Abstract
Autism spectrum disorders (ASD) are a group of disorders characterized by core behavioral features including stereotyped interests, repetitive behaviors and impairments in communication and social interaction. In addition, widespread changes in the immune systems of individuals with ASD have been identified, in particular increased evidence of inflammation in the periphery and central nervous system. While the etiology of these disorders remains unclear, it appears that multiple gene and environmental factors are involved. The need for animal models paralleling the behavioral and immunological features of ASD is paramount to better understand the link between immune system dysregulation and behavioral deficits observed in these disorders. As such, the asocial BTBR mouse strain displays both ASD relevant behaviors and persistent immune dysregulation, providing a model system that has and continues to be instructive in understanding the complex nature of ASD.
Keywords: Autism, ASD, BTBR, Mouse, Immune, Inflammation, Model
1. Introduction
Autism spectrum disorders are a group of neurodevelopmental disorders characterized by restricted interests, repetitive behaviors and impairments in communication and social interaction. Currently 1 in 88 children have been identified as having ASD (CDC, 2012). Despite the high incidence of ASD, the etiology and pathogenesis remain poorly understood. Numerous published findings have identified widespread changes in the immune systems of individuals with ASD both at the systemic and cellular levels (Ashwood et al., 2006). These immune dysfunctions are associated with impairments in core features of ASD as well as aberrant behaviors, decreased adaptability and impaired cognition (Ashwood et al., 2011a,b; Onore et al., 2012).
In individuals with ASD, several lines of evidence point to ongoing inflammation both within the brain (Li et al., 2009; Morgan et al., 2010; Vargas et al., 2005) and in the periphery (Ashwood et al., 2011a; Hashimoto et al., 2011). Work by Vargas et al. demonstrated that in postmortem brains from subjects with ASD there were signs of increased pro-inflammatory cytokines including interleukin (IL)-1β, IL-6, IL-12(p40) and TNFα (Vargas et al., 2005). Moreover, microglia from subjects with ASD display a more activated phenotype in postmortem brain (Morgan et al., 2010) and by PET scan (Suzuki et al., 2013). In addition to immune activation within CNS, circulating levels of cytokines exhibit a profile reminiscent of a proinflammatory immune profile with increased IL-1β, IL-6 and IL-12(p40) production (Ashwood et al., 2011a; Hashimoto et al., 2011; Ricci et al., 2013; Singh, 1996). Increased activation of circulating monocyte cells in the periphery following stimulation with Toll-like receptor (TLR) ligands has also been observed, including changes in gene expression, increased HLA-DR cell surface expression and the release of pro-inflammatory cytokines IL-1β and IL-6 (Enstrom et al., 2010; Jyonouchi et al., 2008, 2011, 2001). Both the circulating levels of these pro-inflammatory cytokines and the degree of monocyte activation are associated with more impaired behaviors in children with ASD (Ashwood et al., 2011a; Enstrom et al., 2010; Onore et al., 2012). These findings notwithstanding, many of the links between ASD and immune system dysregulation are drawn from associative studies that pose compelling correlations between neurodevelopmental disorders and immune dysfunction. However, limitations in experimental design and the myriad of uncontrolled variability, as a result of ethical boundaries placed on human research, requires the use of animal models to effectively link causality to these patterns of association.
Rodent models of human conditions enable scientists to directly test hypotheses generated from evidence drawn from clinical populations. The development of an effective animal model requires extensive investigation into the biological and behavioral pathologies that contribute to the face, construct, and predictive validity of a translational model. These validity measures are crucial for evaluating the relevance of a model for understanding the human condition. For the study of ASD and its associations with immune system dysregulation, researchers have identified a mouse strain, the BTBR mouse, that has strong validity for evaluating the neuro-immunological contributions to ASD-like behaviors.
BTBR mice were derived from an inbred strain carrying the at (nonagouti; black and tan) and wildtype T (brachyury) mutations that were crossed with mice with the tufted (Itpr3tf) allele (http://jaxmice.jax.org/strain/002282.html). As part of the Mouse Phenome Project (MPP), BTBR mice were characterized and found to have neuroanatomical abnormalities such as a hereditary loss of corpus callosum (Wahlsten et al., 2003). However, it was not until work by Moy et al., who characterized the BTBR mice as exhibiting behaviors that had strong face validity to ASD, that the BTBR mouse was brought to the attention of the neurodevelopmental research community (Moy et al., 2007). More recent work by Heo et al. described the immune system of the BTBR mouse as having a number of immunological abnormalities consistent with increased immune activation (Heo et al., 2011). In light of the mounting evidence of immune dysfunction in ASD coupled to the behavioral abnormalities, the BTBR mouse makes for an interesting target to research mechanisms of asocial behaviors as they relate to immune dysfunction (Reviewed in Onore et al., 2012) and may help to better understand the complex nature of ASD.
