Abstract
As epidemiologic studies continue to note a striking increase in rates of autism spectrum disorder (ASD) diagnosis around the world, the lack of identified causative agents in most cases remains a major hindrance to the development of treatment and prevention strategies. Published observations of immune system abnormalities in ASD have increased recently, with several groups identifying fetal protein reactive IgG antibodies in plasma from mothers of children with autism. Furthermore, other gestational immune parameters, including maternal infection and dysregulated cytokine signaling, have been found to be associated with ASD in some cases. While detailed pathogenic mechanisms remain to be determined, the hypothesis that some cases of ASD may be influenced, or even caused, by maternal fetal brain–reactive antibodies or other in utero immune-related exposures is an active area of investigation. This article reviews the current literature in this area and proposes several directions for future research.
Autism spectrum disorders (ASDs) are now the most common neurodevelopmental disorders, with a diagnostic incidence of 1:88. Individuals with ASD display variable impairments in communication and social interaction and have restricted interests that often manifest as repetitive stereotypies. Within ASD, individuals may meet diagnostic thresholds in all 3 behavioral domains, resulting in a diagnosis of autistic disorder (AU); in 1 or 2 of the 3 domains yielding an ASD or Asperger disorder diagnosis; or have related impairments that differ sufficiently to result in a diagnosis of childhood disintegrative disorder or pervasive developmental disorder–not otherwise specified. Currently, these disorders are behaviorally diagnosed using instruments such as the Autism Diagnostic Interview–Revised1 and the Autism Diagnostic Observation Schedule,2 which evaluate the criteria found in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision).3 Work is currently under way to produce an updated Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition), which is scheduled to be released in May 20134 and will likely combine AU, ASD, Asperger disorder, childhood disintegrative disorder, and pervasive developmental disorder–not otherwise specified into the broad category of ASD, which will include measures of clinical specifiers of severity as well as associated clinical features.
Owing to the lack of biochemical diagnostic tests, it is unclear whether the symptoms underlying ASD stem from different etiologies or represent different manifestations of similar genetic or environmental factors. Data from twin studies have supported a strong genetic association with ASD based on high monozygotic twin concordance, and large-scale genetic screens have revealed numerous risk factors,5 each with relatively low penetrance, supporting the hypothesis that the behavioral manifestations of ASD may be a convergent consequence of genetic or environmental interference with any of a large number of critical neurodevelopmental processes. Interestingly, a recent report6 indicates a higher than previously reported concordance among dizygotic twins, resulting in a best-fit model determination of AU risk that attributes a 55% contribution of environmental factors and a 37% contribution of genetic factors. Risk for developing the broader diagnosis of ASD was found to have a nearly identical distribution of risk factors.
The human immune system possesses shared genetic and environmental components, relying on environmental exposures to inform the manifestation of genetically encoded signaling and effector functions. In the context of pregnancy, the maternal immune system has the added burden of nonresponsiveness to paternal as well as fetal-specific antigens, while maintaining the health of the mother and fetus. Thus, the components of the maternal immune system that cross the placenta may be considered to be fetal environmental exposures. Indeed, proper fetal neurodevelopment relies on the precise timing, functional levels, and anatomic localization of many signaling molecules that may be altered by exogenous factors to which the embryo is exposed. One in utero environmental factor that is gaining increased attention is maternal IgG antibodies.
GESTATIONAL ROLES OF MATERNAL IgG
The placenta provides a remarkably selective barrier that allows nutrition and immune factors to transfer to the developing fetus, while restricting the passage of potentially harmful molecules. Among the components of the maternal immune system that enter the fetal compartment, IgG antibodies transfer at high concentrations beginning around midgestation,7 culminating in circulating IgG levels in the newborn that exceed those in maternal circulation owing to neonatal FC receptor–mediated active transport across the placenta. This IgG, which is entirely maternal in origin, is thought to provide the newborn with a transient, protective immunity based on the environmental exposures of the mother until the child is capable of mounting an adaptive immune response. However, in cases of maternal autoimmunity, IgG that recognizes self-proteins also crosses the placenta and can interfere with fetal development as in maternal myasthenia gravis, which can lead to transient neonatal myasthenia gravis8 and, in rare cases, the often fatal disorder arthrogryposis multiplex congenita.9 While such conditions are relatively uncommon and the offending IgG are cleared from the infant’s circulation within the first 6 months of life, situations where IgG alters important developmental processes can lead to permanent defects. For example, in some cases of neonatal lupus erythematosis,10 characteristic maternal antibodies, which bind to the ribosomal Ro ribonucleoprotein, prevent proper heart formation in the developing fetus, thereby requiring corrective surgery in the newborn.
