Abstract
Objective
To examine the association of cord blood leptin with body mass index (BMI) growth velocity from birth to 12 months of age among infants exposed and not exposed to over-nutrition in utero (defined as maternal overweight/obesity or presence of gestational diabetes).
Methods
185 infants enrolled in the Exploring Perinatal Outcomes among Children study (76 exposed and 109 not exposed) had leptin and insulin measured in cord blood. Longitudinal weight and length measures in the first 12 months of life (average 4 per participant) obtained from medical records were used to compute BMI growth rates. Mixed models were used to examine associations of cord blood leptin with growth.
Results
Compared with unexposed infants, those exposed had significantly higher cord blood insulin (8.64 v. 6.97 uU/ml, P <0.01) and leptin levels (8.89 v. 5.92 ng/ml, P = 0.05) as well as increased birth weights (3438.04 v. 3306.89 g, P = 0.04). There was an inverse relationship between cord leptin levels and BMI growth from birth to 12 months of age (P = 0.005); however, exposure to over-nutrition in utero did not significantly modify this association (P = 0.59).
Conclusion
We provide support of a possible operational feedback mechanism by which lower cord blood leptin levels are associated with faster infant growth in the first year of life. Our data do not tend to support the hypothesis that this mechanism is altered in infants exposed to over-nutrition in utero; however our sample is too small to provide sufficient evidence. Larger epidemiological studies are needed to elucidate the mechanisms responsible for increased propensity for obesity in exposed offspring.
Keywords: childhood obesity, diabetes, fetal overnutrition, insulin, leptin, maternal obesity
Introduction
As the prevalence of obesity continues to rise, novel research hypotheses are being examined in hopes of finding key modifiable behaviors for preventive strategies. Several studies have demonstrated that the path toward obesity may start as early as during the intrauterine period. Exposure to maternal diabetes1,2 and or/maternal obesity in utero were shown to be associated with increased obesity later in life, and a more rapid body mass index (BMI) growth velocity throughout childhood, especially as these offspring enter puberty.3
The mechanisms responsible for the increased growth and adiposity among children exposed to maternal diabetes and/or obesity in utero remain unclear. The fetal over-nutrition hypothesis4,5 proposes that in utero exposure to altered maternal fuels (including glucose, free fatty acids and triglycerides) in obese and/or diabetic pregnancies may alter fetal hormones that regulate feeding behavior, growth and adiposity.6 Leptin, a hormone secreted by adipocytes and by the placenta, is associated with fetal growth,7 infant growth,8–10 adipose tissue development11 and insulin secretion.12 This endocrine feedback loop, called the adipoinsular axis, links the brain and endocrine pancreas with other peripheral insulin-and leptin-sensitive tissues in the control of feeding behavior, metabolic regulation and energy balance.12 When body fat stores are low, leptin levels are also low, which will decrease satiety, therefore promoting growth and stimulating insulin production.12 In both animals and humans, inefficient leptin action (i.e., leptin-resistance) leads to hyperphagia, decreased fat oxidation, insulin resistance and obesity.12,13 Thus, induction of leptin resistance may be hypothesized as a potential mechanism for later development of obesity in offspring exposed to over-nutrition in utero.
To address this hypothesis we examined the association between cord blood leptin levels and BMI growth velocity from birth to 12 months of age among infants exposed and not exposed to over-nutrition in utero. We hypothesized that infants exposed to over-nutrition will have higher cord blood leptin and insulin levels. Further, we hypothesized that the inverse relationship between cord blood leptin and BMI growth velocity in the first 12 months will be altered in exposed offspring suggesting impaired leptin action.
Methods
Participants
These analyses are based on a sample of 185 mother-infant pairs (54 non-white and 131 white) participating in the retrospective cohort study Exploring Perinatal Outcomes among Children (EPOCH). All were members of the Kaiser Permanente Colorado Health Plan (KPCO) at birth, were living in Colorado over the study period, and had stored cord blood samples available. The study was approved by the local Institutional Review Board and all participants provided written informed consent.