2. Behavioral phenotype
The BTBR mouse has strong face validity for a range of behavioral deficits that are analogous to those observed in individuals with ASD. Most notably, this mouse is characterized by reductions in sociability and increased repetitive/compulsive behaviors. The low sociability level of the BTBR mouse is most apparent in the three-chamber social approach task. These mice spend equal time approaching and investigating a novel object compared to a novel social stimulus (i.e. novel mouse) (McFarlane et al., 2008), a behavioral response well replicated across numerous laboratories. These reductions in social approach are accompanied by reductions in social motivational processes as BTBR mice fail to form a conditioned place preference for contexts associated with social stimuli (Pearson et al., 2012). While this study suggests that motivation may be a factor contributing to low sociability, the extent to which BTBR mice respond to non-social rewards (e.g. amphetamines, food) remains unknown. Moreover, these deficits in social motivation may reflect dysfunction in the acquisition or retention of social-specific memory cues.
In addition to reduced social approach, the BTBR mouse is characterized by a reduction in the display of a range of species-typical social behaviors. At a juvenile age, BTBR mice spend significantly less time engaging in social interactions including reductions in sniffing and following behaviors compared to the ‘typical’ C57BL/6J mouse (McFarlane et al., 2008). These reductions in social investigation persist throughout adolescence (Scattoni et al., 2013) and into adulthood (Defensor et al., 2011; Pobbe et al., 2010). Interestingly, when juvenile BTBR mice are reared with more social C57BL/6J mice, the characteristic social deficits are significantly diminished (Yang et al., 2011), highlighting the importance of social peer interactions as a potent influence on social behavior development.
The recently published DSM-5 reformulated the core behavioral domains of ASD by integrating language and communication impairments as a component of social competency, redefining the deficits as social communication impairments (APA, 2013). This restructuring compels researchers to scrutinize the translational value of mouse behavior as they relate to ASD-like behaviors. For the BTBR mouse, variations in the type and frequency of ultrasonic vocalizations were previously proposed as a model for the language and communication deficits observed in humans (Scattoni et al., 2008; Wohr et al., 2011). However, little is known about the functional significance of mouse vocalizations, particularly in adults, or whether these calls have communicative value. By redefining language deficits in the context of social communication, mouse behaviorists are better suited to explore communication deficits that are more ethologically valid and species specific. For example, although BTBR mice elicit fewer vocalizations, these mice produce calls in response to the presence and/or absence of a female similar to the responses observed in the social C57BL/6J mouse (Yang et al., 2013). Moreover, female BTBR mice are noted to display typical maternal care behaviors (Yang et al., 2007) despite differences in call patterns and number of calls elicited from pups (Scattoni et al., 2008). By examining differences in ultrasonic vocalizations in the context of social behavioral responses, as established by the DSM-5, the translational validity of these differences in vocalization patterns in the BTBR mouse as an analogous model for the communication deficits in humans remains under question. Perhaps more relevant though, are differences in species-typical scent marking behavior in BTBR mice in response to social stimuli. Given that olfactory cues are the predominant sensory modality used for exploring social and environmental stimuli in mice, scent-marking behavior may provide a more robust behavioral measure for identifying deficits in social communication. Interestingly, the BTBR mouse displays fewer scent marking behaviors in response to a social stimulus compared to the more social C57BL/6J mouse, and these reductions are accompanied by fewer vocalizations emitted in response to the scent of female-urine (Roullet et al., 2011; Wohr et al., 2011). Together, reductions in social motivation and social approach along with reduced scent marking and altered vocalizations highlight the strong face validity the BTBR mouse holds for modeling the social communication deficits that are at the core of the ASD diagnosis.
Another core feature of ASD is the presence of repetitive patterns of behavior, restricted interests, and insistence on sameness. The BTBR mouse possesses a range of behavioral traits that model both the repetitive motor patterns and inflexible adherence to routines. The most widely noted motor stereotypy in the BTBR mouse is the excessive time spent engaging in self-grooming behaviors compared to other inbred strains (McFarlane et al., 2008; Pearson et al., 2011; Pobbe et al., 2010). An in-depth analysis of the microstructural components of grooming behavior revealed that the BTBR mouse engages in more frequent and longer durations of all grooming behaviors. Moreover, grooming bouts do not occur in the species-typical cephalo-caudal sequence, underscoring the atypical nature of these stereotypies (Pearson et al., 2011). Another motor stereotypy noted in the BTBR mouse is the increasing frequency of compulsive marble burying observed across several laboratories (Amodeo et al., 2012; Schwartzer et al., 2013). Marble burying behavior is used as an index for perseverative behaviors analogous to the restricted patterns of behavior in ASD (Thomas et al., 2009). Importantly, these motor stereotypies in the BTBR mouse are met with deficits in reversal learning and perseverative behaviors across several cognitive tasks. In the Morris Water Maze, BTBR mice show intact spatial learning similar to the C57BL/6J mouse but fail to show quadrant preference during later reversal learning phases (Moy et al., 2007). Similarly, BTBR mice make significantly more perseverative errors in a modified T-maze task (Guariglia and Chadman, 2013). This lack of reversal learning was further characterized in probabilistic learning tasks when correct choices were only reinforced during 80% of the trials (Amodeo et al., 2012). This more ecologically relevant paradigm closely models the cognitive inflexibility observed in individuals with ASD (Solomon et al., 2011).
