Abstract
Cilia are force-generating and -sensing organelles that serve as mechanical interfaces between the cell and the extracellular environment. Cilia are present in tissues that adaptively respond to mechanical loading and fluid flow, and defects in ciliary function can lead to diseases affecting these tissues. As might be expected for a mechanical interface, the formation of cilia is, itself, regulated by mechanical forces, and these links between mechanics and ciliary formation are providing new entry points for dissecting the regulatory pathways of ciliogenesis.
Keywords: cilia, biomechanics, developmental biology, hydrodynamics, calcium, mucus
Once thought to be vestigial in most tissues, cilia are emerging as major players in virtually all aspects of physiology and development. Cilia are microtubule-based organelles that are found in almost all cells of the human body and that are also present throughout the eukaryotes, with higher plants as notable exceptions. The discovery during the past decade and a half that cilia can perform sensory and signaling functions has focused tremendous interest in these aspects of cilia. At the same time, cilia are clearly mechanical devices, capable of generating and sensing forces. Here, we will consider the roles that mechanical forces play in cilia function and especially in ciliogenesis.
Cilia: Motile and immotile
The fundamental structure of a cilium is a cylindrical array of nine doublet microtubules, nucleated by a centriole called as basal body, which push out an extension of the plasma membrane (for a review, see Satir and Christensen 2007). The microtubule doublet array, together with all the associated proteins that hold it together, is known as the axoneme. Two distinct types of cilia may be distinguished as motile and immotile. Motile cilia have a more complex axoneme, in which the microtubule doublets are augmented by a pair of singlet microtubules running up the middle of the cilium, known as the central pair. Protein complexes known as radial spokes run between the central pair and the outer doublets. In motile cilia, dynein motors known as inner and outer dynein arms are attached to the doublet microtubules and drive the bending of the cilium by walking along one doublet while being attached to another, therefore causing the doublets to slide relative to one another.
Cilia as generators and sensors of mechanical force
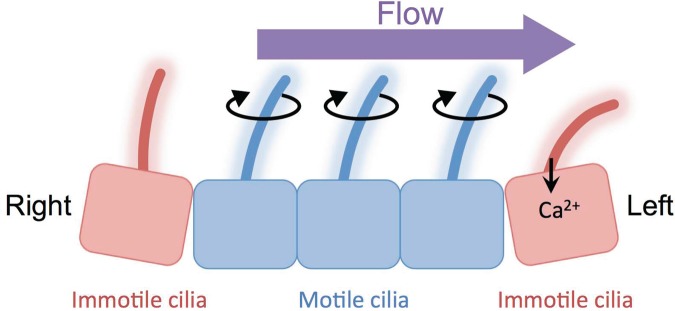
Cilia perform numerous functions in physiology and development, many of which are mechanical in nature. Cilia in the airway, oviduct, embryonic node, and ependyma generate extracellular flows of mucus and other fluids. In adults, these flows are required for proper tissue function—for example, the clearing of mucus from the airway—whereas in developing organisms, these flows provide directional cues for left–right symmetry breaking and cell migration (Sawamoto et al. 2006, Marshall and Kintner 2008, Schneider et al. 2010, Yoshiba and Hamada 2014). Cilia do not just generate mechanical forces but also sense forces. In the mouse node, there are two different populations of cilia: motile and immotile cilia (figure 1; McGrath et al. 2003, Babu and Roy 2013, Yoshiba and Hamada 2014). Motile cilia generate leftward fluid flow in the node (Nonaka et al. 2005, Okada et al. 2005), and perinodal immotile cilia sense the direction of fluid flow via a mechanosensory calcium ion (Ca2+) channel containing the polycystin-2 (PC2) protein (also known as PKD2 and TRPP2; Yoshiba et al. 2012). Leftward fluid flow is converted to Ca2+ signals that are thought to specify the left-side fate (McGrath et al. 2003, Takao et al. 2013, Yoshiba and Hamada 2014). In the kidney, the same Ca2+ channel protein, PC2, allows cilia to detect the fluid flow of the kidney filtrate and to generate a Ca2+ signal that responds to flow (Nauli et al. 2003, Praetorius and Spring 2003). This was most directly demonstrated by Praetorius and Spring (2001), who showed that intracellular Ca2+ levels were increased when cilia were bent by a micromanipulator. This flow detection mechanism by PC2 and cilia is also found in cholangiocytes and endothelial cells (Masyuk et al. 2006, AbouAlaiwi et al. 2009). PC2 is therefore the most prominent mechanosensory protein currently under discussion in the context of cilia, and, given any situation in which flow or mechanical forces affect cilia or cilia-mediated pathways, the most obvious question to ask will be whether PC2 is required (Patel and Honore 2010). The importance for PC2 in cilia-mediated mechanical sensing is also important from the perspective of disease, as will be discussed below.
Figure 1.
Cilia generate and sense forces. Two types of cilia are present in the mouse node and are important for deciding left–right asymmetry. Motile cilia generate leftward directional flow in the node. Immotile cilia at the edge of the node sense flow. The polycystin-2 channel on cilia responds to flow and converts it to calcium ion (Ca2+) signals.
The generation of forces by motile cilia requires the coordination of thousands of dynein arms, which are motor protein complexes associated with the doublet microtubules of axonemes, so as to generate a coordinated bending motion (King and Kamiya 2009). The mechanisms by which these dyneins are coordinated remain incompletely understood but are thought to involve mechanical coupling between dynein arms, so that the chemomechanical cycle of one dynein is influenced by forces exerted by the other arms. This type of coupling has been demonstrated in studies showing that applied bending forces can modulate dynein arm activity (Morita and Shingyoji 2004).
