Abstract
Serotonin receptors are the product of 15 distinct genes, 14 of which are G protein-coupled receptors. These receptors are expressed in a wide range of cell types, including distinct neuronal populations, and promote diverse functional responses in multiple organ systems. These receptors are important for mediating the in vivo effects of their cognate neurotransmitter, serotonin, as well as the endogenous tryptamines. In addition, the actions of many drugs are mediated, either directly or indirectly, through serotonin receptors, including antidepressants, antipsychotics, anxiolytics, sleep aids, migraine therapies, gastrointestinal therapeutics and hallucinogenic drugs. It is becoming increasingly evident that serotonin receptors can engage in differential signaling that is determined by the chemical nature of the ligand and that ligands that demonstrate a predilection for inducing a particular signaling cascade are considered to have “functional selectivity”. The elucidation of the cellular signaling pathways that mediate the physiological responses to serotonin and other agonists is an active area of investigation and will be an onward-looking focal point for determining how to effectively and selectively promote beneficial serotonergic mimicry while avoiding unwanted clinical side effects. This review highlights the modulation of serotonin 2A, 2C, and four receptors by β-arrestins, which may represent a fulcrum for biasing receptor responsiveness in vivo.
Keywords: G protein-coupled receptor, functional selectivity, 5-hydroxytryptamine (5-HT), receptor trafficking, homologous desensitization
Introduction
The serotonin receptors, also known as the 5-hyrdoxytryptamine (5-HT) receptors, are expressed in the periphery as well as in the central nervous system and are activated by the neurotransmitter serotonin. The receptor family is divided into seven main types, comprising 15 genetically unique receptors. All of the serotonin receptors are G protein-coupled receptors (GPCR), with the exception of the serotonin 3 receptor (5-HT3R), which is a ligand-gated ion channel. Serotonin receptors expressed in the central nervous system are involved in mediating diverse biological processes, including the modulation of mood, cognition, learning, memory, aggression, feeding behavior, sleep, and pain processing and transmission mechanisms. Receptors expressed in the periphery mediate smooth muscle contraction, platelet aggregation, gastrointestinal function and also impact upon pain processing. Further, activation of serotonin receptors modulates the release of a number of neurotransmitters and hormones. Considering their potential for influencing wide-ranging functions throughout the body, the serotonin receptors are prominent targets for pharmacotherapies, including antidepressants, antipsychotics, appetite suppressants, migraine therapies and drugs for the treatment of gastrointestinal disorders. They are also the direct target of abused drugs, such as the serotonergic hallucinogens lysergic acid diethylamide (LSD) and dimethyltryptamines (DMT) and also indirect targets of psychostimulants such as cocaine and amphetamines.
Canonical regulation of GPCR signaling by β-arrestins
The canonical model of GPCR regulation posits that the agonist bound receptor is regulated through interactions with β-arrestins (reviewed in Ferguson, 2001; Pierce and Lefkowitz, 2001; Luttrell and Lefkowitz, 2002). Upon activation, GPCRs are phosphorylated by GPCR kinases (GRKs), thus initiating the termination of G protein-mediated signaling and promoting the recruitment of β-arrestins to the receptors. The interaction between β-arrestins and GPCRs has been measured by confocal microscopy, co-immunoprecipitation, bioluminescence/fluorescence resonance energy transfer (BRET/FRET), gene reporter and enzyme fragment complementation assays (Barak et al., 1997; Hamdan et al., 2005; Groer et al., 2007; von Degenfeld et al., 2007; Drake et al., 2008; McDonald and Bohn, 2010). There are two forms of β-arrestin, β-arrestin1 and β-arrestin2, which are for the most part, ubiquitously expressed. The two isoforms have very similar amino acid structures and in some cases can substitute in function for each other; however, there is some indication that certain GPCRs may be preferentially regulated by one isoform over the other (Oakley et al., 2000; Kohout et al., 2001).
Following β-arrestin binding to an agonist–receptor complex, structural evidence indicates that β-arrestins sterically block the receptor–G protein interaction and thus play a critical role in the process of homologous desensitization (Gurevich and Gurevich, 2006). Therefore, if the β-arrestin interaction is disrupted, the potential for receptor desensitization may be diminished and relative agonist efficacy may be enhanced. The Lefkowitz group has shown, using mouse embryonic fibroblasts (MEF) derived from genetically modified mice lacking both β-arrestins (βarr1/2-KO MEF), that agonist-induced signaling of β2 adrenergic receptor to adenylyl cyclase and angiotensin II receptor to phospholipase C is enhanced (Kohout et al., 2001). This group has also demonstrated this phenomenon utilizing siRNA gene silencing, wherein β-arrestin ablation enhances agonist induced G protein-signaling through these receptors in HEK293 cells (Ahn et al., 2003).
Role of β-arrestins in facilitating GPCR signaling
In addition to their roles in the desensitization of GPCRs, β-arrestins have also been shown to mediate receptor signaling by acting as scaffolding elements. In this manner, β-arrestins can couple GPCRs to signaling proteins in a manner that is independent of G protein mediated activation (Lefkowitz and Shenoy, 2005; Kovacs et al., 2009). Cell culture studies have shown that β-arrestins can facilitate signaling for many pathways, including the MAP kinase family of serine/threonine kinases, specifically ERK1/2, p38 kinase and c-Jun N-terminal kinases, the Src family of tyrosine kinases, phosphatidylinositol 3 kinase (PI3-K), and protein kinase B (Akt) (Daaka et al., 1998; Luttrell et al., 1999; McDonald et al., 2000; Luttrell and Lefkowitz, 2002). β-arrestin-mediated signaling has been demonstrated for a wide range of GPCRs, including the neurokinin-1, angiotensin, parathyroid hormone, vasopressin, β-adrenergic, chemokine and dopamine receptors (DeWire et al., 2007). Further, a study by Stalheim et al. (2005) demonstrated that β-arrestins are involved in both the desensitization of G protein coupling and the activation of ERK1/2 for the protease-activated receptor-2 (PAR2) in vitro. However, mutation of the C-terminal tail of the receptor, a proposed β-arrestin interaction site, only affected the β-arrestin-mediated activation of ERK1/2, suggesting that the pro-signaling and signaling-arresting functions of β-arrestin are controlled by distinct interactions with the GPCRs.
β-arrestin-mediated internalization of GPCRs
Another important step in the β-arrestin-mediated regulation of GPCRs is the internalization of the agonist-bound receptor into intracellular vesicles. Depending on which path the receptor takes following internalization (whether it facilitates coupling to signaling components or acts to isolate the receptor from signaling components), β-arrestin-mediated internalization can positively or negatively impact GPCR signaling. Interactions with β-arrestins facilitate receptor endocytosis for many GPCRs, including the β-adrenergic, angiotensin, muscarinic, dopamine, mu-opioid and serotonin 2 A receptors (5-HT2AR) (reviewed in Ferguson, 2001; Schmid et al., 2008). Both β-arrestin1 and β-arrestin2 have been shown to directly interact with clathrin and the β2-adaptin subunit of the AP-2 complex (Goodman et al., 1996, Laporte et al., 1999; Kim and Benovic, 2002). The recruitment of these proteins then promotes the assembly of the receptors into clathrin-coated endocytic vesicles. Not surprisingly, interfering with β-arrestin/GPCR interactions has been shown to impact receptor trafficking. For example, mutant β-arrestin proteins inhibit β2-adrenergic receptor internalization (Ferguson et al., 1996). Studies in cell lines demonstrate that the extent of agonist-induced receptor internalization can be influenced by the levels of GRK and β-arrestin protein expressed in the cells, such that overexpression of the proteins can facilitate internalization (Ferguson et al., 1996; Menard et al., 1997; Zhang et al., 1998). The importance of β-arrestin-mediated trafficking of GPCRs is clearly demonstrated in cell lines that lack β-arrestins, wherein certain receptors fail to internalize in response to agonist (Kohout et al., 2001; Stalheim et al., 2005). Receptor endocytosis can serve as a means to compartmentalize receptor signaling, facilitate de-phosphorylation and resensitization or to shuttle the receptor towards a degradation pathway. Therefore, β-arrestins are key components in determining the function and life cycle of a G protein coupled receptor (Shenoy and Lefkowitz, 2003).