Despite the mounting evidence supporting an ASD-like phenotype in the BTBR mouse, it is important to consider what other neurobehavioral processes may be contributing to the marked reductions in sociability and increased repetitive and perseverative behaviors. One potential confound to consider is the role anxiety may contribute to the social behavior deficits and perseverative behaviors in the BTBR mouse. To date, there are numerous conflicting reports using a range of behavioral assays stating both elevated anxiety-like responses (Benno et al., 2009; Pobbe et al., 2011) and low stress responses (Silverman et al., 2010) as well as no observable difference in anxiety-like behaviors compared to the C57BL/6J mouse (Karvat and Kimchi, 2012; McFarlane et al., 2008; Moy et al., 2007).
In addition to anxiety, individuals with ASD often have comorbid cognitive impairments and intellectual disabilities, although these deficits are not necessary for the ASD diagnosis. Recent advances in the development of operant touchscreen technology has afforded researchers the ability to explore differences in complex cognitive processes including executive function, pattern separation, and memory formation (Bussey et al., 1994, 2008; Horner et al., 2013). By pairing computer assisted-touchscreens to classic operant conditioning chambers, researchers have developed a host of paradigms that are analogous to tasks used in measuring cognition in human and non-human primate studies. This fully-automated and standardized approach to measuring cognitive function enables researchers to identify specific processing deficits that may otherwise be ambiguous in traditional rodent mazes. In the BTBR mouse, touchscreen operant paradigms have revealed impairments in task-switching paradigms that require cognitive flexibility between multiple sets of rules (Rutz and Rothblat, 2012). Specifically, while BTBR mice are able to inhibit prepotent responding during an automatic rule-switching task, they exhibit deficits in reversal task performance that is dependent on the active use of contextual information. In addition, Silverman et al. developed a transitive inference paradigm and identified deficits in higher order discrimination thinking in the BTBR mouse that closely parallels the transitive inference deficits observed in individuals with ASD (Silverman et al., 2013; Solomon et al., 2011). In this task, mice are trained to discriminate between a series of hierarchical visual stimulus pairs and later tested on their ability to transfer these learned relationships to novel pairs. While the BTBR mice successfully discriminate between novel pairs in close proximity on the hierarchy, they exhibit marked deficits in inferences when stimulus pairs were farther apart on the hierarchy (Silverman et al., 2013). Interestingly, differences in cognitive performance between the BTBR and C57BL/6J mouse are accompanied by impairments in attention tasks as well. In the 5-choice serial time task, BTBR mice show deficits in impulse control and decreased motivation similar to the characteristic phenotype of attention deficit/hyperactivity disorder (APA, 2013; McTighe et al., 2013), a neurodevelopmental disorder often comorbid with ASD. Given this range of behavioral deficits reported in the BTBR mouse, including the core ASD-like features of social impairments and stereotypical repetitive behaviors as well as anxiety, cognitive and attentional deficits, the characteristic phenotype of this mouse model presents a useful translational tool for identifying plausible biological markers and endophenotypes that contribute to the etiology and pathologies associated with ASD.
3. Immune findings in BTBR mice
Among the numerous immunological findings in ASD discovered to date, reports of increased levels of pro-inflammatory markers remain the most consistently observed. These include increases in pro-inflammatory cytokines such as IL-1β, TNFα and IL-6, and chemokines such as MCP-1 in plasma/sera, cerebral spinal fluid (CSF) and post mortem brain tissue (Ashwood et al., 2011a; Emanuele et al., 2010; Li et al., 2009; Suzuki et al., 2011; Vargas et al., 2005). As noted, many of these studies highlight a connection between increased immune dysregulation or activation with more impaired behaviors (Onore et al., 2012). These findings suggest a pro-inflammatory immune profile is prevalent in the ASD population or at the very least there is a subgroup of the ASD population where ongoing immune activation may be linked to more impaired behavioral symptomatology.