The two fundamental mechanical properties of cilia that are most relevant to their ability to sense mechanical forces is their length and their rigidity. Cilia have different lengths in different tissues and cell types, ranging from 1 to 100 microns but generally being around 10 microns long. The flexural rigidity of cilia has been measured in the motile cilia of the mollusk gill using micromanipulation approaches and has been found to have a value in the range of 10−18 Newton meters squared (Nm2) (Baba 1972). Baba (1972) calculated that such a flexural rigidity could be accounted for if the outer doublet microtubules were physically connected to each other and each had a Young's modulus with an order of magnitude of 5 gigapascals, a value quite consistent with the reported mechanical measurements of microtubules (Gittes et al. 1993). An analysis of the bending of the immotile sensory cilia of the kidney in response to fluid flow indicated a much lower flexural rigidity (Schwartz et al. 1997, Young et al. 2012), which suggests that the machinery of ciliary motility may contribute to mechanical rigidity, although this comparison would be stronger if the same methods had been used to measure the rigidity in both cases. In mammalian sperm, accessory structures, such as the outer dense fibers and fibrous sheath, surround axonemes and are thought to provide increased stiffness (Fawcett 1975).
The length of cilia is intimately linked to their ability to generate and respond to forces. For this reason—and also because of their relatively simple linear shapes—cilia have served as simple model systems for understanding the general question of organelle size regulation (Rafelski and Marshall 2008). The process by which cilia assembly, ciliogenesis, is highly complex, requiring the orchestrated production of hundreds of different proteins, which are selectively imported through a diffusion filter at the base of the cilium (for a review, see Avasthi 2013) and then carried into the growing structure by an active process known as intraflagellar transport (IFT; Ishikawa and Marshall 2011). Biomechanical analysis is therefore interesting not only in terms of ciliary function, but it also provides a distinct set of tools for probing the mechanism of ciliogenesis, orthogonal to the current molecular genetic methods. Although molecular genetic methods such as mutation or RNA interference are more specific in their targets than mechanical perturbation, they suffer from a major drawback in that the kinetics of the perturbation are extremely slow compared with the timescale required to build a cilium. Therefore, one typically observes cilia in a steady-state response to the perturbation, which not only hinders the analysis of the dynamic response to the perturbation, which is often crucial for testing quantitative models but also runs the risk that by the time observations are being made, the cells may have already activated compensating pathways. In contrast, mechanical stimuli can be applied and removed at an extremely short timescale relative to the timescale of ciliogenesis and ciliary length changes. Therefore, although mechanical methods may lack molecular specificity, they gain tremendous temporal sensitivity. Obviously, a combination of mechanical and molecular perturbations will be the most powerful approach.
The relationship between mechanical force and ciliary orientation in tissues
In ciliated epithelial sheets or tubes, the cilia need to beat in the correct direction in order to generate a coherent fluid flow that will be physiologically useful. For example, if the cilia in the airway were to be oriented in the opposite direction from their normal orientation, they might drive mucus down into the lungs, instead of out of them, and this would be lethal. Obviously, the orientation of cilia in such tissues has to be driven by long-range developmental cues that specify tissue orientation, but experiments have shown that ciliary orientation is also affected by the fluid flow that the cilia, themselves, generate (Mitchell et al. 2007, Guirao et al. 2010), which leads to a positive feedback loop. This feedback loop is thought to help coordinate ciliary orientation so that cilia beat in a consistent direction. However, the ultimate input to this feedback system would be long-range developmental cues, such as planar cell polarity (Marshall and Kintner 2008).
Ciliary orientation is clearly important in tissues that generate flow; however, ciliary orientation is also important for immotile cilia that sense mechanical forces. Chondrocytes have primary cilia that project into developing cartilage, and these cilia are mechanically sensitive (Wann et al. 2012). The chondrocyte cilia have defined orientations in different regions of cartilage, always aligned perpendicular to the articular surface of the joint cartilage, although they are sometimes facing the articular surface and sometimes facing away from it, and these directional biases are seen only in load-bearing regions (McGlashan et al. 2007, Farnum and Wilsman 2011). As with cartilage, in bone, cilia also appear to be playing a mechanical sensing role (Nguyen and Jacobs 2013); however, the orientation of cilia relative to the direction of load has not yet been analyzed in the same level of detail as it has in cartilage. In these systems, it is not known why, from a teleological perspective, the organism would want to have its cilia aligned in a particular direction. Given the studies with kidney cilia, it would seem that cilia are mechanosensors that respond to forces applied perpendicular to their long axis. A testable hypothesis, therefore, is that chondrocyte cilia may be required to sense forces applied in a direction parallel to the articular surface.
There are two distinct mechanisms that could explain the correlation of ciliary orientation or length with mechanical forces in a tissue: Either developmental signals such as planar polarity cue direct ciliogenesis in a manner appropriate to the forces that the tissue will subsequently experience or the mechanical forces, themselves, directly influence the process of ciliogenesis. The role of mechanical forces in directing morphogenesis and differentiation is an important question in developmental biology that is still quite murky, and it may not be as easy to distinguish developmental signals from mechanical forces. For example, mechanical tension in epithelial sheets is able to bias the direction of oriented cell division (Mao et al. 2013) and cell migration (Beloussov et al. 2000). These tissue-level studies are recapitulated in single cells, in which, for example, the orientation of externally applied tension can dictate the orientation of cell division (Fink et al. 2011). These effects of mechanical forces on cilia represent a specific instance of the more general role that mechanical forces are now thought to play in regulating cellular processes such as cell division or differentiation, whereby forces or mechanical properties of the extracellular matrix or substrate can exert strong influences on cell fate and behavior (Discher et al. 2005, 2009, Lopez et al. 2008). Because ciliogenesis involves so many aspects of cell biology, including cytoskeletal and trafficking systems, it stands to reason that forces could affect ciliogenesis, and, indeed, a growing number of experiments are now pointing to such an effect.