The use of β-arrestin knockout mice to study GPCR regulation in vivo
Since β-arrestins can regulate GPCR function by multiple mechanisms, it has been critically important to evaluate the physiological relevance of such regulation. The use of β-arrestin knockout mice (βarr1-KO or βarr2-KO) has been integral in determining how the responsiveness of certain GPCRs can be impacted upon by the removal of a particular β-arrestin in vivo (reviewed in Schmid and Bohn, 2009). In the knockout mice, some agonist-induced behaviors are enhanced, suggesting that for these receptor-mediated behaviors, β-arrestin may be acting as a negative regulator. For example, mice lacking β-arrestin2 display enhanced antinociception and greatly diminished antinociceptive tolerance compared to wild-type (WT) mice following treatment with the mu-opioid receptor agonist, morphine (Bohn et al., 1999; 2000; 2002; 2004). In addition, DAMGO-induced [35S]-GTPγS binding demonstrates that G protein coupling is enhanced in membranes from the spinal cords, periaqueductal gray and brainstem of βarr2-KO mice (Bohn et al., 1999; 2000; 2002). These studies suggest that in the absence of β-arrestins, the agonist may display enhanced relative efficacy in activating the receptor and to produce the behaviors. Similarly, β-adrenergic receptor agonists produce exaggerated hemodynamic responses in βarr1-KO mice compared to their WT littermates suggesting that the β2-adrenergic receptor is more responsive to agonist without the dampening actions of β-arrestin1 (Conner et al., 1997).
Knockout mouse studies have also demonstrated that β-arrestins can facilitate GPCR-mediated actions. In these cases, a diminished behavioral response to a GPCR agonist may indicate a disruption of β-arrestin-mediated signaling. For example, the Limbird laboratory demonstrated that α2-adrenergic receptor agonists induce sedation to a lesser extent in βarr2-KO mice than in WT mice, suggesting a signal-promoting role for β-arrestin2 (Wang et al., 2004). The D2-dopamine receptor was the first GPCR shown to couple to β-arrestin2 to promote signaling in vivo and the stimulation of this receptor by dopamine or dopaminergic drugs leads to the formation of an Akt, β-arrestin2 and protein phosphatase 2A (PP2A) signaling complex in the mouse striatum. Furthermore, the disruption of this complex formation in the βarr2-KO mice is correlated with a reduction in the stimulant effects of amphetamine in these animals (Beaulieu et al., 2005). The Caron laboratory went on to show that some of the antipsychotic effects of lithium can be attributed to a disruption of the β-arrestin2/Akt/PP2A signaling complex in WT mice (Beaulieu et al., 2008). These studies suggest that decreased behavioral responses in βarr2-KO mice may be attributed to a disruption in β-arrestin2-mediated signaling. More recently, β-arrestin2 has been shown to be important in facilitating 5-HT2AR signaling in cells and in vivo and this will be discussed in greater detail below (Schmid et al., 2008).
Functional selectivity of GPCR signaling
Agonist binding to a GPCR can stimulate the activation of multiple signaling pathways, including both G protein-mediated and β-arrestin-mediated/G protein-independent pathways. Moreover, the extent of the activation of each of the downstream pathways depends upon the ligand, with some ligands promoting activation of one pathway over another and vice versa. This concept of an agonist selectively stabilizing a receptor conformation, causing the receptor to preferentially interact with a subset of the multiple signaling pathways to which it is coupled, has been termed functional selectivity or biased agonism. (Figure 1) (Kenakin, 1995; Kobilka and Deupi, 2007; Urban et al., 2007). Ligands can also bias receptors for interacting with β-arrestins. For example, morphine, methadone and fentanyl are all agonists at the mu-opioid receptor; however, while methadone and fentanyl robustly recruit both β-arrestins to the receptor, morphine appears to preferentially recruit β-arrestin2 (Bohn et al., 2004). In addition to being ligand-directed, receptor-mediated signaling can also be directed by the cellular microenvironment in which the receptor is expressed. This also has been demonstrated for the muopioid receptor, wherein treatment with morphine does not lead to the recruitment of β-arrestin2 to the receptor in HEK293 cells, but does cause β-arrestin2 translocation in MEF cells and in HEK293 cells overexpressing GRK2 (Bohn et al., 2004).
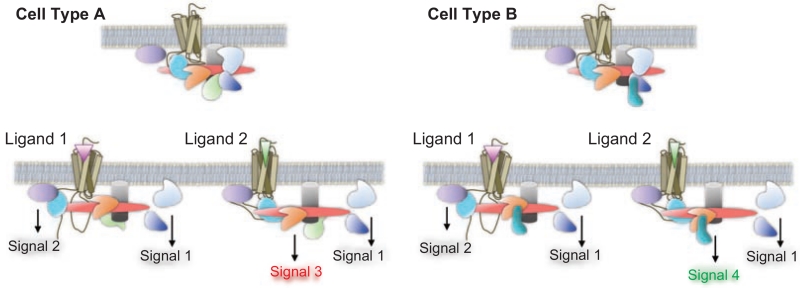
Figure 1.
Functional selectivity in GPCR signaling is determined by agonist as well as the cellular complement of proteins expressed in residence with the receptor. The diagram depicts a single GPCR type expressed in two distinct cell types (left: cell type A; right: cell type B). The different cell types express proteins in proximity to the receptor; some of which are the same between cell types and some of which are different (proteins are depicted as colored cartoon shapes clustered with the receptor). Upon agonist activation, the receptor engages some proteins in a conserved manner to produce the same signal regardless of the ligand or the cell type (Signal 1). However, some signaling cascades are specifically engaged by a particular ligand regardless of cell type (Signal 2) while other cascades are unique to the ligand and the cell type (Signal 3 and Signal 4). (See colour version of this figure online at www.informahealthcare.com/bmg)
As GPCRs, the serotonin receptors are subject to regulation by β-arrestins. However, the role of β-arrestins in regulating serotonin receptors has only partially been determined. This review focuses on the modulation of serotonin 2A receptor (5-HT2AR), serotonin 2C receptor (5-HT2CR) and serotonin 4 receptor (5-HT4R) responsiveness by β-arrestins.
Serotonin 2A receptor signaling and regulation via β-arrestins
The 5-HT2AR is expressed in both the periphery and in the central nervous system and is involved in mediating many physiological responses, including platelet aggregation, vascular and nonvascular smooth muscle contraction and the modulation of mood and perception (Roth et al., 1998). Dysregulation of the 5-HT2AR has been implicated in a variety of mental health disorders, including depression, suicide and some of the symptoms of schizophrenia (Maes and Meltzer, 1995; Roth and Meltzer, 1995). Therefore, the 5-HT2AR is a prominent target for drug therapy; for example 5-HT2AR blockade has been shown to mediate, at least in part, the effects of atypical antipsychotic drugs such as clozapine, risperidone and olanzapine (Meltzer, 2002). In addition, serotonergic drugs that cause hallucinations in humans produce their pharmacological effects by activating the 5-HT2AR (Aghajanian and Marek, 1999; Nichols, 2004). Interestingly, not all agonists at the 5-HT2AR induce hallucinations in humans (Pieri et al., 1978; Nichols, 2004), suggesting that agonists to the 5-HT2AR can promote diverse functional consequences. Moreover, studies by the Sealfon and Gingrich laboratories have shown that hallucinogens can elicit differential downstream gene response profiles than non-hallucinogens acting at the 5-HT2AR (Gonzalez-Maeso et al., 2007).
