Abstract
Animals use a nervous system for locomotion in some stage of their life cycle. The nematode Caenorhabditis elegans, a major animal model for almost all fields of experimental biology, has long been used for detailed studies of genetic and physiological locomotion mechanisms. Of its 959 somatic cells, 302 are neurons that are identifiable by lineage, location, morphology, and neurochemistry in every adult hermaphrodite. Of those, 75 motoneurons innervate body wall muscles that provide the thrust during locomotion. In this Overview, we concentrate on the generation of either forward- or backward-directed motion during crawling and swimming. We describe locomotion behavior, the parts constituting the locomotion system, and the relevant neuronal connectivity. Because it is not yet fully understood how these components combine to generate locomotion, we discuss competing hypotheses and models.
Keywords: animal behavior, locomotion, neurobiology, Caenorhabditis elegans
Locomotion, the act of self-propulsion, is a fundamental animal behavior. Almost all animals use a nervous system for locomotion in some stage of their life cycle (with the telling exceptions of sponges and placozoa). Searching for food, conspecifics, or improved conditions; catching prey; escaping from predators; migrating; and dispersing are all animal behaviors that require locomotion. The nematode Caenorhabditis elegans is a major animal model for almost all fields of experimental biology. It was the first animal model to offer a complete description of a developmental lineage, a nervous system, and a genome. Of its 959 somatic cells, 302 are neurons that are identifiable by lineage, location, morphology, and neurochemistry in every adult hermaphrodite. Of those, 75 motoneurons innervate body wall muscles that provide the thrust during locomotion. Considerable advances have been made in the neurobiology of sensory transduction and integration. Complementing this knowledge with the neuronal mechanisms that underlie locomotion can lead to a fully integrated model of a complete nervous system, from sensory input to behavioral output.
In this Overview, we concentrate on the generation of either forward- or backward-directed locomotion during crawling and swimming. Interesting topics such as steering and navigation remain outside of the intended scope. We focus on the adult hermaphrodite animal, because the vast majority of research on the locomotion of C. elegans was performed on that life stage; when appropriate, we also discuss findings specific to the larval nervous systems. There are three distinct, complementary levels of analysis required for understanding a system (Marr 1982): a computational level (what are the input and output of the system?), a hardware implementation level (what are the components of the system, and how are they connected?), and an algorithmic level (how is the computation implemented?). We follow this scheme and describe locomotion behavior, the parts of the locomotion system and their connectivity, and hypotheses formulated to explain how these components generate locomotion.
Locomotion behavior
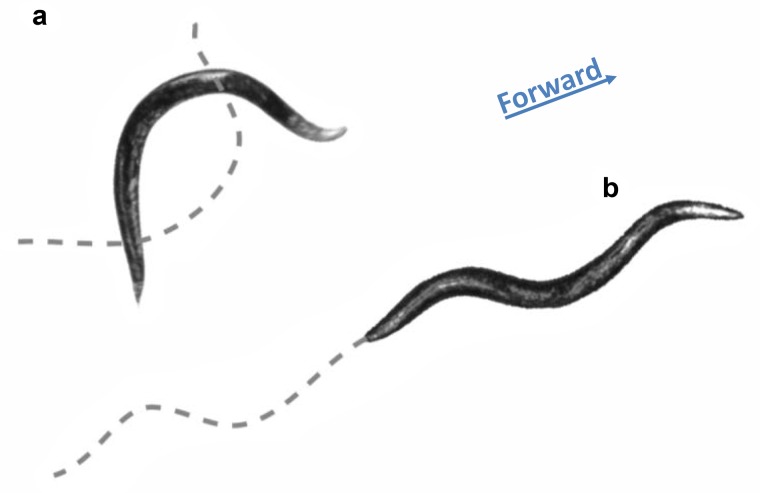
Similar to other nematodes, C. elegans locomotes in an undulatory fashion (Gray J and Lissmann 1964, Burr and Robinson 2004). It generates thrust by propagating dorsoventral body bends along its body against the direction of locomotion (Gray J and Lissmann 1964). When the animal swims in a liquid of a viscosity comparable to that of water, the wavelength of undulation is almost twice the body length, which produces alternating C-shape conformations (figure 1a) at a frequency of about 2 hertz (Hz; Pierce-Shimomura et al. 2008, Berri et al. 2009, Fang-Yen et al. 2010, Vidal-Gadea et al. 2011). Although swimming is sometimes loosely referred to as “thrashing,” it is a directional behavior that enables navigation, such as in chemotaxis assays (Pierce-Shimomura et al. 2008). When the nematode is presented with high mechanical loads, such as those on the surface of an agar gel or in a 10,000-fold more viscous liquid, the wavelength of the undulating body shortens to less than a single body length, which produces a typical S shape (figure 1b), undulating at approximately 0.3 Hz. Therefore, the motor program is shaped by physical interactions between the body and its environment through proprioceptive feedback. Varying the mechanical load imposed by the environment between these two extremes reveals a gradual transition of the corresponding wavelengths and frequencies (Berri et al. 2009, Fang-Yen et al. 2010, Lebois et al. 2012). This continuum, as opposed to discrete gaits, suggests that a single motor program shaped by physical forces and proprioceptive feedback underlies locomotion. Nevertheless, the two gait extremes were found to be pharmacologically and genetically separable: Dopamine induces crawling in a low-viscosity environment, whereas serotonin induces swimming in shallow liquid (Vidal-Gadea et al. 2011). Furthermore, pharyngeal pumping was correlated with levels of crawling but not swimming (Vidal-Gadea et al. 2011). Therefore, it is likely that biogenic amines mediate the perception of the mechanical properties of the environment to modulate proprioception, as well as directly modulating the locomotor program. Such intricate means of modulation may be of particular importance in natural environments that are less uniform than are those in the laboratory both spatially and temporally.
Figure 1.
Typical body posture of an adult hermaphrodite Caenorhabditis elegans during swimming after it was submerged in saline (a) and crawling on an agar surface (b). The dotted lines denote the midline at half of a locomotion cycle earlier in the case of swimming (a) and of a single locomotion cycle earlier in the case of crawling (b). Note that the amount of displacement per cycle during swimming is smaller but the frequency of undulation (not shown) is higher than that during crawling. The animals’ heads are pointing toward the right (see the arrow marked Forward) and the body bends are dorsoventral, with the lateral aspect toward the viewer.
If we ignore steering, the locomotion behavior of C. elegans on short timescales (on the order of 1 second) can be heuristically divided to four categories: (1) forward locomotion, which consists of the propagation of dorsoventral body bends, from the anterior to the posterior, originating at the head; (2) backward locomotion, which consists of the propagation of dorsoventral body bends from the posterior to the anterior; (3) dwelling, which consists of a large variety of nondirectional dynamics of dorsoventral body bends; and (4) quiescence, the complete absence of motion (Gray JM et al. 2005, Raizen et al. 2008, Stephens et al. 2011, Gallagher et al. 2013). Each of these microcategories can persist exclusively for many seconds (Fujiwara et al. 2002, Flavell et al. 2013, Iwanir et al. 2013). However, it is a matter of ongoing debate whether some or all of these categories correspond to discrete behavioral microstates or to a continuum of tunable behaviors and, if so, how many such behavioral states there may be (Gallagher et al. 2013). In addition, macrostates can be observed, whereby distinct proportions of behavioral microcategories are maintained for prolonged periods, up to the order of an hour (Nagy et al. 2013). The existence of these macrostates suggests that any short-term description of locomotion would be incomplete. Nevertheless, techniques for high-resolution tracking of locomotion patterns for prolonged periods are not commonly implemented. A major technical bottleneck arises from the need to analyze in a timely fashion the vast amount of experimental data produced by prolonged, continuous, high-resolution assays. Progress in this area will require incorporating big-data methods to supplement or even replace traditional tabletop methods for the curation, storage, analysis, and sharing of behavioral data.