Placental IgG Transport and BBB Formation
Another layer of complexity is added to this system by the blood-brain barrier (BBB). The BBB consists primarily of endothelial tight junctions and provides a selective filter for central nervous system (CNS) entrance to cells and molecules from vascular circulation, allowing only certain components to enter and generally preventing access of inflammatory agents.11 Although IgG is normally restricted from the adult CNS, it may access developing brain targets prior to the complete formation of the BBB. The timing of BBB development in the fetus is complex12 and develops its exclusion properties primarily in response to Wnt/β-catenin signaling from perivascular astrocytes. Numerous associated signaling molecules participate in this cascade, several of which have genetic variants that have been implicated as autism susceptibility factors. Among these is the Engrailed 2 gene,13 which functions to regulate sensitivity to exogenous factors from maternal circulation that cross the placenta. Such genetic susceptibilities may underlie the mechanism of maternal antibody involvement in autism development.
Building on the concept of genetic susceptibility to an immune-mediated trigger of autism development, a decreased ability to restrict the production of self-reactive antibodies, in other words, a deficit in immune tolerance, may provide the basis for the production of self-reactive antibodies. One pathway that influences maintenance of self-tolerance in a dose-dependent fashion is the MET receptor tyrosine kinase and its associated signaling cascade.14,15 Interestingly, a functional MET polymorphism, which leads to decreased protein levels, has been associated with autism in several independent genetic studies.16–18 Furthermore, recent work by our laboratory indicates that this polymorphism is significantly associated with maternal antifetal brain antibodies found in mothers of children with autism.19
Alternatively, maternal fetal brain–reactive antibodies may arise in the context of an immune response to an infection. In this case, epitopes on the pathogen could elicit antibody production in association with a danger signal.20 B cells with receptor specificity for fetal antigens would likely not be eliminated through central tolerance mechanisms because of the sequestered nature of CNS antigens. This phenomenon of molecular mimicry has been demonstrated to precipitate the symptoms of rheumatic fever after infection with group A streptococcus21 through the induction of self-reactive antibodies and T cells. The resultant immune-mediated disorder causes variable inflammation of the CNS, joints, skin, and heart.
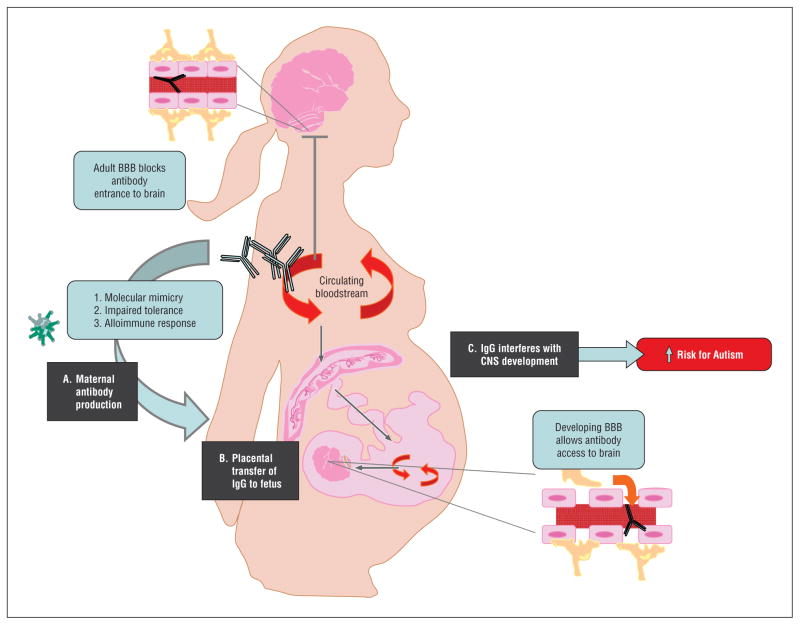
Regardless of the ontogeny of autism-associated maternal antibodies, there is a general consensus that the resulting pathogenic mechanism likely proceeds through 1 of 3 established mechanisms: (1) blockade or interference with the function of the target molecules22; (2) acting as an agonist and activating a receptor or signaling cascade23; or (3) through the induction of inflammation and the consequent effects on CNS development (Figure).24
Figure.
