Abstract
The renal autonomic nervous system may contribute to hypertension and vascular disease. Although the effects of renal artery denervation on blood pressure lowering are controversial, there may be other beneficial vascular effects independent of blood pressure lowering. Bilateral renal denervation (RDN) or sham operation (SO) was performed in 14-week-old male apolipoprotein E–deficient mice on a Western diet starting at 10 weeks of age. Efficacy of RDN was confirmed by reduction of renal norepinephrine levels (SO: 3.8±0.1 versus RDN: 1.7±0.3 ng/mL; P<0.01) at 6 weeks after procedure. Compared with SO, RDN had no effect on blood pressure (SO: 101.0±2.4 versus RDN: 97.5±1.6 mm Hg; P=0.25), total cholesterol (SO: 536.7±28.5 versus RDN: 535.7±62.9 mg/dL; P=0.99), or triglycerides (SO: 83.7±3.5 versus RDN: 86.9±10.2 mg/dL; P=0.78). Quantification of atherosclerosis at 20 weeks of age demonstrated reduced atherosclerosis in mice receiving RDN compared with SO (arterial tree oil-red-O surface staining RDN: 4.2±0.5% versus SO: 6.3±0.7%; P<0.05). Reduced atherosclerosis was associated with increased smooth muscle cell content in atherosclerotic plaques (RDN: 13.3±2.1 versus SO: 8.1±0.6%; P<0.05). Serum levels of aldosterone, monocyte chemoattractant protein-1, and 8-isoprostane were lower in mice that received RDN compared with sham-operated mice (aldosterone; RDN: 206.8±33.2 versus SO: 405.5±59.4 pg/mL, P<0.05; monocyte chemoattractant protein-1; RDN: 51.7±7.9 versus SO: 91.71±4.6 pg/mL, P<0.05; 8-isoprostane; RDN: 331.9±38.2 versus SO: 468.5±42.0 pg/mL, P<0.05). RDN reduces progression of atherosclerosis in apolipoprotein E–deficient mice. These changes are associated with reduced aldosterone levels, monocyte chemoattractant protein-1, and markers of oxidative stress.
Keywords: atherosclerosis, hypertension, oxidative stress, sympathetic nervous system
Renal denervation (RDN) may be useful in some patients with refractory hypertension.1 However, a recent controlled trial which included a sham RDN procedure did not show a significant reduction in blood pressure in patients with resistant hypertension.2 It may be that these results were because of incomplete denervation, but this assertion will require further clinical investigation, possibly utilizing different devices and a biomarker reflecting successful denervation. Apart from the effects on blood pressure, preclinical studies have suggested that RDN may have additional systemic beneficial effects. For example, RDN has been shown to improve glucose metabolism and insulin sensitivity in humans.3 In stroke-prone, hypertensive rats, RDN was shown to be protective against oxidative stress and brain injury.4 Because of the potentially broad, pressor-independent effects of sympathetic activation toward multiple vascular processes, we tested the effects of bilateral RDN on progression of atherosclerosis in a normotensive murine model of hyperlipidemia.
Methods
Animals
Male apolipoprotein E–deficient (ApoE−/−) mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Mice were housed under specific pathogen-free conditions in static microisolator cages with tap water ad libitum in a temperature-controlled room with a 12:12-hour light/dark cycle and were fed a Western diet (TD88137, Harlan, WI) starting at 10 weeks of age. All animal use protocols complied with the Principle of Laboratory and Animal Care established by the National Society for Medical Research and were approved by the University of Michigan Committee on Use and Care of Animals.
Renal Denervation
Bilateral RDN (n=8) or sham operation (SO; n=8) was performed in 14-week-old, male, ApoE−/− mice that had been on Western diet for 4 weeks prior to surgery. Mice were anesthetized with IP injection of sodium pentobarbital (67 mg/kg). The surgical procedure was performed as described previously.5 Briefly, the abdomen was opened through a ventral midline laparotomy. The small intestine was gently moved aside and draped with sterile saline-soaked gauze. The renal nerve bundles were visualized by locating the abdominal aorta and renal arteries. Visible renal nerves, fat, and connective tissue were cleared from the renal vessels. Then renal vessels were painted with 10% phenol in an alcohol solution to destroy the remaining nerves. For SO mice, the kidneys were exposed as with the RDN procedure, but renal nerves were kept intact. After surgery, mice were maintained on the Western diet for an additional 6 weeks before experiment termination.
Blood Pressure Measurement
Blood pressure was measured 6 weeks after RDN or SO in nonanesthetized mice by tail plethysmography using the BP-2000 Blood Pressure Analysis System (Visitech System, Apex, NC). Blood pressure was also measured via carotid arterial catheterization at the time of sacrifice, as previously described.6 Briefly animals were anesthetized with urethane (1.0 g/kg, IP). Body temperature was maintained at 37°C on a controlled heating pad. Blood pressure was recorded via a microtip catheter in the right common carotid artery using a data acquisition system powerlab 8/30 and chart software (AdInstruments, Colorado Springs, CO).