BTBR mice have shown a number of immune abnormalities many of which are also observed in children with ASD. These include alterations in both the innate and adaptive arms of the immune system. Similar to observations made in postmortem brains of subjects with ASD (Li et al., 2009; Vargas et al., 2005), BTBR mice show elevated expression of cytokines in the brain (Heo et al., 2011). These include increases in IL-1β (as well as other IL-1 family members such as IL-18 and IL-33), IL-6, and IL-12. These changes vary in different regions of the brain. However, increases in the IL-1 family of cytokines were observed in most regions sampled. IL-1β as well as its family members have been found to be elevated in ASD in a number of studies and associated with more impaired behaviors (Ashwood et al., 2011a; Croonenberghs et al., 2002a; Enstrom et al., 2010; Jyonouchi et al., 2001; Li et al., 2009; Ricci et al., 2013). This suggests that the IL-1 family may contribute to either the pathology of ASD or to a particular behavioral phenotype. In BTBR mice a similar finding was observed, with the level of IL-33 in the brain correlating with more impaired behaviors (Heo et al., 2011). Moreover, BTBR mice show signs of increased microglia activation as measured by MHC-II expression (Heo et al., 2011) as well as increased numbers of microglia (Zhang et al., 2013). This parallels findings in ASD showing increased activation markers for microglia (iba1 and HLA-DR) in postmortem brains (Li et al., 2009; Morgan et al., 2010; Vargas et al., 2005) and by PET scan (Suzuki et al., 2013). Thus activation or proliferation of microglia might play an important role in behaviors relevant to ASD. However, given the current techniques used to measure microglia markers, it remains unclear whether these neuroimmune alterations result from dysregulation of preexisting microglia or due to infiltration of macrophages from the periphery.
Macrophages and microglia are capable of polarizing into two major subtypes, categorized as M1 or M2 (Sica and Mantovani, 2012). The “classical” or M1 subtype typically releases large quantities of pro-inflammatory cytokines and promotes cell-mediated immunity characterized by production of high levels of IL-12, and low levels of IL-10. “Alternatively activated” or M2 macrophages function to resolve inflammation in wound healing and to clear cellular debris following an inflammatory event. M2 macrophages produce very low levels of IL-12 and high amounts of anti-inflammatory molecules including IL-10 and TGFβ (Sica and Mantovani, 2012). A prominent M1 skewing is evident in BTBR mice, with macrophages from BTBR mice producing significantly more IL-12 and less IL-10 than C57BL/6J mice (Onore et al., 2013). In addition, production of IL-1β by macrophages stimulated under M1 skewing conditions (IFN-γ and LPS) are associated with more impaired social behaviors; increased production of IL-12 in unstimulated cultures was also associated with more severe grooming behaviors, whereas increased production of IL-10 in response to LPS was correlated with less grooming (Onore et al., 2013). Similarly, M1 macrophage skewing and increased production of IL-12 was found in offspring born to dams exposed to maternal immune activation (MIA) induced by the viral mimic Polyinosinic–polycytidylic acid [poly(I:C)] (Onore et al., 2014). This and other MIA models also underscore the neurodevelopmental links between the immune and central nervous system as offspring of MIA dams exhibit both ASD-like behavioral deficits and dysregulated immune responses similar to those observed in BTBR mice (Hsiao et al., 2012; Schwartzer et al., 2013). In addition, these models suggest a role for epigenetic changes as causative factors for the ASD-associated behaviors as these immune findings are evident in bone marrow progenitors in both the BTBR mouse and mice from the MIA model (Hsiao et al., 2012; Onore et al., 2013; Onore et al., 2014).
An M1 polarization is consistent with increased IL-12 observed in plasma and brain specimens observed in individuals with ASD, and may play a role in the pathophysiology (Ashwood et al., 2011a; Singh, 1996; Vargas et al., 2005). IL-12 is a potent activator of T- and natural killer cells, which in turn produce IFN-γ. The aberrant production of IFN-γ is associated with a number of autoinflammatory and autoimmune diseases. In ASD, the increased production of IFN-γ in the supernatants of peripheral blood mono-nuclear cells (PBMC) (Croonenberghs et al., 2002b), natural killer cells (Enstrom et al., 2009), and increased plasma levels (Singh, 1996) has been observed in some children. This increased IFN-γ production by PBMC has been associated with increased ADOS and ADI-R severity scores (Ashwood et al., 2011a). In addition, increased levels of IFN-γ but not IL-4 have been observed in postmortem brain specimens from individuals with ASD (Li et al., 2009). There is also evidence from archived sera taken from mothers during the second trimester of their pregnancy that IFN-γ is significantly increased during this period and its increase is associated with increased risk of ASD (Goines et al., 2011). Taken together these data on increased IFN-γ would suggest that an environment exists in children with ASD that preferentially promotes M1 cells and may be linked to phenotypic severity.