Schematic representation of maternal antibody-associated autism. A, Maternal antibodies that bind to fetal brain proteins may arise through (1) an immune response to a molecular mimic, (2) impaired tolerogenic mechanisms, or (3) alloimmunization to fetal antigens during a previous pregnancy. B, The placenta actively transports maternal IgG into fetal circulation from midgestation through parturition. C, Normally, the adult blood-brain barrier (BBB) excludes circulating IgG from the brain parenchyma, while the developing fetal brain is variably permissive to maternal IgG during gestation. The presence of maternal fetal brain–reactive IgG during critical windows of neurodevelopment appear capable of precipitating adverse offspring behavioral manifestations including autism. CNS indicates central nervous system.
Maternal IgG and Neurodevelopment
The fetal BBB is not yet fully formed during embryonic development and appears to be variably permissive to factors that enter the fetal circulation across the placenta, including IgG. Women with systemic lupus erythematosus, which is associated with autoantibodies recognizing both the N-methyl-D-aspartate receptor and double-stranded DNA, often have children with learning disabilities whereas children born to fathers with systemic lupus erythematosis do not, supporting a pathogenic role for the antibodies during fetal brain development. Studies in a murine systemic lupus erythematosus model of gestational antibody transfer reveal specific deposition of maternal anti–N-methyl-D-aspartate IgG in the developing neocortex at 60- to 70-fold higher concentration than in the maternal brain.23 Furthermore, on behavioral testing, offspring of mouse dams harboring such antibodies displayed impaired performance on a variety of behavioral tests that varied based on antibody titer.
Maternal IgG in Autism
Observational Studies
Interest in a possible association between maternal antibodies and ASD began with the observation that gestational exposure to fetal paternally derived lymphocyte antigens elicited an antibody response in some mothers, which was associated with pregnancy complications and autism in a small cohort (Table).25 These maternal antibodies, which caused complement-dependent cytotoxicity to lymphocytes of the child, were assumed to stem from exposure to paternal antigens during a previous pregnancy similarly to the genesis of anti-Rh antibodies.
Based on some intriguing pilot work from Dalton et al,31 we began our search for an association between maternal autoantibodies and autism. Using samples from the Childhood Autism Risk from Genes and the Environment Study,33 a large case-control cohort recruited through the Children’s Center for Environmental Health at the University of California, Davis, we went on to demonstrate IgG reactivity against fetal brain proteins at approximately 37 kDa and 73 kDa in plasma from 7 of 61 mothers (11.5%) of children with ASD but not in 62 mothers of typically developing children (P = .006) or 40 mothers of children with non-ASD developmental delay (P = .04).27 Zimmerman et al26 also performed a pilot study involving 11 mothers of children with autism and control mothers, examining maternal serum for autoantibodies to brain proteins derived from rats. Reactivity was observed to targets in rat prenatal (gestational day 18) but not to postnatal (day 8) or adult brain, which was not directed at previously identified neuronal targets of autoimmunity.
Building on these findings, Singer et al29 highlighted that this reactivity is primarily to fetal and not adult brain proteins in a larger sample set, observing reactivity to a 36-kDa human fetal brain antigen in approximately 10% of mothers of children with autism vs 2% of control mothers (P = .04). The striking consistency between the outcomes of both the Braunschweig27 and Singer29 studies indicate that antibody reactivity to antigens at approximately 36 to 39 kDa and 73 kDa was significantly more prevalent among mothers of children with autism. Observed in approximately 10% to 12% of mothers of children with autism, this antibody reactivity is thus one of the most prevalent biomarkers for autism discovered to date. Furthermore, it is interesting to note that both studies found a significant association between maternal anti-fetal brain reactivity and having a child with a regressive form of autism, yielding P values of .002 and .03 to .04 in the Braunschweig27 and Singer29 studies, respectively. This observation may reflect a subset of ASD cases whose clinical course is related to maternal antibody exposure.