Verification of RDN
To verify successful RDN, renal norepinephrine (NE) content was measured 6 weeks after RDN or SO using an NE ELISA kit (RM Diagnostics, Colorado Springs, CO). Briefly, the kidney was homogenized in 0.01N HCl in the presence of EDTA (1 mmol/L) and sodium metabisulfite (4 mmol/L) followed by centrifugation at 18 000g for 10 minutes at room temperature. Two hundred microliters of supernatant was collected for NE content which was performed according to manufacturer's instruction. For plasma measurement of NE, 20 μL of plasma from ApoE−/− mice 6 weeks after SO or RDN was assayed.
For immunofluorescence staining of nerve fibers distal to excision site, the intrarenal arteries from ApoE−/− mice 6 weeks after RDN or SO were harvested and fixed in 10% zinc formalin. Arteries were then incubated in PBS with blocking serum diluted in Triton X-100 (1%) for 1 hour. They were then incubated overnight at 4°C with a primary antibody against a marker of sympathetic nerves (rabbit polyclonal anti-tyrosine hydroxylase, 1:200; EMD Millipore, Billerica, MA). Arteries were then washed with PBS and incubated for 1 hour at room temperature with fluorescence-conjugated secondary antibody. Labeled sympathetic nerves on intrarenal arteries were examined using a Nikon TE200-E microscope. The intensity of fluorescence signal was quantified as arbitrary units using NIH ImageJ software.
Atherosclerosis Analysis
Quantification of atherosclerosis was performed as previously described.7 Briefly, mice were euthanized under IP pentobarbital anesthesia (100 mg/kg), and arterial trees were perfused at physiological pressure and fixed in 10% zinc formalin. Arterial trees were then stained with oil-red-O and pinned on wax trays to quantify the atherosclerotic surface area occupied in the aortic arch, brachiocephalic artery, common carotid arteries, and subclavian arteries. The lesion area was expressed as a percentage of total surface area examined. Paraffin-embedded heart including aortic root was sectioned through the aortic sinus. A series of 5 μm sections were obtained at the level of the aortic sinus, and 4 cross sections were analyzed from each mouse. Sections were stained with hematoxylin and eosin for quantification of lesion area normalized by respective medial area of aorta. The lesion area was defined as the area between the endothelial cell layer and internal elastic lamina. The macrophage and actin content were quantified with corresponding antibodies to Mac-3 (1:100, BD Biosciences, San Jose, CA), or α-smooth muscle cell actin (1:1000, Cedarlane Laboratories, Burlington, NC). Collagen content was examined with Sirius red (Sigma, St. Louis, MO). 8-isoprostane, angiotensin II, and monocyte chemoattractant protein-1 (MCP-1) content were quantified with corresponding antibodies to 8-isoprostane (1:200, Abcam, Cambridge, MA), angiotensin II (1:400, Novus Biologicals, Littleton, CO), or MCP-1 (1:50, Abcam, Cambridge, MA). Positive staining area was expressed as percentage of the total lesion area. All images were analyzed by automatic detection of positive staining intensity using Nikon MetaMorph software. Brachiocephalic arteries were also cross-sectioned and analyzed. Four sections at 500 μm intervals from each sample were analyzed using Nikon MetaMorph software. Lesion area was expressed as percentage of cross-sectional lumen area.
Measurements of Serum Samples
Serum samples were collected via retro-orbital bleeding using capillary tubes 6 weeks after surgery. Circulating concentrations of MCP-1, interleukin-1β (R&D Systems, Minneapolis, MN), 8-isoprostane (Cayman Chemical, Ann Arbor, MI), renin (Molecular Innovations, Novi, MI), angiotensin II, and aldosterone (Enzo Life Sciences, Farmingdale, NY) were measured with corresponding ELISA kits following manufacturers’ instructions. Plasma renin activity was determined by a renin assay kit (Sigma, St. Louis, MO) following the manufacturer's instruction.
Cholesterol and triglycerides were measured in the Chemistry Core of the Michigan Diabetes Research and Training Center using Enzymatic-Colorimetric kits (Roche, Indianapolis, IN). Blood glucose levels were measured after overnight fasting with a glucometer using test strips (Bayer HealthCare LLC, Mishawaka, IN). Fasting insulin levels were measured with an ELISA kit (Crystal Chemical Inc, Wakefield, MA). Insulin resistance was determined using homeostasis model assessment-estimated insulin resistance index by homeostasis model assessment-estimated insulin resistance equation: [homeostasis model assessment-estimated insulin resistance = fasting insulin (μIU/mL) × fasting glucose (mmol/L)/22.5].8
Kidney Content of Renin, Angiotensin II, and Aldosterone
100 mg kidney was homogenized in 250 mL PBS followed by centrifugation at 18 000g for 10 minutes at room temperature. Then supernatant was collected to measure content of renin, angiotensin II, or aldosterone using corresponding ELISA kits according to the manufacturer's instruction.