Interestingly, when pregnant BTBR dams undergo maternal immune activation, their offspring show more severe behavioral impairments as well as more persistent immune alterations that may reflect elevated maternal immune responses in the treated dams due to genetic and environmental effects. In the offspring of BTBR dams treated with MIA there were increased signs of ex vivo inflammation. This was observed in cultured splenocytes that produced increased levels of IL-6, IL-17, and TNFα following stimulation with phorbol myristate acetate/ionomycin compared with BTBR offspring of dams treated with saline. These findings were associated with more impaired repetitive behaviors and increased ultrasonic vocalizations. The mechanism by which MIA affects BTBR dams is not known. Currently, there is no evidence that NFκB signaling, the major signaling pathway involved in production of pro-inflammatory cytokines, is altered in BTBR animals. Analysis of NFκB phosphorylation in BTBR mice cortex and cerebellum, as assessed by ELISA, shows no significant differences from C57BL/6J animals (Malik et al., 2011). This may suggest that other cellular dysfunctions yet described in BTBR mice are present and are related to their abnormal immune profiles. However, the dynamics and triggers for increased cytokine production have not been determined.
When BTBR mice were crossed with C57BL/6J mice, they showed improvements in both immune and behavioral phenotypes such that they were midway between the C57BL/6J and BTBR parent strains (Heo et al., 2011). Remarkably, when BTBR embryos were transferred into C57BL/6J dams, significant improvements in both social and repetitive behaviors were observed (Zhang et al., 2013). This may suggest that a maternal environmental factor or factors present in BTBR dams is needed to elicit the asocial behavioral observations of the BTBR mouse in addition to the genetic make-up of these animals. Given the inflammatory immune profiles in BTBR mice, it is easy to speculate that this profile during gestation results in features similar to MIA treated mice. However, it is not known if the immune profile of pregnant BTBR dams remains elevated compared to that of non-pregnant animals. Regardless of the maternal environment, BTBR mice implanted in C57BL6/J dams showed increased repetitive behaviors relative to C57BL/BJ mice, although there were still quantitative decreases in repetitive behaviors in BTBR fetuses that developed in these dams. Interestingly, C57BL/6J animals showed no increase in repetitive behaviors when developed in a BTBR dam (Zhang et al., 2013). It is important to note that significant social improvements have also been observed in BTBR mice cross-fostered with C57BL/6J dams, whereas no significant improvements were seen in repetitive grooming behaviors (Yang et al., 2011). Thus it is unclear how much of the behavioral improvements seen in the embryonic cross experiments can be attributed to social environment or changes in the maternal immune environment. Taken together, these observations would suggest that a combination of maternal environment, inherent immune dysregulation, as well as other genetic susceptibility factors, probably relating to neuronal developmental regulation, all work together to produce the BTBR behavioral phenotype.
Aside from differences in the innate immune system, evidence suggests dysregulation in adaptive immune responses of BTBR mice, although the picture is less clear. Heo et al. (2011) demonstrated that BTBR mice produce substantially more antibodies than C57BL/6J mice, despite having fewer relative numbers of B-cells. This is contrary to what is observed in children with ASD who exhibit decreased immunoglobulin production (Heuer et al., 2008) and increased numbers of B-cells (Hashimoto et al., 2011). In BTBR mice antibody titers were increased in the brain where some of the antibodies appeared to be reactive with “self” neuronal tissue (Heo et al., 2011). What drives the increased production of circulating antibodies/auto-antibodies is not known; however, increased levels of factors promoting antibody production such as IL-6 have been observed (Onore et al., 2013; Schwartzer et al., 2013). Other changes in the BTBR immune system include elevated numbers of CD4+ and CD8+ T-cells in peripheral immune organs, including spleen, mesenteric lymph node, and peripheral blood (Heo et al., 2011). Importantly, increased T-cell numbers are not a feature of children with ASD (Hashimoto et al., 2011). Therefore, further assessment of dynamic cellular T and B-cell responses in BTBR mice are required.
