Abstract
In developed countries, the majority of all violent crime is committed by a small group of antisocial recidivistic offenders, but no genes have been shown to contribute to recidivistic violent offending or severe violent behavior, such as homicide. Our results, from two independent cohorts of Finnish prisoners, revealed that a monoamine oxidase A (MAOA) low-activity genotype (contributing to low dopamine turnover rate) as well as the CDH13 gene (coding for neuronal membrane adhesion protein) are associated with extremely violent behavior (at least 10 committed homicides, attempted homicides or batteries). No substantial signal was observed for either MAOA or CDH13 among non-violent offenders, indicating that findings were specific for violent offending, and not largely attributable to substance abuse or antisocial personality disorder. These results indicate both low monoamine metabolism and neuronal membrane dysfunction as plausible factors in the etiology of extreme criminal violent behavior, and imply that at least about 5–10% of all severe violent crime in Finland is attributable to the aforementioned MAOA and CDH13 genotypes.
INTRODUCTION
Violent crime is a major issue that affects the quality of life even in stable and wealthy societies. In industrialized countries, the majority of all violent crime is committed by a relatively small group of antisocial recidivistic offenders,1,2 and more than 50% of severe antisocial behavior is attributable to genetic factors.3 The classic study by Mednick et al.,4 reported a significant correlation between adoptees and their biological parents for property crimes, but not for violent crimes. However, a recent study using an enormous Swedish nationwide adoption database with a long follow-up period found convincing evidence that the criminal records of biological parents predicted both violent and non-violent criminality among their adopted away children.5 Two decades ago, it was observed that a rare mutation leading to a complete deficiency of monoamine oxidase A (MAOA) was associated with impulsive and aggressive behavior in a Dutch kindred.6 Thus far, only two studies have reported an association between a specific gene and criminal violent offending.7,8 In the study by Caspi et al.,7 55 (12%) of the boys who were studied had a combination of the low-activity MAOA promoter genotype and childhood maltreatment, which accounted for 44% of the violent convictions in their cohort.7 Although this finding has not been replicated, and the majority of violent convictions in this cohort were not severe, such as homicide or attempted homicide, this MAOA variant has become widely called as a ‘warrior gene’. Recently, it was reported that this finding has actually started to influence the attitudes on court sentences in the US.9 A meta-analysis that included 11 000 individuals showed a significant interaction between the low-activity MAOA genotype and childhood adversities on a subsequent antisocial outcome.10 However, the largest study on this issue, with more than 4000 individuals, could not confirm the hypothesis that this MAOA genotype moderates the relationship between childhood maltreatment and antisocial behavior, but found statistically non-significant evidence for a main effect of MAOA genotype on having disposition toward violence.11 Thus, the issue of a ‘warrior gene’ has remained controversial.
In the study by Bevilacqua et al.,8 a stop codon in HTR2B was associated with substance abuse and a risk of committing impulsive crimes such as homicide, batteries and arsons, and impulsive behavior in mice. This finding indicated that HTR2B has a role in impulsivity, but it was not possible to conclude whether the functional variant was associated with substance abuse or violent behavior per se. In conclusion, no genes nor their polymorphisms have been shown to explain severe violent behavior in humans, nor contribute to recidivistic violent offending. Therefore, we conducted a candidate gene study on both MAOA and HTR2B, and performed a genome-wide association study (GWAS) in cohorts of Finnish violent offenders compared with the general population.
MATERIALS AND METHODS
Participants
The clinical and sociodemographic characteristics of the participants in this study are shown in Table 1.
Table 1.
