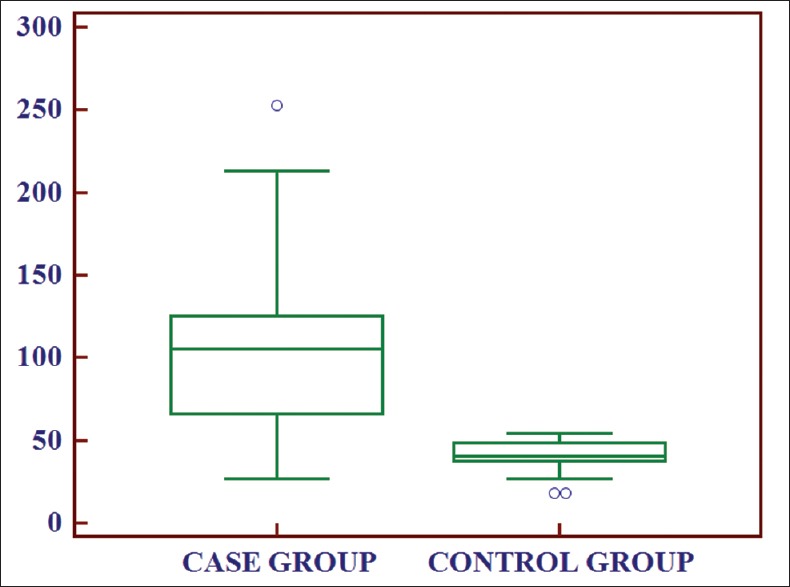
Box and whisker plot showing comparison of ischemia modified albumin levels between case and control group
Figure 4.
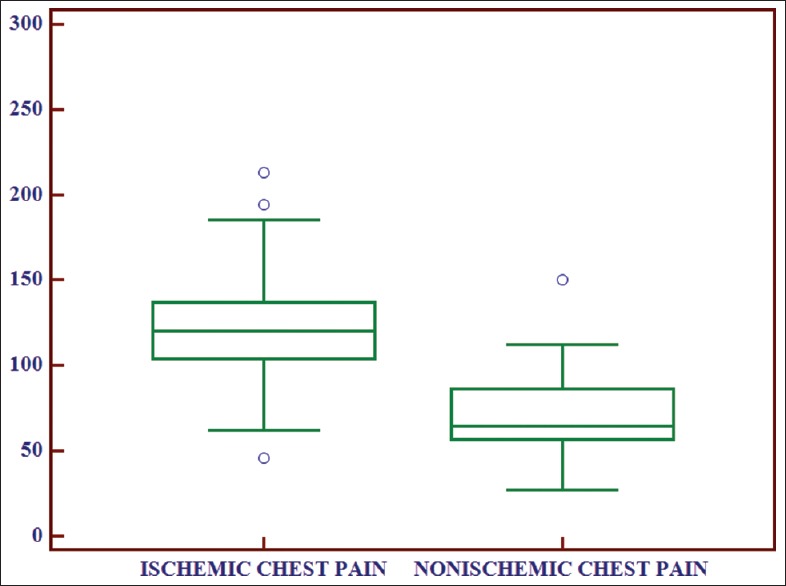
Box and whisker plot showing comparison of ischemia modified albumin levels between ischemic chest pain and nonischemic chest pain group
Receiver operating characteristic curves were constructed to test the performance of IMA and hs-cTnT for rule-in and rule-out of AMI and earlier prediction in patients with an initially less hs-cTnT result that turned positive within 6–24 h after admission. Sensitivities, specificities, negative predictive, and positive predictive values (NPV and PPV) were calculated from ROC curves. Statistics was used to compare the AUC of the ROC curves. The AUC for IMA and hs-cTnT in initial 6 h was 0.932 (0.87–0.97; P < 0.001) and 0.797 (0.71–0.86; P < 0.001), Respectively [Figure 5]. On considering the cut-off points as >65.23 for IMA and >14 for hs-cTnT which were obtained from ROC curve, the statistical data are shown in Table 6. Sensitivity (95.6%) and NPV (95.8%) was higher for hs-cTnT while specificity (81.1%) and PPV (74.4%) was higher in for IMA. By combining IMA with hs-cTnT, the sensitivity, specificity, PPV, and NPV were 95.7%, 81.1%, 88.6%, and 92.5%, respectively. The PLR was 5.06 and NLR was 0.05.