4. Future directions
A considerable amount of research has been performed using the BTBR mouse model, at genetic, behavioral, and immunological levels. This mouse strain represents one of the most described animal models that exhibit autism-relevant behaviors to date. The BTBR mouse model does, however, present several challenges for future research. Despite efforts to trace specific causal factors in BTBR mice, the complex genetic and physiologic nature of BTBR mice has made this a difficult task. Since genetic trait markers for the behavioral deficits do not exist for the BTBR mouse, crosses with other stains to utilize the myriad of genetic and molecular tools currently available to mouse research are difficult. This is in part because any cross of BTBR with other strains to date has shown improvements in the phenotypic behaviors. Further work on BTBR genetics will have to be performed in order to overcome these issues, potentially by utilizing multiple backcrosses to obtain genetic variants of BTBR while monitoring for the presence of BTBR characteristic behavioral traits in littermates. Immunological studies have some advantages here. The work by the Lawrence laboratory has established that immunological transfers from C57BL/6J mice into BTBR are possible without imposing graft versus hosts’ responses, which will allow for further studies of the role the immune system plays in strain-specific behavioral profiles (Zhang et al., 2013). Although the traditional toolbox of molecular techniques is limited in BTBR animals, their complex nature and apparent multifactorial presentation of autism relevant phenotypes make it a unique model for studying idiopathic autism.
While much of the literature has focused on the parallels between the BTBR mouse and ASD, it is important to note that many of these key immunological and behavioral characteristics manifest in other neurodevelopment disorders. For example, schizophrenia and ASD share overlapping behavioral deficits in sociability as well as commonalities in dysregulated immune responses (Gibney and Drexhage, 2013). Moreover, both of these disorders are hypothesized to manifest as a result of maternal inflammation suggesting common neuroimmunological pathologies across diseases (Meyer et al., 2011). These similarities in the human condition limit our ability to effectively translate animal models to specific neuropsychiatric disorders. For the BTBR mouse, it remains unclear whether the behavioral and biological similarities observed in this mouse strain represent pathologies unique to ASD or if these deficits more effectively model global pathologies that contribute to altered brain and behavior development across neuropsychiatric disorders. An endophenotype approach to animal models in which biological and behavioral parallels to humans are used to identify common mechanisms to disease may better serve the preclinical field. Therefore, a deeper investigation into the validity of the BTBR strain as a model specific to ASD is warranted before gaining predictive validity as an effective translational tool.
5. Conclusion
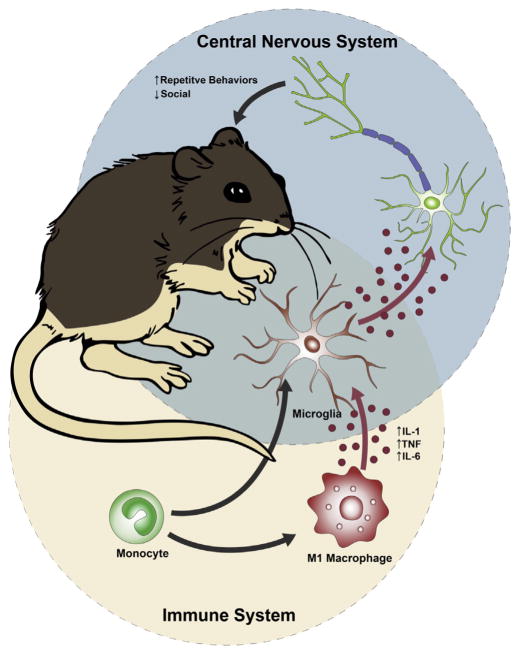
The BTBR mouse model has many behavioral and immunological features relevant to ASD. Investigation into the immune abnormalities of this inbred strain may help to understand the role immune dysregulation plays in behavioral responses. The inflammatory environment created by M1 macrophages in these animals parallels many of the immunological finding in human subjects with ASD. A skewed innate immune response seems to increase behavioral deficits in both the social and repetitive domains. These deficits appear to result at least in part from maternal immune dysfunction and its effect on the developing fetus. Experiments to further exacerbate the maternal immune environment results in increased impairments in BTBR animals, and the transfer of BTBR embryos into C57BL/6J dams abrogates much of the behavioral phenotype present. However, repetitive behaviors remain increased in BTBR animals even when they develop in a C57BL/6J dam, suggesting that several additional factors contribute to the BTBR phenotype than simply the maternal environment. Indeed, inflammatory markers in adult BTBR animals correlate with increased repetitive behaviors, suggesting that persistent immune dysregulation in juvenile and adult animals also contributes to the behavioral phenotype in BTBR mice. Although not fully understood, work in other immune behavioral models would suggest that peripheral immune dysfunction can work in two ways: peripheral cytokines/chemokines can enter into the CNS at low or high levels during disease states, where they can directly affect neuronal development and function; or alternatively, these cytokines/chemokines can activate perivascular macrophages or microglia which in turn produce downstream factors which can alter neuronal function (Fig. 1). Similarly, as alteration in mitochondrial function have been observed in the MIA mouse model (Giulivi et al., 2013), mitochondrial functions should be investigated in the BTBR mouse as well, given that these changes could lead to increased oxidative stress and neuroinflammation (James et al., 2004). Future work into the immune dysfunction of BTBR animals should help to elucidate their role in aberrant behavior and neurodevelopment.