To expand and confirm the previous findings, we have gone on to profile 560 additional mothers recruited into the Childhood Autism Risk from Genes and the Environment Study30 since our original report. To identify the expression timing of the fetal brain antigens, we tested maternal antibody reactivity to brain protein from 3 gestational ages in the Rhesus macaque. Observing the highest relative expression levels in late gestation (day 152), we screened our expanded cohort for maternal fetal brain–reactive antibodies. A refined Western blotting technique allowed us to resolve an additional band migrating near the 37-kDa band at approximately 39 kDa. The prevalence of maternal antibodies to the 37 kDa/73 kDa and 39 kDa/73 kDa bands remained the same as in our original study, and reactivity to the 37 kDa/73k Da band pair remained exclusive to mothers of children with autism. When compared with children with ASD whose mothers did not have maternal fetal brain–reactive antibodies, behavioral tests revealed that children with ASD with mothers harboring reactivity to the 37-kDa and 73-kDa bands had significantly impaired (P = .005) use of expressive language on the Mullen Scales of Early Learning,30,34 while children with ASD born to mothers with the 39-kDa and 73-kDa band patterns had significantly increased (P = .047) irritability scores on the Aberrant Behavior Checklist.30,35 These results support the hypothesis that maternal fetal brain–reactive antibodies may underlie certain behavioral features of some cases of ASD.
A central critique of studies linking maternal fetal brain–reactive antibodies and ASD has been that blood collection for antibody determination typically occurs after a child’s diagnosis and may represent antibody specificities that developed after the birth of the child. To address this issue, archived midpregnancy blood samples in a large case-control study were screened for reactivity toward the fetal brain. Findings from this study confirmed the gestational presence of maternal fetal brain–reactive antibodies more commonly among mothers of children with autism, particularly those recognizing the 39-kDa and 73-kDa antigens.28
Behavioral Models
Pioneering murine model studies described in 196836 demonstrated that human IgG is transferred across the mouse placenta and into fetal circulation. This observation, combined with the knowledge that several autoimmune disorders involve pregnancy complications, lead to the development of mouse models of gestational transfer of self-reactive IgG for lupus,37 fetal arthrogryposis,38 bullous pemphigoid,39 and epidermolysis bullosa acquisita.40 The possibility that maternal antibody transfer may precipitate neurologic disturbances in offspring led Dalton et al31 to perform a pilot study using intraperitoneal administration of serum from a mother of one child with autism and one child with a severe language delay into pregnant mice. After observing that IgG antibodies from this mother stained cerebellar Purkinje neurons, large brainstem neurons, and cells from a neuroblastoma line, the group went on to note significant reductions in exploratory behavior (P = .02) in juveniles and impaired motor control (P < .001). The behavioral manifestations were hypothesized to originate in the cerebellum, prompting nuclear magnetic resonance studies that observed reduced cerebellar metabolites (P < .05) in adults after in utero IgG exposure. The combination of cellular staining and altered offspring behavior seen in this study suggests, at least in this individual, that the noted behavioral consequences, which are reminiscent of features of ASD, may have originated from the direct actions of brain-reactive maternal antibodies.
Despite the sophistication of rodent behavioral tests, the core features of autism are difficult to assess in such model systems. Furthermore, the kinetics of maternal-fetal IgG transfer differ markedly between mice and humans,41 with substantial variation in the neurodevelopmental processes occurring during maternal IgG exposure. To address some of these issues, Martin et al32 administered intravenous injections of purified pooled IgG from mothers of multiple children with autism or control mothers at 3 separate times spanning gestation. Reduced postweaning peer contact (P = .04) and increased nonsocial active behavior (P = .04) was observed among the offspring of dams treated with maternal AU IgG, while hyperactivity was observed during individual housing conditions (P = .01), suggestive of autism-related behavior. Furthermore, dramatic whole-body stereotypic behaviors were observed in the offspring of AU IgG–treated dams in both solo (P = .003–.01) and social (P = .01–.03) testing paradigms. These behaviors, which included repetitive back flipping and pacing, recapitulate some of the self-directed stereotypical actions characteristic of autism and were observed throughout the testing ages.