The clinical and sociodemographic characteristics of the study participants
| A. Discovery cohort |
Non-violent offenders (ASP, substance abuse, maltreated) |
Violent offenders (ASP, substance abuse, maltreated) |
Extremely violent offenders (ASP, substance abuse, maltreated) |
Controls (ASP, substance abuse, maltreated) |
| GWAS (N) | ||||
| Males | 124 (71, 108, 22) | 339 (266, 327, 86) | 56 (47, 54, 15) | 3424 (NA, NA, NA) |
| Females | 12 (12, 11, 4) | 21 (21, 21, 10) | 2 (2, 2, 2) | 2559 (NA, NA, NA) |
| All | 136 (83, 119, 26) | 360 (287, 348, 96) | 58 (49, 56, 17) | 5983 (NA, NA, NA) |
| Age (mean±s.d.) | 37.3±10.7 | 37.4±8.8 | 36.7± 9.2 | 57.5± 13.3 |
| Regenotyping (N) | ||||
| Males | 176 (100, 155, 28) | 472 (368, 447, 124) | 75 (63, 73, 20) | 891 (NA, NA, NA) |
| Females | 27 (26, 23, 7) | 37 (35, 35, 13) | 3 (3, 3, 3) | 986 (NA, NA, NA) |
| All | 203 (126, 178, 35) | 509 (403, 482, 137) | 78 (66, 76, 23) | 1877 (NA, NA, NA) |
| Age (mean±s.d.) | 39.9±10.6 | 36±9.2 | 36.9± 8.5 | 50.7± 11.1 |
| B. Replication cohort (N) | Homicide offenders | Controls | ||
| All males | 103 (59, 96, NA) | 1786 (NA, NA, NA) | ||
| Age (mean±s.d.) | 29.4±8.2 | 53.3± 15.6 | ||
Abbreviations: APD, antisocial personality disorder; GWAS, genome-wide association study; NA, not applicable.
CRIME (Discovery cohort)
To obtain a comprehensive and representative cohort of Finnish offenders who had committed several severe violent crimes, we conducted a survey in the 19 largest prisons in Finland to identify such individuals. By using the National Prison Register, we obtained information on all prisoners serving their sentence in these prisons. We specifically asked for participation from those prisoners with Finnish origin who had committed at least two crimes that led to prison sentences as indications of an antisocial lifestyle. A total of 1004 prisoners were screened, of which, 184 (18.4%) refused to participate in the study and 26 (2.6%) were excluded because of a psychosis diagnosis. Altogether, the CRIME cohort included 794 prisoners. Those individuals who had committed only non-violent crimes were classified as non-violent offenders (n = 215). Those with at least one violent crime conviction were classified as violent offenders (n = 538) and those having committed at least 10 violent crimes were classified as extremely violent offenders (n = 84). The subgroup of extremely violent offenders overlapped completely with the violent offenders. Violent crime status was unknown for 41 individuals who were excluded from the cohort. Violent offences included murder, attempted murder, manslaughter, attempted manslaughter, other types of homicide and battery. Non-violent crimes were typically drunk-driving, drug-related crimes or property crimes. Offenders having committed only sexual crimes were excluded. The participants were interviewed with Structured Clinical Interview for DSM-IV- Disorders (SCID) to exclude individuals with a psychosis diagnosis, and to assess whether or not the subject fulfilled criteria for antisocial personality disorder. Also, any history of substance abuse (alcohol, heroin, buprenorphine, amphetamine, cannabis, other) was obtained through a questionnaire, which included also questions about maltreatment during childhood (e.g., good circumstances, indifferent parents or severe maltreatment such as family violence). The history of criminal convictions was obtained from the National Crime Register.
The subjects provided a written informed consent. This study was approved by the Ethics Committee for Pediatrics, Adolescent Medicine and Psychiatry, Hospital District of Helsinki and Uusimaa, and Criminal Sanctions Agency. All the subjects who participated in the study received a voucher of 20 euros for their participation.
Health 2000 control cohort
The Health 2000 study (http://www.terveys2000.fi/doc/methodologyrep.pdf) is a Finnish nationwide survey collected in 2000–2001. The study subjects were selected from the Social Insurance Institution (SII) of Finland (Kela) based on the assumption that these individuals would reflect the main demographic distributions of the Finnish population. The main aims of this survey were to characterize the public health problems in Finland, in terms of both physical and mental health and work-related traits. The study consisted of home interviews and a health examination that included a laboratory examination conducted at a local health center. Blood samples for DNA extraction were also collected. In total, Health 2000 included 8028 individuals from the general population who were over 30 years of age (54% were female). Of these, 2124 (26%) belonged to a GenMets sub-cohort who were genotyped by whole-genome chips. Originally, the GenMets sample was collected to study metabolic syndrome, where half of the individuals had metabolic syndrome and the other half were age- and gender-matched controls.12 A written informed consent was obtained from participants. This part of the study was approved by the ethics committee of the Helsinki University Central Hospital.