The field of analysis of behavioral data is rapidly evolving, and several approaches have been suggested for mathematically describing C. elegans locomotion. Determining phenotypes manually using heuristic classifications such as hypo- or hyperkinesis or by scoring changes in ad hoc defined features has been immensely fruitful, despite the limited experimental resolution and the possibility of unknowingly introducing a bias. Centroid tracking data from high-throughput assays were analyzed using hidden Markov models to identify locomotion states (Flavell et al. 2013, Gallagher et al. 2013). Body shape data of sufficiently high spatial and temporal resolution showed that the space of shapes adopted by C. elegans is low dimensional, which reveals an underlying simplicity of seemingly complex locomotion dynamics (Stephens et al. 2011, Feeny et al. 2013). Moreover, the quantitative description of behavior arising from the building blocks spanning the space of observed body postures, termed eigenworms, provided a framework for unbiased scoring of previously undetectable phenotypes (Stephens et al. 2011, Brown AEX et al. 2012).
A different high-resolution approach was intended to preserve the intuitive importance of individual body bends while still relying on machine vision in order to minimize bias (Nagy et al. 2013). This (computationally intensive) approach emphasized the notion that the generation, propagation, and decay of individual body bends are fundamental primitives of C. elegans locomotion, which are expected to have identifiable physiological correlates (Gray JM et al. 2005, Von Stetina et al. 2005, Boyle et al. 2012, Wen et al. 2012). The optimization in computational complexity offered by the eigenworm model comes at the expense of a natural relationship between its building blocks and the physiological activity in the neural circuits of the animal, as compared with a model based directly on the dynamics of body bends. Depending on the question at hand, these two approaches may be complementary. Notably, all of the approaches discussed above discard global information, such as absolute position and orientation, and thereby implicitly assume that C. elegans does not maintain or use such parameters.
Continuum mechanics
The shape of the adult nematode is a slim cylinder, tapered at both ends, about 50 microns in diameter and 1 millimeter (mm) long. At this size, it is smaller than the capillary length of the water–air interface (which is approximately 2 mm); when it is in a fluid, C. elegans swims in a low (less than 1) Reynolds number regime (Sznitman et al. 2010), in which the viscous forces are greater than the inertial forces. Intuitively, under these conditions, the animal will stop moving almost instantly once it ceases to produce force. The stiffness and the elastic modulus of the static body of a wild-type adult animal were found to be approximately 0.60 Newtons per meter and in the range of 100–200 kilopascals, respectively (Fang-Yen et al. 2010, Sznitman et al. 2010, Petzold et al. 2011, Backholm et al. 2013). Hydrostatic pressure contributes modestly to stiffness. Puncturing the cuticle decreases the body stiffness by about 18% (Park et al. 2007), and the animal continues undulating after being punctured (our observation). Manipulating the contraction of muscles pharmacologically or optogenetically has suggested that the resting muscle tone is a major contributor to the resting body stiffness (Petzold et al. 2011). The forces produced by the coordinated action of body wall muscles during locomotion have also been measured. During swimming, the body delivers propulsive thrusts on the order of a few nanonewtons (Sznitman et al. 2010), whereas during crawling, the adult nematode produces forces in the range of 1–9 micronewtons (Doll et al. 2009, Johari et al. 2013).
Muscle cells
Ninety-five body wall muscle cells are staggered in two rows in each of four quadrants along the anterior–posterior axis (figure 2c). As in other nematodes, steering in C. elegans is achieved by differential activation of the 20 anteriormost muscle cells (in the head and neck), whereas thrust is produced by dorsoventral bending of the entire body. The quadrants of head and neck muscles are independently innervated by nerve-ring motoneurons and can turn freely relative to the anterior–posterior axis of the body (Hall and Altun 2008). In contrast, along the neck and the rest of the body, the muscles from the two subventral quadrants send thin processes called muscle arms into the ventral nerve cord, whereas those from the two subdorsal quadrants send arms into the dorsal cord (White et al. 1976, Von Stetina et al. 2005). In addition to chemical neuromuscular junctions, the muscle arms seem to also be electrically coupled by gap junctions where they meet in the nerve cords (Liu Q et al. 2006). This pattern of innervation is consistent with the observation that the vast majority of locomotion patterns involve the generation and propagation of dorsoventral bends. Maneuvers that require a distinct activation of muscles in adjacent quadrants have been observed during transitions between larval stages (Singh and Sulston 1978). However, the cellular mechanisms that enable the independent control of each quadrant are still unknown.
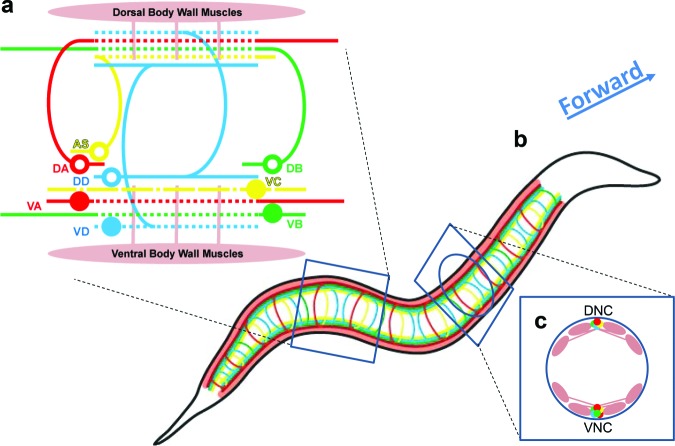
Figure 2.
The neuromuscular system for locomotion. (a) Motoneurons of eight classes innervate the dorsal (top) and ventral (bottom) groups of muscle cells that send muscle arms into the dorsal and ventral cords. All soma (the colored circles) and most synaptic inputs to motoneurons are in the ventral nerve cord, whereas neuromuscular junctions (the dashed lines) are in the dorsal and ventral cords. Five classes of motoneurons (AS, DA, DB, DD, and VD) send commissures from the dorsal to the ventral cords. For simplicity, only one motoneuron of each class is presented. However, four classes (VA, VB, VD, and AS) have about twice as many neurons as the others (DA, DB, DD, and VC). The most prevalent synapses are from cholinergic to GABAergic motoneurons (not shown), but there are other synapses and gap junctions among the motoneurons and to motoneurons more anterior and posterior (table S1; White et al. 1976, Haspel and O'Donovan 2011). (b) The motoneurons and muscle cells repeat along the body (the head is on the right) to compose the complete neuromuscular system. (c) Muscle cells are organized in four quadrants, each consisting of two staggered rows (sagittal view) next to the dorsal and ventral nerve cords (DNC and VNC, respectively).
The body wall muscle cells are rhomboid in shape and are obliquely striated. As in other nematodes, the muscles are anchored along their entire length and not only at their ends (Burr and Robinson 2004, Hall and Altun 2008). The muscles’ contractile force is distributed by organelles (the dense bodies and M lines) that mechanically link actin filaments to the extracellular matrix and cuticle (Francis and Waterston 1991, Lecroisey et al. 2007). Cholinergic and GABAergic (i.e., choline-producing and ©-aminobutyric acid–producing, respectively) neuromuscular junctions (Richmond and Jorgensen 1999) occur en passant on the axon of the motoneuron through thin arms that protrude from the muscle main mass (Hall and Altun 2008) inside the ventral or dorsal nerve cord or on the nerve ring. This morphology suggests that the muscle cell membrane cannot be passive, because synaptic current would dissipate along the muscle arms and on arrival at the much larger body. Indeed, body wall muscles produce calcium-mediated action potentials (Liu P et al. 2011, Gao and Zhen 2011). Muscle contraction follows bursts of action potentials that are induced by a cholinergic input and inhibited GABAergically (Liewald et al. 2008, Liu P et al. 2013). However, an additional intrinsic, homeostatically regulated mechanism for muscle activity is suggested by two observations: (1) that muscular action potential frequency recovers (rather than stops) within seconds of a pharmacological block of a cholinergic input, a GABAergic input, or both inputs (Liu P et al. 2011) and (2) that animals freeze in their current posture (rather than assume a straight posture) when cholinergic motoneurons are acutely hyperpolarized (Wen et al. 2012). In both cases, muscle activity seems to resume in the absence of cholinergic input.