Data collection
Infant weight and recumbent length, gestational age, and maternal pre-pregnancy BMI were obtained from the KPCO perinatal database. The KPCO database links maternal prenatal and delivery medical records with infant perinatal data. Infant BMI was calculated as weight in kilograms divided by length in meters squared. Only individuals with three or more BMI measures within the first year of life were included in the analysis, yielding 185 mother-infant dyads.
Cord blood hormones measures
Cord blood hormones were obtained using frozen plasma from cord blood samples stored at birth. The plasma leptin concentration was measured by using a Millipore radio-immunoassay with a sensitivity of 0.5 ng/ml. The concentration of plasma insulin was determined by using a Millipore radioimmunoassay with a sensitivity of 3 uU/ml. Laboratory analyses were performed at the University of Colorado Hospital laboratory and the Children’s Hospital laboratory (Aurora, CO, USA). With each assay three controls are conducted; Millipore leptin controls 1 and 2 from the Millipore leptin kit and BioRad Immunoassay Plus control level 1. BioRad Lyphocheck Unassayed Chemistry Control (human) level 1 and 2 are used as an assay validation and assessed again established ranges.
Exposure definition
Exposure to over-nutrition in utero was defined as maternal pre-pregnancy overweight/obesity (BMI ≥25 kg/m2) or presence of gestational diabetes (GDM). Overweight women were included in exposure group as an association of cord blood leptin and growth trajectory has been shown in women with pre-pregnancy BMI of ≥25 kg/m2.14,15 Maternal pre-pregnancy BMI was calculated from the KPCO-measured weight before the last menstrual cycle preceding pregnancy and measured height. Physician-diagnosed GDM was ascertained from the KPCO perinatal database. GDM was coded as present if diagnosed through the standard KPCO screening protocol (described below) and absent if screening was negative. Since the 1990s, KPCO has routinely screened for GDM in all non-diabetic pregnancies using a two-step standard protocol. At 24–28 weeks, all pregnant women are offered screening with a 1-h, 50-g glucose tolerance test (OGTT). Patients with blood glucose value ≥ 140 mg/dl undergo a diagnostic 3-h, 100-g diagnostic OGTT. GDM is diagnosed when two or more glucose values during the diagnostic OGTT meet or exceed the criteria for a positive test, as recommended by the National Diabetes Data Group.16
Statistical analysis
Baseline covariates were compared between exposed and unexposed groups using either a Satterthwaite t-test for a difference in means for continuous covariates or a two-sided Fisher’s exact test for a difference in proportions for dichotomous covariates. The associations of covariates with infant adiposity were examined using mixed models. Models had infant BMI as the outcome. The primary determinants of interest included cord-blood leptin levels, exposure to over-nutrition status (yes/ no), and exposure by leptin interaction. Other covariates considered in the models included: infant age, age squared, sex, race/ethnicity, breast feeding status, gestational age at birth and all possible age interactions. To account for the correlation within subjects across time, the model included a random effect for age and age squared. Second order interactions were examined first, followed by linear interactions and finally the fixed effects of interest. All analyses were conducting using SAS 9.3 (SAS Cary, NC). Tests of association using the mixed model were conducted with REML estimation and Kenward-Roger (Biometrics 1997) degrees of freedom. The best mixed model fit was selected using a backwards stepwise model selection method with orthogonal polynomials in age.
Results
A total of 76 infants were exposed to over-nutrition in utero (27 from GDM and 49 from maternal overweight/obesity) and 109 infants were unexposed. The median number of longitudinal BMI measurements per infant was 4 (minimum 3, maximum 17). Table 1 describes the characteristics of the sample stratified by exposure status. Exposed infants had significantly higher cord blood insulin (8.64 v. 6.97 uU/ml, P < 0.01) and leptin levels (8.89 v. 5.92 ng/ml, P = 0.05). Exposed infants also had increased birth weights (3438.04 v. 3306.89 g, P = 0.04). By design, mothers of exposed infants had higher pre-pregnant BMI levels (28.31 v. 21.41 kg/m2, P < 0.001).
