Abstract
Purpose: Nefopam is a widely available analgesic for the management of pain. The aim of this study was to reveal the effect of regional hyperthermia of the abdominal area on the pharmacokinetics of nefopam. Materials and methods: A randomised, single-dose, crossover, open-label study was conducted to reveal the effect of hyperthermia using modulated electro-hyperthermia on the pharmacokinetics of nefopam. The pharmacokinetics of orally administered nefopam without hyperthermia was studied in 12 healthy volunteers and then 7 days later they were treated with nefopam plus modulated electro-hyperthermia to the abdominal area for 1 h. Blood samples were collected up to 24 h after the drug administration. From the blood concentration-time curve, the maxinum plasma concentration (Cmax), time to Cmax (Tmax) and the area under the curve (AUC) were obtained. The safety and tolerability of these treatments were also assessed. Results: The geometric mean ratios (GMRs) ((nefopam + modulated electro-hyperthermia)/nefopam) and the associated 90% confidence intervals (CIs) for Cmax, AUClast and AUCinf were 1.2804 (1.1155∼1.4696), 1.0512 (0.9555∼1.1566) and 1.0612 (0.9528∼1.1819), respectively. The increase in Cmax was statistically significant, and Tmax was significantly shortened. Conclusions: The significant increase in Cmax and decrease in Tmax indicated that modulated electro-hyperthermia increased the absorption of the orally administered nefopam, thereby transitionally increasing the blood concentration of the drug. The AUC is an important parameter that contributes to the therapeutic effect of drugs. The lack of significant change in AUC suggests that modulated electro-hyperthermia may increases the absorption of orally administered drugs without increasing the systemic adverse effect of the drugs.
Keywords: Clinical trial modulated electro-hyperthermia, nefopam, pharmacokinetics
Introduction
Nefopam (3,4,5,6-tetrahydro-5-methyl-1-phenyl-1 h-2,5-benzoxazocine hydrochloride) is a widely used analgesic to ease acute and chronic pain since it was discovered more than 40 years ago[1–3]. Nefopam was first used for muscle relaxation to avoid spasms [3] and it has become a popular analgesic prescribed to ease acute and chronic pain. Nefopam is unrelated chemically or pharmacologically to any of other analgesic compounds. Nefopam is a non-opioid that affects the uptake of serotonin, norepinephrine and dopamine [2,3]. However, its analgesic mechanism has not yet been fully revealed although nefopam has been shown to affect the serotonin-type neurons of the opioid system [4]. Nefopam has been shown to be more effective than narcotic analgesics: 30 mg of nefopam produces an analgesic effect equivalent to that of 300 mg of aspirin or approximately 90 mg of morphine [2,4].
It has been well known that mild temperature hyperthermia increases the blood circulation and permeability in the target organs or tissues [5]. Therefore, we hypothesised that hyperthermia of the abdominal region might increase the absorption of orally administered nefopam, and thereby it increases the efficacy of the drug. As the first step of our clinical study, we investigated the effects of regional hyperthermia of the abdominal area on the pharmacokinetics of nefopam in healthy volunteers.
For the heating of the abdominal area we used modulated electro-hyperthermia with EHY-2000 (Oncotherm, Troisdorf, Germany), which has been used to treat a variety of human cancers [6–8]. This treatment device is designed to selectively heat malignant tumours and tumour cells via modularly delivering 13.56 MHz RF [7,9,10]. Impressive clinical response has been reported for a variety of human cancers including brain tumours [6,8].
The present study demonstrated that regional hyperthermia of the abdominal area of healthy volunteers increased the uptake of orally administered nefopam. These results of a randomised, single-dose, crossover, and open-label clinical trial should be useful in determining the optimal drug dose and other factors for the effective use of nefopam in combination with hyperthermia.
This study was registered with the identification number KCT 0001301 at the Clinical Research Information Service in Republic of Korea (CRIS, http://cris.nih.go.kr).
Materials and methods
Study design
We conducted a randomised, single-dose, crossover, open-label study to investigate the effects of electro-hyperthermia on the pharmacokinetics (PK) of nefopam in healthy volunteers of both genders and of various ages. As the characteristics of an exploratory study are different from those of a conventional clinical trial in terms of statistical hypothesis evaluation, a minimum number of subjects, total 12 subjects, were used. Nefopam was administered orally in Acupan capsules (Pharmbio Korea, Seoul, Republic of Korea).