Motoneurons
The neck and head musculature is innervated by 28 motoneurons of 9 classes: RIM, RIV, RMF, RMG, and RMH (bilaterally symmetric); RME, SMB, and URA (four members in each class); and IL1 neurons (six members) (White et al. 1986). The neural circuits in the head and the neck provide most of the steering during locomotion, but little is known about their activity. Along the neck and body, 75 motoneurons innervate body wall muscle such that each muscle is innervated by multiple motoneurons. These neurons are divided by their morphology into eight distinct classes (White et al. 1976, 1986, Chen BL et al. 2006, Hall and Altun 2008). Four classes innervate ventral muscles (12-VA, 11-VB, 6-VC, and 13-VD), and four innervate dorsal muscles (11-AS, 9-DA, 7-DB, and 6-DD). Although the morphology of these neurons and muscle cells has been studied in detail, their physiological activity is not fully characterized. In particular, little is known about the electrophysiological properties of the motoneurons and their synapses. Each nerve cord motoneuron has a cell body that is about 2 microns in diameter, located in the ventral nerve cord, and two neurites: a dendrite within the nerve cord that contains postsynaptic specializations and an axon that forms neuromuscular junctions. For dorsal and inhibitory motoneurons, a lateral commissure brings the axon to the dorsal nerve cord (no cell bodies reside in the dorsal nerve cord; figure 2a).
Although electrophysiological measurements have been performed in C. elegans, genetically encoded calcium indicators are more commonly used for measuring the physiological activity in its muscles and neurons. The neuronal activity of all six classes of cholinergic motoneurons has been measured in restricted, tethered, and freely moving animals (Haspel et al. 2010, Faumont et al. 2011, Kawano et al. 2011, Wen et al. 2012), but recording of GABAergic motoneuron classes during locomotion has not yet been reported. The physiological imaging of motoneurons resulted in apparent discrepancies: One study showed signals that correlated with the direction of locomotion (B motoneurons during forward and A motoneurons during backward locomotion) but not with local bending (Haspel et al. 2010), a second showed a correlation with local bending but not with the direction of locomotion (Faumont et al. 2011), and a third showed a correlation with both (Kawano et al. 2011). Notably, these studies differed in the amount of restraint of the animals and in the indicator used. Plausibly, all excitatory motoneurons exhibit activity that correlates with dorsoventral bending at the side they innervate; A and B motoneurons also exhibit slower baseline activity that correlates with the direction of locomotion.
The unique characteristics of each class of motoneurons may assist in understanding their respective roles in locomotion. The B motoneurons (11 ventral B [VB] and 7 dorsal B [DB]) have a short neurite in the ventral nerve cord that receives most of the synaptic inputs. The neurite leads to a cell body; in the case of the DB motoneurons, a commissure from the cell body crosses to the dorsal nerve cord. Both dorsal and ventral B motoneurons proceed with an axon that provides neuromuscular junctions posterior to the cell body and a long, posterior neurite devoid of synaptic specializations. It was suggested (Lou Byerly and Richard L. Russell, personal communications, cited by White et al. 1986) that the long neurite serves as a stretch sensor for the locomotion motoneurons. Although the long neurites point in the intuitively wrong direction to propagate a body bend (the bends propagate from head to tail for forward locomotion, and the B motoneurons point toward the tail), it was demonstrated that such propagation is feasible computationally and that its direction can be dictated by the time lag of distal sensory feedback (Bryden and Cohen 2008). It was also demonstrated that VB or DB motoneurons depolarize in correlation with a respective ventral or dorsal bend about 200 microns anterior to their soma (Wen et al. 2012). This observation suggested that B motoneurons receive proprioceptive feedback and that the stretch receptors may be located in the DB commissure rather than in the long neurites. Additional cells or structures may also contribute to proprioceptive feedback; it was not demonstrated that this process is cell autonomous.
Ventral and dorsal B motoneurons make cholinergic excitatory neuromuscular junctions with four or six ventral or dorsal, respectively, body wall muscle cells, posterior to their cell body. Their main synaptic outputs (cholinergic and probably excitatory) to other motoneurons are to inhibitory D motoneurons that innervate the opposing side: VB innervates a DD, whereas DB innervates two VDs. Fewer synapses are made from DB to AS and DD and from VB to VA and VD. B motoneurons form gap junctions with neighboring motoneurons of their class and with the interneuron pair AVB. Another interneuron pair, PVC forms cholinergic (and possibly other) synapses with the B motoneurons (Duerr et al. 2008). Ablation of DB motoneurons in the larva impaired forward locomotion (Chalfie et al. 1985). Consistently, B motoneurons exhibit higher levels of calcium during forward than during backward locomotion (Haspel et al. 2010, Kawano et al. 2011), which is strong evidence that they are involved in forward locomotion.
The A motoneurons (12 ventral A [VA] and 9 dorsal A [DA]) are morphological mirror images of the B motoneurons. Accordingly, their neuromuscular junctions and long neurites point anteriorly. However, unlike B motoneurons, their response to being stretched has not been demonstrated (Wen et al. 2012). Similar to B motoneurons, VA and DA classes are cholinergic and form excitatory neuromuscular junctions with four ventral or six dorsal body wall muscle cells, respectively, anterior to their cell body; VA innervates an inhibitory dorsal DD, whereas DA innervates two inhibitory ventral VDs. Weaker synapses are formed by DA to DB and DD and by VA to VD. DA motoneurons form gap junctions with the VA neuron anterior to them and with the AS motoneurons posterior to them. The VA motoneurons form gap junctions with the AS motoneurons anterior to them and half of them form a gap junction with the DA neuron posterior to them. Both ventral and dorsal A motoneurons receive chemical synapse from the interneuron pairs AVD and AVE and form gap junctions with the interneuron pair AVA and SABD (only anterior portion for AVE and SABD). The elimination of backward locomotion following DA motoneuron ablation in the larva (Chalfie et al. 1985) and higher observed levels of activity during backward locomotion (Haspel et al. 2010, Kawano et al. 2011) suggest that A motoneurons are involved in backward locomotion.
The GABAergic D motoneurons (13 ventral D [VD] and 6 dorsal D [DD]) are shaped like a capital letter H, with a commissure that connects a dorsal and a ventral branches. Both classes have a cell body in the ventral nerve cord; but whereas VD motoneurons have a dorsal dendrite and ventral neuromuscular junctions, DD motoneurons have a ventral dendrite and dorsal neuromuscular junctions. Each D motoneuron is connected with gap junctions to the adjacent neurons of its class. The D motoneurons are not innervated by interneurons; instead, they receive cholinergic inputs from other motoneurons (White et al. 1976, Petrash et al. 2013). Each VD motoneuron receives input from the AS, DA, and DB motoneurons that innervate the opposing muscle cells. Similarly, DD motoneurons receive input from the VA and VB motoneurons that innervate the opposing muscle cells but also from RID, a single dorsal motoneuron with sparse neuromuscular junction. Finally, both VD and DD motoneurons receive cholinergic synapses from VC motoneurons. In addition to their synaptic output at neuromuscular junctions, DD motoneurons also innervate the opposing VD motoneurons, whereas VD motoneurons also innervate the local VA and VB motoneurons.
Therefore, D motoneurons are situated to provide dorsoventral cross-inhibition: DD motoneurons could coactivate with contracting ventral muscle cells and inhibit the opposing dorsal muscles. Almost symmetrically, VD motoneurons could coactivate with contracting dorsal muscle cells and inhibit the opposing ventral muscle cells, as well as the VA and VB motoneurons that innervate these muscles. However, it seems that forward locomotion does not strictly require this cross-inhibition. Only backward locomotion is compromised in nematode strains that do not synthesize GABA or when D motoneurons are ablated (Mclntire et al. 1993). These so-called shrinker animals produce a bilateral instead of alternating dorsoventral contraction when they are stimulated to move backward.