Morphology of Kidney
To examine the morphology of kidneys after RDN or SO, the kidneys were fixed in 10% zinc formalin 6 weeks after surgery. A coronary cross section containing the hilum of the kidney was embedded in paraffin. A series of 5 μm sections were obtained and stained with hematoxylin and eosin for morphometric quantification of glomeruli. For glomeruli counts, 3 cross sections were analyzed from each mouse. Ten fields from the subcapsular and juxtamedullary regions in each section were randomly selected, and glomeruli were counted to determine the average number of glomeruli per section. Bowman's space area was determined by subtracting the area around the outer surface of glomerular capillary bed from the area around the inner surface of Bowman's capsule. Twenty glomeruli per kidney were measured to determine the average Bowman's space area. All measurements were performed using NIH ImageJ software.
Statistical Analysis
All data are presented as mean±standard error. Statistical analysis was performed using GraphPad Prism. Results were analyzed using unpaired t test for comparison between 2 groups. For multiple comparisons, results were analyzed using 1-way ANOVA followed by Tukey post-test analysis. P values <0.05 were considered statistically significant.
Results
Efficacy of RDN Procedure
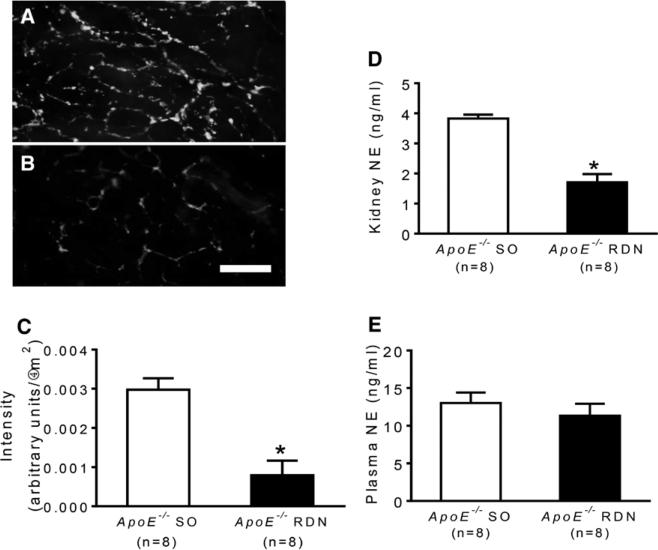
Six weeks after RDN or SO, the intrarenal sympathetic nerve fibers were analyzed for tyrosine hydroxylase by immunofluorescence staining. Intrarenal arteries from RDN mice showed reduced immunofluorescence staining compared with those from SO mice (Figure 1A–1C). The efficacy of RDN was confirmed by measuring NE content in kidney. Renal NE levels were significantly reduced after RDN compared with SO mice (Figure 1D); however, plasma levels of NE were similar between the groups (Figure 1E).
Figure 1.
Representative photomicrographs of whole-mount immunofluorescence staining of tyrosine hydroxylase (TH) on isolated intrarenal arteries distal to renal denervation (RDN) excision site from ApoE−/− mice after sham operation (SO; A) or RDN (B). C, Quantification of intensity of TH staining on renal arteries. D, Levels of norepinephrine (NE) in kidneys of ApoE−/− mice after SO or RDN. E, Plasma levels of NE in ApoE−/− mice after SO or RDN. *P<0.01 compared with sham control. Scale: 50 μm.
To assess the morphometric changes of kidney after SO or RDN, the average number of glomeruli and Bowman's space area were measured. The average number of glomeruli was similar between the 2 groups (SO, 8.5±0.5/mm2; RDN, 8.3±0.3/mm2; P=0.7). No differences were detected in Bowman's space area between these groups (SO, 0.72±0.1×103 mm2; RDN, 0.8±0.1×103 μm2; P=0.16).
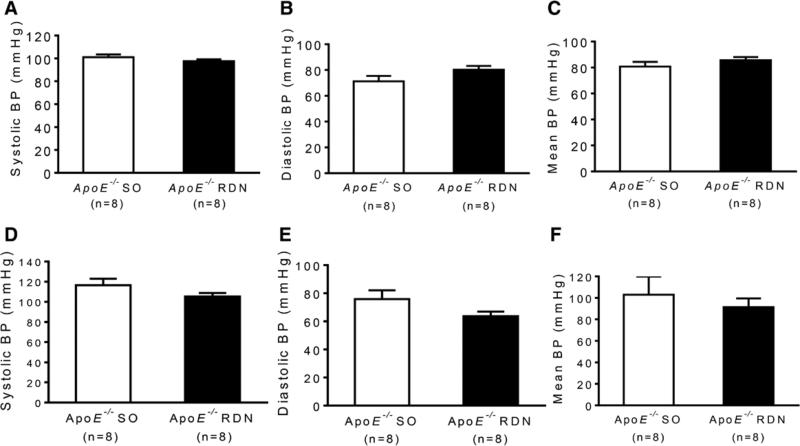
No differences were noted in body weight, fasting glucose, insulin, homeostasis model assessment-estimated insulin resistance, total cholesterol, triglycerides (Table), or blood pressure measured by tail-cuff (Figure 2A–2C) or catheterization (Figure 2D–2F) between RDN and SO groups.
Table.