Figure 5.
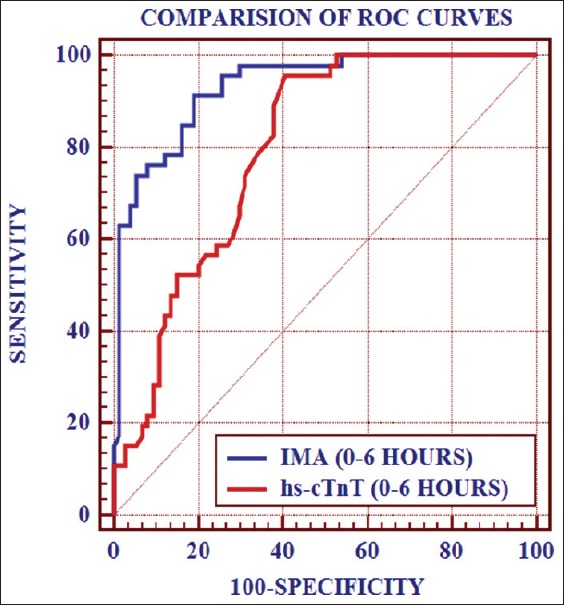
Comparison of receiver operating characteristic curves of ischemia modified albumin and high sensitive cardiac troponin T
Table 6.
Sensitivity, specificity, PPV, NPV, PLR, NLR, and AUC of IMA and hs-cTnT (individually and after combining both parameters) during initial 6 h of presentation at the optimum cut-off value obtained from the ROC taking significant rise in hs-cTnT after 6-12 h as standard marker
Discussion
An ideal diagnostic test should have the ability to triage majority of patients without MI for safe discharge rapidly and correctly to ensure efficient appropriation of reserves in the emergency department settings with low threshold for “missed MI's.” The current scenario is changing to high sensitive troponin assays which have 10–100 folds lower detection limits than conventional assays. With a decrease in the diagnostic cut-off by implementing more sensitive and precise assays, sensitivity is further increased but specificity is decreased owing to detection of more acute, subacute, and chronic cardiac diseases not related to ACS.[8,9]
Under these circumstances, necessity calls for the availability of such a marker which helps in the rule in and rule out criteria for acute myocardial infarction especially in early presenters. IMA is a novel biomarker that has shown to improve the diagnosis in many studies and can support the early diagnosis of myocardial infarction.[10] Hence, in this study, we have reported the diagnostic utility of IMA and compared that with the hs-cTnT assay for the evaluation of patients with ACS.
In the present study, when serum IMA levels were compared between group 1 (ischemic chest pain) and group 2 (nonischemic chest pain), the levels of IMA were significantly higher in ischemic chest pain group as compared to nonischemic chest pain group (P = 0.012). Similarly, P < 0.001 on comparing levels between ischemic chest pain group and control group. Sensitivity and NPV were higher for hs-cTnT while specificity and PPV were higher in for IMA. The combination of IMA with hs-cTnT yielded an AUC of 0.884 (95% confidence interval [CI]: 0.820–0.948, P < 0.001) with better sensitivity, specificity, PPV, and NPV as well. Our results are in complete agreement with Patil et al.,[11] Gurumurthy et al.,[12] Ertekin et al.,[13] and Pan et al.[14] as sensitivity, specificity, PPV, and NPV of the present study were found to be similar with those studies.