The 11 dorsal AS motoneurons are similar in shape to DA motoneurons, except that they lack the long anterior–dorsal neurite. They form cholinergic (probably excitatory) neuromuscular junctions with two dorsal muscle cells and induce inhibition of the opposing ventral muscle (through numerous synapses to VD). In addition, they form gap junctions with VA and DA and the locomotory interneurons AVA. They also receive cholinergic input from all classes of locomotory interneurons: AVA, AVB, AVD, AVE, and PVC (White et al. 1976, 1986). The only published recordings of AS activity show a correlation with dorsal bending but not with the direction of locomotion (Faumont et al. 2011). Therefore, the role of AS motoneurons in locomotion is unclear, and they are commonly not incorporated in models and studies of the locomotion network, although they may support the generation or maintenance of motor patterns and body undulations.
The role of the six ventral VC motoneurons in locomotion is also unclear. Two VCs (4 and 5) innervate the vulva muscles and are involved in egg laying. The other VCs (1–3 in the anterior part and 6 in the posterior) innervate ventral muscle cells very sparsely and may have a small direct contribution to the excitation of muscles. The VC motoneurons are interconnected with gap junctions and are likely coactive. The anterior VC1, -2, and -3 innervate the inhibitory motoneurons VD and DD throughout the anterior portion (White et al. 1986, Haspel and O'Donovan 2011), whereas the connectivity of the posterior VC6 is unknown. If its connectivity is similar to that of the anterior VCs, it may innervate the posterior VDs and DDs. Therefore, the VC motoneurons might be coactive, exciting each other and the inhibitory D motoneurons.
Input from sensory and interneurons
There are five main lateral pairs of premotor interneurons that innervate the A, B, and AS motoneurons (supplemental table S1). These are AVA, AVB, AVD, AVE, and PVC—commonly referred to as command (Chalfie et al. 1985) or locomotion interneurons. Of these, AVB and PVC, which connect to B and AS motoneurons, were demonstrated to be active and nonoscillating during forward locomotion (Faumont et al. 2011, Kawano et al. 2011) and were shown to affect but not abolish forward locomotion when ablated (Chalfie et al. 1985). Similar activity and ablation results were demonstrated for AVA, AVD, and AVE, which connect to A and AS motoneurons during backward locomotion (Chalfie et al. 1985, Faumont et al. 2011, Kawano et al. 2011). Even when all the locomotion interneurons are ablated, animals still produce forward and backward waves of muscle contraction, although they are slow; uncoordinated; and, at times, simultaneously in both directions (Zheng et al. 1999, Kawano et al. 2011). A few other neurons have sparse input to the motoneurons. Other sensory and interneurons affect locomotion (Tsalik and Hobert 2003, Piggott et al. 2011) through the locomotion interneurons but do not make direct synaptic contact with the locomotion motoneurons. A single proprioceptive neuron, DVA (Li et al. 2006), which is sensitive to overall curvature of the animal's body, affects the cholinergic synapses of A and B motoneuron through neuropeptidergic modulation (Hu et al. 2011). Finally, neuromodulation through monoamines and neuropeptides affects the dynamics of locomotion (Bargmann 2012, Donnelly et al. 2013, Flavell et al. 2013).
Anatomical connectivity
The only existing organismwide connectivity data set (a connectome) is that of C. elegans. The relatively small nervous system, detailed knowledge of anatomy, and timely development in electron microscopy techniques allowed the reconstruction of most neurons, chemical synapses, and gap junctions (White et al. 1986). This 15-years-long feat has attracted wide attention from both computational and experimental neuroscientists. The original connectivity data set resulted in the identification of 5958 chemical synapses (1207 of which are neuromuscular junctions) and 1106 gap junctions in the adult nematode (White et al. 1986, Hall and Altun 2008, Varshney et al. 2011; Steven Cook and David Hall, Albert Einstein College of Medicine, personal communication, 18 September 2013). However, the connectivity data set was focused on the head and tail ganglia, and a portion of the ventral and dorsal nerve cords that contain the locomotor motoneurons was reconstructed in only a single hermaphrodite animal (White et al. 1986, Varshney et al. 2011). Moreover, the nerve cords were reconstructed only halfway along the body, and the data for the region posterior to the vulva are still incomplete. The sparse data for the posterior parts of the nerve cords are from a male nematode, and the degree to which neuroanatomy varies between the sexes remains somewhat unclear. Currently, connectivity data is partial or missing for 39 of the 302 hermaphrodite neurons, including 28 of the 75 locomotor motoneurons. In two studies (Chen BL et al. 2006, Varshney et al. 2011), consistency was improved and the data set was annotated. Recently, the original micrographs were digitized and reconstructed and many connections were identified that were originally missed (Cook et al. 2013). However, Cook and colleagues (2013) did not resolve the posterior gap in the data. The connectivity data for the anterior section exhibit repeating patterns when they are mapped according to muscle innervations (Haspel and O'Donovan 2011). This feature has been used to extrapolate and predict the missing connections (Haspel and O'Donovan 2011, 2012).
Finally, the muscle arms were reconstructed only within the ventral nerve cord and not all the way to the muscle (White et al. 1986). B. L. Chen and colleagues (2006) assumed that the arms are perpendicular to the ventral nerve cord and occur in the middle third of the muscle cell. This assumption allowed a hypothetical assignment of muscle to neuromuscular junction. Although it is plausible, the assumption excludes the possibility of any lateral asymmetry. Several initiatives are under way to gather new connectivity data from multiple animals. To make such data valuable to the study of locomotion, neuromuscular junctions must be assigned to specific muscles by reconstructing the muscle arms. A detailed structure could provide a framework in which hypotheses would be formulated and tested (Bargmann 2012, Bargmann and Marder 2013). Still, regardless of how detailed it is, the structure alone does not provide the functional context, which would have to be independently measured.
Hypotheses and published models of locomotion
The algorithmic process implemented by the C. elegans neuromuscular system to produce locomotion behavior remains unclear. The undulatory motion of the nematode is driven by a dorsoventral difference in muscle contraction that produces a propagating dorsoventral body bend. This motor pattern can be divided into two elements that need to be understood: the generation of rhythmic alternating bends and the propagation of these bends along the body. Either or both of these elements could be produced by sensory feedback or central pattern generation, the two prominent circuit components in the generation of locomotor patterns. A central pattern generator (CPG; box 1) is a feed-forward component in the generation of locomotion in all vertebrates and invertebrates studied to date, which suggests that it might also be relevant for C. elegans locomotion. In that case, pattern generation may arise through interactions among currents in individual neurons (pacemaker oscillator neurons), or through interactions among neurons (network-based rhythmicity). Existing models can be divided into three competing hypotheses by the role and location of CPGs and sensory feedback in generating and propagating rhythmic alternating bends along the body (figure 3):
Box 1. Central pattern generator.
Central pattern generators (CPGs) are autonomous groups of neurons or neural networks that produce patterned, rhythmic neural output in the absence of sensory or descending inputs that carry specific timing information (Marder and Bucher 2001). This concept has proven to be useful in the study of many motor systems, because CPGs were found to underlie the production of most rhythmic motor patterns, such as breathing, walking, flying, and swimming (Marder and Calabrese 1996, Grillner 2006). It was even suggested that CPGs are required to generate the ongoing activity of the mammalian cortex (Yuste et al. 2005). A CPG circuit can produce a rhythmic pattern on the basis of a variety of mechanisms and designs. It can rely on cellular properties (i.e., pacemaker cells) or connectivity (e.g., reciprocal inhibition) or a combination of the two. It can range in size from a single pacemaker cell (Chen CF et al. 1971) to a complex interconnected network.