Subjects
At least eight subjects were required to obtain 90 % statistical power. Considering the dropout rate, a total of 12 subjects consisted of for men and eight women were enrolled in this study. The inclusion criteria for this study were chosen to allow the enrolment of a wide range of patients in the study:
Healthy adults aged 19–53 (this is unique because we did not include gender or age restrictions).
Body mass index (BMI) range 18.0–24.8 kg/m2.
No history of a congenital or chronic disease over the prior 3 years, and no findings of pathological symptoms or other disorders at the time of the screening.
Results of the laboratory screening including haematology, blood chemistry, and urine tests were used to determine the eligibility of the subjects for this study.
After being fully briefed on the study objectives and study content the patients voluntarily agreed to participate in the study by signing an informed consent form.
Willing to and capable of following all of the procedures during the study period.
The exclusion criteria were strict, thereby allowing the enrolment of a unified cohort of healthy volunteers.
This study was approved by the Institutional Review Board of Chonbuk National University Hospital (Jeonju, Republic of Korea) and was conducted according to the Declaration of Helsinki for biomedical research involving human subjects and the Guidelines for Good Clinical Practice. A detailed explanation of the study was provided to all subjects, and written informed consent was obtained from all participants prior to screening.
Methods
All subjects were orally administered one tablet of nefopam hydrochloride in the form of an Acupan 30 mg tablet. The first sequence received nefopam alone, whereas second sequence received nefopam with modulated electro-hyperthermia. Based on the results of a previous study [11] on the wash-out period (the time until the drug is excreted from the body) the time between the first sequence and sequence of nefopam from the human body was set at 7 days.
Blood samples for the PK analysis were collected before drug administration (0 h) and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12 and 24 h after drug administration for a total of 12 blood samples. The blood was collected using heparinised tubes, and stored temporarily in an icebox. Within 30 min after sampling, the tubes were centrifuged for 10 min at 2,700 rpm at 4 °C. Plasma (1 mL) was transferred into two cryotubes containing 1.0 mL of 5% formic acid (98%) diluted in water. The remaining plasma was divided into two aliquots, frozen at −20 °C for 24, and then stored at −70 °C until further analysis. The serum concentration of nefopam was analysed using a validated liquid chromatography mass spectrometry method and blood concentration time-curves for nefopam were obtained by plotting the nefopam concentrations in blood as a function of time after nefopam administration. The AUClast, primary area under the curve from nefopam administration to the final time point was obtained. The AUCinf, area under the curve extrapolated to infinitive time was calculated from the AUClast and the elimination rate constant as follows: AUCinf = AUClast + Ct/λz. The maximum concentration of nefopam in the blood (Cmax), the time when the Cmax occurred (Tmax) after nefopam administration and the half-life (t1/2) of blood nefopam were measured. The clearance rate removal of nefopam per unit time after oral administration (CL)) and the volume of distribution (Vd) (the quantity of nefopam divided by the blood concentration assuming that the drug is distributed evenly in the body after oral administration) of nefopam were also measured. Patient vital signs and possible adverse effects (AE) were also monitored.
Modulated electro-hyperthermia
The modulated electro-hyperthermia treatment was applied using an EHY-2000 clinical heating device set at a 13.56 MHz carrier frequency and amplitude modulated according to a time fractal pattern. The modulated electro-hyperthermia was performed for 60 min starting half an hour after nefopam administration. The subjects were positioned supine on a water-mattress electrode. A circular upper electrode 30 cm in diameter was coupled over the abdominal area covering the entire liver, both kidneys and at least more than 90% of the intestines. The power output was 100 W for the first 10 min, 120 W over the next 10 min and 150 W for the remaining treatment time. Self-calibration of the device was performed before every treatment. The body temperature, blood pressure and pulse rate of each subject were measured before, during and after the experiment. Body temperature was measured using an infrared ear thermometer (IRT 4020, Braun Gmbh, Kronberg, Germany), and temperature of the abdominal skin surface below the circular upper electrode probe was measured using a non-contact infrared thermometer transmitter (Thermo Checker DT-060, Easytem, Gyeonggi-do, Republic of Korea). Adverse events (AEs) were monitored throughout the study. AEs were determined by investigator inquiry and by spontaneous patient reports. AEs were recorded in terms of symptoms and signs, duration and severity (mild, moderate, and severe). The clinical safety parameters, including the blood glucose levels, vital signs, 12-lead ECG results and clinical laboratory tests, were performed at pre-determined intervals.