Mechanical forces affect ciliogenesis
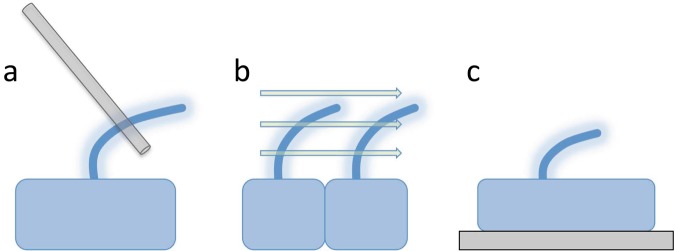
Studying the mechanobiology of cilia requires methods to apply forces to ciliated cells in a controlled fashion and to monitor the effect on cilia. Several methods for applying forces to ciliated cells are illustrated in figure 2. One of the most direct methods for applying force is using either microneedles or optical traps to apply force directly to cilia, as was discussed above in the study of cilia-mediated mechanosensation. This method has the advantage that the force can be applied directly onto the cilia of a single cell but is hard to apply to a large number of cells. Furthermore, such a method tends to apply the force focally at a single point, which is fine for bending cilia but, in many cases, it is desirable to apply a force more globally on the whole cell.
Figure 2.
Methods for applying forces to cilia. (a) Direct application of mechanical forces onto the cilia themselves—for example, using glass needles or optical tweezers. This method has the advantage that the exact spot of force application can be controlled. (b) Fluid flow. This method has the advantage that it may mimic the types of forces present in many physiological systems, such as the endothelium or kidney tubules. (c) Stretching the cell, either by adhering the cell to a patterned substrate that forces the cell to increase its spread surface area or by physically stretching the substrate after the cell has adhered.
One of the technically simplest ways to apply forces to cultured cells is by fluid flow. Human umbilical vein endothelial cells, grown in a flow chamber with continuous flow, were used to show that increased shear stress causes cilia to disassemble (Iomini et al. 2004). Although the mechanism of this induced disassembly has not been determined, one interesting link is provided by the fact that a key protein involved in intraflagellar transport, IFT74, disappears from cilia after 2 hours of fluid flow (Iomini et al. 2004). This might suggest a regulatory link from mechanical sensing pathways to the IFT pathway, although this needs to be explored more thoroughly. Endothelial cells are ciliated inside blood vessels, and although the function of these cilia in blood vessel development or physiology is not known, there are some hints that the presence of cilia correlates with disrupted fluid flow, possibly playing a role in atherosclerosis (see below for a discussion of this link). The ability of increased flow to induce ciliary resorption shown in these experiments would provide a mechanistic basis for the correlations seen between cilia and flow in the aorta and carotid artery (Van der Heiden et al. 2011).
Resnick and Hopfer (2007) applied a very simple approach to studying the mechanical forces on cilia: growing a layer of mouse kidney cells in an orbital shaker to generate fluid flow over their surface. They found that this flow causes ciliary length to decrease, an effect likely due to the shear forces induced by the fluid flowing over the cell layer. The decrease in ciliary length due to fluid flow was found to require the polycystins PC1 (also known as PKD1) and PC2 and downstream signaling mediated by Ca2+ signaling pathways (Besschetnova et al. 2010). These researchers also reported a decrease in intraflagellar transport speed, but it is not clear whether this is a cause of the ciliary shortening or a consequence, because the speed of the IFT particles is known to be a function of length (Engel et al. 2009).
In general, the study of fluid shear forces and their influence on cilia will be of great importance for understanding the developmental biology and physiological homeostasis of tissues in which fluid can flow, such as in blood vessels, kidney tubules, oviduct, airway, and brain ventricles. If the response of cilia to flow is a physiologically relevant behavior, the response might not be observed in cilia derived from tissues such as cartilage in which fluid is not normally flowing. Fluid shear also induced the shortening of cilia in osteoblasts (Delaine-Smith et al. 2014). One interpretation is that the flow-induced shortening might reflect a general shortening response to externally applied forces, and those forces can be generated by a variety of means of which fluid shear is just one example.
Fluid shear is one type of force that ciliated cells are subject to, but it is not the only one. Stretching and compression are alternative mechanical forces present in many tissues. For example, as was discussed above, chondrocyte cilia are aligned relative to the articular surface of cartilage, but only in load-bearing regions, which suggests a possible role of mechanical forces as signals for ciliary formation or orientation. Direct experiments with compressive forces applied to chondrocytes embedded in agarose three-dimensional culture have provided evidence that compressive force can reduce both the length of cilia and the fraction of cells that are ciliated (McGlashan et al. 2010). One origin of forces in a living organism is tissue deformation during development. As an embryo develops, epithelial sheets can bend and deform in ways that will subject cells to tensile and compressive forces, depending on their position within the tissue. An effect of tensile forces on ciliogenesis was suggested by experiments in which cells were grown on micropatterned substrate patches of different area, causing cells to spread over the patch and therefore take on different spread areas. Larger spread areas therefore cause cells to mimic a shape that would be seen in tissues that were stretched out. Such an increase in spread area also caused the cilia that were present to be shorter (Pitaval et al. 2010). Suggesting that tensile forces, as with compressive forces, might inhibit ciliary formation. Pitaval and colleagues (2010) found that actin cytoskeleton function was required for the response of cilia to the stretched area, which is consistent with the idea that actin is an integrator of global cellular mechanics. Actin has been implicated in ciliogenesis in large-scale screens (Kim et al. 2010) although the mechanistic role of actin in ciliogenesis remains completely unknown.