The tumultuous history of the CPG concept from the early 1900s (Brown TG 1912) to its acceptance in the 1960s (Wilson 1961) and the success of in vitro preparations seemingly suggested a dichotomy between an autonomous, free-running, central clock and distributed circuits driven by sensory input. However, in order to be adaptive in its natural setting, it is advantageous for a CPG to integrate sensory input. Indeed, CPGs incorporate receptive and proprioceptive inputs from the central nervous system and the sensory periphery. Such sensory feedback can modulate the overall activity of the CPG or tweak the output cycle by cycle (Cang and Friesen 2002, Grillner 2006, Gjorgjieva et al. 2013).
It is beneficial to regard the CPG as a prediction generator—an internal model that feeds forward an estimation made by the nervous system of the timing and coordination required during the current cycle of behavior (Kuo 2002). A CPG tuned to a baseline frequency by natural selection provides an advantageous starting point for incorporating sensory information into the process of determining behavioral output. In noisy, unexpected environments, the baseline frequency would require more adjustment based on sensory feedback. Conversely, in a more predictable environment or in the case of a fast behavior (where there is no time within a cycle for feedback), the CPG would be more prominent.
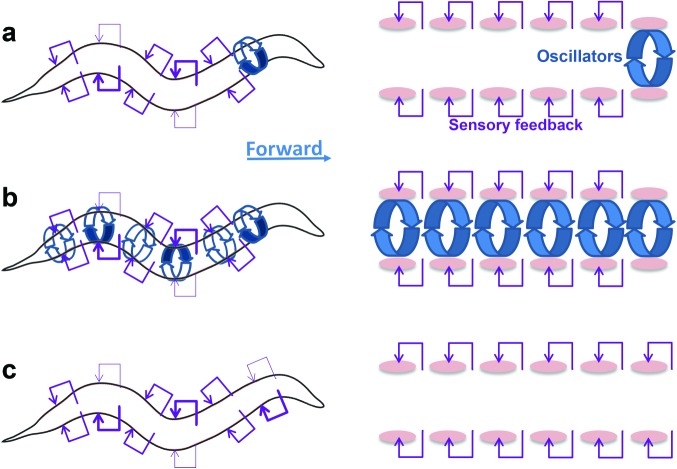
Figure 3.
Three competing hypotheses for the production of locomotor patterns are distinguished by the presence and location of oscillators and sensory feedback: (a) During forward locomotion, the neck generates rhythmic dorsoventral bends, and physical, neuronal, or sensory feedback mechanisms propagate them along the body. (b) Coupled oscillators along the body generate and propagate rhythmic dorsoventral bends, and sensory feedback modulates the resulting motor patterns. (c) Sensory feedback extending along the body generates and propagates rhythmic dorsoventral bends. The diagrams on the right show the main differences among the hypotheses in the location of muscles (the pink ovals), oscillators (the round blue arrows), and sensory feedback (the purple arrows) along the anterior–posterior axis (the head is to the right). The diagrams on the left demonstrate the approximate location of increased neuromuscular activity (thick arrows for sensory feedback, round arrows for oscillators) along the body of the nematode (the head is to the right; the blue arrow designates the forward direction).
The first hypothesis is that oscillators in the neck generate rhythmic dorsoventral bends, and physical, neuronal, or sensory feedback mechanisms propagate them along the body (figure 3a). The generation of forward locomotion in these models relies on the existence of a CPG in the head. In the first computational model developed for locomotion of C. elegans (Niebur and Erdös 1991), periodic dorsoventral motion of the head initiates movement and sets the frequency of undulation. The environment must be sufficiently stiff to support the sinusoidal shape of the nematode and generate forward thrust, setting propagation speed by strong lateral forces. Although the model does not require sensory input for locomotion, introducing stretch receptor input from body curvature produces smoother output. More recently, Wen and colleagues (2012) similarly showed that a purely mechanical model, based on a harmonic oscillator in the neck and anterior proprioception along the body, can account for the two modes of forward locomotion—crawling and swimming, depending on the viscosity of the environment. These models were successful in capturing some behaviors but were not intended to resolve a detailed neural implementation.
A more detailed neural model for forward crawling was proposed by Karbowski and colleagues (2008), with two neural networks corresponding to the head and body and components that were based on some neuroanatomical data. A CPG in the head also generates the main oscillatory rhythm in this model; the rhythm is transmitted to the body through two interneuron pairs (AVB and PVC). However, the activity of these neurons has since been demonstrated not to correlate with locomotion undulations (Faumont et al. 2011, Kawano et al. 2011). Moreover, anterior-to-posterior wave propagation was implemented by proprioceptive processes extending posteriorly, in contrast to recent findings (Wen et al. 2012). Although the Karbowski and colleagues (2008) model proposes a network implementation of the head CPG, a specific network motif is not strictly required for generating oscillations; a CPG can be driven even by a single pacemaking cell. Cell autonomous oscillations of membrane potential require at least two competing currents with specific temporal dynamics: a depolarizing current, such as a sodium leak (Pierce-Shimomura et al. 2008), or a hyperpolarization activated cation current counteracted by a depolarization-activated hyperpolarizing current, such as a voltage-activated potassium current. However, rhythmic patterns that arise from cellular properties remain unexplored to date.
The models included in this hypothesis were not implemented for backward locomotion. Rather, it is implicit that a similar circuit composed of a CPG in the tail, A motoneurons, and proprioceptive feedback may produce it. To establish the validity of the key hypothesis of these models—namely, the existence of a CPG in the head or tail—would require experiments that target neural circuitry in the these areas. Inhibiting head neurons by optogenetics or similar methods or selectively inhibiting subsets of head neurons could provide insight into their involvement in locomotion.
The second hypothesis is that coupled oscillators along the body generate and propagate rhythmic dorsoventral bends, and sensory feedback modulates the resulting motor patterns (figure 3b). Models in animals other than C. elegans rely on a rhythm being produced by a set of coupled CPGs (Marder and Bucher 2001, Grillner and Jessell 2009) and modulated by sensory feedback from stretch receptors (Grillner et al. 1995, Cang and Friesen 2002, Gjorgjieva et al. 2013). Models of this kind produce a robust but flexible output. The direction of propagation of the activity can be controlled by increasing the endogenous frequency of the most anterior or the most posterior oscillator to entrain the chain. If a chain of coupled oscillators underlies C. elegans locomotion, each oscillator could be made of pacemaker neurons, network motifs, or muscle cells. One possibility is that some classes of motoneurons have pacemaking properties (Angstadt and Stretton 1989). The arrangement of each motoneuron class into an array of at least 6 (DD) and as many as 13 (VD) roughly uniformly distributed cell bodies along the ventral nerve cord could provide an anatomical substrate for such a chain. The coupling could be implemented through gap junctions among members of some motoneuron classes (e.g., D and B classes). Other motoneurons could be indirectly connected to cells of their own class through interneurons or through motoneurons from a different class. Each oscillator along the body could also be implemented by a small local network of motoneurons, because the ventral nerve cord is organized into repeated units that control specific muscles (Haspel and O'Donovan 2011). Alternately, generation of oscillations could arise from the muscle cells themselves (as proposed for Ascaris suum by Stretton et al. 1985). Gap junctions among muscle cells (Liu P et al. 2011) could provide the coupling between oscillators and dictate the phase relationship. In this scenario, motoneurons would modulate the motor pattern and control speed, amplitude, and direction of bend propagation or would provide sensory feedback. Similar roles for muscle and motoneurons were described for the asynchronous flight muscle in flies (Dickinson and Tu 1997).
In order for these models to account for the different body wavelengths observed in environments of different mechanical loads; additional layers of regulation, such as sensory feedback and neuromodulation would have to be considered (Vidal-Gadea et al. 2011). However, even in the absence of sensory feedback, mechanical forces from the environment can contribute directly to the output of these models (Majmudar et al. 2012). However, the existence of a set of CPGs along the nematode body has not been supported. Barring experiments that use genetic, pharmacological, or optogenetic methods to isolate, inhibit, or reset putative oscillators while their effects on the motor output are recorded, the coupled oscillators hypothesis is weakly supported.