Statistics
Statistical analysis was conducted using SAS software (version 9.3, SAS Institute, Cary, NC, USA). To compare the Cmax, AUClast, and AUCinf values between the two treatment groups, the geometric mean ratio (GMR) and 90% confidence intervals (CIs) were calculated using analysis of variance (ANOVA) based on a mixed effects model that considered the sequence, period, and treatment as fixed effects and the subject as a random effect. The 90% CIs for parameters were within the bioequivalence limit of 0.8 to 1.25.
Results
The 12 healthy subjects consisted of four men and eight women. The age distribution was 19–53 years, and the BMI range was 18.0–24.8 kg/m2. The individual anthropomorphic data for all of the enrolled individuals are shown in Table 1.
Table 1. Mean demographic data of the subjects who enrolled in the study (n = 12).
| Parameter |
|||||
|---|---|---|---|---|---|
| Sex | Age (years) | Height (cm) | Weight (kg) | BMI (kg/m2) | |
| Male 4 Female 8 | |||||
| Mean | 33.7 | 162.4 | 57.8 | 21.7 | |
| Minimum | 19 | 155.4 | 45.5 | 18 | |
| Maximum | 53 | 177.5 | 75.4 | 24.8 | |
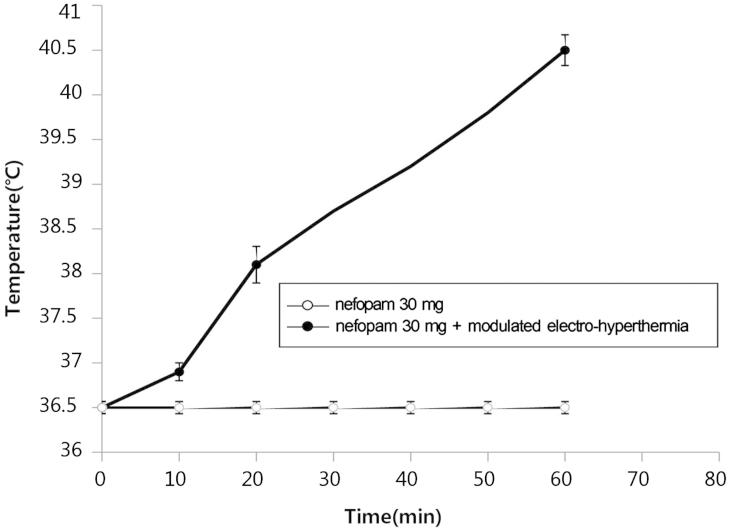
Body temperature before the experiment ranged from 36.4–36.9 °C (mean 36.5 °C) and that after modulated electro-hyperthermia ranged from 36.9–37.8 °C (mean 37.4 °C), indicating an average rise of 0.9 °C. The temperature of the abdominal skin surface underneath the upper electrode before heating ranged from 35.2–36.1 °C (mean 35.5 °C) and it increased to 39.8–40.9 °C (mean 40.5 °C), indicating the average surface temperature increase by about 4 °C (Figure 1 and Table 2).
Figure 1.
Temperature measurements of the abdominal area were repeated immediately after the first modulated electro-hyperthermia and ranged from 39.8–40.9 °C (mean 40.5 °C), indicating an average surface temperature increase of 4 °C.
Table 2. The temperature of the abdominal area of participants who underwent modulated electro-hyperthermia (n = 12).
| Parameter (°C) |
||||
|---|---|---|---|---|
| 0 min | 10 min | 20 min | 60 min | |
| Mean | 36.5 | 36.9 | 38.1 | 40.5 |
| Minimum | 36.4 | 36.6 | 37.4 | 39.8 |
| Maximum | 36.9 | 37.4 | 38.6 | 40.9 |
0 min, the abdominal temperature was measured before modulated electro-hyperthermia; 10 min, 20 min, and 60 min, the abdominal temperatures were measured 10 min (100 W), 20 min (120 W), and 60 min (150 W) after modulated electro-hyperthermia, respectively.
