Abstract
Objectives
To assess potential improvement in SCD risk prediction by adding selected risk markers from the 12-lead ECG to measurement of the left ventricular ejection fraction (LVEF).
Background
Novel strategies to improve risk stratification for sudden cardiac death (SCD) are needed. Given the modest odds associated with most individual risk markers, combining multiple markers may be a useful approach.
Methods
From the ongoing Oregon Sudden Unexpected Death Study, SCD cases with pre-event LVEF available were compared to matched control subjects with coronary artery disease. Resting heart rate, QRS duration (QRSD), and JTc intervals were measured from archived ECGs prior and unrelated to the SCD event. Independent odds of SCD for individual and combined ECG markers were calculated.
Results
SCD cases (n= 317; 67.9 ± 12.9 years) were more likely than controls (n=317; 67.9 ± 12.8 years) to have LVEF ≤ 35% (26% vs. 11%). Mean heart rate, QRSD, and JTc were significantly higher in cases (all p<0.0001). In adjusted analyses, higher heart rate [OR 2.6 (1.8 – 3.7)], QRSD [OR 1.5 (1.0 – 2.5)] and JTc [OR 2.3 (1.6 – 3.4)] were independently associated with SCD. When ECG markers were combined, SCD odds progressively increased with one [OR 3.4 (2.1 – 5.4)] and ≥ 2 elevated markers [OR 6.3 (3.3 – 12.1)]. Addition of ECG markers to an adjusted model with LVEF improved discrimination (C statistic 0.724 vs. 0.642) and net reclassification (by 22.7%) (p<0.0001).
Conclusions
Combining selected 12-lead ECG markers with LVEF improves SCD risk prediction, and warrants further investigation in prospective studies.
Keywords: sudden cardiac death, risk, electrocardiogram, ejection fraction
Introduction
Despite concerted efforts in the field of resuscitation over the past few decades, out of hospital sudden cardiac arrest is fatal in the vast majority of individuals (>90%) within the first few minutes of the event (1). Therefore, efforts at prediction and prevention of sudden cardiac death (SCD) remain vital. While the implantable cardioverter defibrillator (ICD) is an effective intervention to reduce arrhythmic mortality (2,3), the challenge lies in identifying the high risk individual who will optimally benefit from it due to concerns of cost as well as potential harm (4). Left ventricular (LV) systolic dysfunction, which has almost exclusively been used as the clinical predictor of SCD risk lacks adequate discriminating power, highlighting the need for alternative approaches to risk stratification (5).
Though several other risk markers have been evaluated (6–9), these await incorporation into improved risk stratification algorithms. One of the statistical challenges in identifying effective novel markers is that high odds ratios are required to improve risk prediction at the individual level (10), almost never achievable by single markers. Combining multiple risk markers to additively improve risk may therefore represent the next logical step in improving SCD risk prediction (5,11). Ideally, such markers should be derived from widely available, preferably inexpensive clinical tools.
Prospective cohort studies are a robust setting for the evaluation of risk predictors. However, given the low annual incidence of SCD in the population, very large cohorts are needed to obtain adequate numbers which poses practical challenges. Community-based case-control studies with prospective ascertainment of SCD offer the advantage of being able to obtain feasible numbers for analysis within a reasonable time and therefore can be a valuable complementary approach to cohort studies (12). Though prior studies have evaluated the concept of using multiple markers to enhance SCD risk prediction (13–16), there is a lack of studies in the general population. From the Oregon Sudden Unexpected Death Study (Oregon-SUDS), we have previously reported on the utility of certain parameters from the 12-lead ECG which could represent simple, non-invasive markers to aid SCD risk stratification (17–19). However, we have not previously evaluated a combination of these ECG markers together. Therefore, in the present study, we tested the hypothesis that combining multiple select risk markers from the surface ECG could have cumulative effects on the risk of SCD and improve the risk prediction model beyond LVEF.
Methods
Case Ascertainment
Subjects with SCD were identified from the ongoing Oregon Sudden Unexpected Death Study (Oregon SUDS), a community-based study of SCD in the Portland, Oregon metropolitan region (population ~1 million). Methods have been previously published (20,21). Briefly, individuals of any age who suffer an out-of-hospital sudden cardiac arrest are identified through collaboration with the region’s emergency medical response system (EMS), the Medical Examiner’s office, and local hospitals. Cardiac arrests are adjudicated and included in the study as SCD only after comprehensive review of the arrest circumstances recorded by the EMS response team, the patient’s medical history prior to the SCD, and autopsy data if available. If they meet criteria, survivors of cardiac arrest are included as SCD cases. SCD is defined as a sudden unexpected pulseless condition of likely cardiac origin, occurring after a rapid witnessed collapse; unwitnessed deaths occurring within 24 hours of the patient being seen alive in a normal state of health were also included (22). Subjects were excluded if they had a prior terminal illness (e.g. terminal cancer), or any identifiable non-cardiac etiology of sudden death, such as stroke, pulmonary embolism, trauma, violent death, overdose, drowning or suicide. While patients with a history of congestive heart failure were included in the case population, the adjudication process maximizes the likelihood the cardiac arrest cases with rapid, unexpected collapse and likely cardiac etiology were included. Patients on hospice or patients with end-stage heart failure with progressive deterioration in the days preceding arrest were excluded.