Patil et al.[11] conducted a study on 102 patients of acute chest pain in which IMA and troponin I were estimated. Ischemia modified albumin (P < 0.05) and troponin I (P < 0.001) concentrations were significantly higher in acute myocardial infarction and in UA than in the healthy controls. The sensitivity and specificity of ischemia modified albumin for the detection of ACS was 88% and 93% as compared to 87% and 75%, respectively, for troponin I. The combined use of ischemia modified albumin and troponin I significantly enhanced the sensitivity to 96%. The area which was under the ROC curve of ischemia modified albumin in ACS was 0.90.
In a study conducted by Gurumurthy et al.,[12] 540 patients were included, and IMA was estimated. Mean serum IMA levels (U/mL) in patients with STEMI (92.1 ± 10.6), NSTEMI (87.3 ± 5.95), and UA (88.9 ± 6.16) were significantly higher than noncardiac chest pain (77.9 ± 6.69) and also healthy subjects (54.7 ± 17.2) (P < 0.001). ROC curve analysis of IMA in serum revealed that the cut-off value above which IMA can be considered positive was 84.4 U/L. The area under the curve was found to be 0.933 with 95% CI (0.911–0.954) (P < 0.0001). The sensitivity and specificity were found to be 88 and 89%, respectively.
Ertekin et al.[13] carried out a study on 30 ACS patients in which statistically significant higher IMA values were determined in the patient groups compared to the control group (P < 0.001 for both groups). ROC curve analysis of IMA in serum was done. The area under the curve was found to be 0.898. The sensitivity, specificity, PPV, and NPV were found to be 83%, 90%, 89%, and 84%, respectively. The PLR was 2.25 and NLR was 0.17.
Pan et al.[14] conducted a similar study on 169 patients in which IMA level was detected by ACB test. AUC was 0.754. As the cut-off point for IMA in this study was 70.4 U/mL, the sensitivity, specificity, PPV and NPV of IMA were 79.8%, 65.2%, 77.7%, and 69.7% respectively. The sensitivity and NPV of IMA combined with the conventional cardiac marker panel for the diagnosis of ACS were 93.4% and 86.0%, respectively. In this study, the sensitivity, specificity, PPV, and NPV of a combination of IMA and MPO were 93.5%, 97.2%, 98.1%, and 90.7%. While in case of combining IMA with hs-cTnT, the corresponding values were 95.7%, 81.1%, 88.6%, and 92.5%.
For effective management in the emergency department, NPV is most essential as negative hs-cTnT value helps to rule out ACS. The gain in sensitivity may be particularly important in patients with a short duration from symptom onset to admission. A negative hs-cTnT test has a high NPV, and may thus serve as an exclusionary test early in the diagnostic process. It was the main advantage of choosing hs-cTnT instead of conventional cTnT.
As multiple cardiac and noncardiac conditions are associated with mild-to-moderate hs-cTnT elevations, serial testing will become increasingly important for identifying those patients with true ACS.[8] By the time, hs-cTnT levels rise on serial dilution irreversible injury occurs in the myocardium. Hence, an added value by early and specific marker like IMA would help in the decision-making power of myocardial ischemia and can help in early intervention. If treatment of AMI is done within 1 h (the golden hour), mortality can be reduced from 9% to 3%, if delay of 3–4 h, mortality could be 5 times higher.[15] IMA levels increase within 30 min of an ischemic episode which has significant advantages over hs-cTnT (which takes 3–6 h to rise to measurable circulating levels after MI) suggesting IMA may be useful in a triage. When using traditional cardiac markers like troponins, there is a substantial delay before the factor is expressed after cardiac damage, and many ischemic episodes do not lead to increased troponin levels. Since hs-cTnT has higher sensitivity and NPV while IMA has higher specificity and PPV, IMA and hs-cTnT comprise an excellent combination which can be used in the early diagnosis of ACS. When the physician is trying to decide whether to send the patient home or keep him in the hospital, the information from these tests will be useful.