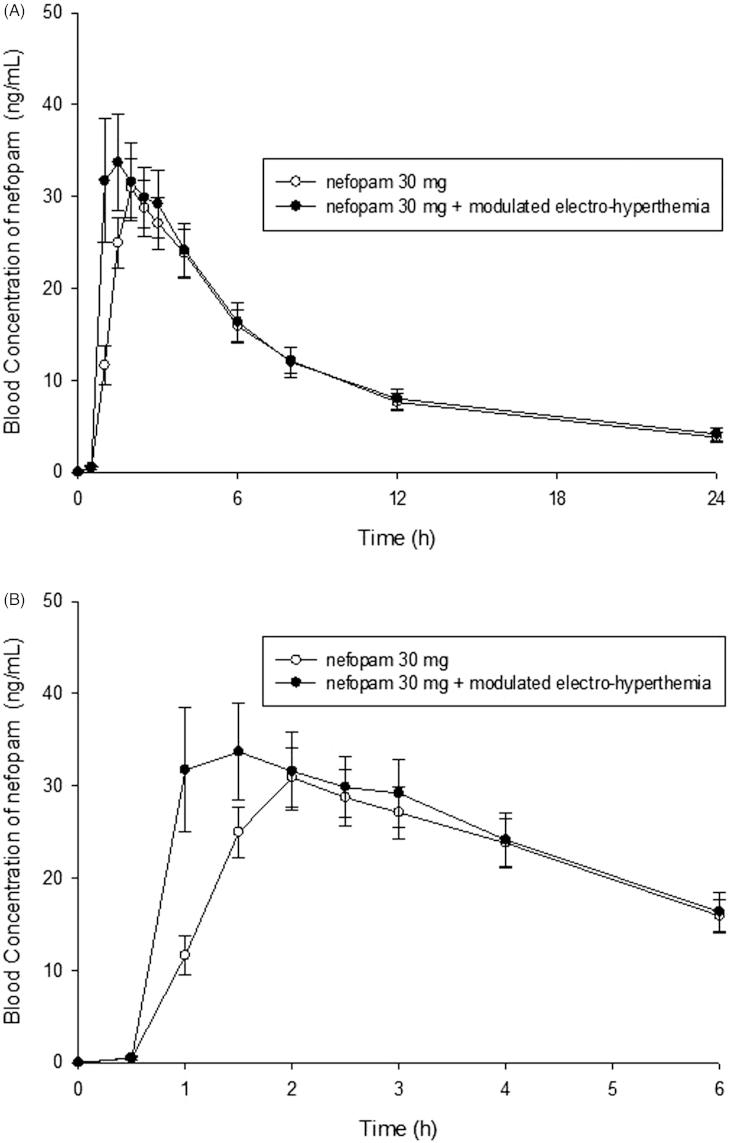
The arithmetic mean values of the Cmax, AUClast, AUCinf, Tmax, t1/2, CL/F and Vd/F of the nefopam alone and nefopam + modulated electro-hyperthermia group are shown in Figure 2 and Table 3. It is shown that Cmax significantly increased by 28%, whereas AUClast and AUCinf were not significantly changed. The GMRs ((nefopam + modulated electro-hyperthermia)/nefopam alone) with the associated 90% CIs for Cmax, AUClast and AUCinf, are shown in Table 4. The 90% CIs for parameters were within the bioequivalence limit of 0.8–1.25. The geometric mean ratio (point estimate) between the nefopam + modulated electro-hyperthermia and the nefopam alone was 1.2804 for Cmax, and 1.0512, and 1.0612 for AUClast and AUCinf,, respectively (Table 4). These results were showing no significant effect of modulated electro-hyperthermia on the AUCs whereas the regional heating significantly increased Cmax. The t1/2 (terminal half-life of blood concentration in blood), CL/F (apparent total clearance of the drug for the plasma, and Vd/F (apparent volume of distribution) were not significantly altered by hyperthermia.