Control ascertainment
Control subjects were enrolled from the same geographic area from the following sources in order to have representation of individuals with both stable and non-stable coronary artery disease (CAD): (1) patients who called 911 and received ambulance transport for symptoms of chest pain or dyspnea but who did not have a cardiac arrest; (2) patients who visited the cardiology clinic or who had angiography (with ≥50% stenosis noted on a coronary artery) at one of the participating hospitals; and (3) members of the region’s Kaiser Permanente health maintenance organization (HMO). Medical records were retrieved and reviewed for all potential control subjects to determine whether the patient had a history of CAD. Control subjects were enrolled if they provided consent, and were found to have documented CAD based on available medical records, as defined below, and no history of ventricular arrhythmias.
Definition of coronary artery disease
Coronary artery disease in both case and control subjects was defined as ≥50% stenosis by angiogram, history of myocardial infarction, or documented revascularization (CABG or PCI) documented in medical records, prior to arrest for cases and prior to or at ascertainment for controls.
Study design
A case-control design was used to compare SCD cases to control subjects with CAD. All SCD cases were included, regardless of the presence or absence of prior documented CAD. Population-based evaluation indicates that approximately half of SCD cases have previously recognized CAD before occurrence of SCD (23). Among SCD patients without prior documented CAD, published autopsy studies indicate that ≥80% of SCD patients age ≥50 have significant CAD at the time of their arrest (24). Thus, in the community, the vast majority of SCD cases occur in the setting of associated significant CAD. The present analysis included individuals with documented CAD prior to arrest / ascertainment (65% of cases and 75% of controls), and individuals whose sentinel event (first documented CAD) resulted in either SCD (35% of cases) or a non-fatal coronary event or coronary procedure (25% of controls). This study design allows for the investigation of factors related to SCD in individuals with CAD, and has the possibility of identifying factors associated with arrhythmic death rather than with CAD in general.
Subject inclusion criteria
Cases and controls (Feb. 2002 – Dec. 2013) were included in the analysis if they were age ≥18, had physician records available for review, had LVEF assessment, and had an ECG in sinus rhythm prior and unrelated to the SCD with heart rate, QRS, JT, QT, and RR intervals. Eligible cases and controls were then frequency matched by sex and age (in 5-year intervals), which resulted in 317 cases and 317 controls for analysis. Compared to cases excluded from analysis, included cases were somewhat older (68 vs. 65 years), with a higher prevalence of medically-documented diabetes, hypertension, and history of myocardial infarction and documented coronary disease, though body mass index was similar.
Assessment and definition of ECG markers, ejection fraction, and clinical variables
Heart rate and QRS duration were computer-read; JT, QT, and RR intervals were manually-read using digital on-screen software (Datinf Measure: DataInf GmbH; Tubingen, Germany). Ejection fraction was obtained from an echocardiogram, angiogram, or MUGA scan obtained from clinical records. For analysis, if more than one EF assessment or ECG was available, the EF or ECG closest to and prior to arrest / ascertainment was chosen. Assessment of EF occurred a median of 415 and 115 days prior for cases and controls, respectively; ECGs were a median of 274 and 140 days prior, respectively. For controls without pre-ascertainment tests available, post-ascertainment EF assessment (median 1 day post) or ECG (median 227 days post) was accepted. Overall, over two-thirds of assessment was within 2 years of the SCD / ascertainment. Clinical history of hypertension and diabetes were obtained from the clinical record closest to arrest / ascertainment.
Statistical analysis
Left ventricular function was categorized as severely reduced (EF ≤35%) or not severely reduced (EF >35%), and also modeled as a continuous variable in sensitivity analyses. ECG parameters were categorized into quartiles based on the distribution among control subjects, with values at or above the 75th percentile compared to values below the 75th percentile, and also modeled as continuous variables in sensitivity analyses. For univariate analysis, case-control differences were compared using independent samples t-tests and the Pearson chi-square statistic.
Multivariable logistic regression was used to estimate the association of SCD with EF, adjusting for age as a continuous variable, sex, diabetes, and hypertension. In subsequent models, ECG markers were added. In three separate models, EF and ECG markers were entered (i) separately as dichotomous variables (EF ≤35% vs. >35%, and ≥75th vs. <75th percentile for heart rate, JTc, and QRS); (ii) separately as continuous variables (EF, heart rate, JTc, and QRS); and (iii) jointly as categorical variables to model the cumulative joint effects of the ECG markers. In all multivariable models, JTc, rather than QTc, was used as an indicator of ventricular repolarization because JTc can be measured even when QRSD is prolonged, and thus allows for simultaneous modeling of ventricular depolarization (QRSD) and repolarization (JTc). For the cumulative modeling, each individual was considered to fall into a “high risk” ECG marker group if at least two of their ECG markers were above the 75th percentile, into a “low risk” group if all three ECG markers were below the median, or a “moderate risk” ECG marker group (if at least one ECG marker was above the median value, but at most one ECG marker was above the 75th percentile). We evaluated model fit with the Hosmer-Lemehow goodness of fit test (25), and model discrimination with the C statistic (26). We calculated the sensitivity and specificity of models with and without ECG markers using the classification table output from SAS PROC LOGISTIC, using a predicted probability of ≥0.5 to indicate high risk. Furthermore, we calculated net reclassification improvement and integrated discrimination improvement, comparing the models with and without ECG variables (27). We interpreted significant improvement in the C statistic with the addition of ECG markers to indicate improved prediction of SCD. Likewise, if the NRI and IDI indicated improved reclassification and discrimination with the addition of ECG markers, this would indicate potential utility of ECG markers for improved risk prediction.