The third hypothesis is that sensory feedback extending along the body generates and propagates rhythmic dorsoventral bends (figure 3c). This hypothesis assumes that rhythm generation and propagation is completely independent of a CPG, unlike other animal models such as lamprey, leech, and Drosophila larva. The generation and propagation of oscillatory bending through proprioceptive feedback was first suggested (but not implemented) in a model in which the control of motoneurons by the locomotion interneurons was investigated (Niebur and Erdös 1993). This work relied on electrophysiological properties of neurons that were taken from A. suum, a larger nematode with a similar nervous system (Niebur and Erdös 1993). Bryden and Cohen (2008) developed a neurally controlled model in which different modules along the body could independently generate oscillations, as opposed to initiating them in the head. Each module generated oscillations through the interaction of neural components and sensory feedback, assuming a constant input current from an interneuron. Sensory input from local and proximal modules mediated the propagation of bends with a phase relationship that could be adjusted through the time lag of the feedback. Proprioceptive feedback was crucially required in this model for rhythm generation and was shown to enable appropriate phase lags for crawling, even in a minimal circuit of B motoneurons without cross-inhibition (Bryden and Cohen 2008).
To capture the continuum between forward crawling and forward swimming, Boyle and colleagues (2012) incorporated neuromuscular control by a sensory feedback mechanism (necessary for the production of undulations) with a physical model of the body and the environment. Despite including only B and D motoneurons and an AVB interneuron, the model captured forward swimming and crawling behaviors, as well as intermediate patterns of locomotion, as a function of the viscosity of the environment. This model produced robust oscillations across a wide range of frequencies. In its current form, the model assumes long-range posterior proprioceptive input to B motoneurons, but this framework could be modified to accommodate the subsequently reported anterior and shorter-range proprioception (Wen et al. 2012). The key novel assumptions of the Boyle and colleagues (2012) model are that the muscles are tonically active and that the B motoneurons (or other components of the pathway) act as bistable neurons exhibiting hysteresis rather than graded responses. Evidence for the bistability has been experimentally reported in C. elegans interneurons and muscle cells, but not yet in ventral cord motoneurons (Mellem et al. 2008, Wen et al. 2012). Importantly, a dorsoventral asymmetry was strictly required in this model. It was implemented through a VD–VB connection, necessary for resetting the activation of the ventral side (which had a lower activation threshold). The models included in this hypothesis were not implemented for backward locomotion.
What do we learn from the models?
Computational models demonstrate what is possible given a set of assumptions. Therefore, it is not surprising that all published models for C. elegans locomotion produce an approximation of the undulatory motor output, because this is a preliminary requirement. Useful models also generate predictions that can be tested with experiments, such as those we suggest above. The models span a range from purely neural (Niebur and Erdös 1993, Bryden and Cohen 2008, Karbowski et al. 2008) to purely mechanical (Niebur and Erdös 1991, Majmudar et al. 2012) and from minimal circuits incorporating only forward motoneurons (Boyle et al. 2012) to detailed ones incorporating most known circuit elements (Karbowski et al. 2008, Haspel and O'Donovan 2011). We argue that each level of description is useful, depending on the questions at hand. At this stage, a key goal for the field is to identify the biologically relevant framework for rhythm generation and propagation, perhaps prior to elucidating the details of its implementation. Answering these questions will present new and exciting puzzles: How does endocrine or paracrine signaling modulate the neuromuscular system? What are the long-term dynamics of locomotion during development, the reproductive period, and aging? How is sensory information integrated into the locomotion circuits to achieve navigation? How is locomotion coupled with additional behaviors such as egg laying, defecation, or nictation?
The L1 larva
Only three (of eight, figure 2a) motoneuron classes are present in the first larval stage (L1): two cholinergic classes that innervate dorsal muscle (DA and DB) and one GABAergic class, DD, that at this stage innervates ventral muscles. The DD motoneurons rearrange their neuromuscular junctions to innervate dorsal muscle at the L1 to L2 molt (White et al. 1978). Although the hypotheses for generation and propagation of body bends are applicable in this smaller nervous system, this presents a conundrum: How can cholinergic (presumably excitatory) dorsal and GABAergic (presumably inhibitory) ventral neuromuscular junctions produce a ventral bend? We propose four possible answers: (1) Structural ventral spring: If all three motoneuron classes contribute to a dorsal bend (by exciting dorsal muscles or inhibiting ventral ones) against a passive ventral bend, the animal can assume all bending angles between those bends (Chen SP et al. 2011). (2) Hypertonic ventral muscle: An imbalance in baseline activity causes the dorsal muscle to be relaxed until activated by DA and DB and the ventral muscle to be contracted until it is inhibited by DD. (3) Excitatory GABA: As was demonstrated for other developing nervous systems (Ben-Ari et al. 2007), GABAergic synapses can deliver excitatory signals when an elevated level of intracellular chloride ions creates an efflux through the chloride-permeable receptors. In this case, DD motoneurons may contribute to a ventral bend, whereas DA or DB motoneurons contribute to a dorsal one (Han et al. 2011). (4) SAB motoneurons: The three SAB motoneurons innervate anterior ventral body wall muscle in the L1 larva (White et al. 1986) and could potentially account for the ventral activation needed for locomotion in the L1 larvae.
In summary, much progress has been made in understanding the computational (behavioral output), hardware (components), and some algorithmic aspects of the locomotion of C. elegans. Of these, the algorithmic aspects of locomotion remain the least well understood. The relative simplicity of the dynamics of forward and backward locomotion and of the anatomy of the nematode are amenable to detailed measurements of the behavior and properties of neurons and muscles and to various levels of modeling. However, many cellular and synaptic properties of the motoneurons have not been experimentally measured to date, and the degree of variability between animals (or over time in a single animal) is largely unknown. Models incorporating novel experimental findings while avoiding superfluous computational complexity will be key to an algorithmic understanding of locomotion.
Supplementary Material
Acknowledgments
We thank Netta Cohen, Jorge Golowasch, Farzan Nadim, Dirk Bucher, and Eric Fortune for reading and discussing the manuscript and Daphne Soares for her help with figures and useful comments. We also thank David Hall and Steven Cook for personal communications.
Supplemental material
The supplemental material is available online at http://bioscience.oxfordjournals.org/lookup/suppl/doi:10.1093/biosci/biu058/-/DC1.