Figure 2.
(A) The mean plasma concentration-time profiles of nefopam after the uptake of nefopam alone or in combination with modulated electro-hyperthermia. The data are presented as means ± standard error. (B) The mean plasma concentration-time profiles of nefopam after the uptake of nefopam alone or in combination with modulated electro-hyperthermia from 0 h to 6 h.
Table 3. Pharmacokinetic (PK) parameters of nefopam (n = 12).
| Nefopam 30 mg |
Nefopam 30 mg + modulated electro-hyperthermia |
||||
|---|---|---|---|---|---|
| PK parameter | Mean | SE | Mean | SE | p value |
| Cmax (ng/mL) | 32.18 | 3.16 | 42.42 | 5.32 | 0.0087 |
| AUClast (h × ng/mL) | 253.72 | 29.49 | 276.56 | 36.41 | 0.3654 |
| AUCinf (h × ng/mL) | 308.98 | 38.65 | 342.52 | 48.13 | 0.3411 |
| Tmax (h) | 2.08 | 0.21 | 1.58 | 0.23 | 0.0470 |
| t1/2 (h) | 8.47 | 0.82 | 9.40 | 0.97 | 0.1656 |
| CL/F (L/h) | 140.39 | 49.65 | 142.80 | 53.70 | 0.3411 |
| Vd/F (L) | 1198.71 | 140.19 | 1254.50 | 155.07 | 0.6194 |
Values are presented as the arithmetic mean. SE, standard error; Cmax, maxinum plasma concentration; AUClast, area under the plasma concentration-time curve until the final time point; AUCinf, area under the curve extrapolated to infinity; Tmax, time to Cmax; t1/2, terminal half-life; CL/F, apparent total clearance after oral administration; Vd/F, apparent volume of distribution after oral administration.
Table 4. Statistical analysis of nefopam (n = 12).
| Geometric LS mean |
Geometric LS mean ratio |
|||
|---|---|---|---|---|
| Pharmacokinetic parameter | Nefopam30 mg | Nefopam 30 mg + modulated electro-hyperthermia | Point estimate | 90% confidence interval |
| Cmax (ng/mL) | 29.65 | 37.97 | 1.2804 | 1.1155–1.4696 |
| AUClast (h × ng/mL) | 217.92 | 229.09 | 1.0512 | 0.9555–1.1566 |
| AUCinf (h × ng/mL) | 262.44 | 278.51 | 1.0612 | 0.9528–1.1819 |
These values are presented as the geometric means (CV%). The 90%CIs for parameters were within the bioequivalence limit of 0.8 to 1.25. The point estimate is geometric mean ratio. Cmax, maximum plasma concentration; AUClast, area under the curve until the final time point; AUCinf, area under the curve extrapolated to infinity
least-squares mean (LS).
We observed no significant changes in the clinical laboratory results, which included the 12-lead ECG, vital signs, and physical examination. Two of the subjects complained of nausea and dizziness after receiving nefopam alone and one subject felt mild fatigue after nefopam + modulated electro-hyperthermia. Importantly, all the subjects recovered from the adverse events within 2 h after treatment without further complications.
Discussion
In the present study, mild heating of the abdominal area of healthy volunteers with modulated electro-hyperthermia significantly increased the maximum blood concentration (Cmax) and reduced the time for reaching the maxim blood concentration (Tmax) of the orally administered nefopam, a widely used analgesic drug [2–4].
We used the modulated electro-hyperthermia heating unit to mildly heat the abdominal area of healthy volunteers. The characteristics of the EHY-2000 heating unit have been described previously [7,10]. In the present study we did not measure the temperature in the internal organ, but observed that the body temperature increased by about 0.9 °C and the temperature of the skin surface under the circular electrode with 30 cm diameter increased by about 4 °C at the end of 1 h heating (Figure 1). The significant increase in Cmax (peak concentration of nefopam in blood), and the significant decrease in Tmax (time to reach Cmax after drug administration) clearly indicated that the absorption of the orally administered drug to blood stream was significantly increased by the modulated electro-hyperthermia of abdominal area. The significant increase in Cmax did not affect the hepatic metabolism or renal metabolism of the drug as indicated by the lack of significant change in the t1/2, CL/F and Vd/F (Table 3).