Results
Univariate results
Among the 317 cases and 317 controls (Table 1), cases were more likely to have diabetes (50% vs. 31%, p<0.0001) and lower EF (49% vs. 56%, p<0.0001). Cases also had significantly elevated ECG markers (p<0.0001), with a heart rate 9 beats per minute faster than controls (77 vs. 68), a longer QRS duration (106 vs. 99), a prolonged JTc interval (351 vs. 335), and QTc interval (458 vs. 428). Severely reduced LV systolic function (EF ≤35%) was much more common among cases (26% vs. 11%), while ECG markers at or above the 75th percentile were observed in a significantly higher proportion of cases (p≤.002, Table 1).
Table 1.
Demographics, comorbidities, and ECG parameters in matched cases and controls with EF assessment from the Oregon Sudden Unexpected Death Study (SUDS)
| Cases (n=317) | Controls (n=317) | p value | |
|---|---|---|---|
| Age* | 67.9 ± 12.9 | 67.9 ± 12.8 | -- |
| Male* | 200 (63%) | 200 (63%) | -- |
| Hypertension | 249 (79%) | 235 (74%) | 0.22 |
| Diabetes | 159 (50%) | 96 (31%) | <0.0001 |
| History of MI | 167 (53%) | 142 (45%) | 0.047 |
| Beta blocker use | 191 (61%) | 196 (65%) | 0.29 |
| EF (mean ± SD) | 49 ± 16 | 56 ± 13 | <0.0001 |
| Heart rate (mean ± SD) | 77 ± 18 | 68 ± 14 | <0.0001 |
| QRS (mean ± SD) | 106 ± 26 | 99 ± 20 | <0.0001 |
| JTc (mean ± SD) | 351 ± 42 | 335 ± 33 | <0.0001 |
| QTc† (mean ± SD) | 458 ± 41 | 428 ± 30 | <0.0001 |
| EF ≤35% | 81 (26%) | 35 (11%) | <0.0001 |
| ECG parameters ≥75th percentile‡ | |||
| Heart rate (≥ 76 bpm) (n, %) | 159 (50%) | 83 (26%) | <0.0001 |
| QRS (≥ 106 ms) (n, %) | 124 (39%) | 87 (27%) | 0.002 |
| JTc (≥ 355 ms) (n, %) | 136 (43%) | 77 (24%) | <0.0001 |
| QTc† (≥ 448 ms) (n, %) | 131 (55%) | 63 (24%) | <0.0001 |
Cases and controls frequency matched on age (5-year categories) and sex
For QTc, cases n=240, controls n=267. QTc was not calculated for subjects with QRS < 120, according to convention
Cut-point of ≥75th percentile in controls shown in parentheses
Cumulative effect of ECG markers
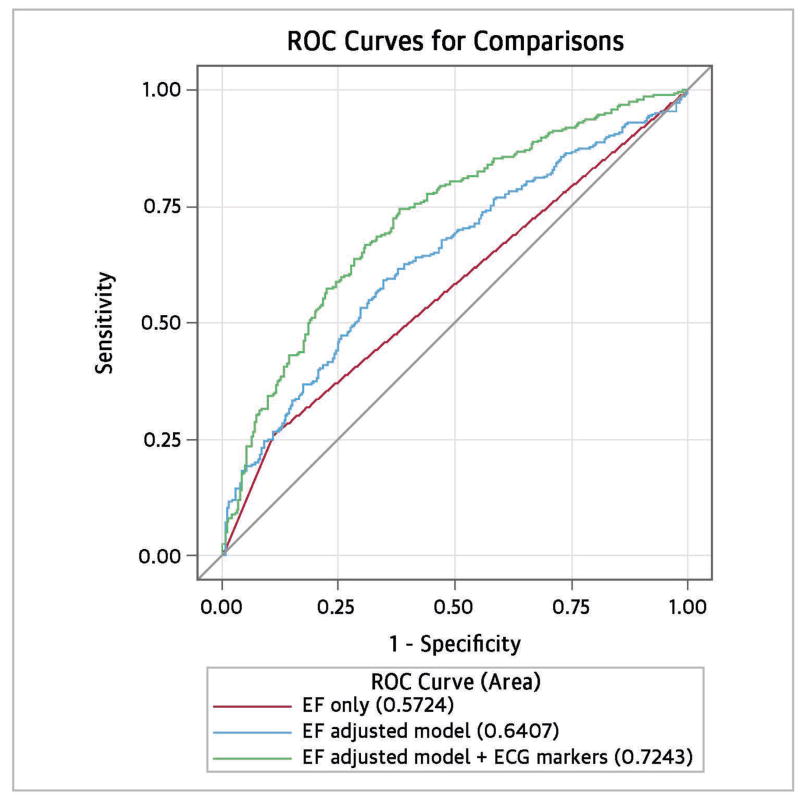
In multivariable modeling, EF ≤35% was associated with a 2.8-fold increased odds of SCD (Table 2), which was slightly diminished [odds ratio (OR) 2.6, 95% confidence interval (CI) 1.7 – 4.1] after adjusting for age, sex, diabetes, and hypertension. When the dichotomized ECG markers were added to the adjusted model, EF was further diminished, but remained a relatively strong and significant predictor of SCD (OR 2.0, 95% CI 1.3 – 3.2). Each of the ECG markers (≥75th vs. <75th percentile) was also significantly associated with SCD: heart rate OR 2.6 (95% CI 1.8 – 3.7), JTc interval OR 2.3 (95% CI 1.6 – 3.4), and QRS duration OR 1.5 (95% CI 1.0 – 2.5). We tested for potential interaction of beta blockers with EF, heart rate, QRS, and JTc intervals. There was no significant interaction between beta blocker use and any of the markers (p≥0.62). Addition of the ECG markers significantly improved model discrimination; the C statistic for the EF-adjusted model plus ECG markers (0.724) was significantly higher than the EF-adjusted model without ECG markers (0.642, p<0.0001) (Figure 1). As an indication of internal validity, the Akaike Information Criterion (AIC) of the model with EF plus ECG markers was considerably lower than that of the model with only EF (764.64 vs. 843.84), indicating better model calibration.
Table 2.
Multivariable model with dichotomous EF and ECG parameters (317 cases, 317 controls)
| Odds Ratios and 95% CI | |||
|---|---|---|---|
|
| |||
| EF only | Adjusted model | Adjusted model with ECG parameters | |
| EF ≤35% | 2.8 (1.8 – 4.3) | 2.6 (1.7 – 4.1) | 2.0 (1.3 – 3.2), p=0.0001 |
| Heart rate ≥ 75th% | -- | -- | 2.6 (1.8 – 3.7), p<0.0001 |
| QRS ≥ 75th% | -- | -- | 1.5 (1.0 – 2.5), p=0.03 |
| JTc ≥ 75th % | -- | -- | 2.3 (1.6 – 3.4), p<0.0001 |
| C statistic | 0.573 | 0.642 | 0.724† |
| Nagelkerke§ R2 | 0.05 | 0.09 | 0.19 |
Odds ratios presented for presence of high-risk markers, as defined.
Adjusted model includes age, sex, hypertension, and diabetes.
C statistic (ROC curve) is significantly better for the EF adjusted model plus ECG parameters than for the EF adjusted model without ECG parameters (p<0.0001).
Nagelkerke Pseudo-R2 calculates the improvement in the predictive model compared to a model with intercept only (no predictor variables).
Figure 1.
Addition of ECG parameters improves prediction of SCD.
ROC curves comparing logistic regression models for SCD. Model with LVEF alone had poorest performance; model with LVEF adjusted for age, gender, diabetes, and hypertension was better. The adjusted model with LVEF + ECG risk markers had significantly better discrimination (C statistic 0.7243 vs. 0.6407, p<0.0001).
Using a predicted probability of 0.50 as a cut-off to identify “high risk” patients, the model with only EF had a sensitivity of 60.6% and a specificity of 62.5%, while the EF plus ECG model had a sensitivity of 63.1% and a specificity of 69.4%. Using a predicted probability cut-off of 0.40 in the full model resulted in a sensitivity of ≥80%, while a cut-off of 0.60 resulted in a specificity of ≥80%. In analyses when EF and ECG markers were modeled as continuous variables, results were similar; EF and each ECG marker was significantly associated with SCD (p≤0.002), and the C statistic significantly improved with addition of the ECG markers (p=0.0001).