References cited
- Angstadt JD, Stretton AOW. Slow active potentials in ventral inhibitory motor neurons of the nematode Ascaris. Journal of Comparative Physiology A. 1989;166:165–177. doi: 10.1007/BF00193461. [DOI] [PubMed] [Google Scholar]
- Backholm M, Ryu WS, Dalnoki-Veress K. Viscoelastic properties of the nematode Caenorhabditis elegans, a self-similar, shear-thinning worm. Proceedings of the National Academy of Sciences. 2013;110:4528–4533. doi: 10.1073/pnas.1219965110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI. Beyond the connectome: How neuromodulators shape neural circuits. BioEssays. 2012;34:458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Marder E. From the connectome to brain function. Nature Methods. 2013;10:483–490. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa J-L, Tyzio R, Khazipov R. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiological Reviews. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Berri S, Boyle JH, Tassieri M, Hope IA, Cohen N. Forward locomotion of the nematode C. elegans is achieved through modulation of a single gait. HFSP Journal. 2009;3:186–193. doi: 10.2976/1.3082260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JH, Berri S, Cohen N. Gait Modulation in C. elegans: An integrated neuromechanical model. Frontiers in Computational Neuroscience. 2012;6:10. doi: 10.3389/fncom.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AEX, Yemini EI, Grundy LJ, Jucikas T, Schafer WR. A dictionary of behavioral motifs reveals clusters of genes affecting Caenorhabditis elegans locomotion. Proceedings of the National Academy of Sciences. 2012;110:791–796. doi: 10.1073/pnas.1211447110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TG. The factors in rhythmic activity of the nervous system. Proceedings of the Royal Society B. 1912;85:278–289. [Google Scholar]
- Bryden J, Cohen N. Neural control of Caenorhabditis elegans forward locomotion: The role of sensory feedback. Biological Cybernetics. 2008;98:339–351. doi: 10.1007/s00422-008-0212-6. [DOI] [PubMed] [Google Scholar]
- Burr AHJ, Robinson AF. Locomotion behavior. In: Gaugler R, Bilgrami AL, editors. Nematode Behavior. CABI; 2004. pp. 25–62. [Google Scholar]
- Cang J, Friesen WO. Model for intersegmental coordination of leech swimming: Central and sensory mechanisms. Journal of Neurophysiology. 2002;87:2760–2769. doi: 10.1152/jn.2002.87.6.2760. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. Journal of Neuroscience. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BL, Hall DH, Chklovskii DB. Wiring optimization can relate neuronal structure and function. Proceedings of the National Academy of Sciences. 2006;103:4723–4728. doi: 10.1073/pnas.0506806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CF, Von Baumgarten R, Takeda R. Pacemaker properties of completely isolated neurones in Aplysia californica. Nature New Biology. 1971;233:27–29. doi: 10.1038/newbio233027a0. [DOI] [PubMed] [Google Scholar]
- Chen SP, Tang A, Wen Q, Samuel ADT . Poster presented at the 18th International C. elegans Meeting. Los Angeles: University of California; 2011. How larvae wiggle: Functional analysis of the L1 larva locomotor circuit. 22–26 June 2011. [Google Scholar]
- Cook S, Brittin C, Jarrell T, Hall D, Emmons S. Abstract presented at the 19th International C. elegans Meeting. Los Angeles: University of California; 2013. Two minds of a worm: Comparison of the L4 and adult hermaphrodite connectomes. 26–30 June 2013. [Google Scholar]
- Dickinson MH, Tu MS. The function of dipteran flight muscle. Comparative Biochemistry and Physiology A. 1997;116:223–238. [Google Scholar]
- Doll J, Harjee N, Klejwa N, Kwon R, Coulthard SM, Petzold B, Goodman MB, Pruitt BL. SU-8 force sensing pillar arrays for biological measurements. Lab on a Chip. 2009;9:1449–1454. doi: 10.1039/b818622g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly JL, Clark CM, Leifer AM, Pirri JK, Haburcak M, Francis MM, Samuel ADT, Alkema MJ. Monoaminergic orchestration of motor programs in a complex C. elegans behavior. PLOS Biology. 2013;11 doi: 10.1371/journal.pbio.1001529. (art. e1001529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr JS, Han H-P, Fields SD, Rand JB. Identification of major classes of cholinergic neurons in the nematode Caenorhabditis elegans. Journal of Comparative Neurology. 2008;506:398–408. doi: 10.1002/cne.21551. [DOI] [PubMed] [Google Scholar]
- Fang-Yen C, Wyart M, Xie J, Kawai R, Kodger T, Chen S[P], Wen Q, Samuel ADT. Biomechanical analysis of gait adaptation in the nematode Caenorhabditis elegans. Proceedings of the National Academy of Sciences. 2010;107:20323–20328. doi: 10.1073/pnas.1003016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faumont S, et al. An image-free opto-mechanical system for creating virtual environments and imaging neuronal activity in freely moving Caenorhabditis elegans. PLOS ONE. 2011;6 doi: 10.1371/journal.pone.0024666. (art. e24666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeny BF, Sternberg PW, Cronin CJ, Coppola CA. Complex orthogonal decomposition applied to nematode posturing. Journal of Computational and Nonlinear Dynamics. 2013;8 (art. 041010) [Google Scholar]
- Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, Bargmann CI. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell. 2013;154:1023–1035. doi: 10.1016/j.cell.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R, Waterston RH. Muscle cell attachment in Caenorhabditis elegans. Journal of Cell Biology. 1991;114:465–479. doi: 10.1083/jcb.114.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M, Sengupta P, McIntire SL. Regulation of body size and behavioral state of C. elegans by sensory perception and the EGL-4 cGMP-dependent protein kinase. Neuron. 2002;36:1091–1102. doi: 10.1016/s0896-6273(02)01093-0. [DOI] [PubMed] [Google Scholar]
- Gallagher T, Bjorness T, Greene R, You Y-J, Avery L. The geometry of locomotive behavioral states in C. elegans. PLOS ONE. 2013;8 doi: 10.1371/journal.pone.0059865. (art. e59865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Zhen M. Action potentials drive body wall muscle contractions in Caenorhabditis elegans. Proceedings of the National Academy of Sciences. 2011;108:2557–2562. doi: 10.1073/pnas.1012346108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorgjieva J, Berni J, Evers JF, Eglen SJ. Neural circuits for peristaltic wave propagation in crawling Drosophila larvae: Analysis and modeling. Frontiers in Computational Neuroscience. 2013;7 doi: 10.3389/fncom.2013.00024. (art. 24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Lissmann HW. The locomotion of nematodes. Journal of Experimental Biology. 1964;41:135–154. doi: 10.1242/jeb.41.1.135. [DOI] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. Proceedings of the National Academy of Sciences. 2005;102:3184–3191. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: The cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: Searching for simplicity in spinal locomotor networks. Current Opinion in Neurobiology. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Deliagina T, Ekeberg O, el Manira A, Hill RH, Lansner A, Orlovsky GN, Wallén P. Neural networks that co-ordinate locomotion and body orientation in lamprey. Trends in Neurosciences. 1995;18:270–279. [PubMed] [Google Scholar]
- Hall DH, Altun ZF. C. Elegans Atlas. Cold Spring Harbor Laboratory Press; 2008. [Google Scholar]
- Han B, Bellemer A, Koelle M. Poster presented at the 18th International C. elegans Meeting. Los Angeles: University of California; 2011. Identification of chloride transporters that regulate GABA signaling. 22–26 June 2011. [Google Scholar]
- Haspel G, O'Donovan MJ. A perimotor framework reveals functional segmentation in the motoneuronal network controlling locomotion in Caenorhabditis elegans. Journal of Neuroscience. 2011;31:14611–14623. doi: 10.1523/JNEUROSCI.2186-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel G, O'Donovan MJ. A connectivity model for the locomotor network of Caenorhabditis elegans. Worm. 2012;1:125–128. doi: 10.4161/worm.19392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel G, O'Donovan MJ, Hart AC. Motoneurons dedicated to either forward or backward locomotion in the nematode Caenorhabditis elegans. Journal of Neuroscience. 2010;30:11151–11156. doi: 10.1523/JNEUROSCI.2244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Pym ECG, Babu K, Vashlishan Murray AB, Kaplan JM. A neuropeptide-mediated stretch response links muscle contraction to changes in neurotransmitter release. Neuron. 2011;71:92–102. doi: 10.1016/j.neuron.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanir S, Tramm N, Nagy S, Wright C, Ish D, Biron D. The microarchitecture of C. elegans behavior during lethargus: Homeostatic bout dynamics, a typical body posture, and regulation by a central neuron. Sleep. 2013;36:385–395. doi: 10.5665/sleep.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johari S, Nock V, Alkaisi MM, Wang W. On-chip analysis of C. elegans muscular forces and locomotion patterns in microstructured environments. Lab on a Chip. 2013;13:1699–1707. doi: 10.1039/c3lc41403e. [DOI] [PubMed] [Google Scholar]