The 5-HT2AR has been shown to interact with β-arrestins both in vitro and in vivo, implicating these proteins in the regulation of the receptor. The Roth group has demonstrated that both β-arrestin1 and β-arrestin2 are able to interact with fusion proteins encoding the third intracellular loop of the rat 5-HT2AR (Gelber et al., 1999). The 5-HT2AR is highly expressed on pyramidal neurons in the prefrontal cortex (Willins et al., 1997; Cornea-Hebert et al., 1999; 2002) and both β-arrestin1 and β-arrestin2 are expressed in frontal cortex as well (Attramadal et al., 1992; Gurevich et al., 2002). To assess 5-HT2AR co-expression with β-arrestins in vivo, Gelber et al. (1999) utilized dual-label fluorescence confocal microscopy on tissue sections from the rat prefrontal cortex. Both β-arrestin1 and β-arrestin2 are shown to be co-expressed with the 5-HT2AR in some, but not all, cortical pyramidal neurons. Further analysis of β-arrestin1 expression revealed some colocalization with the 5-HT2AR in intracellular vesicles (Gelber et al., 1999). These studies indicate that the 5-HT2AR can interact with β-arrestins in vivo and suggest that these interactions may have relevance to the regulation of the receptor in the frontal cortex.
Given that β-arrestins have been found to be colocalized with the 5-HT2AR in cortical neurons, studies have explored whether β-arrestins are recruited to the receptor in response to agonist treatment. Serotonin induces marginal translocation of β-arrestin1 to the plasma membrane of HEK293 cells transfected with the 5-HT2AR and β-arrestin1 tagged with GFP (βarr1-GFP) as determined by confocal microscopy. β-arrestin2 is also recruited to the 5-HT2AR following agonist treatment, as the 5-HT2AR agonist quipazine induces robust translocation of βarr2-GFP to the plasma membrane (Bhatnagar et al., 2001). Further, a myc-tagged β-arrestin2 (myc-βarr2) co-immunoprecipitates with the 5-HT2AR following treatment with serotonin (Bhattacharya et al., 2010). In Figure 2 we show that serotonin also induces β-arrestin2 translocation to 5-HT2AR expressed in HEK293 cells. Similarly, we have recently demonstrated by co-immunoprecipitation, that treatment with 5-hydroxytryptophan (5-HTP), the precursor to serotonin, induces associations between the 5-HT2AR and β-arrestin2 in the mouse frontal cortex (Schmid and Bohn, 2010).
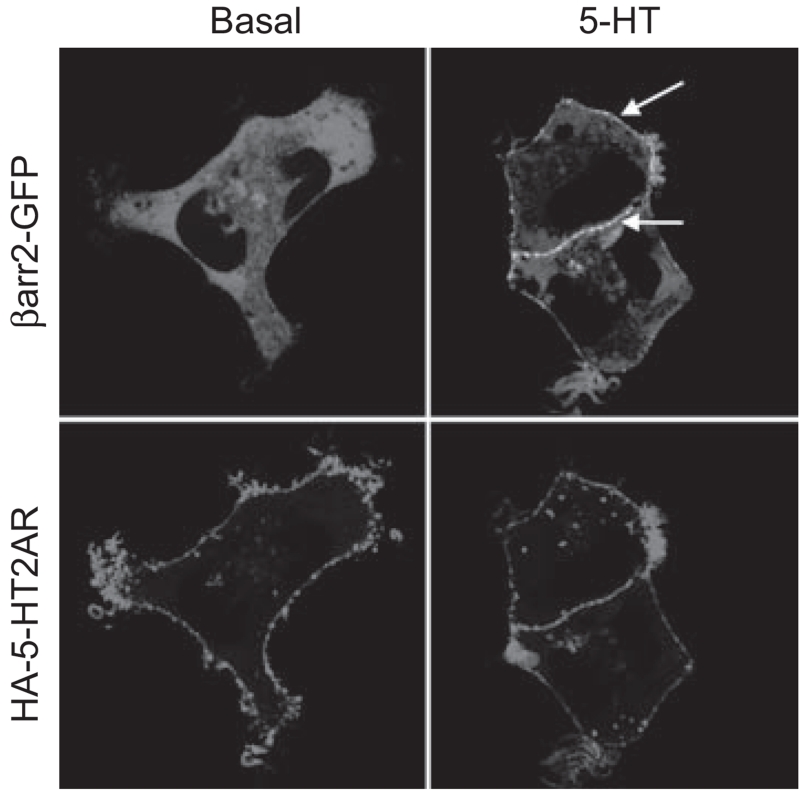
Figure 2.
Serotonin induces βarr2-GFP translocation to 5-HT2AR. HEK293 cells were transfected with HA-tagged mouse 5-HT2AR (HA-5-HT2AR) and GFP-tagged β-arrestin2 (βarr2-GFP). The cells were serum-starved for 2 hours and labeled with an HA-AlexaFluor 594 antibody for 15 minutes prior to live cell imaging with a confocal microscope. In the absence of agonist, βarr2-GFP is distributed throughout the cytosol. Treatment with serotonin (5-HT) (1 μM in 2 μM ascorbate) for 5 minutes leads to βarr2-GFP translocation to the plasma membrane outlining the cells as indicated by the arrows (top). Alexa-Fluor HA staining (bottom) is shown to demonstrate the location of the 5-HT2AR in the membrane.
β-arrestins as negative regulators of the 5-HT2AR
The 5-HT2AR has been shown to couple with Gαq, Gαi/o and Gα12/13 proteins both in vitro and in vivo (reviewed in Nichols, 2004). Stimulation of the Gαq pathway results in the activation of phospholipase C (PLC), the generation of inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG), and the release of calcium from intracellular stores (Conn and Sanders-Bush, 1984; 1986; Roth et al., 1984). Short-term exposure to agonist leads to a marked decrease in phosphoinositide (PI) hydrolysis in vitro (Ivins and Molinoff, 1991; Roth et al., 1995) and therefore PI hydrolysis is often used to study the desensitization state of the 5-HT2AR. Prior to the availability of siRNA or genetic knockout cell models, dominant negative β-arrestins (βarr1319-418; βarr2284-409), which encode only for the C-terminal tail of the protein and contain the clathrin-binding domain and not the GPCR binding domain, have been utilized to inhibit the function of endogenously expressed β-arrestins in vitro (Krupnick et al., 1997; Orsini and Benovic, 1998; Hanley and Hensler, 2002). This construct is expected to compete in receptor-pit assembly, and thereby disrupt normal trafficking and desensitization of the GPCR; for example, co-expression of βarr1319-418, potentiates the isoproterenol-induced desensitization of the β2-adrenergic receptor (Zhang et al., 1997; Gray et al., 2001). Studies of 5-HT2AR expressed in HEK293 cells tested the involvement of β-arrestin-regulation in the desensitization of quipazine-stimulated PI hydrolysis and found that βarr1319-418 has no effect on quipazine-induced desensitization of PI hydrolysis, nor did it affect the resensitization of the pathway (Gray et al., 2001). Though β-arrestin1 does not seem to be required for the desensitization of the 5-HT2AR in HEK293 cells, its function to negatively regulate the 5-HT2AR may be cell-type specific. For instance, in C6 glioma cells which endogenously express the 5-HT2AR, the βarr1319-418 dominant negative mutant potentiated quipazine-induced 5-HT2AR receptor desensitization and inhibited resensitization of receptor-mediated PI hydrolysis (Gray et al., 2001).
Facilitation of 5-HT2AR signaling by β-arrestins
β-arrestins can promote the activation of GPCR signaling by scaffolding components of the cascades to the receptors (DeWire et al., 2007). Studies in our laboratory have demonstrated 5-HT2AR signaling through β-arrestins at the biochemical level (Schmid et al., 2008). Serotonin leads to the activation of ERK1/2 that is greatly potentiated in WT MEFs stably expressing the 5-HT2AR as compared to serotonin effects on 5-HT2AR expressed in βarr1/2-KO MEFs (Schmid et al., 2008). In animal studies, serotonin activates ERK1/2 in frontal cortex of WT but not βarr2-KO mice, suggesting that serotonin utilizes a β-arrestin2-dependent mechanism to activate this cascade in vivo (Schmid et al., 2008). In neuronal cultures, the 5-HT2AR agonist, α-methylserotonin, was shown to induce ERK1/2 phosphorylation in the dendrites of cortical neurons in a manner that requires β-arrestins as siRNA mediated knockdown of either β-arrestin inhibited the activation of ERK1/2 by α-methylserotonin (Yuen et al., 2008). Interestingly, the presence of a cell-permeable dynamin inhibitory peptide also blocked the increase in ERK1/2 phosphorylation by α-methylserotonin, suggesting that 5-HT2AR endocytosis may be essential for β-arrestin-dependent, serotonin-induced ERK1/2 phosphorylation. Recently, we have found in cortex as well as in cortical cultures, that serotonin activates Akt via Src- and PI3-K-dependent mechanism that requires β-arrestin2 (Schmid and Bohn, 2010).