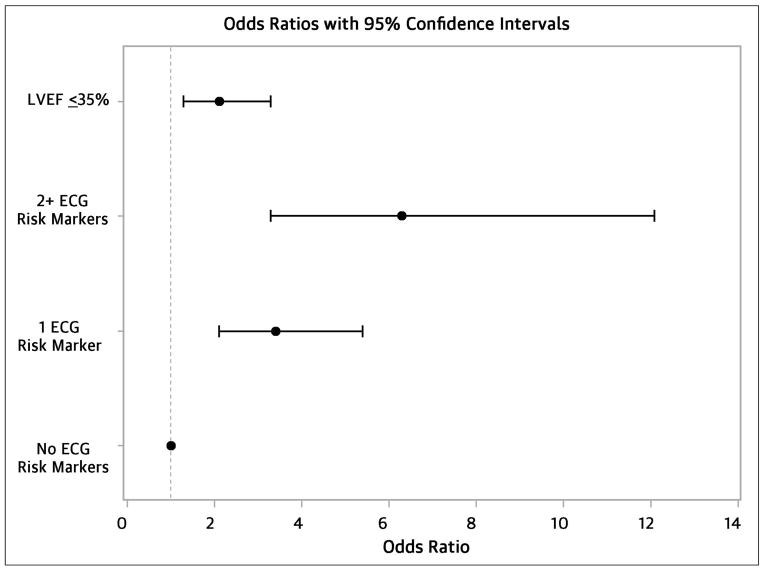
When we examined the cumulative joint effects of the ECG markers (Table 3; Central Figure, Panel A), individuals with a “high risk” ECG marker profile (≥2 markers at or above the 75th percentile) had a 6-fold increased odds of SCD (OR 6.3, 95% CI 3.3 – 12.1) compared to those with “low risk” (all ECG markers below the median). Those with “moderate risk” (at least one marker above the median, but no more than one marker at or above the 75th percentile) were at a 3-fold increased odds (OR 3.4, 95% CI 2.1 – 5.4) compared to the “low risk” group. In this model, low EF remained significantly associated with SCD (OR 2.1, 95% CI 1.3 – 3.3).
Table 3.
Multivariable adjusted model with EF and ECG parameters (317 cases, 317 controls)
| Odds Ratio (95% Confidence Interval) | |
|---|---|
| EF ≤35% | 2.1 (1.3 – 3.3), p=0.002 |
| ECG markers: | |
| ≥ 2 above 75th percentile | 6.3 (3.3 – 12.1), p<0.0001 |
| ≥ 1 above median, but ≤1 above 75th percentile | 3.4 (2.1 – 5.4), p<0.0001 |
| All below median | Ref |
| C statistic | 0.700 |
| Nagelkerke§ R2 | 0.17 |
Adjusted model includes age, sex, hypertension, and diabetes.
ECG markers in patients with EF >35%
We tested the association of the ECG markers with SCD in the majority subgroup with EF >35% (236 cases, 281 controls). Heart rate (OR 2.8, 95% CI 19 – 4.1) and JTc (OR 2.4, 95% CI 1.6 – 3.5) remained significant (p<0.0001), while QRS was borderline (OR 1.5, 95% CI 0.97 – 2.2, p=0.07); C statistic = 0.705.
Net reclassification improvement
Table 4 shows a reclassification table (27) for the adjusted logistic regression models with and without the ECG markers, modeled as in approach (i) above. Each cell shows the number of subjects falling into the low (<0.50), moderate (0.50–0.69), and high (≥70) categories of predicted probabilities before and after adding ECG variables to the model. E.g., in the first row, 79 cases fell into the low category in both models; 43 moved from the low category to the moderate category when ECG markers were added; and 3 moved from the low category to the high category. Addition of the ECG variables to the multivariable model resulted in an overall improvement of classification among cases, with a net of 61 cases moving into higher probability categories. Among controls, addition of the ECG variables resulted in a net of 14 cases moving into lower probability categories. Overall, the net reclassification improvement (NRI) was 22.7%, z = 4.81, p<0.0001. The integrated discrimination improvement (IDI) measures improvement, comparing the new to the old model, in average sensitivity minus the decrease in average specificity, and was 7.7% (p<0.0001).
Table 4.
Reclassification table showing predicted probabilities for SCD odds from adjusted logistic regression model without and with ECG variables.
| Model without ECG variables* | Model with ECG variables | |||
|---|---|---|---|---|
| Cases | <0.50† | 0.50 – 0.69† | ≥ 0.70† | Total |
| <0.50 | 79 | 43 | 3 | 125 (39%) |
| 0.50 – 0.69 | 29 | 61 | 54 | 144 (45%) |
| >=0.70 | 0 | 10 | 38 | 48 (15%) |
| Total | 108 (34%) | 114 (36%) | 95 (30%) | 317 |
| Controls | <0.50 | 0.50 – 0.69 | >= 0.70 | Total |
| <0.50 | 175 | 21 | 2 | 198 (62%) |
| 0.50 – 0.69 | 46 | 47 | 14 | 107 (34%) |
| >=0.70 | 0 | 5 | 7 | 12 (4%) |
| Total | 221 (70%) | 73 (23%) | 23 (7%) | 317 |
Net reclassification improvement = 22.7%, z = 4.811, p <0.0001
Logistic model included the following terms: EF ≤35% vs. >35%, age, sex, diabetes, and hypertension. In the model with ECG markers, each marker (heart rate, JTc, and QRS) was entered separately as a dichotomous variable (≥75th vs. <75th percentile).
Predicted probabilities from the logistic regression model. Note that when the predicted probability is 0.5, then the two outcomes (SCD or no SCD) are equally likely.
Green colors indicate an improvement in prediction of SCD odds with addition of ECG markers (movement from lower to higher predicted probability for cases, and from higher to lower predicted probability for controls); red colors indicate worsening prediction (movement from higher to lower predicted probability for cases, and from lower to higher predicted probability for controls).