Metabolic Parameters of ApoE−/− SO and ApoE−/− RDN Mice
| Parameter | ApoE−/− SO | ApoE−/− RDN |
|---|---|---|
| Body weight, g | 29.9±1.5 | 31.3±0.8 |
| Blood glucose, mg/dL | 77.0±6.3 | 95.6±8.0 |
| Plasma insulin, ng/mL | 0.38±0.2 | 0.54±0.2 |
| HOMA-IR | 0.08±0.05 | 0.14±0.06 |
| Total cholesterol, mg/dL | 536.7±28.5 | 535.7±62.9 |
| Triglyceride, mg/dL | 83.7±3.5 | 86.9±10.2 |
HOMA-IR indicates homeostasis model assessment-estimated insulin resistance; RDN, renal denervation; and SO, sham operation.
Figure 2.
Blood pressure (BP) of ApoE−/− mice after sham operation (SO) or renal denervation (RDN). Systolic, diastolic, and mean arterial pressure measured by tail-cuff (A–C) and catheterization of carotid artery (D–F).
Effect of RDN on Atherosclerosis
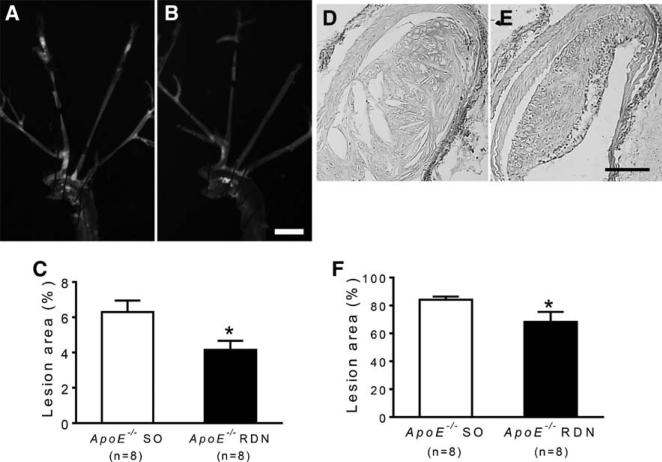
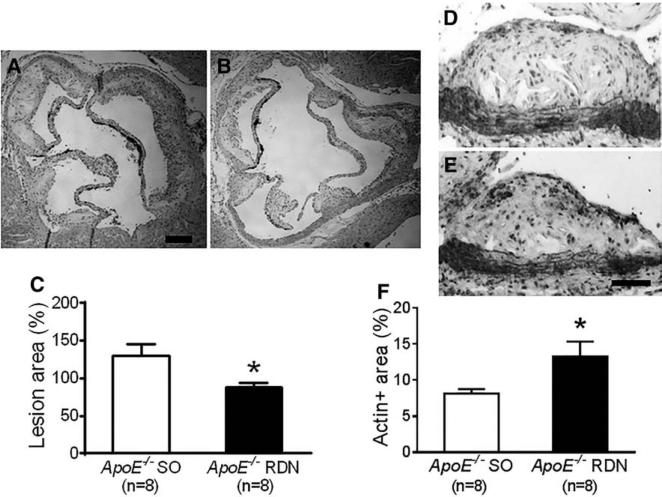
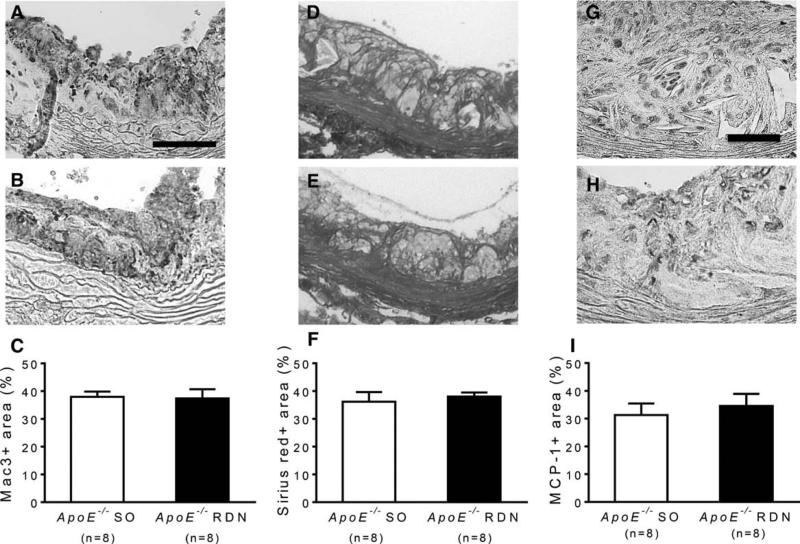
After 10 weeks of Western diet and 6 weeks after RDN or SO, the atherosclerosis was quantified by oil-red-O staining of aortic trees in ApoE−/− mice. The oil-red-O-positive lesion area was significantly reduced in mice after RDN compared with the SO (Figure 3A–3C). The cross-sectional lesion area involving the proximal brachiocephalic artery was also reduced in mice receiving RDN compared with SO (Figure 3D–3F). Consistently, the atherosclerotic lesion area involving the aortic root was significantly reduced in mice after RDN compared with SO (Figure 4A–4C). Reduced lesion area after RDN was associated with increased smooth muscle cell content as determined by α-smooth muscle cell actin immunostaining (Figure 4D–4F). Macrophage content determined by Mac-3 staining (Figure 5A–5C) and collagen content determined by Sirius red staining (Figure 5D–5F) were similar between the RDN and sham groups. No differences were noted in MCP-1-positive areas between these groups (Figure 5G–5I).