β-arrestin-mediated regulation of 5-HT2AR internalization
Similar to other GPCRs, agonist stimulation leads to the internalization of the 5-HT2AR, both in vitro and in vivo (Berry et al., 1996; Gray and Roth, 2001). The involvement of β-arrestins in regulating agonist-induced internalization events has also been considered. The Roth group demonstrated that treatment of HEK cells with serotonin induces internalization of the 5-HT2AR, a process that is blocked by the dominant negative dynamin K44A (Bhatnagar et al., 2001). Interestingly, the β-arrestin dominant negative mutants (βarr1319-418; βarr2284-409) have no affect on quipazine-induced internalization of a GFP-tagged 5-HT2AR (5-HT2AR-GFP), as analyzed by confocal microscopy. Further, quantification by a cell-surface biotinylation assay showed that βarr1319-418 has no effect on the serotonin-induced 5-HT2AR internalization either (Bhatnagar et al., 2001). However, it is not clear if these studies reflect the lack of involvement of β-arrestin or if they demonstrate the failure of the dominant negative mutant β-arrestins to effectively compete with this aspect of β-arrestin regulation.
In contrast to the studies reported above in HEK293 cells, studies from our laboratory have demonstrated that the 5-HT2AR can be internalized in a β-arrestin-dependent manner, both in primary neuronal cultures and in MEFs, which perhaps underscores the importance of the cellular environment in determining the function of the receptor in response to ligand (Schmid et al., 2008). As previously reported, we found that the 5-HT2AR is predominantly localized in intracellular vesicles in neurons cultured from the frontal cortex of WT mouse neonates (Gelber et al., 1999; Xia et al., 2003). However, in neurons cultured from βarr2-KO mice the 5-HT2AR primarily resides on the cell surface (Schmid et al., 2008). This lack of 5-HT2AR internalization is rescued by transfection of βarr2-GFP in the βarr2-KO neurons. The differential localization of the 5-HT2AR depending upon the expression of β-arrestin2 was also observed in MEFs. In the absence of serum, the 5-HT2AR is localized to the cellular membrane of both WT and βarr1/2-KO MEFs while treatment with serotonin induces 5-HT2AR internalization in WT but not in βarr1/2-KO MEFs (Figure 3; Xia et al., 2003; Schmid et al., 2008).
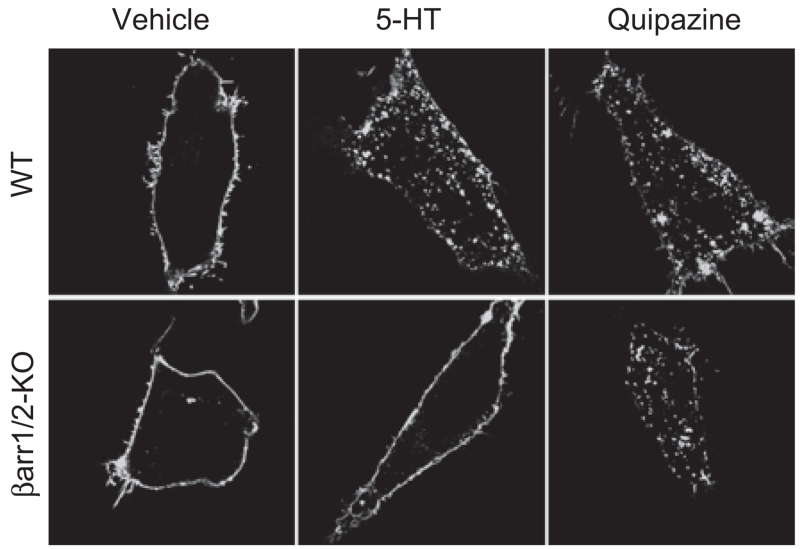
Figure 3.
Agonist-induced internalization of 5-HT2AR-YFP expressed in WT and βarr1/2-KO MEFs. WT and βarr1/2-KO MEFs were transfected with YFP-tagged 5-HT2AR (5-HT2AR-YFP) and serum-starved for 2 hours. Under these conditions, the 5-HT2AR-YFP is localized to the plasma membrane following treatment with vehicle (2 μM ascorbate) in both cell types. Treatment of WT cells with serotonin (5-HT, 1μM) or quipazine (1μM) induces receptor internalization within 30 minutes. While serotonin fails to internalize the receptor in the βarr1/2-KO MEFs (Schmid et al., 2008), quipazine leads to internalization of the 5-HT2AR in the absence of β-arrestins.
While β-arrestins can interact with the 5-HT2AR to affect internalization, they are not absolutely required for agonist-induced internalization in all cell lines. To assess whether the 5-HT2AR can be internalized via a β-arrestin-dependent pathway in HEK293 cells, Gray et al. (2003) co-expressed a constitutively active mutant of β-arrestin1 with the 5-HT2AR and assessed trafficking of the receptor. The βarr1R169E mutant binds to GPCRs regardless of the phosphorylation state of the receptor, resulting in enhanced desensitization and internalization of the receptors in the absence of agonist (Gurevich et al., 1995; Gray-Keller et al., 1997; Kovoor et al., 1999). Co-expression of βarr1R169E with the 5-HT2AR resulted in translocation of the mutant to the plasma membrane in the absence of agonist as assessed by confocal microscopy. Further, co-immunoprecipitation studies reveal agonist-independent associations between the flag-tagged 5-HT2AR with the mutant β-arrestin1. In addition, βarr1R169E expression induced significant constitutive internalization of the 5-HT2AR and decreased basal 5-HT2AR signaling, as assessed by PI hydrolysis (Gray et al., 2003). These studies further indicate that the 5-HT2AR can be internalized by β-arrestins; however, the extent of their involvement appears to be cell-type specific.
Functional selectivity of 5-HT2AR agonists and β-arrestin regulation
While β-arrestins can play an integral role in the desensitization and internalization of the 5-HT2AR, their function may be influenced by the complement of intracellular proteins that are available to help initiate or modulate their interactions with the receptor. The chemical nature of the agonist also determines the role that β-arrestins play in the regulation of the 5-HT2AR (Figure 1). In WT MEFs expressing 5-HT2AR, agonists, such as serotonin, 2,5-dimethoxy-4-iodoamphetamine (DOI) and quipazine, induce internalization of the receptor (Figure 3; Schmid et al., 2008). However, serotonin does not induce 5-HT2AR internalization in the βarr1/2-KO MEFs, while the effects of DOI and quipazine in the KO MEFs are identical to that seen in the WT MEFs (Figure 3; Schmid et al., 2008). These findings underscore the impact that different ligands can make upon determining the dependence upon β-arrestins for inducing receptor internalization.
The differential contribution of β-arrestins is also evident when assessing agonist-directed signaling pathways. While both DOI and serotonin activate ERK1/2 in 5-HT2AR expressing WT MEFs, the extent of ERK activation is fourfold greater for serotonin. However, serotonin and DOI activate ERK1/2 in βarr1/2-KO MEFs to the same extent, which is similar to the levels induced by DOI in the WT cells, suggesting a β-arrestin-bias in the actions of serotonin at the receptor for activating ERK1/2. The contribution of the Gαq protein-coupled pathway to ERK stimulation was determined by treating MEFs with the PLC inhibitor, U73122; this was found to partially block serotonin-mediated ERK1/2 activation and fully block DOI effects in WT MEFs. In the βarr1/2-KO MEFs, U73122 pretreatment completely inhibits ERK1/2 phosphorylation induced by both agonists. Taken together, these data suggest that serotonin can promote ERK1/2 phosphorylation via a β-arrestin-mediated and a Gαq/PLC-mediated pathway. Further, this is an example of ligand-directed signaling at the 5-HT2AR, as DOI induced activation of ERK1/2 occurs solely through the PLC-mediated pathway in these cells (Schmid et al., 2008).