The mechanisms underlying the increase in Cmax and decrease in Tmax by mild hyperthermia of the abdominal area is unclear. However, it would be reasonable to suggest that the regional hyperthermia of the abdominal area increased the temperature in the internal organs, particularly the gastro-intestinal (GI) system, thereby increasing the blood circulation in the organs and improving the absorption of the drug. It is also possible that an elevation of temperature in the GI system improved the permeability of the GI tract. The increase in cutaneous temperature, as indicated by the 4 °C increase in skin surface temperature under the 30-cm diameter electrode, may have caused marked increase in cutaneous blood perfusion in the heated area [5,12,13]. It is highly likely that significant increase in regional temperature leads to an increase in systemic blood circulation and influences the pharmacokinetics of drugs. Collectively, we may hypothesise that heating the abdominal area increased blood circulation in the GI system as well as that in whole body, and thereby it increased the absorption of orally administered nefopam.
Interestingly, local heating has been known to reduce or even block pain [14–16]. Increase in the oxygen supply and increase in the elimination of carbon dioxide as well as other metabolic wastes via heat-induced increase in blood circulation may account for the heat-induced reduction of local pain [14]. A clinical investigation on the potential increase in the analgesic effect of nefopam by mild local hyperthermia is in progress in our institute.
Although the present study was on the pharmacokinetics of nefopam, the result of the present study may have significant implication for the treatment of cancer with hyperthermia in combination with chemotherapy [17–21] since regional heating may significantly increase body temperature and alter the pharmacokinetics of chemotherapeutic agents.
In the present study, we used modulated electro-hyperthermia to regionally heat the abdominal area of patients and observed a significant increase in the body temperature and the uptake of orally administered nefopam. It remains to be elucidated whether or not similar increase in the uptake of orally administered drug would occur with other types of hyperthermia devices.
Conclusion
In conclusion, the increase in Cmax and decrease in Tmax indicated that the absorption of nefopam is increased by modulated electro-hyperthermia of the abdominal area. The AUC is an important parameter that influences the therapeutic effect of drugs [22]. The lack of significant changes in the AUC suggested that regional heating of the abdominal area increased the absorption of orally administered drugs, thereby transitionally increasing blood concentration of drugs without increasing the adverse effect of the drug.
Declaration of interest
This study was supported by Pharmbio Korea Co., Ltd, Seoul, Republic of Korea and HOSPI Care Co., Ltd, Gyeonggi-do, Republic of Korea. The authors alone are responsible for the content and writing of the paper.
References
- Aymard G, Warot D, Démolis P, Giudicelli JF, Lechat P, Le Guern ME, et al. Comparative pharmacokinetics and pharmacodynamics of intravenous and oral nefopam in healthy volunteers. Pharmacol Toxicol. 2003;92:279–86. doi: 10.1034/j.1600-0773.2003.920605.x. [DOI] [PubMed] [Google Scholar]
- Kakkar M, Derry S, Moore RA, McQuay HJ. Single dose oral nefopam for acute postoperative pain in adults. Cochrane Database Syst Rev. 2009;3:CD007442. doi: 10.1002/14651858.CD007442.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klohs MW, Draper MD, Petracek FJ, Ginzel KH, Re ON. Benzoxazocines: A new chemical class of centrally acting skeletal muscle relaxants. Arzneimittelforschung. 1972;22:132–133. [PubMed] [Google Scholar]
- Marazziti D, Rotondo A, Ambrogi F, Cassano GB. Analgesia by nefopam: Does it act through serotonin? Drugs Exp Clin Res. 1991;17:259–61. [PubMed] [Google Scholar]