Table 1.
Descriptive characteristics (means/S.D.) of study participants, by exposure to over-nutrition in utero
| Unexposed (n = 109) | Exposed (n = 76) | P-value | |
|---|---|---|---|
| Sex (% female) | 53% | 53% | 0.94 |
| Race/ethnicity (% non-Hispanic white) | 75% | 64% | 0.11 |
| Maternal pre-pregnant BMI (kg/m2) | 21.41 ± 1.90 | 28.31 ± 5.34 | <0.001 |
| Birth weight (grams) | 3306.89 ± 393.6 | 3438.04 ± 430.23 | 0.04 |
| Gestational age at birth (weeks) | 39.12 ± 1.30 | 39.15 ± 1.40 | 0.89 |
| Cord blood leptin (ng/ml) | 5.92 ± 4.40 | 8.89 ± 8.06 | 0.05 |
| Cord blood insulin (uU/ml) | 6.97 ± 5.20 | 8.64 ± 5.88 | <0.01 |
BMI, body mass index.
Table 2 represents the association between covariates and infant BMI during the first year of life. Associations were estimated using the most parsimonious mixed model fit. The best fit model included infant BMI during the first year of life as the outcome and the following predictors: age, age squared, cord blood leptin, exposure status, cord blood leptin by age interaction and infant birth weight. Predicted BMI was calculated using the best model fit and the observed average covariate values of the population.
Table 2.
Association of covariates with infant BMI growth in the first 12 months of age
| Covariate | Estimate | Confidence Intervals (lower, upper) | P-value |
|---|---|---|---|
| Time (months) | 0.041 | 0.66, 0.82 | <0.0001 |
| Time squared (months2) | −0.003 | −0.05, −0.03 | <0.0001 |
| Cord blood leptin (uU/ml) | 0.01 | −0.01, 0.04 | 0.2619 |
| Time by cord blood leptin interaction | 0.002 | −0.01, 0.00 | 0.0046 |
| Exposure status (BMI >25 kg/m2 or GDM) | −0.30 | −0.57, −0.03 | 0.0295 |
| Sex (male as referent group) | −0.21 | −0.48, 0.07 | 0.1439 |
| Birth weight (g) | 0.0002 | 0.00, 0.00 | <0.0001 |
BMI, body mass index; GDM, gestational diabetes.
The model- predicted BMI for a male infant was slightly higher than the model- predicted BMI for a female infant. The average BMI in the first year of life for a male infant in the exposed group with a cord leptin level of 7.5 ng/ml, and a birth weight of 3360 g was 13.5 kg/m2 (95% CI: 13.3–13.7). The average BMI for a female infant in the exposed group with a cord leptin level of 7.5 ng/ml, and a birth weight of 3360 g was 13.4 kg/m2 (95% CI: 13.2–13.6). The average BMI was significantly associated with birth weight. A 100 g increase in birth weight was associated with a 0.12 kg/m2 higher average BMI (95% Cl: 0.09–0.16) in the first year of life.