In addition to studies showing that mechanical forces influence ciliogenesis and length regulation, forces also influence the motile function of cilia. Coordination of dynein arms is necessary to generate the large-scale coherent and propagated bending of cilia. This coordination is thought to involve mechanical feedback from ciliary bending onto the activity of the dynein arms. The mechanical sensors that respond to ciliary deformation during the normal process of ciliary beating would also respond to deformations applied by external forces, and, indeed, externally applied forces can change the beating of cilia (Hill et al. 2010). In the unicellular green alga Chlamydomonas, mechanical agitation alters the flagellar beat frequency (Wakabayashi et al. 2009), and this response requires Ca2+ signalling and appears to affect outer dynein arm activity. In the cilia of the zebrafish kidney, mechanical stretching alters the beat frequency of the cilia, which, unlike the case in mammalian kidney, are motile (Hellman et al. 2010). Motile and immotile cilia have substantial differences in their protein composition (Pazour et al. 2005, Ishikawa et al. 2012), so it is probable that the molecules involved in sensing force in the regulation of motile cilia beating will be distinct from those that sense mechanical forces and bending in sensory cilia, such as those of the kidney. However, the PC2 mechanosensory channel can influence the beating waveform of cilia and flagella (Yang et al. 2011), which raises the possibility that this key mechanical regulatory molecule is a fundamental part of the force response system in motile cilia, although forces also influence dynein activity in demembranated flagella in which the PC2 channel would not play a role (Morita and Shingyoji 2004). Therefore, the signals regulating ciliary motility in response to forces are likely to be quite complex.
If mechanical forces applied to cells affect ciliogenesis, there are two possible ways this could happen. Because cilia are, themselves, mechanosensory, the forces may act directly on the cilia. For example, increased surface tension in spread cells could make it more difficult for cilia to elongate, because the growth of cilia requires pushing out a plasma membrane protrusion. Cilia formation requires the migration of the centrosome to the cell surface, and both the change in spread area (Rodríguez-Fraticelli et al. 2012) and that in extracellular fluid shear (Kotsis et al. 2008) are known to influence centrosome positioning and movement, so that one possible way in which forces impede cilia formation is by preventing the centriole from migrating to the correct position on the upper surface of the cell. However, cilia are complex systems regulated by a large number of signaling pathways (Miyoshi et al. 2011, Avasthi and Marshall 2012, Yuan and Sun 2012). It is therefore quite possible for mechanical forces applied to the cell body to affect ciliogenesis indirectly, via the numerous signalling pathways that regulate cilia. Mechanical stresses applied to cells in culture are shown to increase intracellular cyclic adenosine monophosphate (Fitzgerald and Hughes-Fulford 1999, Meyer et al. 2000), to increase intracellular Ca2+ levels (in cardiomyocytes; Ruwhof et al. 2001), and also to stimulate signaling in extracellular signal-regulated kinases 1 and 2 and mitogen-activated protein kinases in MC3T3-E1 cells (Kanno et al. 2007). All of these pathways are known to regulate ciliogenesis and ciliary length control, so if mechanical forces applied to cells alter these pathways, they could thereby affect cilia just by changing the activation status of signaling pathways. It is also possible that some of the intracellular trafficking pathways involved in building and maintaining cilia may be mechanically responsive. For example, it is now appreciated that building a cilium requires substantial membrane trafficking, and this trafficking can be affected by plasma membrane tension and surface area. Learning exactly how cells sense mechanical forces and transduce this information to the ciliary assembly pathway is a tremendous challenge for the future that will require an integrated combination of approaches from systems biology and mechanobiology.
Why cilia are a promising system for the future investigation of mechanics in development
The idea that mechanical forces can influence tissue development has been around for a long time and represents an ongoing extension of the ideas first espoused by Thompson (1917) that physical forces sculpt the form of living organisms. But, despite the potential importance of physical forces, far less research has been devoted to the study of mechanobiology of development compared with molecular and genetic studies of the patterning molecules that regulate animal and plant form. Partly, this is because it is difficult to apply forces or to measure forces in a living context. Cilia are particularly convenient because, in many cell types, they protrude from cells, making it possible to apply forces directly to them (Praetorius and Spring 2001). Also, because cilia are essentially elastic beams, it is even possible to infer forces’ acting on them by analyzing their shape.
A second advantage that cilia have is that they give us directional information. Most of the current read-outs for mechanical influences on development are essentially scalar; for example, analyzing stem cell differentiation pathways as a function of applied forces gives a scalar read-out of cell fate that has no intrinsic direction. In contrast, cilia provide a vector read-out, because we can interrogate not only the length and function of the cilia but also the direction of their orientation. The easy visualization of cilia, even in living animals and tissues (Ou et al. 2005, O'Connor et al. 2013), makes these organelles amenable to analysis in vivo.