- Song CW. Effect of local hyperthermia on blood-flow and microenvironment: A review. Cancer Res. 1984;44(Suppl10):S4721–30. [PubMed] [Google Scholar]
- Wismeth C, Dudel C, Pascher C, Ramm P, Pietsch T, Hirschmann B, et al. Transcranial electro-hyperthermia combined with alkylating chemotherapy in patients with relapsed high-grade gliomas – phase I clinical results. J Neurooncol. 2010;98:395–405. doi: 10.1007/s11060-009-0093-0. [DOI] [PubMed] [Google Scholar]
- Szasz A, Iluri N, Szasz O. Local hyperthermia in oncology. To choose or not to choose? In: Huilgol N, editor. Hyperthermia. Rijeka, Croatia: In Tech; 2013. pp. 1–82. [DOI] [Google Scholar]
- Fiorentini G, Giovanis P, Rossi S, Dentico P, Paola R, Turrisi G, et al. A phase II clinical study on relapsed malignant gliomas treated with electro-hyperthermia. In Vivo. 2006;20:721–4. [PubMed] [Google Scholar]
- Andocs G, Szasz O, Szasz A. Oncothermia treatment of cancer: From the laboratory to clinic. Electromagn Biol Med. 2009;28:148–65. doi: 10.1080/15368370902724633. [DOI] [PubMed] [Google Scholar]
- Szasz A. Challenges and solutions in oncological hyperthermia. Thermal Med. 2013;29:1–23. [Google Scholar]
- Ahmad M, Yaqoob M, Murtaza G. Study of pharmacokinetics and comparative bioavailability of nefopam 30 mg tablets in twelve fasting healthy Pakistani male young subjects: Single-dose, randomized, two-period, two-treatment, and two-way cross-over design. Med Princ Pract. 2012;21:271–6. doi: 10.1159/000333560. [DOI] [PubMed] [Google Scholar]
- Song CW, Park HJ, Lee CK, Griffin R. Implication of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia. 2005;21:761–7. doi: 10.1080/02656730500204487. [DOI] [PubMed] [Google Scholar]
- Ausmus PL, Wilke AV, Frazier DL. Effects of hyperthermia on blood flow and cis-diamminedichloroplatinum(II) pharmacokinetics in murine mammary adenocarcinomas. Cancer Res. 1992;52:4965–8. [PubMed] [Google Scholar]
- http://www2.massgeneral.org/painrelief/pain%20topics/heat_cold.pdf
- Seegenschmiedt MH, Vernon CC. A Historical perspective on hyperthermia in oncology. In: Seegenschmiedt MH, Fessenden P, Vernon CC, editors. Thermoradiotherapy and Thermochemiotherapy: Biology, Physiology, Physics. Volume 1. Berlin: Springer Verlag; 1996. pp. 3–46. [Google Scholar]
- Kim YP, Choi Y, Kim S, Park YS, Oh IJ, Kim KS, et al. Conventional cancer treatment alone or with regional hyperthermia for pain relief in lung cancer: A case-control study. Complement Ther Med. 2015;23:381–7. doi: 10.1016/j.ctim.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010;11:561–70. doi: 10.1016/S1470-2045(10)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed F, Marchettini P, Stuart OA, Urano M, Suqarbaker PH. Thermal enhancement of new chemotherpeutic agents at moderate hyperthermia. Ann Surg Oncol. 2003;10:463–8. doi: 10.1245/aso.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Fotopoulou C, Cho CH, Kraetschell R, Gellermann J, Wust P, Lichtenegger W, et al. Regional abdominal hyperthermia combined with systemic chemotherapy for the treatment of patients with ovarian cancer relapse: Results of a pilot study. Int J Hyperthermia. 2010;26:118–26. doi: 10.3109/02656730903369200. [DOI] [PubMed] [Google Scholar]
- Inman BA, Stauffer PR, Craciunescu OA, Maccarini PF, Dewhirst MW, Vujaskovic Z. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int J Hyperthermia. 2014;30:171–5. doi: 10.3109/02656736.2014.882021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angele MK, Albertsmeier M, Prix NJ, Hohenberger P, Abdel-Rahman S, Dieterle N, et al. Effectiveness of regional hyperthermia with chemotherapy for high-risk retroperitoneal and abdominal soft-tissue sarcoma after complete surgical resection: A subgroup analysis of a randomized phase-III multicenter study. Ann Surg. 2014;260:749–56. doi: 10.1097/SLA.0000000000000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Guidance for Industry. Drug Interaction Studies – Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. Silver Spring, MD: Food and Drug Administration; 2012. [Google Scholar]