Figure 3.
Effect of renal denervation (RDN) on atherosclerosis of aortic tree. Representative photomicrographs of aortic tree stained with oil-red-O from ApoE−/− mice after sham operation (SO; A) and RDN (B). C, Quantification of atherosclerosis expressed by % surface area of aortic arch covered with lipid-rich atherosclerotic lesions. Representative photomicrographs of cross sections of brachiocephalic arteries stained with hematoxylin and eosin from ApoE−/− mice after SO (D) and RDN (E). F, Quantification of lesion area expressed by % of arterial lumen area. *P<0.05 compared with sham control. Scale: 2 mm for A and B, 100 μm for D and E.
Figure 4.
Effect of renal denervation (RDN) on atherosclerosis at aortic root. Representative photomicrographs of lesions at aortic root stained with hematoxylin and eosin from ApoE−/− mice after sham operation (SO; A) and RDN (B). C, Quantification of lesion area normalized by respective medial area of aorta. Representative photomicrographs of α-smooth muscle cell actin staining of lesions at aortic root from ApoE−/− mice after SO (D) and RDN (E). F, Quantification of α-smooth muscle cell actin–positive area expressed by % of lesion area at aortic root. *P<0.05 compared with sham control. Scale: 200 μm for A and B, 100 μm for D and E.
Figure 5.
Immunostaining of Mac-3, collagen, and monocyte chemoattractant protein-1 (MCP-1) in atherosclerotic lesions at aortic root. Representative photomicrographs of Mac-3 staining of lesions at aortic root from ApoE−/− mice after sham operation (SO; A) and renal denervation (RDN; B). C, Quantification of Mac-3-positive area expressed by % of lesion area. Representative photomicrographs of Sirius red staining of lesions at aortic root from ApoE−/− mice after SO (D) and RDN (E). F, Quantification of Sirius red–positive area expressed by % of lesion area. Representative photomicrographs of MCP-1 staining of lesions at aortic root from ApoE−/− mice after SO (G) and RDN (H). I, Quantification of MCP-1-positive area expressed by % of lesion area. Scale: 100 μm for A, B, D, and E. 50 μm for G and H.
Circulating Levels of Inflammatory Cytokines
To determine the effect of RDN on a panel of circulating biomarkers, the serum levels of MCP-1 and interleukin-1β were measured from ApoE−/− mice 6 weeks after RDN or SO. The levels of MCP-1 were significantly reduced after RDN compared with those after SO (51.7±7.9 versus 91.7±14.6 pg/mL; n=8 per group; P<0.05). No differences were detected in the levels of interleukin-1β (SO, 109.3±26.0 pg/mL; RDN, 70.3±22.6 pg/mL; n=8 per group; P=0.3).
Effect of RDN on Renin, Angiotensin II, Aldosterone, and Oxidative Stress
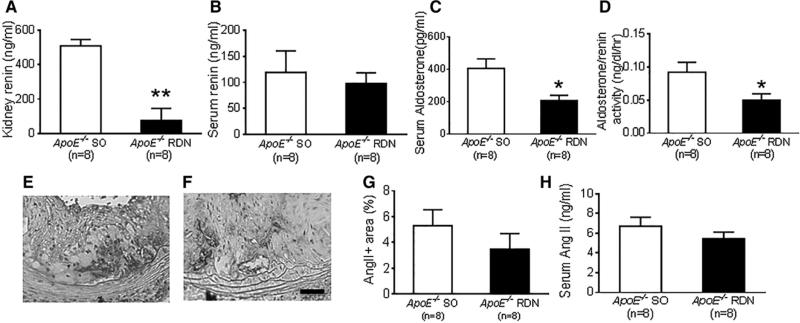
Six weeks after RDN or SO, kidney levels of renin measured by ELISA were significantly reduced in mice with RDN compared with SO group (Figure 6A). No differences were detected in serum levels of renin between these groups (Figure 6B). The serum levels of aldosterone were significantly reduced in mice with RDN compared with SO (Figure 6C), while they were similar in kidneys (SO, 84.1±13.4 pg/mL; RDN, 109.7±12.1 pg/mL; n=8 per group; P=0.12). Plasma renin activity was similar between these groups (SO, 411.1±14.7 ng/mL per hour; RDN, 408.7±21.8 ng/mL per hour; n=8 per group; P=0.93); however, the aldosterone-to-renin ratio was significantly reduced in mice after RDN compared with mice after SO (Figure 6D).
Figure 6.