This differential 5-HT2AR signaling was also assessed in vivo where DOI and 5-HTP treatment induces ERK1/2 phosphorylation in the frontal cortex of WT mice. Consistent with the studies in the MEF cells, only DOI induces ERK1/2 phosphorylation in the cortex of βarr2-KO mice, suggesting that there is a disruption of serotonin-induced 5-HT2AR signaling to ERK when β-arrestin2 is absent (Schmid et al., 2008). These differences between the ligands were also reflected in behavioral responses, where serotonin (injected as the precursor, 5-hydroxy-L-tryptophan) produces a head twitch response in WT but not in βarr2-KO mice, while DOI produces the behavior in both genotypes, suggesting that the deletion of β-arrestin2 only impacts serotonin-but not DOI-mediated signaling (Schmid et al., 2008).
While the differences in agonist-directed internalization and signaling parallel the responses induced in vivo, a direct causal link between the internalization event and the signaling and behavioral events has yet to be made. However, other studies examining regulatory proteins in addition to β-arrestins, including PSD-95, may help to shed more light on the interplay between these systems (Abbas et al., 2009). In regard to the interplay between the signaling and the behavior, we have recently found that serotonin and 5-HTP induce a β-arrestin/Src/Akt2 signaling complex at the 5-HT2AR and that the formation of this complex is necessary for the head twitch response induced by these agonists. In contrast, N-methyltryptamines do not induce the formation of this signaling complex, and head twitch responses caused by these compounds are β-arrestin-independent. Furthermore, disruption of the signaling cascade, by inhibiting PI3-K or Src, prevents serotonin-induced Akt activation in neurons and attenuates serotonin-mediated head twitch responses in normal mice (Schmid and Bohn, in press).
Taken together, these studies demonstrate that the 5-HT2AR interacts with β-arrestins and that these interactions can dictate receptor signaling, trafficking and behavioral responses. Moreover, the studies demonstrate that ligands at the 5-HT2AR can be engineered to possess functional selectivity that can bias receptor signaling and trafficking. This may thereby be a means to impact upon the behavioral responses triggered by this receptor. While the variations seen in cellular model systems emphasize the need to study drug responses at the receptor in vivo, this approach is complicated by the fact that all serotonin receptors are activated by serotonin and that these GPCRs are likely regulated by β-arrestins. The serotonin 2C (5-HT2CR) and the serotonin 4 (5-HT4R) receptors have also been studied for their regulation by β-arrestins and these findings are discussed below.
Serotonin 2C receptor signaling and regulation via β-arrestins
The 5-HT2CR is expressed in numerous brain regions, including the choroid plexus, cortex, basal ganglia, hippocampus and hypothalamus and has been shown to play an essential role in the regulation of mood (Giorgetti and Tecott, 2004; Millan, 2005). The 5-HT2CR is the therapeutic target for many disorders, including obesity, schizophrenia, anxiety, depression, drug addiction and Parkinson’s disease (reviewed in Berg et al., 2008; Iwamoto et al., 2009). Like the 5-HT2AR, the 5-HT2CR has been shown to couple to multiple G proteins and activates PLC, PLA2, phospholipase D (PLD) and ERK1/2 (reviewed in Leysen, 2004; Werry et al., 2006).
Facilitation of 5-HT2CR signaling by β-arrestins
Activation of 5-HT2CR leads to ERK1/2 phosphorylation in several cell lines (Werry et al., 2005; Labasque et al., 2008). Moreover, serotonin activation of 5-HT2CR can lead to β-arrestin-dependent and -independent activation of ERK1/2. Using siRNA to knockdown Gαq, Gα13 and Gαi/o proteins, the Marin group showed that serotonin-mediated 5-HT2CR activation of ERK1/2 occurs via a G protein-independent pathway; pretreatment with the PLC inhibitor U73122 or with pertussis toxin also has no effect on ERK1/2 activation in this HEK293 cell line. Interestingly, siRNA to β-arrestin1 and β-arrestin2, as well as a dominant negative to β-arrestin1 (βarr1319-418), inhibit serotonin-induced the ERK1/2 phosphorylation (Labasque et al., 2008). Further, a YFP-tagged β-arrestin2 (βarr2-YFP) co-immunoprecipitates with the 5-HT2CR following a five minute treatment with serotonin. A dominant negative or siRNA to calmodulin reduced ERK1/2 phosphorylation in HEK293 cells and, interestingly, reduced the interaction between βarr2-YFP and the receptor. These data suggest that the 5-HT2CR activates ERK1/2 in a β-arrestin-dependent manner and that calmodulin and β-arrestins may act in concert to fully activate this signaling pathway in this cell line (Labasque et al., 2008).
β-arrestin-mediated regulation of 5-HT2CR internalization
The second intracellular loop of the 5-HT2CR undergoes mRNA editing events, which produces distinct isoforms of the receptor that have varying degrees of constitutive activity (Burns et al., 1997; Niswender et al., 1998; Liu et al., 1999). Trafficking profiles of the edited receptors differ such that while the non-edited 5-HT2CR (5-HT2C-INI receptor) is constitutively internalized, the edited versions, such as the 5-HT2C-VGV receptor, are mainly localized to the plasma membrane. While internalization of the edited receptors can be induced by agonist stimulation, a study by the Caron laboratory demonstrated that the unedited version of the 5-HT2CR is constitutively internalized due to its interactions with β-arrestins and that this receptor is highly associated with βarr2-GFP even in the absence of agonist (Marion et al., 2004). Interestingly, mutation of proline 158 in the second intracellular loop of the receptor to an alanine relocalizes the receptor to the cellular membrane and dramatically decreases its constitutive interactions with β-arrestin2 (Marion et al., 2006). These studies demonstrate that the 5-HT2CR is capable of binding to β-arrestins in vitro and implicate that β-arrestins are responsible for the differences in constitutive activity observed for the various 5-HT2CR edited products.
Serotonin 4 receptor signaling and regulation via β-arrestins
The involvement of GRKs and β-arrestins in the desensitization and internalization of serotonin receptors has also been demonstrated for the 5-HT4R. The 5-HT4Rs are highly expressed in the limbic structures of the central nervous system, where they have been implicated in learning and memory, feeding control, the stress response and neurodegenerative diseases (Bockaert et al., 2004). They are also highly expressed in the myenteric plexus and circular muscle layers of the colon, where they have been implicated in the pathogenesis of irritable bowel syndrome (Kim, 2009). The 5-HT4R has also been identified and studied for its role in regulating atrial fibrillation (Ouadid et al., 1992; Yusuf et al., 2003). Furthermore, there are spliced variants of the 5-HT4R, which are differentially expressed (Blondel et al., 1998; Claeysen et al., 1998).
Negative regulation of 5-HT4R by β-arrestins
The 5-HT4Rs predominantly couple to Gαs proteins and stimulate adenylyl cyclase (Dumuis et al., 1988); cellular studies have demonstrated a prominent role for GRKs in the desensitization of the 5-HT4R in vitro. Ponimaskin et al. (2005) show that serotonin stimulation of 5-HT4AR in transfected S. frugiperda (Sf.9) cells leads to a dose-dependent phosphorylation of serine residues on the C-terminal domain of the receptor in a manner that is independent of second messenger-dependent kinases (such as PKA and PKC). Moreover, overexpression of GRK2 significantly decreases serotonin-induced cAMP generation in these cells, suggesting a negative regulatory role for GRK2 at the 5-HT4R. A similar effect was also seen for variants of the 5-HT4R, including the 5-HT4A, 5-HT4B, 5-HT4E and 5-HT4F receptors, which displayed less G protein-coupling when GRK2 was overexpressed in COS-7 cells (Barthet et al., 2005).