Discussion
Our findings suggest that selected high-risk markers from the electrocardiogram may improve prediction of SCD risk when used in combination with the LVEF. While earlier analyses from the Oregon SUDS have demonstrated that each of the ECG markers alone is associated with SCD risk, in the present study, we show that a combination of these markers has a cumulative effect and could identify individuals at even higher risk than that conferred by a single marker. Using a matched population of SCD cases and CAD controls, we confirmed the independent predictive ability of these selected ECG risk markers. Each marker (heart rate, JTc, and QRSD), modeled independently and adjusting for EF and clinical characteristics, was associated with an increased odds of SCD. Next, we demonstrated that consideration of multiple markers together results in much higher odds for SCD (over 6-fold higher with two abnormally high markers) compared to the presence of a single marker, which suggests that using several markers may additively improve SCD risk prediction. Furthermore, when we compared a risk prediction model with only LVEF to a model which also incorporates these ECG markers, both the discrimination and the net reclassification significantly improved. While the C statistics for the full models with ECG markers were moderately good (0.70 – 0.72), this was a significant improvement above the models with only EF and clinical characteristics (0.64). Addition of the ECG markers to the risk prediction model modestly improved sensitivity and specificity compared to the model with EF alone. All of the above support the premise that identification of individuals with “high electrocardiographic risk” has the potential to improve on SCD risk prediction beyond LVEF. Subgroup analyses in the majority subgroup with EF >35% showed that the ECG markers remained associated with a similar increased risk of SCD as well as improvement in model discrimination.
These results provide encouraging proof-of-concept for combining LVEF with widely available ECG markers to improve SCD risk stratification. LVEF, though the only risk marker used at present, is a modest predictor of SCD (21,28). Combining EF with other parameters could be a useful strategy for improving predictive ability. For example, consideration of abnormal heart rate turbulence in addition to low EF doubled the sensitivity of SCD prediction compared with low EF alone in a population of MI survivors (29).
In the present study we evaluated risk prediction in an SCD population that included individuals across a broad spectrum of LV systolic function, from severely reduced to normal. Prior studies investigating an approach of combining multiple risk variables to create a “risk score” have mainly focused on subjects with EF ≤ 35%. Using follow-up data from the MUSTT study, Buxton et al. proposed an arrhythmic death/cardiac arrest risk score which incorporated the variables of inducible VT, history of CHF, low EF, NSVT > 10 days post CABG and IVCD or LBBB (14). An interesting finding from this study was that patients with only low LVEF as a risk predictor may be at relatively lower risk of mortality compared to patients with EF > 30% but with other risk markers. In a related, but different strategy, the MADIT-II risk score used a combination of clinical, laboratory and ECG variables to identify subjects who would benefit maximally from ICD implantation (16), which essentially identifies candidates specifically at greater risk of arrhythmic death among those with low EF. The Duke SCD risk score also assessed a selected population of patients undergoing coronary angiography and proposed a set of clinical and angiographic variables in combination with low EF to predict SCD (15). These results emphasize the need to actively pursue novel markers beyond LVEF which can enhance risk prediction.
Use of ECG parameters is especially appealing since the ECG is noninvasive, relatively inexpensive, and a widely available tool with which clinicians are universally familiar. While several ECG risk markers have been described in the literature, we selected markers which we have found to be previously and consistently associated with SCD in our population (17–19). Several studies have shown promising results in using multiple ECG markers to improve cardiac risk assessment in the real world. In a population-based study of elderly Danish subjects, simple ECG markers such as Q waves, ST-T changes and left ventricular hypertrophy predicted fatal and non-fatal cardiovascular events at follow-up (30). Similarly, using ECGs from health system databases, QRS score (reflecting myocardial scar burden) and the QRS-T angle identified patients at high risk of mortality among those with preserved LVEF (31). Ostman-Smith and colleagues constructed an ECG risk score mainly derived from QRS amplitudes which correlated with SCD with high sensitivity and specificity in patients with hypertrophic cardiomyopathy (32). Collectively these findings indicate the significant potential of the 12-lead ECG to enhance SCD risk prediction in the population.
Since SCD is not a single disease condition, but rather the end result of the interaction of multiple deleterious processes, it stands to reason that strategies for prevention will need to rely on cumulative information from many risk markers, potentially reflecting different aspects of the disease process (33). While the present study has focused on the ECG, this likely represents one piece of a multi-pronged risk approach to enhancement of risk stratification. The use of markers derived from other measurements of risk is also likely to contribute to the process. For example, the Framingham risk score demonstrated that a combination of clinical variables such as age, diabetes, hypertension, cholesterol and smoking was effective in broadly predicting CVD events (34). Other novel avenues such as plasma and genetic biomarkers (35) (36) and nuclear imaging (37) could play a valuable role. The overall goal would be to exploit the additive or synergistic effects of multiple risk factors to identify individuals at highest risk for preventive interventions, so as to be both effective and cost-efficient.