Expanding these findings to a larger group of mothers of children with autism, Singer et al24 administered pooled, purified IgG from 63 mothers of children with autism to pregnant mice, following an intraperitoneal injection strategy. In agreement with previous findings, behavioral alterations were noted in the offspring of mice treated with autism-associated maternal IgG, including hyperactivity, increased anxiety, and decreased social interaction (all P < .001). The observation of increased numbers of microglia following the course of antibody administration suggests that their effects may be mediated through inflammatory processes initiated by antibody binding, a conclusion further supported by elevated brain-derived neurotrophic factor levels (P = .03).
It is interesting to note that murine models of genetically determined behavioral disorders sharing some clinical overlap with ASD, such as fragile X and Rett syndromes,42 have yielded similar behavioral findings to those just described. These observations support the concept that the behavioral features characteristic of ASD may represent a convergent phenotype arising from a variety of factors, including immunologic, genetic, or environmental. Thus, the various model systems that recapitulate features of autism each shed light on sensitive processes that are necessary for proper neurodevelopment.
ADDITIONAL GESTATIONAL IMMUNE ASSOCIATIONS WITH ASD
Maternal infection and inflammation during pregnancy has long been known to negatively impact fetal development. Gestational rubella infection, particularly during the first trimester, often leads to congenital rubella syndrome, which manifests with severe behavioral consequences including mental retardation and autism43 as well as myriad physical abnormalities such as hydrocephaly and cardiac malformations. In much of the world, instances of congenital rubella syndrome have been effectively eliminated through rubella vaccination in the form of the measles, mumps, and rubella vaccine.44 Maternal inflammation during pregnancy resulting from other viral or bacterial infections has been linked with autism and schizophrenia in offspring and has been proposed to stem from altered cytokine signaling during pregnancy. This phenomenon has been extensively modeled in animal studies using lipopolysaccharide, a bacterial cell wall component that activates toll-like receptor 4, and poly I:C, a double-stranded RNA viral mimic that activates toll-like receptor 3.45 Both lipopolysaccharide and poly I:C initiate strong innate immune responses and induce production of the pro-inflammatory cytokine interleukin 6, which is known to induce placental inflammatory processes46 and has been shown to mediate the neurodevelopmental effects of gestational inflammation.47 It is possible that such inflammatory processes could be related to the production of maternal antibodies that recognize fetal antigens through maternal-fetal cross talk48 or that maternal antibody to antigen interactions may precipitate inflammation-induced neurodevelopmental alterations similarly to bacterial or viral challenge.
FUTURE DIRECTIONS
The dominant model of maternal antibody–associated autism maintains that pathogenic maternal antibodies initiate their effects through antigen recognition, thus making the identification of the target molecules of utmost importance. It is interesting to note that among the studies that have identified the presence of brain-reactive maternal antibodies among mothers of children with autism, most have observed bands of reactivity by Western blot in the 36 to 39 kDa range. Furthermore, the finding of increased relative antigen levels in preparations of fetal brain protein support the possibility that the antibodies recognize a developmentally regulated or fetal isoform that may simultaneously explain the behavioral manifestations in offspring as well as the lack of maternal symptoms. Of additional interest is that these maternal antibodies have been observed both in retrospective studies involving archived samples as well as in samples taken after child diagnosis around 3 years of age, suggesting that they are persistent in individuals who possess them. To our knowledge, no study to date has investigated whether subsequent children born to mothers with brain-reactive antibodies have a risk for developing autism that is higher than published familial recurrence rates of approximately 19%.49 Although only a subset of mothers of children with autism possesses brain-reactive antibodies and the children themselves do not appear to generate similar maternal fetal brain–reactive antibodies,50 the identification of the target antigens may also shed light on pathways whose perturbation from genetic polymorphism or environmental exposure lead to the behavioral outcome of autism. Advancements in treatment and management of established autoimmune diseases provide strategies for the potential prevention of the consequences of fetal exposure to autism-associated maternal antibodies.
Table 1.