Ciliopathies and mechanics
The most obvious disease that may represent a defect in mechanobiology of cilia is polycystic kidney disease (PKD), a common genetic cause of kidney failure in which the kidney becomes full of large fluid-filled cysts that impair function. PKD is thought to arise when cilia are not able to detect the flow of kidney filtrate, with the kidney tissue then attempting to compensate by increasing proliferation and dilating the tubules, eventually leading to the formation of cysts (Harris and Torres 2009). A common genetic cause of PKD is a mutation in the PC2 (polycystin) protein, which, as was discussed above, is required for flow-responsive Ca2+ signaling by cilia. Therefore, in a human patient with a mutation in PC2, their cilia will not signal flow information appropriately, which will lead to disease. The jck (juvenile cystic kidneys) mouse model for PKD is defective in Nek8, a PC2-interacting kinase, and it has been shown that this kinase is also required for sensing fluid shear forces (Manning et al. 2013), which further supports the link between force sensing and PKD. Although we think of this disease as resulting from a failure of cilia to detect forces acting on them, this may also be a case in which forces acting on the tissue can influence formation of cilia. As is shown in figure 3, fluid flow in epithelial tubes creates several forces on the ciliated cells that have already been shown to alter ciliogenesis (see above). We may, therefore, in the kidney, be dealing with a highly complex mechanical system in which fluid flow and pressure modulate the production of the very organelles that are responsible for sensing these forces.
Figure 3.
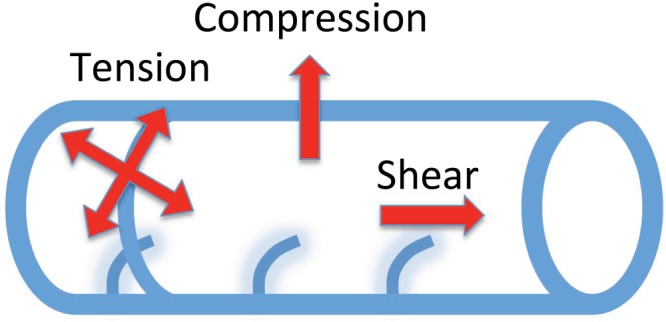
Forces acting on ciliated cells in an epithelial tube because of fluid flow and internal pressure. Pressure inside the tube creates compression forces acting across the wall of the tube and tension forces acting within the plane of the epithelium. Either of these can, in principle, be sensed and used to modulate ciliogenesis. Fluid flow also creates shear forces acting on the cilia. The degree to which these three types of forces play a role in regulating ciliogenesis in developing tissues is a major unanswered question.
Another disease in which cilia may play a mechanics-related role is atherosclerosis. Cilia distribution on the endothelium of the aorta and carotid artery correlates closely with regions of turbulent blood flow, and these same regions are sites of the deposition of atherosclerotic plaques (Van der Heiden et al. 2008). This correlation does not show causality, but a further experiment in which flow was artificially perturbed showed that cilia increased in new regions of disrupted flow (Van der Heiden et al. 2008), strongly implying that altered fluid flow increases ciliogenesis. The same study also showed that mice with a genetic deficiency in apolipoprotein E, which increases atherosclerosis because of an impaired clearance of very low-density lipoprotein remnants, which leads to higher low-density lipoprotein in the bloodstream, show an increase in the number of cilia on the endothelium, which raises the possibility that the onset of atherosclerosis can trigger cilia formation. It is interesting to consider whether the presence of cilia in regions disposed to atherosclerosis could play a causal role in disease. For example, the cilia could physically trap cells, debris, or lipoprotein particles, although this has yet to be directly demonstrated. An alternative is that the presence or absence of cilia may depend on whether cilia are required only for highly sensitive flow measurements, such that in regions of higher shear forces, cilia are not required because mechanosensitive molecules on the cell surface are able to measure the flow without needing cilia. Direct evidence of cilia as mechanical flow sensors in endothelial cells has recently been presented on the basis of live imaging in zebrafish, showing that endothelial cilia bend in response to flow, that the deflection angle correlates with endothelial cell calcium signals, and that these signals are perturbed when ciliogenesis or PC2 channel expression are defective (Goetz et al., 2014).
Finally, respiratory diseases involving changes in the rheological properties of mucus are likely to involve alterations in the mechanical interactions between cilia and the mucus. It is known that mucus viscosity changes the beat frequency of cilia in mammalian respiratory cells for mucociliary clearance (Sanderson and Dirksen 1986, Bloodgood 2010). Airway diseases, such as chronic obstructive pulmonary disease, are associated with changes in ciliary beating (Yaghi et al. 2012), as well as mucus production, and we need better models for understanding whether these are related. Because the metachronal synchronization of ciliary beating is mediated by hydrodynamic coupling, metachrony should be strongly affected by alterations in the mechanical properties of the mucus (Gheber et al. 1998, Guirao and Joanny 2007, Horváth and Sorscher 2008, Elgeti and Gompper 2013). We therefore expect that genetic perturbation to mucus production could dramatically affect cilia motility, possibly allowing mucus abnormality to produce symptoms that mimic ciliary dysfunction.
This may be the explanation for Young's syndrome, which seems to combine aspects of ciliary and mucus defects (Verra et al. 1991, de Iongh et al. 1992). But in addition to the immediate affect of mucus rheology alterations on ciliary beating motility (Sanderson and Dirksen 1986, Andrade et al. 2005), the fact that mechanical forces can also affect ciliogenesis and ciliary length, as well as the coordinated orientation of ciliary beating direction, means that there is the potential for even temporary alterations in mucus rheology to have a long-lasting effect on ciliary assembly, length, and orientation. This may constitute the mechanistic basis for secondary ciliary dyskinesia, a defect in ciliary motility that arises because of respiratory insults. Environmental insults, such as pollution or smoking, or pathological insults, such as bacterial infection, could end up affecting the mechanobiology of ciliated cells, leading to effects on cilia that persist after the pathological insult has been removed. Understanding such effects and how to combat them in a clinical setting will require a much more detailed knowledge of the links between cellular mechanics, extracellular mechanics, and ciliary assembly.