- Karbowski J, Schindelman G, Cronin CJ, Seah A, Sternberg PW. Systems level circuit model of C. elegans undulatory locomotion: Mathematical modeling and molecular genetics. Journal of Computational Neuroscience. 2008;24:253–276. doi: 10.1007/s10827-007-0054-6. [DOI] [PubMed] [Google Scholar]
- Kawano T, Po MD, Gao S, Leung G, Ryu WS, Zhen M. An imbalancing act: Gap junctions reduce the backward motor circuit activity to bias C. elegans for forward locomotion. Neuron. 2011;72:572–586. doi: 10.1016/j.neuron.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Kuo AD. The relative roles of feedforward and feedback in the control of rhythmic movements. Motor Control. 2002;6:129–145. doi: 10.1123/mcj.6.2.129. [DOI] [PubMed] [Google Scholar]
- Lebois F, Sauvage P, Py C, Cardoso O, Ladoux B, Hersen P, Di Meglio J-M. Locomotion control of Caenorhabditis elegans through confinement. Biophysical Journal. 2012;102:2791–2798. doi: 10.1016/j.bpj.2012.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecroisey C, Ségalat L, Gieseler K. The C. elegans dense body: Anchoring and signaling structure of the muscle. Journal of Muscle Research and Cell Motility. 2007;28:79–87. doi: 10.1007/s10974-007-9104-y. [DOI] [PubMed] [Google Scholar]
- Li W, Feng Z, Sternberg PW, Xu XZS. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liewald JF, Brauner M, Stephens GJ, Bouhours M, Schultheis C, Zhen M, Gottschalk A. Optogenetic analysis of synaptic function. Nature Methods. 2008;5:895–902. doi: 10.1038/nmeth.1252. [DOI] [PubMed] [Google Scholar]
- Liu P, Chen B, Wang Z-W. Gap junctions synchronize action potentials and Ca2+ transients in Caenorhabditis elegans body wall muscle. Journal of Biological Chemistry. 2011;286:44285–44293. doi: 10.1074/jbc.M111.292078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Chen B, Wang Z-W. Postsynaptic current bursts instruct action potential firing at a graded synapse. Nature Communications. 2013;4 doi: 10.1038/ncomms2925. (art. 1911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Chen B, Gaier E, Joshi J, Wang Z-W. Low conductance gap junctions mediate specific electrical coupling in body-wall muscle cells of Caenorhabditis elegans. Journal of Biological Chemistry. 2006;281:7881–7889. doi: 10.1074/jbc.M512382200. [DOI] [PubMed] [Google Scholar]
- Majmudar T, Keaveny EE, Zhang J, Shelley MJ. Experiments and theory of undulatory locomotion in a simple structured medium. Journal of the Royal Society Interface. 2012;9:1809–1823. doi: 10.1098/rsif.2011.0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Current Biology. 2001;11:R986–R996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiological Reviews. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- Marr D. Vision: A Computational Approach. Freeman; 1982. [Google Scholar]
- Mclntire SL, Jorgensen E, Kaplan J, Horvitz HR. The GABAergic nervous system of Caenorhabditis elegans. Nature. 1993;364:337–341. doi: 10.1038/364337a0. [DOI] [PubMed] [Google Scholar]
- Mellem JE, Brockie PJ, Madsen DM, Maricq AV. Action potentials contribute to neuronal signaling in C. elegans. Nature Neuroscience. 2008;11:865–867. doi: 10.1038/nn.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy S, Wright C, Tramm N, Labello N, Burov S, Biron D. A longitudinal study of Caenorhabditis elegans larvae reveals a novel locomotion switch, regulated by Gαs signaling. eLife. 2013;2 doi: 10.7554/eLife.00782. (art. e00782) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebur E, Erdös P. Theory of the locomotion of nematodes: Dynamics of undulatory progression on a surface. Biophysical Journal. 1991;60:1132–1146. doi: 10.1016/S0006-3495(91)82149-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebur E, Erdös P. Theory of the locomotion of nematodes: Control of the somatic motor neurons by interneurons. Mathematical Biosciences. 1993;118:51–82. doi: 10.1016/0025-5564(93)90033-7. [DOI] [PubMed] [Google Scholar]
- Park SJ, Goodman MB, Pruitt BL. Analysis of nematode mechanics by piezoresistive displacement clamp. Proceedings of the National Academy of Sciences. 2007;104:17376–17381. doi: 10.1073/pnas.0702138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrash HA, Philbrook A, Haburcak M, Barbagallo B, Francis MM. ACR-12 ionotropic acetylcholine receptor complexes regulate inhibitory motor neuron activity in Caenorhabditis elegans. Journal of Neuroscience. 2013;33:5524–5532. doi: 10.1523/JNEUROSCI.4384-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold BC, Park S-J, Ponce P, Roozeboom C, Powell C, Goodman MB, Pruitt BL. Caenorhabditis elegans body mechanics are regulated by body wall muscle tone. Biophysical Journal. 2011;100:1977–1985. doi: 10.1016/j.bpj.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce-Shimomura JT, Chen BL, Mun JJ, Ho R, Sarkis R, McIntire SL. Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proceedings of the National Academy of Sciences. 2008;105:20982–20987. doi: 10.1073/pnas.0810359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott BJ, Liu J, Feng Z, Wescott SA, Xu XZS. The neural circuits and synaptic mechanisms underlying motor initiation in C. elegans. Cell. 2011;147:922–933. doi: 10.1016/j.cell.2011.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You Y, Sundaram MV, Pack AI. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451:569–572. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- Richmond JE, Jorgensen EM. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nature Neuroscience. 1999;2:791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RN, Sulston JE. Some observations on molting in Caenorhabditis elegans. Nematologica. 1978;24:63–71. [Google Scholar]
- Stephens GJ, Bueno de Mesquita M, Ryu WS, Bialek W. Emergence of long timescales and stereotyped behaviors in Caenorhabditis elegans. Proceedings of the National Academy of Sciences. 2011;108:7286–7289. doi: 10.1073/pnas.1007868108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stretton AOW, Davis RE, Angstadt JD, Donmoyer JE, Johnson CD. Neural control of behaviour in Ascaris. Trends in Neurosciences. 1985;8:294–300. [Google Scholar]
- Sznitman J, Purohit PK, Krajacic P, Lamitina T, Arratia PE. Material properties of Caenorhabditis elegans swimming at low Reynolds number. Biophysical Journal. 2010;98:617–626. doi: 10.1016/j.bpj.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalik EL, Hobert O. Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. Journal of Neurobiology. 2003;56:178–197. doi: 10.1002/neu.10245. [DOI] [PubMed] [Google Scholar]
- Varshney LR, Chen BL, Paniagua E, Hall DH, Chklovskii DB. Structural properties of the Caenorhabditis elegans neuronal network. PLOS Computational Biology. 2011;7 doi: 10.1371/journal.pcbi.1001066. (art. e1001066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gadea A, et al. Caenorhabditis elegans selects distinct crawling and swimming gaits via dopamine and serotonin. Proceedings of the National Academy of Sciences. 2011;108:17504–17509. doi: 10.1073/pnas.1108673108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stetina SE, Treinin M, Miller DM., III The motor circuit. International Review of Neurobiology. 2005;69:125–167. doi: 10.1016/S0074-7742(05)69005-8. [DOI] [PubMed] [Google Scholar]
- Wen Q, et al. Proprioceptive coupling within motor neurons drives C. elegans forward locomotion. Neuron. 2012;76:750–761. doi: 10.1016/j.neuron.2012.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the ventral nerve cord of Caenorhabditis elegans. Philosophical Transactions of the Royal Society B. 1976;275:327–348. doi: 10.1098/rstb.1976.0086. [DOI] [PubMed] [Google Scholar]
- White JG, Albertson DG, Anness MAR. Connectivity changes in a class of motoneurone during the development of a nematode. Nature. 1978;271:764–766. doi: 10.1038/271764a0. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wilson DM. The central nervous control of flight in a locust. Journal of Experimental Biology. 1961;38:471–490. [Google Scholar]
- Yuste R, MacLean JN, Smith J, Lansner A. Opinion: The cortex as a central pattern generator. Nature Reviews Neuroscience. 2005;6:477–483. doi: 10.1038/nrn1686. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Maricq AV. Neuronal control of locomotion in C. elegans is modified by a dominant mutation in the GLR-1 ionotropic glutamate receptor. Neuron. 1999;24:347–361. doi: 10.1016/s0896-6273(00)80849-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.