Table. Compiled Results of Observational and Behavioral Studies Associating Maternal Antibodies With Autisma
| Antibody Target | MW | Prevalence, % | Maternal IgG Population, No. |
|---|---|---|---|
| Observational Studies | |||
| Child/paternal lymphocytes25 | Undetermined | 54 (against child’s or father’s cells) | AU = 11; TD = 20 |
| Rat fetal/juvenile/adult brain26 | Multiple low MW and 250 kDa | 100 (2 patterns) | AU = 11; TD = 10 |
| Human fetal brain27 | 37 kDa and 73 kDa pair | 12 | AU = 61; DD = 40; TD = 62 |
| Human fetal brain28 | 39 kDa and 39 kDa/73 kDa pair | 7 (for 39 kDa) | AU = 84; DD = 49; TD = 160 |
| Fetal/adult human and rat brain29 | 36 kDa, 39 kDa, and 61 kDa | 10 (36 kDa for human fetal brain) | AU = 100; TD = 100 |
| Fetal rhesus macaque brain30 | 37 kDa/73 kDa and 39 kDa/73 kDa pairs | 7 (37/73 kDa) and 4 (39/73 kDa) | AU = 201; ASD = 71; TD = 185; DD = 102 |
| Species | Significant Outcomes | Administration Route; Gestational Age; Amount per Injection; Serum or IgG | Maternal IgG Population |
|---|---|---|---|
| Animal Behavioral Studies | |||
| Rat31 | Decreased exploration Impaired motor control IHC Purkinje/neuroblastoma staining Decreased choline and creatinine levels |
Intraperitoneal; embryonic day (for rodents) 10–17; 0.5 mL; serum | AU = 1; TD = 4 (all multiplex) |
| Rhesus macaque32 | Reduced peer contact Increased nonsocial activity Hyperactivity Whole-body stereotypies |
Intravenous; gestational day (for primates) 27, 41, and 55; 15–20 mg; IgG | AU = 12; TD = 7 (all multiplex) |
| Mouse24 | Hyperactivity Increased anxiety Reduced social interaction Embryonic day 18 microglial activation Increased brain BDNF levels |
Intraperitoneal; embryonic day 13–18; 1.25–1.4 mg; IgG | AU = 63; TD = 63 (mixed parity) |
Abbreviations: ASD, autism spectrum disorder; AU, autism; BDNF, brain-derived neurotrophic factor; DD, developmental delay (nonautism); MW, molecular weight; TD, typically developing.
Observational studies of maternal antibody binding to fetal brain proteins have often noted maternal IgG reactivity to antigens of approximately 36 to 39 kDa, focusing candidate protein identification on this MW range. Behavioral studies have recapitulated some of the features of autism in model systems and have yielded substantially consistent results.
Acknowledgments
Funding/Support: This work was supported by grants 1P01ES11269-01 and 1 R01-ES015359 from the National Institute of Environmental Health Sciences and grant R829388 from the US Environmental Protection Agency. The Autism Speaks Foundation also supported work cited in this review (JV).
Footnotes
Financial Disclosure: Dr Braunschweig received postdoctoral training grant MH073124 from the National Institute of Mental Health (SJ Rogers, principal investigator).
Additional Contributions: We thank Paula Garay, PhD, for her artistic assistance with the figure for this review.
Author Contributions: Study concept and design: Braunschweig and Van de Water. Acquisition of data: Braunschweig and Van de Water. Analysis and interpretation of data: Braunschweig and Van de Water. Drafting of the manuscript: Braunschweig and Van de Water. Critical revision of the manuscript for important intellectual content: Braunschweig. Obtained funding: Van de Water. Administrative, technical, and material support: Braunschweig and Van de Water. Study supervision: Van de Water.
References
- 1.Lord C, Pickles A, McLennan J, et al. Diagnosing autism: analyses of data from the Autism Diagnostic Interview. J Autism Dev Disord. 1997;27(5):501–517. doi: 10.1023/a:1025873925661. [DOI] [PubMed] [Google Scholar]
- 2.Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. J Child Psychol Psychiatry. 2002;43(6):807–821. doi: 10.1111/1469-7610.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- 4.American Psychiatric Association. [Accessed August 20, 2011];DSM-5 development: autism spectrum disorder. http://www.dsm5.org/ProposedRevision/Pages/proposedrevision.aspx?rid=94#.