Future challenges
The links among cilia, mechanics, and disease raise the stakes for understanding the mechanobiology of ciliogenesis. If we understood how mechanical forces and cilia interact, we might be able to develop completely new methods of therapy for diseases such as cystic kidneys or atherosclerosis based on modulating mechanosensory or ciliogenesis pathways. But until we understand exactly how mechanical forces are coupled to cilia, it will be extremely difficult to move beyond correlative studies. The key is to learn what molecular pathways link mechanical stimuli to ciliogenesis and ciliary function, which will then allow these links to be systematically removed by genetic means. If the signaling pathways can be blocked, it will then be possible to test the causal role of mechanical effects on ciliogenesis. However, such studies are always fraught with concerns about the pleiotropic effects of any genetic lesion. There is, therefore, a persistent need for methods to apply and modulate mechanical forces inside living tissues. Methods to measure forces by fluorescence resonance energy transfer–based mechanosensors in living cells are becoming available (Meng and Sachs 2012), and these will also be extremely important for learning more about the relation of mechanics to ciliogenesis.
It is also interesting to consider whether mechanical methods could be used for therapeutic purposes. For example, could micropumps be implanted to augment weak or abnormal fluid flows? Could fluid rheology be tuned to compensate for ciliary weakness? Alternatively, the pharmacological modulation of mechanosensory signaling pathways could provide an avenue of attack of ciliary diseases. Even if such strategies turn out not to be effective, pursuing them would undoubtedly yield a wealth of new insights into both cilia and tissue biomechanics.
Acknowledgments
We acknowledge the financial support of National Institutes of Health grant no. GM097017 for our studies of ciliary and flagellar length control.
References cited
- AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circulation Research. 2009;104:860–869. doi: 10.1161/CIRCRESAHA.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade YN, Fernandes J, Vázquez E, Fernández-Fernández JM, Arniges M, Sánchez TM, Villalón M, Valverde MA. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. Journal of Cell Biology. 2005;168:869–874. doi: 10.1083/jcb.200409070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avasthi P. Signaling: Sifting at the ciliary base. Nature Chemical Biology. 2013;9:414–415. doi: 10.1038/nchembio.1272. [DOI] [PubMed] [Google Scholar]
- Avasthi P, Marshall WF. Stages of ciliogenesis and regulation of ciliary length. Differentiation. 2012;83:S30–S42. doi: 10.1016/j.diff.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba SA. Flexural rigidity and elastic constant of cilia. Journal of Experimental Biology. 1972;56:459–467. doi: 10.1242/jeb.56.2.459. [DOI] [PubMed] [Google Scholar]
- Babu D, Roy S. Left–right asymmetry: Cilia stir up new surprises in the node. Open Biology. 2013;3 doi: 10.1098/rsob.130052. art. 130052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloussov LV, Louchinskaia NN, Stein AA. Tension-dependent collective cell movements in the early gastrula ectoderm of Xenopus laevis embryos. Development Genes and Evolution. 2000;210:92–104. doi: 10.1007/s004270050015. [DOI] [PubMed] [Google Scholar]
- Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Current Biology. 2010;20:182–187. doi: 10.1016/j.cub.2009.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood RA. Sensory reception is an attribute of both primary cilia and motile cilia. Journal of Cell Science. 2010;123:505–509. doi: 10.1242/jcs.066308. [DOI] [PubMed] [Google Scholar]
- De Iongh R, Ing A, Rutland J. Mucociliary function, ciliary ultrastructure, and ciliary orientation in Young's syndrome. Thorax. 1992;47:184–187. doi: 10.1136/thx.47.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaine-Smith RM, Sittichokechaiwut A, Reilly GC. Primary cilia respond to fluid shear stress and mediate flow-induced calcium deposition in osteoblasts. FASEB Journal. 2014;28:430–439. doi: 10.1096/fj.13-231894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang Y-L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Discher D[E], Dong C, Fredberg JJ, Guilak F, Ingber D, Janmey P, Kamm RD, Schmid-Schönbein GW, Weinbaum S. Biomechanics: Cell research and applications for the next decade. Annals of Biomedical Engineering. 2009;37:847–859. doi: 10.1007/s10439-009-9661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgeti J, Gompper G. Emergence of metachronal waves in cilia arrays. Proceedings of the National Academy of Sciences. 2013;110:4470–4475. doi: 10.1073/pnas.1218869110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel BD, Ludington WB, Marshall WF. Intraflagellar transport particle size scales inversely with flagellar length: Revisiting the balance-point length control model. Journal of Cell Biology. 2009;187:81–89. doi: 10.1083/jcb.200812084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnum CE, Wilsman NJ. Orientation of primary cilia of articular chondrocytes in three-dimensional space. Anatomical Record. 2011;294:533–549. doi: 10.1002/ar.21330. [DOI] [PubMed] [Google Scholar]
- Fawcett DW. The mammalian spermatozoon. Developmental Biology. 1975;44:394–436. doi: 10.1016/0012-1606(75)90411-x. [DOI] [PubMed] [Google Scholar]
- Fink J, et al. External forces control mitotic spindle positioning. Nature Cell Biology. 2011;13:771–778. doi: 10.1038/ncb2269. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J, Hughes-Fulford M. Mechanically induced c-Fos expression is mediated by cAMP in MC3T3-E1 osteoblasts. FASEB Journal. 1999;13:553–557. doi: 10.1096/fasebj.13.3.553. [DOI] [PubMed] [Google Scholar]