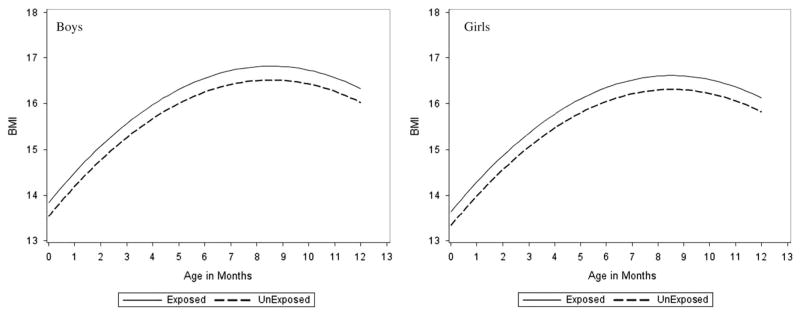
BMI was significantly higher in infants exposed to over-nutrition in utero as compared with those not exposed. Infants exposed to maternal pre-pregnant overweight/obesity or GDM were on average 0.30 kg/m2 of BMI larger than the unexposed infants (95% Cl: 0.03–0.57). However, the effect of exposure to over-nutrition was consistent over time (no significant interaction between exposure status and time), suggesting that exposure to over-nutrition in utero does not influence the BMI rate of change in the first year of life. A fixed effect of exposure on infant BMI growth is shown in Fig. 1. The figures show the predicted infant BMI during the first year of life for an infant with a cord leptin blood level of 7.5 ng/ml and a birth weight of 3360 g by sex and exposure status. The effect of exposure on BMI was not modified by sex.
Fig. 1.
Infant body mass index (BMI) by gestational age (month) according to exposure status. Mean infant BMI curves adjusted for covariates of interest by exposure to maternal diabetes in utero from birth. Confidence intervals not provided.
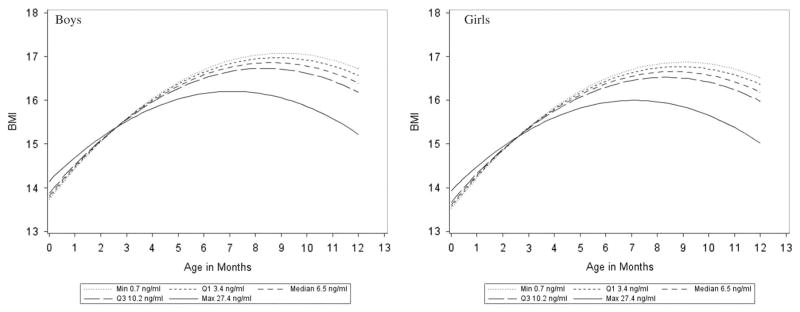
The predicted BMI growth curve during the first year of life was influenced by cord blood leptin level. There was an inverse relationship between cord blood leptin levels and rate of change in BMI in the first year of life (P = 0.005 for the interaction between cord blood leptin and time). Fig. 2 demonstrate the effect of cord blood leptin level on infant BMI growth. The figures plot the predicted BMI for boys and girls during the first year of life for infants with an average birth weight of 3360 g stratified by exposure to over-nutrition in utero status, for a range of cord leptin blood levels. The range of cord blood levels include the minimum, first quartile, median, third quartile and maximum observed in the data. The lower the levels of cord blood leptin, the faster the BMI growth trajectories were during the first year of life. For example, for an infant with a cord blood leptin level of 0.7 ng/ml the model predicted a 3.0 kg/m2 increase in BMI across the first year of life (95% Cl: 2.5–3.5). For an infant with cord blood leptin level of 7.5 ng/ml the model predicted a 2.6 kg/m2 increase in BMI across the first year of life (95% Cl: 2.3–3.0). Finally, the relationship between cord blood leptin levels and BMI rate of change was not modified by exposure status (test for interaction, P = 0.59). Results were consistent when analyses were conducted separately for maternal overweight/obesity and maternal GDM status.
Fig. 2.
Infant body mass index (BMI) by gestational age (month) across varying cord leptin levels. Model predicted mean infant BMI adjusted for covariates of interest across cord blood leptin levels (min, Q1, media, Q3, and max) observed in the study. Prediction intervals not provided.