Limitations
Our study was prospective, community-based and utilized multiple-source ascertainment and physician adjudication to accurately identify SCD cases. However, a study of this nature is subject to certain limitations. In order to evaluate a feasible number of subjects, the analysis was performed retrospectively, from archived medical records and tests performed prior to the SCD event. We meticulously collected all available lifetime clinical history to enable robust phenotyping of cases. Our analysis was restricted to individuals with ECG and echocardiographic information available in medical records prior to arrest and hence may not be generalizable to the entire population. However, this is a necessary limitation of a study of this nature, as any novel SCD risk stratification approach needs to take EF into account. Furthermore, patients who present for cardiac screening prior to SCD represent a clinically relevant subgroup for which there may be a real-world opportunity for preventive intervention. We did not assess an exhaustive set of ECG variables but chose a limited number of markers previously known to be associated with SCD in order to demonstrate the concept. Future studies assessing other potentially relevant ECG markers will be needed. While we looked at markers at a single point in time, risk is a dynamic phenomenon subject to temporal change and more studies will be needed to address this aspect. Prior to clinical application with a view to improving primary prevention, the specificity of any risk prediction model for SCD (as opposed to non-sudden death) will need to be evaluated; however, we looked at markers which have been previously well-studied with respect to SCD risk and all have very plausible pathophysiologic links to arrhythmogenesis. Furthermore, while the 75th percentile of the ECG markers in the Oregon SUDS control population was used as an indicator of “high risk”, the distribution of heart rate, QRS, and JTc may differ in other populations, and the most appropriate cut-off value for risk prediction may ultimately be different. Finally, further prospective studies are needed to confirm our findings in independent populations and to investigate the utility of combining other ECG as well as non-ECG parameters to identify the best set of high yield markers to improve SCA risk stratification.
Conclusion
The combined use of selected ECG risk variables, namely, resting heart rate, QRS duration and JTc resulted in improved SCD risk prediction when combined with low LVEF. These findings provide proof of concept for SCD cumulative risk prediction as well as a specific set of widely available and easily measured ECG markers that could be tested in prospective cohort studies.
Figure 2.
ECG parameters (heart rate, QRS, and QTc) are significantly associated with SCD even when adjusting for LVEF.
Odds of SCD were increased with EF ≤35% and with increasing number of ECG risk markers (heart rate, QRS, QTc) above the 75th percentile. Multivariable logistic regression model adjusted for age, gender, diabetes, and hypertension.
Perspectives.
Core Clinical Competencies
Using a set of measurements from the electrocardiogram in combination with echocardiographic ejection fraction can help better identify subjects at risk for sudden cardiac death among those with coronary artery disease.
Translational Outlook
Further prospective studies are needed to evaluate this concept in diverse populations as well as with other risk markers in order to improve risk stratification for SCD and ultimately develop new strategies for prevention.
List of Abbreviations
- SCD
Sudden Cardiac Death
- ECG
Electrocardiogram
- EF
Ejection Fraction
- LV
Left Ventricular
- ICD
Implantable Cardioverter Defibrillator
- CAD
Coronary Artery Disease
Footnotes
Disclosures: Authors have no relationships with industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA : the journal of the American Medical Association. 2008;300:1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. The New England journal of medicine. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. The New England journal of medicine. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. The New England journal of medicine. 2008;359:1009–17. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberger JJ, Buxton AE, Cain M, et al. Risk stratification for arrhythmic sudden cardiac death: identifying the roadblocks. Circulation. 2011;123:2423–30. doi: 10.1161/CIRCULATIONAHA.110.959734. [DOI] [PubMed] [Google Scholar]
- 6.Camm AJ, Pratt CM, Schwartz PJ, et al. Mortality in patients after a recent myocardial infarction: a randomized, placebo-controlled trial of azimilide using heart rate variability for risk stratification. Circulation. 2004;109:990–6. doi: 10.1161/01.CIR.0000117090.01718.2A. [DOI] [PubMed] [Google Scholar]
- 7.Silverman ME, Pressel MD, Brackett JC, et al. Prognostic value of the signal-averaged electrocardiogram and a prolonged QRS in ischemic and nonischemic cardiomyopathy. The American journal of cardiology. 1995;75:460–4. doi: 10.1016/s0002-9149(99)80581-5. [DOI] [PubMed] [Google Scholar]
- 8.Costantini O, Hohnloser SH, Kirk MM, et al. The ABCD (Alternans Before Cardioverter Defibrillator) Trial: strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. Journal of the American College of Cardiology. 2009;53:471–9. doi: 10.1016/j.jacc.2008.08.077. [DOI] [PubMed] [Google Scholar]
- 9.Reinier K, Dervan C, Singh T, et al. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart rhythm : the official journal of the Heart Rhythm Society. 2011;8:1177–82. doi: 10.1016/j.hrthm.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pepe MS, Janes H, Longton G, et al. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. American journal of epidemiology. 2004;159:882–90. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 11.Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–52. doi: 10.1161/CIRCULATIONAHA.111.023846. [DOI] [PubMed] [Google Scholar]