In addition to their role in negatively regulating 5-HT4R-Gαs coupling, GRKs have also been shown to negatively impact on G protein-independent signaling via this receptor (Barthet et al., 2005). Stimulation of primary colliculi neurons or HEK293 cells with serotonin or a selective 5-HT4R agonist (BIMU8) promotes ERK activation via a Src-mediated mechanism that is independent of PKA, PLC, Gαi/o and β-arrestins (Barthet et al., 2007). Interestingly, overexpression of GRK5 inhibits the phosphorylation of ERK 1/2, but not cAMP production, in both HEK293 cells and colliculi neurons following treatment with either serotonin or BIMU8, an effect which is dependent upon both a serine/threonine cluster in the C-terminal tail of the receptor and β-arrestin1, but not β-arrestin2, expression. Furthermore, the phosphorylation of β-arrestin1 at Ser412 appears to be required for this effect as transfection of wild-type β-arrestin1, but not the S412A mutant, rescues this inhibition of ERK in βarr1/2-KO MEFs (Barthet et al., 2009).
5-HT4R internalization via β-arrestins
Serotonin stimulation also has been shown to promote β-arrestin recruitment to the 5-HT4R and to induce internalization of the receptor. Immunoprecipitation of the 5-HT4R from HEK293 cells and immunoblotting for β-arrestins demonstrated that serotonin induces receptor interactions with β-arrestin1 and β-arrestin2 (Barthet et al., 2009). Further, confocal microscopy reveals that treatment with serotonin leads to recruitment of β-arrestin2 to the 5-HT4AR in COS-7 cells and to the 5-HT4AR, 5-HT4BR and 5-HT4ER in HEK293 cells (Barthet et al., 2005; Ponimaksin et al., 2005). This recruitment of β-arrestin2 to the receptor is dependent upon the serine/threonine clusters in the 5-HT4R C-terminal domains and is inhibited by a dominant negative to GRK2, further implicating GRK-phosphorylation of the receptor as an integral component in the regulation of the 5-HT4R (Barthet et al., 2005). Quantification of receptor internalization by radioligand binding of the 5-HT4R antagonist GR113808 on intact cells suggests that β-arrestin2 co-expression may increase receptor internalization induced by serotonin treatment (Ponimaskin et al., 2005). In addition, expression of a dominant negative β-arrestin1 (βarr1319-418) inhibits serotonin-induced internalization of the 5-HT4AR in HEK293 cells and in colliculus neurons; however, inhibiting β-arrestin2/5-HT4R interactions by the co-expression of a dominant negative GRK2 does not completely block endocytosis in HEK293 cells, suggesting the 5-HT4R may also be internalized by a β-arrestin2-independent mechanism (Barthet et al., 2005).
In humans there are nine splice variants of 5-HT4Rs that are expressed in the central nervous system, each with a unique C-terminal tail. The isoforms have nearly identical pharmacological properties, but the differences in C-terminal splicing dictate the level of receptor constitutive activity as well as interactions with signaling, sorting and regulatory proteins (Claeysen et al., 1999; 2001; Joubert et al., 2004; Barthet et al., 2005). While the data above indicate that 5-HT4Rs can be desensitized and internalized via classical GRK and β-arrestin-dependent mechanisms, a study by Mnie-Filali et al. (2010) suggests that at least two isoforms, 5-HT4AR and 5-HT4BR, depend on different mechanisms for internalization. For example, in HEK293 cells, the flag-tagged 5-HT4AR is constitutively internalized, even after serum-starving the cells. In contrast, the HA-tagged 5-HT4BR is localized predominantly to the cellular membrane in the absence of agonist. These differences in the subcellular distribution of the two isoforms were also observed in primary cortical neurons transfected with the tagged receptors. Interestingly, serotonin-induced internalization of the 5-HT4AR in HEK293 cells was inhibited by expression of dominant negative GRK2 and β-arrestin1 (βarr1319-418), while 5-HT4BR internalization was only partially attenuated by βarr1319-418. This study suggests that although β-arrestins are involved in the trafficking of 5-HT4Rs, there are isoform-specific differences that may impact on the overall regulation of these receptors (Mnie-Filali et al., 2010).
Conclusion
The studies discussed in this review indicate that the regulation of serotonin receptor signaling by β-arrestins is extremely complex. β-arrestins can be desensitizers, facilitators of non-G protein signaling cascades and/or involved in the endocytosis of the receptor depending upon (1) the receptor isoform, (2) the agonist which is bound to the receptor and (3) the cellular environment in which the receptor is expressed. Further, the interactions between β-arrestins and the serotonin receptors may have major functional implications for the in vivo responsiveness of the receptors. Therefore understanding the consequences of β-arrestin interactions with the receptors could aid in the development of pharmacotherapies to selectively target the activation or the inhibition of specific serotonin receptor signaling cascades. This may also be important for fine tuning receptor function: by facilitating signaling in certain cell types, and by avoiding certain cascades in other systems, one might be able to preserve beneficial responses and avoid unwanted side effects. Certainly, from a pharmacological perspective, as we begin to assess receptor function and functional selectivity in vivo, serotonergic signaling has become even more complex.
Footnotes
Declaration of interest
Our contributions to this field are funded by a grant from the National Institute on Drug Abuse (DA025158 to LMB). LMB is a consultant to Trevena, Inc. and Purdue Pharma, LC.
References
- Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, Roth BL. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–7136. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci USA. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM, Snyder SH, Caron MG, Lefkowitz RJ. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992;267:17882–17890. [PubMed] [Google Scholar]
- Barak LS, Ferguson SS, Zhang J, Caron MG. A beta-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J Biol Chem. 1997;272:27497–27500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- Barthet G, Gaven F, Framery B, Shinjo K, Nakamura T, Claeysen S, Bockaert J, Dumuis A. Uncoupling and endocytosis of 5-b hydroxytryptamine 4 receptors. Distinct molecular events with different GRK2 requirements. J Biol Chem. 2005;280:27924–27934. doi: 10.1074/jbc.M502272200. [DOI] [PubMed] [Google Scholar]
- Barthet G, Framery B, Gaven F, Pellissier L, Reiter E, Claeysen S, Bockaert J, Dumuis A. 5-hydroxytryptamine 4 receptor activation of the extracellular signal-regulated kinase pathway depends on Src activation but not on G protein or beta-arrestin signaling. Mol Biol Cell. 2007;18:1979–1991. doi: 10.1091/mbc.E06-12-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthet G, Carrat G, Cassier E, Barker B, Gaven F, Pillot M, Framery B, Pellissier LP, Augier J, Kang DS, Claeysen S, Reiter E, Baneres JL, Benovic JL, Marin P, Bockaert J, Dumuis A. Beta-arrestin1 phosphorylation by GRK5 regulates G protein-independent 5-HT4 receptor signalling. EMBO J. 2009;28:2706–2718. doi: 10.1038/emboj.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Berg KA, Clarke WP, Cunningham KA, Spampinato U. Fine-tuning serotonin2c receptor function in the brain: molecular and functional implications. Neuropharmacology. 2008;55:969–976. doi: 10.1016/j.neuropharm.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry SA, Shah MC, Khan N, Roth BL. Rapid agonist-induced internalization of the 5-hydroxytryptamine2A receptor occurs via the endosome pathway in vitro. Mol Pharmacol. 1996;50:306–313. [PubMed] [Google Scholar]