Conclusion
From this study, it can be concluded that hs-cTnT assay is a useful diagnostic test that can be performed to rule out ACS in patients with chest pain but because of its high sensitivity, it may give false positive results. Various noncardiac ailments contribute to the rise in the levels of hs-cTnT, hence serial sampling becomes important for making a confirmatory diagnosis, especially in early presenters. Results of hs-cTnT may be less confirmatory in patients who present to the emergency department soon after the onset of chest pain as it increases 3–6 h after onset of chest pain. Under these circumstances, early cardiac biomarker like IMA can be used which adds to the diagnostic efficacy of myocardial infarction and early treatment can be initiated to prevent mortality.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 2.McCann CJ, Glover BM, Menown IB, Moore MJ, McEneny J, Owens CG, et al. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J. 2008;29:2843–50. doi: 10.1093/eurheartj/ehn363. [DOI] [PubMed] [Google Scholar]
- 3.Jeremias A, Gibson CM. Narrative review: Alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med. 2005;142:786–91. doi: 10.7326/0003-4819-142-9-200505030-00015. [DOI] [PubMed] [Google Scholar]
- 4.Roongsritong C, Warraich I, Bradley C. Common causes of troponin elevations in the absence of acute myocardial infarction: Incidence and clinical significance. Chest. 2004;125:1877–84. doi: 10.1378/chest.125.5.1877. [DOI] [PubMed] [Google Scholar]
- 5.Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia – A preliminary report. J Emerg Med. 2000;19:311–5. doi: 10.1016/s0736-4679(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 6.Wu AH. Pathology and Laboratory Medicine. 2nd ed. Totowa, NJ: Humana; 2003. Clinical markers. [Google Scholar]
- 7.Bar-Or D, Curtis G, Rao N, Bampos N, Lau E. Characterization of the Co(2+) and Ni(2+) binding amino-acid residues of the N-terminus of human albumin. An insight into the mechanism of a new assay for myocardial ischemia. Eur J Biochem. 2001;268:42–7. doi: 10.1046/j.1432-1327.2001.01846.x. [DOI] [PubMed] [Google Scholar]
- 8.Apple FS. A new season for cardiac troponin assays: It's time to keep a scorecard. Clin Chem. 2009;55:1303–6. doi: 10.1373/clinchem.2009.128363. [DOI] [PubMed] [Google Scholar]
- 9.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–67. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 10.Peacock F, Morris DL, Anwaruddin S, Christenson RH, Collinson PO, Goodacre SW, et al. Meta-analysis of ischemia-modified albumin to rule out acute coronary syndromes in the emergency department. Am Heart J. 2006;152:253–62. doi: 10.1016/j.ahj.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Patil SM, Banker MP, Padalkar RK, Pathak AP, Bhagat SS, Ghone RA, et al. The clinical assessment of ischaemia modified albumin and troponin I in the early diagnosis of the acute coronary syndrome. J Clin Diagn Res. 2013;7:804–8. doi: 10.7860/JCDR/2013/5288.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurumurthy P, Borra SK, Yeruva RK, Victor D, Babu S, Cherian KM. Estimation of Ischemia Modified Albumin (IMA) Levels in Patients with Acute Coronary Syndrome. Indian J Clin Biochem. 2014;29:367–71. doi: 10.1007/s12291-013-0367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ertekin B, Kocak S, Defne Dundar Z, Girisgin S, Cander B, Gul M, et al. Diagnostic value of ischemia-modified albumin in acute coronary syndrome and acute ischemic stroke. Pak J Med Sci. 2013;29:1003–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Pan SM, Tong CY, Lin Q, Yao CL, Zhao J, Deng Z. Ischemia-modified albumin measured with ultra-filtration assay in early diagnosis of acute coronary syndrome. World J Emerg Med. 2010;1:37–40. [PMC free article] [PubMed] [Google Scholar]
- 15.Alhashemi JA. Diagnostic accuracy of a bedside qualitative immunochromatographic test for acute myocardial infarction. Am J Emerg Med. 2006;24:149–55. doi: 10.1016/j.ajem.2005.08.002. [DOI] [PubMed] [Google Scholar]