Discussion
We examined the association between cord blood leptin levels and BMI growth velocity from birth to 12 months of age among infants exposed and not exposed to over-nutrition in utero. We found an inverse relationship between cord blood leptin levels and the rate of change in BMI in the first year of life (P = 0.005), suggesting the possibility of a normal feedback mechanism, already operational during the early postnatal period, with lower baseline leptin levels promoting increased BMI growth velocity. Although exposure to over-nutrition in utero was correlated with higher overall BMI levels in infants, exposure status did not tend to influence BMI growth velocity during this period. Finally, in contrast to our hypothesis, we did not find support that leptin action on BMI growth velocity was altered in the exposed offspring. This may suggest that impaired leptin action in early life is not an important mechanism responsible for the accelerated BMI growth in exposed offspring, at least during the first year of life. Despite the negative findings in this small sample of women, this study is an important first step toward a better understanding of potential mechanisms responsible for the increased propensity for obesity conferred by over-nutrition in utero.
Similar to our findings, a recent study of 588 infants found that a smaller size at birth was associated with lower cord blood leptin levels which in turn were associated with rapid weight gain from 0 to 6 months and a higher BMI at 3 years of age.15 Significantly higher levels of cord blood leptin have been shown in infants exposed to maternal GDM or obesity,17 even after adjustment for birth weight, suggesting that exposure to over-nutrition in utero may result in altered leptin levels and possibly altered leptin action. Indeed, similar to previous studies, EPOCH infants exposed to over-nutrition in utero had significantly higher cord blood insulin and leptin levels as well as larger birth weights.1–3 However, while two previous studies have demonstrated an expected inverse relationship between cord blood leptin and subsequent infant growth,14,15 also demonstrated in our sample of unexposed infants, no previous studies have specifically explored this relationship in offspring exposed to over-nutrition in utero. The recent report from Project Viva14 found that exposure to maternal GDM in utero was associated with a smaller change in weight-for-length z-score from birth to 6 months of age. We did not find a significant association between exposure to maternal GDM/ over-nutrition and rate of offspring BMI growth either in this study (restricted to offspring with available cord blood samples), or in our entire cohort.3 It is important to note, however, that the Project Viva report only included 35 offspring exposed to maternal GDM and had only two measures of offspring weight (at birth and 6 months of age). Moreover, they did not specifically explore whether exposure to maternal GDM modifies the association between cord blood leptin and infant growth, as we did here. The novelty of our study lies specifically in our attempt to address this hypothesis. While we found altered cord blood leptin levels in our exposed infants, we found no support that leptin action on subsequent infant BMI growth in the first year of life is altered in offspring exposed to over-nutrition in utero. This may suggest that exposure to over-nutrition in utero influences offspring obesity risk through other mechanisms than impaired leptin action, or that the effect of impaired leptin action (due to exposure to over-nutrition in utero) on offspring BMI growth velocity only becomes apparent later in life.
Our study has some limitations. Our sample of infants exposed to over-nutrition in utero is relatively small (n =76). We appreciate that to conclusively demonstrate an altered association between leptin levels and rate of growth in the exposed group, much larger samples are needed. However, our study is the first that we are aware of that explored the relationship between cord blood leptin and infant BMI rate of growth in infants exposed and not exposed to over-nutrition in utero, using longitudinal analyses. Moreover, we were not able to directly quantify ‘impaired leptin action’ since, to our knowledge such a measure does not exist at the present time. Finally, it has been shown that BMI growth trajectories in exposed v. not exposed offspring substantially diverge only later in life,3 and therefore, leptin levels and growth trajectories throughout the entire childhood period are needed to fully quantify leptin action on BMI growth velocity in offspring exposed to over-nutrition in utero.
In conclusion, our study provides preliminary support of an operational feedback mechanism by which lower cord blood leptin levels are associated with faster infant growth trajectories. We did not find support that this mechanism is altered in infants exposed to over-nutrition in utero. This research is an important first step toward a better understanding of potential mechanisms responsible for the increased propensity for obesity conferred by over-nutrition in utero. Larger epidemiological and mechanistic studies are needed to fully elucidate these mechanisms.