Effect of renal denervation (RDN) on renin, angiotensin II (Ang II), and aldosterone. A, Kidney levels of renin in ApoE−/− mice after RDN and sham operation (SO). B, Serum levels of renin in ApoE−/− mice after RDN and SO, P=0.64. C, Serum levels of aldosterone in ApoE−/− mice after RDN and SO. D, Aldosterone-to-renin ratio in ApoE−/− mice after RDN and SO. Representative photomicrographs of Ang II staining of lesions at aortic root from ApoE−/− mice after SO (E) and RDN (F). Scale: 50 μm. G, Quantification of Ang II-positive area expressed by % of lesion area, P=0.32. H, Serum levels of Ang II in ApoE−/− mice after RDN and SO, P=0.29. *P<0.05 compared with sham control. **P<0.0001 compared with sham control.
To further examine the effect of RDN on angiotensin II expression, both local and systemic angiotensin II levels were tested. No differences were noted in angiotensin II-positive area in atherosclerotic regions at aortic root between these groups (Figure 6E–6G). Serum angiotensin II levels measured by ELISA were also similar between SO and RDN group (Figure 6H). In kidneys, the levels of angiotensin II were below the detection limit.
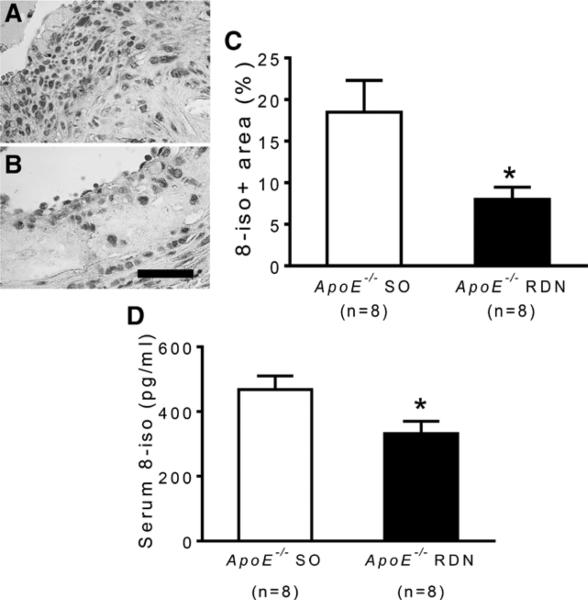
8-Isoprostane, an indicator of oxidative stress, was examined 6 weeks after RDN. 8-Isoprostane staining in atherosclerotic lesions at the aortic root was significantly reduced in mice receiving RDN compared with SO (Figure 7A–7C). Circulating levels of 8-isoprostane were also significantly reduced in mice receiving RDN compared with those receiving SO (Figure 7D).
Figure 7.
Effect of renal denervation (RDN) on levels of 8-isoprostane (8-iso). Representative photomicrographs of 8-iso staining of lesions at aortic root from ApoE−/− mice after sham operation (SO; A) and RDN (B). Scale: 50 μm. C, Quantification of 8-iso-positive area expressed by % of lesion area. D, Serum levels of 8-iso in ApoE−/− mice after RDN and SO. *P<0.05 compared with sham control.
Discussion
The sympathetic nervous system is involved in the regulation of multiple physiological processes.9 Heightened sympathetic activity as indicated by elevated circulating levels of NE is associated with increased mortality.10 As early as 195311 a 5-year survival advantage was demonstrated in hypertensive patients treated with thoraco-lumbar splanchnicectomy, a procedure that results in RDN. The kidney is a particularly relevant organ related to sympathetic nervous system activity as local release of NE from renal sympathetic neurons onto the juxtaglomerular cells leads to release of renin.12 Reduced renal blood flow secondary to local vasoconstriction also results in greater sodium retention.13 These potential effects of renal sympathetic innervation on blood pressure have been exploited with catheter-based approaches to RDN. Several clinical trials have demonstrated that ablation of adventitial renal nerves by delivery of radiofrequency pulses from a catheter positioned in the renal artery is effective in lowering blood pressure in patients with resistant hypertension.14 In contrast, the recent randomized SIMPLICITY 3 trial, in which a randomized sham denervation procedure was included, showed no significant difference in blood pressure between the sham and RDN groups. Although SIMPLICITY 3 is the most definitive trial to date examining the effect of RDN on hypertension, a shortcoming of clinical RDN trials is lack of a reliable measure to prove successful denervation. Additional outcomes in human clinical trials that support a relevant biological effect of catheter-based RDN include reduction in left ventricular hypertrophy.15
Beneficial effects of RDN may extend beyond blood pressure as one human clinical study demonstrated reduction in fasting glucose, insulin, and improved glucose tolerance 3 months after RDN.3 Preclinical studies in rats have also demonstrated that the pressor response to hyperinsulinemia is prevented and reversed by bilateral RDN.16 These findings suggest that there may be a central role for renal sympathetic activation in mediating components of the metabolic syndrome. In a rat model of metabolic syndrome, bilateral RDN significantly reduced plasma renin activity, prevented impairment of endothelial function, performed cardiovascular remodeling, and attenuated markers of oxidative stress.17 The beneficial effects occurred in the absence of changes in adiposity, plasma lipids, or insulin resistance suggesting that beneficial effects of RDN occur by additional pathways.