- Goetz JG, et al. Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell Reports. 2014;6:799–808. doi: 10.1016/j.celrep.2014.01.032. [DOI] [PubMed] [Google Scholar]
- Gheber L, Korngreen A, Priel Z. Effect of viscosity on metachrony in mucus propelling cilia. Cell Motility and the Cytoskeleton. 1998;39:9–20. doi: 10.1002/(SICI)1097-0169(1998)39:1<9::AID-CM2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Gittes F, Mickey B, Nettleton J, Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. Journal of Cell Biology. 1993;120:923–934. doi: 10.1083/jcb.120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao B, Joanny J-F. Spontaneous creation of macroscopic flow and metachronal waves in an array of cilia. Biophysical Journal. 2007;92:1900–1917. doi: 10.1529/biophysj.106.084897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao B, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nature Cell Biology. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- Harris PC, Torres VE. Polycystic kidney disease. Annual Review of Medicine. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman NE, et al. The zebrafish foxj1a transcription factor regulates cilia function in response to injury and epithelial stretch. Proceedings of the National Academy of Sciences. 2010;107:18499–18504. doi: 10.1073/pnas.1005998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DB, Swaminathan V, Estes A, Cribb J, O'Brien ET, Davis CW, Superfine R. Force generation and dynamics of individual cilia under external loading. Biophysical Journal. 2010;98:57–66. doi: 10.1016/j.bpj.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth G, Sorscher EJ. Luminal fluid tonicity regulates airway ciliary beating by altering membrane stretch and intracellular calcium. Cell Motility and the Cytoskeleton. 2008;65:469–475. doi: 10.1002/cm.20273. [DOI] [PubMed] [Google Scholar]
- Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. Journal of Cell Biology. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF. Ciliogenesis: Building the cell's antenna. Nature Reviews Molecular Cell Biology. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Thompson J, Yates JR, 3rd, Marshall WF. Proteomic analysis of mammalian primary cilia. Current Biology. 2012;22:414–419. doi: 10.1016/j.cub.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T. Mechanical stress-mediated RUNX2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. Journal of Cellular Biochemistry. 2007;101:1266–1277. doi: 10.1002/jcb.21249. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG. Functional genomic screens for modulators of ciliogenesis and cilium length. Nature. 2010;464:1048–1051. doi: 10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Kamiya R. Axonemal dyneins: Assembly, structure, and force generation. In: Harris EH, Stern DB, Witman GB, editors. The Chlamydomonas Sourcebook. 2nd ed. Academic Press; 2009. pp. 131–208. [Google Scholar]
- Kotsis F, Nitschke R, Doerken M, Walz G, Kuehn EW. Flow modulates centriole movements in tubular epithelial cells. Pflügers Archiv European Journal of Physiology. 2008;456:1025–1035. doi: 10.1007/s00424-008-0475-8. [DOI] [PubMed] [Google Scholar]
- Lopez JI, Mouw JK, Weaver VM. Biomechanical regulation of cell orientation and fate. Oncogene. 2008;27:6981–6993. doi: 10.1038/onc.2008.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning DK, et al. Loss of the ciliary kinase Nek8 causes left–right asymmetry defects. Journal of the American Society of Nephrology. 2013;24:100–112. doi: 10.1681/ASN.2012050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Tournier AL, Hoppe A, Kester L, Thompson BJ, Tapon N. Differential proliferation rates generate patterns of mechanical tension that orient tissue growth. EMBO Journal. 2013;32:2790–2803. doi: 10.1038/emboj.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF, Kintner C. Cilia orientation and the fluid mechanics of development. Current Opinions in cell biology. 2008;20:48–52. doi: 10.1016/j.ceb.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan SR, Haycraft CJ, Jensen CG, Yoder BK, Pool CA. Articuilar cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biology. 2007;26:234–246. doi: 10.1016/j.matbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- McGlashan SR, Knight MM, Chowdhury TT, Joshi P, Jensen CG, Kennedy S, Poole CA. Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biology International. 2010;34:441–446. doi: 10.1042/CBI20090094. [DOI] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left–right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- Meng F, Sachs F. Orientation-based FRET sensor for real-time imaging of cellular forces. Journal of Cell Science. 2012;125:743–750. doi: 10.1242/jcs.093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer CJ, Alenghat FJ, Rim P, Fong JH, Fabry B, Ingber DE. Mechanical control of cyclic AMP signalling and gene transcription through integrins. Nature Cell Biology. 2000;2:666–668. doi: 10.1038/35023621. [DOI] [PubMed] [Google Scholar]
- Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Kasahara K, Miyazaki I, Asanuma M. Factors that influence primary cilium length. Acta Medica Okayama. 2011;65:279–285. doi: 10.18926/AMO/47009. [DOI] [PubMed] [Google Scholar]