Acknowledgments
Kaar analyzed the data, provided scientific input and wrote the first draft; Brinton provided scientific input, statistical guidance and aided in preparing first draft; Crume and Hamman provided scientific input and final edits; Dabelea designed the study, assisted with hypothesis development, and provided scientific input and final edits. This work was supported by National Institute of Health RO1 DK068001. The study sponsor had no role in the study.
Footnotes
Conflicts of Interest
None.
Ethical Standards
The study was approved by local Institutional Review Board.
References
- 1.Dabelea D, Pettitt DJ. Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J Pediatr Endocrinol Metab. 2001;14:1085–1091. doi: 10.1515/jpem-2001-0803. [DOI] [PubMed] [Google Scholar]
- 2.Pettitt DJ, Bennett PH, Knowler WC, Baird HR, Aleck KA. Gestational diabetes mellitus and impaired glucose tolerance during pregnancy. Long-term effects on obesity and glucose tolerance in the offspring. Diabetes. 1985;34:119–122. doi: 10.2337/diab.34.2.s119. [DOI] [PubMed] [Google Scholar]
- 3.Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941–946. doi: 10.1016/j.jpeds.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29:1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- 5.Lawlor DA, Smith GD, O’Callaghan M, et al. Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the mater-university study of pregnancy and its outcomes. Am J Epidemiol. 2007;165:418–424. doi: 10.1093/aje/kwk030. [DOI] [PubMed] [Google Scholar]
- 6.Dabelea D, Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes. 2011;60:1849–1855. doi: 10.2337/db11-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koistinen HA, Koivisto VA, Andersson S, et al. Leptin concentration in cord blood correlates with intrauterine growth. J Clin Endocrinol Metab. 1997;82:3328–3330. doi: 10.1210/jcem.82.10.4291. [DOI] [PubMed] [Google Scholar]
- 8.Silverman BL, Rizzo T, Green OC, et al. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991;40(Suppl 2):121–125. doi: 10.2337/diab.40.2.s121. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz R, Teramo KA. Effects of diabetic pregnancy on the fetus and newborn. Sem Perinatol. 2000;24:120–135. doi: 10.1053/sp.2000.6363. [DOI] [PubMed] [Google Scholar]
- 10.Ong KK, Ahmed ML, Sherriff A, et al. Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab. 1999;84:1145–1148. doi: 10.1210/jcem.84.3.5657. [DOI] [PubMed] [Google Scholar]
- 11.Jaquet D, Leger J, Levy-Marchal C, Oury JF, Czernichow P. Ontogeny of leptin in human fetuses and newborns: effect of intrauterine growth retardation on serum leptin concentrations. J Clin Endocrinol Metab. 1998;83:1243–1246. doi: 10.1210/jcem.83.4.4731. [DOI] [PubMed] [Google Scholar]
- 12.Kieffer TJ, Habener JF. The adipoinsular axis: effects of leptin on pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2000;278:E1–E14. doi: 10.1152/ajpendo.2000.278.1.E1. [DOI] [PubMed] [Google Scholar]
- 13.Seufert J, Kieffer TJ, Leech CA, et al. Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus. J Clin Endocrinol Metab. 1999;84:670–676. doi: 10.1210/jcem.84.2.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker M, Rifas-Shiman SL, Belfort MB, et al. Gestational glucose tolerance and cord blood leptin levels predict slower weight gain in early infancy. J Pediatr. 2011;158:227–233. doi: 10.1016/j.jpeds.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantzoros CS, Rifas-Shiman SL, Williams CJ, et al. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123:682–689. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and the other categories of glucose intolernance. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 17.Simmons D, Breier BH. Fetal overnutrition in polynesian pregnancies and in gestational diabetes may lead to dysregulation of the adipoinsular axis in offspring. Diabetes Care. 2002;25:1539–1544. doi: 10.2337/diacare.25.9.1539. [DOI] [PubMed] [Google Scholar]