Fig. 1.
The inflammatory profile of BTBR mice contributes to aberrant behavior and neurodevelopment. The inflammatory environment in BTBR mice appears to be driven by innate immune function including the skewing of macrophages to an M1 phenotype, which produce a number of cytokines observed to be upregulated in individuals with autism spectrum disorders (ASD) such as: IL-1β, IL-6, and TNFα. These cytokine are believed to work directly on neurons or through microglia intermediates to alter neuronal function and development.
References
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res. 2012;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA; A.P.A, editor. Diagnostic and Statistical Manual of Mental Disorders. 5. 2013. [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011a;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011b;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: an unusual behavioral phenotype. Behav Brain Res. 2009;197:462–465. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Robbins TW. A novel automated touchscreen procedure for assessing learning in the rat using computer graphic stimuli. Neurosci Res Commun. 1994;15:103–110. [Google Scholar]
- Bussey TJ, Padain TL, Skillings EA, Winters BD, Morton AJ, Saksida LM. The touchscreen cognitive testing method for rodents: how to get the best out of your rat. Learn Memory. 2008;15:516–523. doi: 10.1101/lm.987808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Prevalence of autism spectrum disorders – Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. Morbidity and mortality weekly report. Surveill Summ. 2012;61:1–19. [PubMed] [Google Scholar]
- Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002a;45:1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- Croonenberghs J, Wauters A, Devreese K, Verkerk R, Scharpe S, Bosmans E, Egyed B, Deboutte D, Maes M. Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol Med. 2002b;32:1457–1463. doi: 10.1017/s0033291702006037. [DOI] [PubMed] [Google Scholar]
- Defensor EB, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav Brain Res. 2011;217:302–308. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele E, Orsi P, Boso M, Broglia D, Brondino N, Barale F, di Nemi SU, Politi P. Low-grade endotoxemia in patients with severe autism. Neurosci Lett. 2010;471:162–165. doi: 10.1016/j.neulet.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Enstrom AM, Lit L, Onore CE, Gregg JP, Hansen RL, Pessah IN, Hertz-Picciotto I, Van de Water JA, Sharp FR, Ashwood P. Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain Behav Immun. 2009;23:124–133. doi: 10.1016/j.bbi.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2010;24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney SM, Drexhage HA. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J Neuroimmune Pharmacol. 2013;8:900–920. doi: 10.1007/s11481-013-9462-8. [DOI] [PubMed] [Google Scholar]
- Giulivi C, Napoli E, Schwartzer J, Careaga M, Ashwood P. Gestational exposure to a viral mimetic poly(i:C) results in long-lasting changes in mitochondrial function by leucocytes in the adult offspring. Mediators Inflamm. 2013;2013:609602. doi: 10.1155/2013/609602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, Kharrazi M, Ashwood P, Van de Water J. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: a case-control study. Mol Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guariglia SR, Chadman KK. Water T-maze: a useful assay for determination of repetitive behaviors in mice. J Neurosci Methods. 2013;220:24–29. doi: 10.1016/j.jneumeth.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ashwood P, Corbett BA, Kantor A, Schulman H, Van de Water J, Amaral DG. In search of cellular immunophenotypes in the blood of children with autism. PLoS One. 2011;6:e19299. doi: 10.1371/journal.pone.0019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo Y, Zhang Y, Gao D, Miller VM, Lawrence DA. Aberrant immune responses in a mouse with behavioral disorders. PLoS One. 2011;6:e20912. doi: 10.1371/journal.pone.0020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer L, Ashwood P, Schauer J, Goines P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008;1:275–283. doi: 10.1002/aur.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner AE, Heath CJ, Hvoslef-Eide M, Kent BA, Kim CH, Nilsson SRO, Alsio J, Oomen CA, Holmes A, Saksida LM, Bussey TJ. The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc. 2013;8:1961–1984. doi: 10.1038/nprot.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci USA. 2012;109:12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Geng L, Cushing-Ruby A, Quraishi H. Impact of innate immunity in a subset of children with autism spectrum disorders: a case control study. J Neuroinflammation. 2008;5:52. doi: 10.1186/1742-2094-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi H, Geng L, Streck DL, Toruner GA. Children with autism spectrum disorders (ASD) who exhibit chronic gastrointestinal (GI) symptoms and marked fluctuation of behavioral symptoms exhibit distinct innate immune abnormalities and transcriptional profiles of peripheral blood (PB) monocytes. J Neuroimmunol. 