- Morita Y, Shingyoji C. Effects of imposed bending on microtubule sliding in sperm flagella. Current Biology. 2004;14:2113–2118. doi: 10.1016/j.cub.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Nauli SM, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature Genetics. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Nguyen AM, Jacobs CR. Emerging role of primary cilia as mechanosensors in osteocytes. Bone. 2013;54:196–204. doi: 10.1016/j.bone.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. De novo formation of left–right asymmetry by posterior tilt of nodal cilia. PLOS Biology. 2005;3 doi: 10.1371/journal.pbio.0030268. art. e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor AK, Malarkey EB, Berbari NF, Croyle MJ, Haycraft CJ, Bell PD, Hohenstein P, Kesterson RA, Yoder BK. An inducible ciliaGFP mouse model for in vivo visualization and analysis of cilia in live tissue. Cilia. 2013;2 doi: 10.1186/2046-2530-2-8. art. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Takeda S, Tanaka Y, Izpisúa Belmonte J-C, Hirokawa N. Mechanism of nodal flow: A conserved symmetry breaking event in left–right axis determination. Cell. 2005;121:633–644. doi: 10.1016/j.cell.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–587. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- Patel A, Honore E. Polycystins and renovascular mechanosensory transduction. Nature Reviews Nephrology. 2010;6:530–538. doi: 10.1038/nrneph.2010.97. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. Journal of Cell Biology. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitaval A, Tseng Q, Bornens M, Théry M. Cell shape and contractility regulate diliogenesis in cell cycle-arrested cells. Journal of Cell Biology. 2010;191:303–312. doi: 10.1083/jcb.201004003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. Journal of Membrane Biology. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. Journal of Membrane Biology. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- Rafelski SM, Marshall WF. Building the cell: Design principles of cellular architecture. Nature Reviews Molecular Cell Biology. 2008;9:593–602. doi: 10.1038/nrm2460. [DOI] [PubMed] [Google Scholar]
- Resnick A, Hopfer U. Force-response considerations in ciliary mechanosensation. Biophysical Journal. 2007;93:1380–1390. doi: 10.1529/biophysj.107.105007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Fraticelli AE, Auzan M, Alonso MA, Bornens M, Martín-Belmonte F. Cell confinement controls centrosome positioning and lumen initiation during epithelial morphogenesis. Journal of Cell Biology. 2012;198:1011–1023. doi: 10.1083/jcb.201203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruwhof C, van Wamel JT, Noordzij LA, Aydin S, Harper JC, van der Laarse A. Mechanical stress stimulates phospholipase C activity and intracellular calcium ion levels in neonatal rat cardiomyocytes. Cell Calcium. 2001;29:73–83. doi: 10.1054/ceca.2000.0158. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ, Dirksen ER. Mechanosensitivity of cultured ciliated cells from the mammalian respiratory tract: Implications for the regulation of mucociliary transport. Proceedings of the National Academy of Sciences. 1986;83:7302–7306. doi: 10.1073/pnas.83.19.7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annual Review of Physiology. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Schneider L, et al. Directional cell migration and chemotraxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiology and Biochemistry. 2010;25:279–292. doi: 10.1159/000276562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. American Journal of Physiology. 1997;272:F132–F138. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- Takao D, Nemoto T, Abe T, Kiyonari H, Kajiura-Kobayashi H, Shiratori H, Nonaka S. Asymmetric distribution of dynamic calcium signals in the node of mouse embryo during left–right axis formation. Developmental Biology. 2013;376:23–30. doi: 10.1016/j.ydbio.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Thompson DW. On growth and form. Cambridge University Press; 1917. [Google Scholar]
- Van der Heiden K, et al. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis. 2008;196:542–550. doi: 10.1016/j.atherosclerosis.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Van der Heiden K, Egorova AD, Poelmann RE, Wentzel JJ, Hierck BP. Role for primary cilia as flow detectors in the cardiovascular system. International Review of Cell Molecular Biology. 2011;290:87–119. doi: 10.1016/B978-0-12-386037-8.00004-1. [DOI] [PubMed] [Google Scholar]
- Verra F, Escudier E, Bignon J, Pinchon MC, Boucherat M, Bernaudin JF, de Cremoux H. Inherited factors in diffuse bronchiectasis in the adult: A prospective study. European Respiratory Journal. 1991;4:937–944. [PubMed] [Google Scholar]
- Wakabayashi K-I, Ide T, Kamiya R. Calcium-dependent flagellar motility activation in chlamydomonas reinhardtii in response to mechanical agitation. Cell Motilility and Cytoskeleton. 2009;66:736–742. doi: 10.1002/cm.20402. [DOI] [PubMed] [Google Scholar]
- Wann AKT, Zuo N, Haycraft CJ, Jensen CG, Poole CA, McGlashan SR, Knight MM. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB Journal. 2012;26:1663–1671. doi: 10.1096/fj.11-193649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghi A, Zaman A, Cox G, Dolovich MB. Ciliary beating is depressed in nasal cilia from chronic obstructive pulmonary disease subjects. Respiratory Medicine. 2012;106:1139–1147. doi: 10.1016/j.rmed.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Yang Y, et al. Regulation of flagellar motility by the conserved flagellar protein CG34110/Ccdc135/FAP50. Molecular Biology of the Cell. 2011;22:976–987. doi: 10.1091/mbc.E10-04-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiba S, Hamada H. Roles of cilia, fluid flow, and Ca2+ signaling in breaking of left–right symmetry. Trends in Genetics. 2014;30:10–17. doi: 10.1016/j.tig.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Yoshiba S, et al. Cilia at the node of mouse embryos sense fluid flow for left–right determination via Pkd2. Science. 2012;338:226–231. doi: 10.1126/science.1222538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young Y-N, Downs M, Jacobs CR. Dynamics of the primary cilium in shear flow. Biophysics Journal. 2012;103:629–639. doi: 10.1016/j.bpj.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Sun Z. TORC1-mediated protein synthesis regulates cilia size and function: Implications for organelle size control by diverse signaling cascades. Cell Cycle. 2012;11:1750–1752. doi: 10.4161/cc.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]