A particularly relevant vascular end point related to RDN is atherosclerosis. A recent clinical study in which atherosclerosis of the renal artery was measured by computed tomography angiography revealed no progression after 12 months after RDN while the control group showed increased atherosclerosis.18 There is also a clinical study (NCT01918111) currently recruiting subjects to determine the effect of RDN on multiple end points, including atherosclerosis. Based on the vasculoprotection indicated in preclinical and some clinical studies, attenuated progression of atherosclerosis might be an added benefit. This would be especially revealing if it occurred in the absence of effects on blood pressure, diabetes mellitus, and lipids. To address this hypothesis, we used a model of murine atherosclerosis. Apolipoprotein E–deficient mice develop atherosclerosis, in the absence of hypertension or diabetes mellitus, that is accelerated with a Western diet.19 In this study, efficacy of RDN was confirmed by histological evidence of downstream nerve fiber disruption as well as reduced renal NE levels. In this normotensive murine model, there was no effect of RDN on blood pressure or on systemic levels of NE. In addition, no effects were observed on fasting glucose, insulin, or cholesterol levels. In contrast to previous findings in humans showing improved glucoregulation after RDN,3 plasma glucose and insulin trended higher in mice receiving RDN although this was not a diabetic mouse model. Despite these neutral effects on blood pressure and lipids, RDN was associated with reduction in atherosclerosis based on measurements of lipid staining total surface area as well as cross-sectional analysis at the aortic valves and brachiocephalic arteries. Smooth muscle content was relatively increased in lesions from the RDN mice suggesting greater lesion stability. These changes were associated with the evidence of reduced systemic oxidative stress and Mcp-1 levels. Because aldosterone blockade has been shown to reduce atherosclerosis in primate,20 rabbit,21 and murine models,22,23 and to reduce markers of oxidative stress21–23 and Mcp-1 expression,24 we tested the effect of RDN on serum aldosterone levels. Consistent with the aldosterone hypothesis, serum levels were reduced in this model after RDN.
In conclusion, RDN reduced progression of atherosclerosis in apolipoprotein E–deficient mice. These changes are associated with reduced aldosterone levels, Mcp-1, and markers of oxidative stress. Further studies will be required to define the precise causal mechanisms involved in the atheroprotection associated with RDN.
Perspectives
The sympathetic nervous system plays an important role in hypertension and other vascular disease processes. RDN has been studied in humans with resistant hypertension although its effectiveness seems to be limited based on a recent randomized trial. There may be other vascular benefits associated with RDN that occur even in the absence of blood pressure reduction. This study demonstrates, in a normotensive, hyperlipidemic mouse model, that RDN has a protective effect against atherosclerosis in the absence of blood pressure lowering. Further elucidation of the underlying mechanism(s) by which renal sympathetic activity promotes systemic atherosclerosis may lead to new, less invasive, therapeutic interventions that may be beneficial to a broad population at risk for vascular disease.
Novelty and Significance.
What Is New?
Renal denervation (RDN) leads to reduced aldosterone and oxidative stress in nonhypertensive, atherosclerotic mice.
RDN reduces atherosclerosis without apparent effects on blood pressure.
RDN enhances lesion stability characterized by increased smooth muscle cell content in atherosclerotic plaques.
What Is Relevant?
RDN may provide vascular benefits even in the absence of blood pressure lowering.
Summary
Apolipoprotein E–deficient mice show reduced atherosclerosis after RDN, without effects on blood pressure or cholesterol. These effects are associated with reduced aldosterone levels and oxidative stress. These findings suggest that RDN may provide beneficial effects in subjects prone to vascular disease, even in the absence of blood pressure lowering.
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health (HL088419 to Dr Eitzman).
Footnotes
Disclosures
None.
References
- 1.Lüscher TF, Mahfoud F. Renal nerve ablation after SYMPLICITY HTN-3: confused at the higher level? Eur Heart J. 2014;35:1706–1711. doi: 10.1093/eurheartj/ehu195. doi: 10.1093/eurheartj/ehu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL, SYMPLICITY HTN-3 Investigators A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 3.Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, Hoppe UC, Vonend O, Rump LC, Sobotka PA, Krum H, Esler M, Böhm M. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940–1946. doi: 10.1161/CIRCULATIONAHA.110.991869. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa T, Hasegawa Y, Uekawa K, Ma M, Katayama T, Sueta D, Toyama K, Kataoka K, Koibuchi N, Maeda M, Kuratsu J, Kim-Mitsuyama S. Renal denervation prevents stroke and brain injury via attenuation of oxidative stress in hypertensive rats. J Am Heart Assoc. 