2011;238:73–80. doi: 10.1016/j.jneuroim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- Karvat G, Kimchi T. Systematic autistic-like behavioral phenotyping of 4 mouse strains using a novel wheel-running assay. Behav Brain Res. 2012;233:405–414. doi: 10.1016/j.bbr.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M, Tauqeer Z, Sheikh AM, Wen G, Nagori A, Yang K, Brown WT, Li X. NF-kappaB signaling in the brain of autistic subjects. Mediators Inflamm. 2011;2011:785265. doi: 10.1155/2011/785265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McTighe SM, Neal SJ, Lin Q, Hughes ZA, Smith DG. The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. PLoS One. 2013;8:e62189. doi: 10.1371/journal.pone.0062189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011;69:26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26:383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore CE, Careaga M, Babineau BA, Schwartzer JJ, Berman RF, Ashwood P. Inflammatory macrophage phenotype in BTBR T+tf/J mice. Front Neurosci. 2013;7:158. doi: 10.3389/fnins.2013.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore CE, Schwartzer JJ, Careaga M, Berman RF, Ashwood P. Maternal immune activation leads to activated inflammatory macrophages in offspring. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BL, Bettis JK, Meyza KZ, Yamamoto LY, Blanchard DC, Blanchard RJ. Absence of social conditioned place preference in BTBR T+tf/J mice: relevance for social motivation testing in rodent models of autism. Behav Brain Res. 2012;233:99–104. doi: 10.1016/j.bbr.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BL, Pobbe RL, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, Blanchard RJ. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:228–235. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RL, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+ tf/J mouse strain. Behav Brain Res. 2011;216:446–451. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214:443–449. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci S, Businaro R, Ippoliti F, Lo Vasco VR, Massoni F, Onofri E, Troili GM, Pontecorvi V, Morelli M, Rapp Ricciardi M, Archer T. Altered cytokine and BDNF levels in autism spectrum disorder. Neurotox Res. 2013;24:491–501. doi: 10.1007/s12640-013-9393-4. [DOI] [PubMed] [Google Scholar]
- Roullet FI, Wohr M, Crawley JN. Female urine-induced male mice ultrasonic vocalizations, but not scent-marking, is modulated by social experience. Behav Brain Res. 2011;216:19–28. doi: 10.1016/j.bbr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz HL, Rothblat LA. Intact and impaired executive abilities in the BTBR mouse model of autism. Behav Brain Res. 2012;234:33–37. doi: 10.1016/j.bbr.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Martire A, Cartocci G, Ferrante A, Ricceri L. Reduced social interaction, behavioural flexibility and BDNF signalling in the BTBR T+ tf/J strain, a mouse model of autism. Behav Brain Res. 2013;251:35–40. doi: 10.1016/j.bbr.2012.12.028. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl Psychiatry. 2013;3:e240. doi: 10.1038/tp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Gastrell PT, Karras MN, Solomon M, Crawley JN. Cognitive abilities on transitive inference using a novel touchscreen technology for mice. Cereb Cortex. 2013 doi: 10.1093/cercor/bht293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010;171:1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J Neuroimmunol. 1996;66:143–145. doi: 10.1016/0165-5728(96)00014-8. [DOI] [PubMed] [Google Scholar]
- Solomon M, Smith AC, Frank MJ, Ly S, Carter CS. Probabilistic reinforcement learning in adults with autism spectrum disorders. Autism Res. 2011;4:109–120. doi: 10.1002/aur.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, Yoshihara Y, Wakuda T, Takebayashi K, Takagai S, Matsumoto K, Tsuchiya KJ, Iwata Y, Nakamura K, Tsujii M, Sugiyama T, Mori N. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS One. 2011;6:e20470. doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, Yoshihara Y, Omata K, Matsumoto K, Tsuchiya KJ, Iwata Y, Tsujii M, Sugiyama T, Mori N. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry. 2013;70:49–58. doi: 10.1001/jamapsychiatry.2013.272. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Crabbe JC. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain Res. 2003;971:47–54. doi: 10.1016/s0006-8993(03)02354-0. [DOI] [PubMed] [Google Scholar]
- Wohr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Loureiro D, Kalikhman D, Crawley JN. Male mice emit distinct ultrasonic vocalizations when the female leaves the social interaction arena. Front Behav Neurosci. 2013;7:159. doi: 10.3389/fnbeh.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2011;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao D, Kluetzman K, Mendoza A, Bolivar VJ, Reilly A, Jolly JK, Lawrence DA. The maternal autoimmune environment affects the social behavior of offspring. J Neuroimmunol. 2013;258:51–60. doi: 10.1016/j.jneuroim.2013.02.019. [DOI] [PubMed] [Google Scholar]