- 12.Goldberger JJ, Basu A, Boineau R, et al. Risk stratification for sudden cardiac death: a plan for the future. Circulation. 2014;129:516–26. doi: 10.1161/CIRCULATIONAHA.113.007149. [DOI] [PubMed] [Google Scholar]
- 13.Exner DV, Kavanagh KM, Slawnych MP, et al. Noninvasive risk assessment early after a myocardial infarction the REFINE study. Journal of the American College of Cardiology. 2007;50:2275–84. doi: 10.1016/j.jacc.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 14.Buxton AE, Lee KL, Hafley GE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. Journal of the American College of Cardiology. 2007;50:1150–7. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 15.Atwater BD, Thompson VP, Vest RN, 3rd, et al. Usefulness of the Duke Sudden Cardiac Death risk score for predicting sudden cardiac death in patients with angiographic (>75% narrowing) coronary artery disease. Am J Cardiol. 2009;104:1624–30. doi: 10.1016/j.amjcard.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 16.Goldenberg I, Vyas AK, Hall WJ, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. Journal of the American College of Cardiology. 2008;51:288–96. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 17.Chugh SS, Reinier K, Singh T, et al. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–70. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teodorescu C, Reinier K, Uy-Evanado A, et al. Prolonged QRS duration on the resting ECG is associated with sudden death risk in coronary disease, independent of prolonged ventricular repolarization. Heart rhythm : the official journal of the Heart Rhythm Society. 2011;8:1562–7. doi: 10.1016/j.hrthm.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teodorescu C, Reinier K, Uy-Evanado A, et al. Resting heart rate and risk of sudden cardiac death in the general population: influence of left ventricular systolic dysfunction and heart rate-modulating drugs. Heart rhythm : the official journal of the Heart Rhythm Society. 2013;10:1153–8. doi: 10.1016/j.hrthm.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S community. Journal of the American College of Cardiology. 2004;44:1268–75. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Stecker EC, Vickers C, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. Journal of the American College of Cardiology. 2006;47:1161–6. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 22.Fishman GI, Chugh SS, Dimarco JP, et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–48. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chugh SS, Uy-Evanado A, Teodorescu C, et al. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: The Ore-SUDS (Oregon Sudden Unexpected Death Study) Journal of the American College of Cardiology. 2009;54:2006–11. doi: 10.1016/j.jacc.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adabag AS, Peterson G, Apple FS, et al. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. American heart journal. 2010;159:33–9. doi: 10.1016/j.ahj.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley and Sons; 2000. [Google Scholar]
- 26.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–35. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 27.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–212. [DOI] [PubMed] [Google Scholar]
- 28.Moss AJ, Greenberg H, Case RB, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–5. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 29.Bauer A, Barthel P, Schneider R, et al. Improved Stratification of Autonomic Regulation for risk prediction in post-infarction patients with preserved left ventricular function (ISAR-Risk) European heart journal. 2009;30:576–83. doi: 10.1093/eurheartj/ehn540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jorgensen PG, Jensen JS, Marott JL, et al. Electrocardiographic changes improve risk prediction in asymptomatic persons age 65 years or above without cardiovascular disease. Journal of the American College of Cardiology. 2014;64:898–906. doi: 10.1016/j.jacc.2014.05.050. [DOI] [PubMed] [Google Scholar]
- 31.Strauss DG, Mewton N, Verrier RL, et al. Screening entire health system ECG databases to identify patients at increased risk of death. Circulation Arrhythmia and electrophysiology. 2013;6:1156–62. doi: 10.1161/CIRCEP.113.000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostman-Smith I, Wisten A, Nylander E, et al. Electrocardiographic amplitudes: a new risk factor for sudden death in hypertrophic cardiomyopathy. European heart journal. 2010;31:439–49. doi: 10.1093/eurheartj/ehp443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buxton AE. Risk stratification for sudden death in patients with coronary artery disease. Heart rhythm : the official journal of the Heart Rhythm Society. 2009;6:836–47. doi: 10.1016/j.hrthm.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 34.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 35.Havmoller R, Chugh SS. Plasma biomarkers for prediction of sudden cardiac death: another piece of the risk stratification puzzle? Circulation Arrhythmia and electrophysiology. 2012;5:237–43. doi: 10.1161/CIRCEP.111.968057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damman P, Kampinga MA, van der Horst IC, et al. Multiple biomarkers for the prediction of short and long-term mortality after ST-segment elevation myocardial infarction: the Amsterdam Groningen collaboration. Journal of thrombosis and thrombolysis. 2013;36:42–6. doi: 10.1007/s11239-012-0809-4. [DOI] [PubMed] [Google Scholar]
- 37.Al Badarin FJ, Wimmer AP, Kennedy KF, et al. The utility of ADMIRE-HF risk score in predicting serious arrhythmic events in heart failure patients: incremental prognostic benefit of cardiac 123I-mIBG scintigraphy. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2014;21:756–62. doi: 10.1007/s12350-014-9919-z. quiz 753–55, 763–5. [DOI] [PubMed] [Google Scholar]