- Bhatnagar A, Willins DL, Gray JA, Woods J, Benovic JL, Roth BL. The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J Biol Chem. 2001;276:8269–8277. doi: 10.1074/jbc.M006968200. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Sankar S, Panicker MM. Differences in the C-terminus contribute to variations in trafficking between rat and human 5-HT(2A) receptor isoforms: identification of a primate-specific tripeptide ASK motif that confers GRK-2 and beta arrestin-2 interactions. J Neurochem. 2010;112:723–732. doi: 10.1111/j.1471-4159.2009.06493.x. [DOI] [PubMed] [Google Scholar]
- Blondel O, Gastineau M, Dahmoune Y, Langlois M, Fischmeister R. Cloning, expression, and pharmacology of four human 5-hydroxytryptamine 4 receptor isoforms produced by alternative splicing in the carboxyl terminus. J Neurochem. 1998;70:2252–2261. doi: 10.1046/j.1471-4159.1998.70062252.x. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Compan V, Dumuis A. 5-HT4 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:39–51. doi: 10.2174/1568007043482615. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci. 2002;22:10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol. 2004;66:106–112. doi: 10.1124/mol.66.1.106. [DOI] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Claeysen S, Faye P, Sebben M, Taviaux S, Bockaert J, Dumuis A. 5-HT4 receptors: cloning and expression of new splice variants. Ann NY Acad Sci. 1998;861:49–56. doi: 10.1111/j.1749-6632.1998.tb10172.x. [DOI] [PubMed] [Google Scholar]
- Claeysen S, Sebben M, Becamel C, Bockaert J, Dumuis A. Novel brain-specific 5-HT4 receptor splice variants show marked constitutive activity: role of the C-terminal intracellular domain. Mol Pharmacol. 1999;55:910–920. [PubMed] [Google Scholar]
- Claeysen S, Sebben M, Becamel C, Parmentier ML, Dumuis A, Bockaert J. Constitutively active mutants of 5-HT4 receptors are they in unique active states? EMBO Rep. 2001;2:61–67. doi: 10.1093/embo-reports/kve003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Sanders-Bush E. Selective 5HT-2 antagonists inhibit serotonin stimulated phosphatidylinositol metabolism in cerebral cortex. Neuropharmacology. 1984;23:993–996. doi: 10.1016/0028-3908(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Sanders-Bush E. Regulation of serotonin-stimulated phosphoinositide hydrolysis: relation to the serotonin 5-HT-2 binding site. J Neurosci. 1986;6:3669–3675. doi: 10.1523/JNEUROSCI.06-12-03669.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, Seidman JG. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res. 1997;81:1021–1026. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW, Warner BT. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1963;20:106–120. doi: 10.1111/j.1476-5381.1963.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Watkins KC, Roth BL, Kroeze WK, Gaudreau P, Leclerc N, Descarries L. Similar ultrastructural distribution of the 5-HT(2A) serotonin receptor and microtubule-associated protein MAP1A in cortical dendrites of adult rat. Neuroscience. 2002;113:23–35. doi: 10.1016/s0306-4522(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. beta-arrestin-biased agonism at the beta2-adrenergic receptor. J Biol Chem. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- Dumuis A, Bouhelal R, Sebben M, Cory R, Bockaert J. A non-classical 5-hydroxytryptamine receptor positively coupled with adenylate cyclase in the central nervous system. Mol Pharmacol. 1988;34:880–887. [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Gelber EI, Kroeze WK, Willins DL, Gray JA, Sinar CA, Hyde EG, Gurevich V, Benovic J, Roth BL. Structure and function of the third intracellular loop of the 5-hydroxytryptamine2A receptor: the third intracellular loop is alpha-helical and binds purified arrestins. J Neurochem. 1999;72:2206–2214. doi: 10.1046/j.1471-4159.1999.0722206.x. [DOI] [PubMed] [Google Scholar]
- Giorgetti M, Tecott LH. Contributions of 5-HT(2C) receptors to multiple actions of central serotonin systems. Eur J Pharmacol. 2004;488:1–9. doi: 10.1016/j.ejphar.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- Gray JA, Sheffler DJ, Bhatnagar A, Woods JA, Hufeisen SJ, Benovic JL, Roth BL. Cell-type specific effects of endocytosis inhibitors on 5-hydroxytryptamine(2A) receptor desensitization and resensitization reveal an arrestin-, GRK2-, and GRK5-independent mode of regulation in human embryonic kidney 293 cells. Mol Pharmacol. 2001;60:1020–1030. doi: 10.1124/mol.60.5.1020. [DOI] [PubMed] [Google Scholar]
- Gray JA, Bhatnagar A, Gurevich VV, Roth BL. The interaction of a constitutively active arrestin with the arrestin-insensitive 5-HT(2A) receptor induces agonist-independent internalization. Mol Pharmacol. 2003;63:961–972. doi: 10.1124/mol.63.5.961. [DOI] [PubMed] [Google Scholar]
- Gray-Keller MP, Detwiler PB, Benovic JL, Gurevich VV. Arrestin with a single amino acid substitution quenches light-activated rhodopsin in a phosphorylation-independent fashion. Biochemistry. 1997;36:7058–7063. doi: 10.1021/bi963110k. [DOI] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM. An opioid agonist that does not induce micro-opioid receptor–arrestin interactions or receptor internalization. Mol Pharmacol. 2007;71:549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Dion SB, Onorato JJ, Ptasienski J, Kim CM, Sterne-Marr R, Hosey MM, Benovic JL. Arrestin interactions with G protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, beta 2-adrenergic, and m2 muscarinic cholinergic receptors. J Biol Chem. 1995;270:720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Benovic JL, Gurevich VV. Arrestin2 and arrestin3 are differentially expressed in the rat brain during postnatal development. Neuroscience. 2002;109:421–436. doi: 10.1016/s0306-4522(01)00511-5. [DOI] [PubMed] [Google Scholar]
- Hamdan FF, Audet M, Garneau P, Pelletier J, Bouvier M. High-throughput screening of G protein-coupled receptor antagonists using a bioluminescence resonance energy transfer 1-based beta-arrestin2 recruitment assay. J Biomol Screen. 2005;10:463–475. doi: 10.1177/1087057105275344. [DOI] [PubMed] [Google Scholar]
- Hanley NR, Hensler JG. Mechanisms of ligand-induced desensitization of the 5-hydroxytryptamine(2A) receptor. J Pharmacol Exp Ther. 2002;300:468–477. doi: 10.1124/jpet.300.2.468. [DOI] [PubMed] [Google Scholar]
- Ivins KJ, Molinoff PB. Desensitization and down-regulation of 5-HT2 receptors in P11 cells. J Pharmacol Exp Ther. 1991;259:423–429. [PubMed] [Google Scholar]
- Iwamoto K, Bundo M, Kato T. Serotonin receptor 2C and mental disorders: genetic, expression and RNA editing studies. RNA Biol. 2009;6:248–253. doi: 10.4161/rna.6.3.8370. [DOI] [PubMed] [Google Scholar]
- Joubert L, Hanson B, Barthet G, Sebben M, Claeysen S, Hong W, Marin P, Dumuis A, Bockaert J. New sorting nexin (SNX27) and NHERF specifically interact with the 5-HT4a receptor splice variant: roles in receptor targeting. J Cell Sci. 2004;117:5367–5379. doi: 10.1242/jcs.01379. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- Kim HS. 5-Hydroxytryptamine4 receptor agonists and colonic motility. J Smooth Muscle Res. 2009;45:25–29. doi: 10.1540/jsmr.45.25. [DOI] [PubMed] [Google Scholar]
- Kim YM, Benovic JL. Differential roles of arrestin-2 interaction with clathrin and adaptor protein 2 in G protein-coupled receptor trafficking. J Biol Chem. 2002;277:30760–30708. doi: 10.1074/jbc.M204528200. [DOI] [PubMed] [Google Scholar]
- Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ. Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell. 2009;17:443–458. doi: 10.1016/j.devcel.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Celver J, Abdryashitov RI, Chavkin C, Gurevich VV. Targeted construction of phosphorylation-independent beta-arrestin mutants with constitutive activity in cells. J Biol Chem. 1999;274:6831–6834. doi: 10.1074/jbc.274.11.6831. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Santini F, Gagnon AW, Keen JH, Benovic JL. Modulation of the arrestin-clathrin interaction in cells. Characterization of beta-arrestin dominant-negative mutants. J Biol Chem. 1997;272:32507–32512. doi: 10.1074/jbc.272.51.32507. [DOI] [PubMed] [Google Scholar]