2013;2:e000375. doi: 10.1161/JAHA.113.000375. doi: 10.1161/JAHA.113.000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–556. doi: 10.1161/HYPERTENSIONAHA.107.090696. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Villacorta L, Chang L, Fan Z, Hamblin M, Zhu T, Chen CS, Cole MP, Schopfer FJ, Deng CX, Garcia-Barrio MT, Feng YH, Freeman BA, Chen YE. Nitro-oleic acid inhibits angiotensin II-induced hypertension. Circ Res. 2010;107:540–548. doi: 10.1161/CIRCRESAHA.110.218404. doi: 10.1161/CIRCRESAHA.110.218404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Luo W, Wang J, Guo C, Wolffe SL, Wang J, Sun EB, Bradley KN, Campbell AD, Eitzman DT. Paradoxical protection from atherosclerosis and thrombosis in a mouse model of sickle cell disease. Br J Haematol. 2013;162:120–129. doi: 10.1111/bjh.12342. doi: 10.1111/bjh.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Luo W, Wang J, Guo C, Wang X, Wolffe SL, Bodary PF, Eitzman DT. Obesity-induced endothelial dysfunction is prevented by deficiency of P-selectin glycoprotein ligand-1. Diabetes. 2012;61:3219–3227. doi: 10.2337/db12-0162. doi: 10.2337/db12-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jänig W, Häbler HJ. Specificity in the organization of the autonomic nervous system: a basis for precise neural regulation of homeostatic and protective body functions. Prog Brain Res. 2000;122:351–367. doi: 10.1016/s0079-6123(08)62150-0. [DOI] [PubMed] [Google Scholar]
- 10.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 11.Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc. 1953;152:1501–1504. doi: 10.1001/jama.1953.03690160001001. [DOI] [PubMed] [Google Scholar]
- 12.Kopp UC. Neural Control of Renal Function. Morgan & Clayppol Life Sciences; San Rafael, CA: 2011. [PubMed] [Google Scholar]
- 13.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol. 2011;1:731–767. doi: 10.1002/cphy.c100043. doi: 10.1002/cphy.c100043. [DOI] [PubMed] [Google Scholar]
- 14.Mahfoud F, Lüscher TF, Andersson B, et al. European Society of Cardiology Expert consensus document from the European Society of Cardiology on catheter-based renal denervation. Eur Heart J. 2013;34:2149–2157. doi: 10.1093/eurheartj/eht154. doi: 10.1093/eurheartj/eht154. [DOI] [PubMed] [Google Scholar]
- 15.Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Böhm M, Hoppe UC. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901–909. doi: 10.1016/j.jacc.2011.11.034. doi: 10.1016/j.jacc.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 16.Huang WC, Fang TC, Cheng JT. Renal denervation prevents and reverses hyperinsulinemia-induced hypertension in rats. Hypertension. 1998;32:249–254. doi: 10.1161/01.hyp.32.2.249. [DOI] [PubMed] [Google Scholar]
- 17.Katayama T, Sueta D, Kataoka K, Hasegawa Y, Koibuchi N, Toyama K, Uekawa K, Mingjie M, Nakagawa T, Maeda M, Ogawa H, Kim-Mitsuyama S. Long-term renal denervation normalizes disrupted blood pressure circadian rhythm and ameliorates cardiovascular injury in a rat model of metabolic syndrome. J Am Heart Assoc. 2013;2:e000197. doi: 10.1161/JAHA.113.000197. doi: 10.1161/JAHA.113.000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang ZH, Yang K, Jiang FL, Zeng LX, Jiang WH, Wang XY. The effects of catheter-based radiofrequency renal denervation on renal function and renal artery structure in patients with resistant hypertension. J Clin Hypertens (Greenwich) 2014;16:599–605. doi: 10.1111/jch.12367. doi: 10.1111/jch.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo W, Wang H, Ohman MK, Guo C, Shi K, Wang J, Eitzman DT. P-selectin glycoprotein ligand-1 deficiency leads to cytokine resistance and protection against atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 2012;220:110–117. doi: 10.1016/j.atherosclerosis.2011.10.012. doi: 10.1016/j.atherosclerosis.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takai S, Jin D, Muramatsu M, Kirimura K, Sakonjo H, Miyazaki M. Eplerenone inhibits atherosclerosis in nonhuman primates. Hypertension. 2005;46:1135–1139. doi: 10.1161/01.HYP.0000184640.81730.22. doi: 10.1161/01.HYP.0000184640.81730.22. [DOI] [PubMed] [Google Scholar]
- 21.Rajagopalan S, Duquaine D, King S, Pitt B, Patel P. Mineralocorticoid receptor antagonism in experimental atherosclerosis. Circulation. 2002;105:2212–2216. doi: 10.1161/01.cir.0000015854.60710.10. [DOI] [PubMed] [Google Scholar]
- 22.Keidar S, Hayek T, Kaplan M, Pavlotzky E, Hamoud S, Coleman R, Aviram M. Effect of eplerenone, a selective aldosterone blocker, on blood pressure, serum and macrophage oxidative stress, and atherosclerosis in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2003;41:955–963. doi: 10.1097/00005344-200306000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Keidar S, Kaplan M, Pavlotzky E, Coleman R, Hayek T, Hamoud S, Aviram M. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation. 2004;109:2213–2220. doi: 10.1161/01.CIR.0000127949.05756.9D. doi: 10.1161/01.CIR.0000127949.05756.9D. [DOI] [PubMed] [Google Scholar]
- 24.Han SY, Kim CH, Kim HS, Jee YH, Song HK, Lee MH, Han KH, Kim HK, Kang YS, Han JY, Kim YS, Cha DR. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol. 2006;17:1362–1372. doi: 10.1681/ASN.2005111196. doi: 10.1681/ASN.2005111196. [DOI] [PubMed] [Google Scholar]