- 5.Miles JH. Autism spectrum disorders: a genetics review. Genet Med. 2011;13(4):278–294. doi: 10.1097/GIM.0b013e3181ff67ba. [DOI] [PubMed] [Google Scholar]
- 6.Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol. 1994;1(6):667–669. doi: 10.1128/cdli.1.6.667-669.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’carroll P, Bertorini TE, Jacob G, Mitchell CW, Graff J. Transient neonatal myasthenia gravis in a baby born to a mother with new-onset anti-MuSK-mediated myasthenia gravis. J Clin Neuromuscul Dis. 2009;11(2):69–71. doi: 10.1097/CND.0b013e3181a78280. [DOI] [PubMed] [Google Scholar]
- 9.Hoff JM, Daltveit AK, Gilhus NE. Artrogryposis multiplex congenita: a rare fetal condition caused by maternal myasthenia gravis. Acta Neurol Scand Suppl. 2006;183:26–27. doi: 10.1111/j.1600-0404.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 10.Perez MF, Torres ME, Buján MM, Lanoël A, Cervini AB, Pierini AM. Neonatal lupus erythematosus: a report of four cases. An Bras Dermatol. 2011;86(2):347–351. doi: 10.1590/s0365-05962011000200021. [DOI] [PubMed] [Google Scholar]
- 11.Liebner S, Czupalla CJ, Wolburg H. Current concepts of blood-brain barrier development. Int J Dev Biol. 2011;55(4–5):467–476. doi: 10.1387/ijdb.103224sl. [DOI] [PubMed] [Google Scholar]
- 12.Virgintino D, Errede M, Robertson D, et al. Immunolocalization of tight junction proteins in the adult and developing human brain. Histochem Cell Biol. 2004;122(1):51–59. doi: 10.1007/s00418-004-0665-1. [DOI] [PubMed] [Google Scholar]
- 13.Benayed R, Gharani N, Rossman I, et al. Support for the homeobox transcription factor gene ENGRAILED 2 as an autism spectrum disorder susceptibility locus. Am J Hum Genet. 2005;77(5):851–868. doi: 10.1086/497705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutella S, Bonanno G, Procoli A, et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood. 2006;108(1):218–227. doi: 10.1182/blood-2005-08-3141. [DOI] [PubMed] [Google Scholar]
- 15.Futamatsu H, Suzuki J, Mizuno S, et al. Hepatocyte growth factor ameliorates the progression of experimental autoimmune myocarditis: a potential role for induction of T helper 2 cytokines. Circ Res. 2005;96(8):823–830. doi: 10.1161/01.RES.0000163016.52653.2e. [DOI] [PubMed] [Google Scholar]
- 16.Campbell DB, Sutcliffe JS, Ebert PJ, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103(45):16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson PB, Boccuto L, Skinner C, et al. Further evidence that the rs1858830 C variant in the promoter region of the MET gene is associated with autistic disorder. Autism Res. 2009;2(4):232–236. doi: 10.1002/aur.87. [DOI] [PubMed] [Google Scholar]
- 18.Campbell DB, Li C, Sutcliffe JS, Persico AM, Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 2008;1(3):159–168. doi: 10.1002/aur.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heuer L, Braunschweig D, Ashwood P, Van de Water J, Campbell DB. Association of a MET genetic variant with autism-associated maternal autoantibodies to fetal brain proteins and cytokine expression. Translational Psychiatry. 2011;1(10):e–48. doi: 10.1038/tp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 21.Guilherme L, Kalil J, Cunningham M. Molecular mimicry in the autoimmune pathogenesis of rheumatic heart disease. Autoimmunity. 2006;39(1):31–39. doi: 10.1080/08916930500484674. [DOI] [PubMed] [Google Scholar]
- 22.Cole RN, Ghazanfari N, Ngo ST, Gervásio OL, Reddel SW, Phillips WD. Patient autoantibodies deplete postsynaptic muscle-specific kinase leading to disassembly of the ACh receptor scaffold and myasthenia gravis in mice. J Physiol. 2010;588(pt 17):3217–3229. doi: 10.1113/jphysiol.2010.190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JY, Huerta PT, Zhang J, et al. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. 2009;15(1):91–96. doi: 10.1038/nm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: a pregnant dam mouse model. J Neuroimmunol. 2009;211(1–2):39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Warren RP, Cole P, Odell JD, et al. Detection of maternal antibodies in infantile autism. J Am Acad Child Adolesc Psychiatry. 1990;29(6):873–877. doi: 10.1097/00004583-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerman AW, Connors SL, Matteson KJ, et al. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21(3):351–357. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Braunschweig D, Ashwood P, Krakowiak P, et al. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29(2):226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croen LA, Braunschweig D, Haapanen L, et al. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry. 2008;64(7):583–588. doi: 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neuroimmunol. 2008;194(1–2):165–172. doi: 10.1016/j.jneuroim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Braunschweig D, Duncanson P, Boyce R, et al. Behavioral correlates of maternal antibody status among children with autism [published online October 20, 2011] J Autism Dev Disord. doi: 10.1007/s10803-011-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalton P, Deacon R, Blamire A, et al. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53(4):533–537. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- 32.Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22(6):806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114(7):1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zola H, Swart B, Banham A, et al. CD molecules 2006: human cell differentiation molecules. J Immunol Methods. 2007;319(1–2):1–5. doi: 10.1016/j.jim.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Babcock MJ, Soliman EZ, Ding J, Kronmal AR, Goff DC., Jr Pericardial fat and atrial conduction abnormalities in the Multiethnic Study of Atherosclerosis (MESA) Obesity (Silver Spring) 2011;19(1):179–184. doi: 10.1038/oby.2010.121. [DOI] [PubMed] [Google Scholar]
- 36.Gitlin D, Koch C. On the mechanisms of maternofetal transfer of human albumin and gamma-G globulin in the mouse. J Clin Invest. 1968;47(5):1204–1209. doi: 10.1172/JCI105809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tincani A, Nuzzo M, Motta M, Zatti S, Lojacono A, Faden D. Autoimmunity and pregnancy: autoantibodies and pregnancy in rheumatic diseases. Ann N Y Acad Sci. 2006;1069:346–352. doi: 10.1196/annals.1351.032. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson L, Polizzi A, Morriss-Kay G, Vincent A. Plasma from human mothers of fetuses with severe arthrogryposis multiplex congenita causes deformities in mice. J Clin Invest. 1999;103(7):1031–1038. doi: 10.1172/JCI5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishie W, Sawamura D, Natsuga K, et al. A novel humanized neonatal autoimmune blistering skin disease model induced by maternally transferred antibodies. J Immunol. 2009;183(6):4088–4093. doi: 10.4049/jimmunol.0800389. [DOI] [PubMed] [Google Scholar]
- 40.Kasperkiewicz M, Hirose M, Recke A, Schmidt E, Zillikens D, Ludwig RJ. Clearance rates of circulating and tissue-bound autoantibodies to type VII collagen in experimental epidermolysis bullosa acquisita. Br J Dermatol. 2010;162(5):1064–1070. doi: 10.1111/j.1365-2133.2010.09680.x. [DOI] [PubMed] [Google Scholar]
- 41.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008;13(1):4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- 43.Chess S. Autism in children with congenital rubella. J Autism Child Schizophr. 1971;1(1):33–47. doi: 10.1007/BF01537741. [DOI] [PubMed] [Google Scholar]
- 44.Berger BE, Navar-Boggan AM, Omer SB. Congenital rubella syndrome and autism spectrum disorder prevented by rubella vaccination: United States, 2001–2010. BMC Public Health. 2011;11:340. doi: 10.1186/1471-2458-11-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17(7):389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25(4):604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khosrotehrani K, Leduc M, Bachy V, et al. Pregnancy allows the transfer and differentiation of fetal lymphoid progenitors into functional T and B cells in mothers. J Immunol. 2008;180(2):889–897. doi: 10.4049/jimmunol.180.2.889. [DOI] [PubMed] [Google Scholar]
- 49.Ozonoff S, Young GS, Carter A, et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris CM, Zimmerman AW, Singer HS. Childhood serum anti-fetal brain antibodies do not predict autism. Pediatr Neurol. 2009;41(4):288–290. doi: 10.1016/j.pediatrneurol.2009.04.014. [DOI] [PubMed] [Google Scholar]