- Labasque M, Reiter E, Becamel C, Bockaert J, Marin P. Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C-terminus is essential for G protein-independent, arrestin-dependent receptor signaling. Mol Biol Cell. 2008;19:4640–4650. doi: 10.1091/mbc.E08-04-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Leysen JE. 5-HT2 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:11–26. doi: 10.2174/1568007043482598. [DOI] [PubMed] [Google Scholar]
- Liu Y, Emeson RB, Samuel CE. Serotonin-2C receptor pre-mRNA editing in rat brain and in vitro by splice site variants of the interferon-inducible double-stranded RNA-specific adenosine deaminase ADAR1. J Biol Chem. 1999;274:18351–18358. doi: 10.1074/jbc.274.26.18351. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY. The serotonin hypothesis of major depression. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. [Google Scholar]
- Marion S, Oakley RH, Kim KM, Caron MG, Barak LS. A beta-arrestin binding determinant common to the second intracellular loops of rhodopsin family G protein-coupled receptors. J Biol Chem. 2006;281:2932–2938. doi: 10.1074/jbc.M508074200. [DOI] [PubMed] [Google Scholar]
- Marion S, Weiner DM, Caron MG. RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J Biol Chem. 2004;279:2945. doi: 10.1074/jbc.M308742200. [DOI] [PubMed] [Google Scholar]
- McDonald PH, Bohn LM. Seeking ligand bias: assessing GPCR coupling to beta-arrestins for drug discovery. Drug Discovery Today. 2010 doi: 10.1016/j.ddtec.2010.06.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Mechanism of action of atypical antipsychotic drugs. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Raven Press; New York: 2002. [Google Scholar]
- Menard L, Ferguson SS, Zhang J, Lin FT, Lefkowitz RJ, Caron MG, Barak LS. Synergistic regulation of beta2-adrenergic receptor sequestration: intracellular complement of beta-adrenergic receptor kinase and beta-arrestin determine kinetics of internalization. Mol Pharmacol. 1997;51:800–808. [PubMed] [Google Scholar]
- Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie. 2005;60:441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- Mnie-Filali O, Amraei MG, Benmbarek S, Archer-Lahlou E, Penas-Cazorla R, Vilaro MT, Boye SM, Pineyro G. Serotonin 4 receptor (5-HT4R) internalization is isoform-specific: effects of 5-HT and RS67333 on isoforms A and B. Cell Signal. 2010;22:501–509. doi: 10.1016/j.cellsig.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Sanders-Bush E, Emeson RB. Identification and characterization of RNA editing events within the 5-HT2C receptor. Ann NY Acad Sci. 1998;861:38–48. doi: 10.1111/j.1749-6632.1998.tb10171.x. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Orsini MJ, Benovic JL. Characterization of dominant negative arrestins that inhibit beta2-adrenergic receptor internalization by distinct mechanisms. J Biol Chem. 1998;273:34616–34622. doi: 10.1074/jbc.273.51.34616. [DOI] [PubMed] [Google Scholar]
- Ouadid H, Seguin J, Dumuis A, Bockaert J, Nargeot J. Serotonin increases calcium current in human atrial myocytes via the newly described 5-hydroxytryptamine4 receptors. Mol Pharmacol. 1992;41:346–351. [PubMed] [Google Scholar]
- Pierce KL, Lefkowitz RJ. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci. 2001;2:727–733. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- Pieri L, Keller HH, Burkard W, Da Prada M. Effects of lisuride and LSD on cerebral monoamine systems and hallucinosis. Nature. 1978;272:278–280. doi: 10.1038/272278a0. [DOI] [PubMed] [Google Scholar]
- Ponimaskin E, Dumuis A, Gaven F, Barthet G, Heine M, Glebov K, Richter DW, Oppermann M. Palmitoylation of the 5-hydroxytryptamine4a receptor regulates receptor phosphorylation, desensitization, and beta-arrestin-mediated endocytosis. Mol Pharmacol. 2005;67:1434–1443. doi: 10.1124/mol.104.008748. [DOI] [PubMed] [Google Scholar]
- Roth BL, Meltzer HY. The role of serotonin in schizophrenia. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. [Google Scholar]
- Roth BL, Nakaki T, Chuang DM, Costa E. Aortic recognition sites for serotonin (5HT) are coupled to phospholipase C and modulate phosphatidylinositol turnover. Neuropharmacology. 1984;23:1223–1225. doi: 10.1016/0028-3908(84)90244-2. [DOI] [PubMed] [Google Scholar]
- Roth BL, Palvimaki EP, Berry S, Khan N, Sachs N, Uluer A, Choudhary MS. 5-Hydroxytryptamine2A (5-HT2A) receptor desensitization can occur without down-regulation. J Pharmacol Exp Ther. 1995;275:1638–1646. [PubMed] [Google Scholar]
- Roth BL, Berry SA, Kroeze WK, Willins DL, Kristiansen K. Serotonin 5-HT2A receptors: molecular biology and mechanisms of regulation. Crit Rev Neurobiol. 1998;12:319–338. doi: 10.1615/critrevneurobiol.v12.i4.30. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Physiological and pharmacological implications of beta-arrestin regulation. Pharmacol Ther. 2009;121:285–293. doi: 10.1016/j.pharmthera.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Serotonin, but not N-Methyltryptamines, activates the Serotonin 2A Receptor via a barrestin2/Src/AKT signaling complex in vivo. J Neurosci. 2010;30:13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci USA. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J. 2003;375:503–515. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalheim L, Ding Y, Gullapalli A, Paing MM, Wolfe BL, Morris DR, Trejo J. Multiple independent functions of arrestins in the regulation of protease-activated receptor-2 signaling and trafficking. Mol Pharmacol. 2005;67:78–87. doi: 10.1124/mol.104.006072. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, Von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- von Degenfeld G, Wehrman TS, Hammer MM, Blau HM. A universal technology for monitoring G-protein-coupled receptor activation in vitro and noninvasively in live animals. Faseb J. 2007;21:3819–3826. doi: 10.1096/fj.07-9597com. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhao J, Brady AE, Feng J, Allen PB, Lefkowitz RJ, Greengard P, Limbird LE. Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science. 2004;304:1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]
- Werry TD, Gregory KJ, Sexton PM, Christopoulos A. Characterization of serotonin 5-HT2C receptor signaling to extracellular signal-regulated kinases 1 and 2. J Neurochem. 2005;93:1603–1615. doi: 10.1111/j.1471-4159.2005.03161.x. [DOI] [PubMed] [Google Scholar]
- Werry TD, Christopoulos A, Sexton PM. Mechanisms of ERK1/2 regulation by seven-transmembrane-domain receptors. Curr Pharm Des. 2006;12:1683–1702. doi: 10.2174/138161206776873725. [DOI] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Xia Z, Hufeisen SJ, Gray JA, Roth BL. The PDZ-binding domain is essential for the dendritic targeting of 5-HT2A serotonin receptors in cortical pyramidal neurons in vitro. Neuroscience. 2003;122:907–920. doi: 10.1016/s0306-4522(03)00589-x. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Jiang Q, Chen P, Feng J, Yan Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem. 2008;283:17194–17204. doi: 10.1074/jbc.M801713200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Al-Saady N, Camm AJ. 5-hydroxytryptamine and atrial fibrillation: how significant is this piece in the puzzle? J Cardiovasc Electrophysiol. 2003;14:209–214. [PubMed] [Google Scholar]
- Zhang J, Barak LS, Winkler KE, Caron MG, Ferguson SS. A central role for beta-arrestins and clathrin-coated vesicle-mediated endocytosis in beta2-adrenergic receptor resensitization. Differential regulation of receptor resensitization in two distinct cell types. J Biol Chem. 1997;272:27005–27014. doi: 10.1074/jbc.272.43.27005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci USA. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]