In the present study, the Genmets subpopulation (n = 2124) was used as a control group in the GWAS analyses. Following the discovery analyses and regenotyping, the entire Health 2000 cohort (n = 6600) was further used as a study cohort in the analyses of candidate single nucleotide polymorphisms (SNPs) for the general population.
FINRISK and Corogene control cohorts
The National FINRISK surveys have been conducted in Finland every 5 years since 1972. The first study included population-based samples from the provinces of North Karelia and Kuopio. This survey targeted persons between 30 and 59 years of age and focused on cardiovascular risk factors in the Finnish population. Since then, five more areas have been included, that is, Helsinki and Vantaa, Turku and Loimaa, North Savo, Oulu, and Lappland, and the age limits were expanded to 25–74 years (http://www.ktl.fi/finriski).
The Corogene study consists of 5000 consecutive Finnish patients (N = 5000) who were assigned to coronary angiogram by the Helsinki University Central Hospital from June 2006 to March 2008. Since then, a new cohort of the same size has been collected in 5-year intervals over a period of 12 months for each interval. A total of 2500 patients from the first Corogene cohort, as well as 2500 age-, geographical area- and sex-matched controls from the FINRISK surveys were selected for GWASs, and these were used as controls in the genome-wide association analysis of this study.13
Homicide offender cohort
The replication cohort included 114 violent offenders who had committed at least one homicide, and who underwent forensic mental examination because of the extreme nature of their crimes. All these offenders were males. The DNA samples were collected between 1987 and 1998, as a part of the Finnish-American studies (with NIAAA/NIH) (Behavioral, Biochemical, Endocrine and Genetic Study of Alcohol Abusing Violent Offenders). The violent offenders were studied as inpatients at the University Hospital of Helsinki. Written informed consent was obtained from each participant. Protocols were approved by the Institutional Review Board (IBR) of the US National Institute of Health (NIH) and the National Institute of Mental Health (NIMH), and also by the Office for Protection from Research Risks (OPRR), the University of Helsinki Department of Psychiatry IRB, the University of Helsinki Central Hospital IRB, the Finnish Ministry of Social Affairs and Health and the Ethics Committee of the National Public Health Institute of Finland.
Phenotypes
Violent offending
Violent offending was defined by counting those individuals with at least one sentence for any violent offence, based on data from the National Crime Register.
Extremely violent offending
The characterization of ‘extremely violent offending’ was obtained by first calculating percentiles from the quantitative trait of ‘violent offending’ for the entire sample of criminals, and then dichotomizing that by using the 90th percentile cutoff value in the GWAS (i.e., those convicted of 10 or more severe violent crimes) as the threshold. The 78 offenders who passed genotype quality control had committed a total of 1154 murders, manslaughters, attempted homicides or batteries (median 13, range 10–41). There was an overlap between these traits, as the extremely violent offenders were also included in the violent offender group.
Homicide offenders (replication cohort)
The individuals of the replication cohort were recruited through a forensic mental examination, which is ordered by the Finnish juridical system when a crime is severe, such as homicide, or an exceptionally brutal violent crime. All of these individuals had been convicted for at least one murder or manslaughter. No more detailed information on their criminal history was available.
Secondary outcomes in the population-based sample
In the Health 2000 sample, the diagnosis of lifetime alcohol use disorders (i.e., alcohol dependence and abuse) was obtained through a structured diagnosis-based evaluation on criteria from the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) for psychiatric disorders. A total of 577 individuals (males n = 470, females n = 107) in this sample had alcohol use disorder. An early childhood environment of economical difficulties or severe conflicts was retrospectively self-reported by the participants with the questions such as: ‘Did your childhood family have long-lasting financial problems?’ and ‘Were there severe conflicts in your childhood family?’. The possible response options included ‘Yes’, ‘No’ and ‘I don’t know’. Participants reporting ‘I don’t know’ were excluded from the analyses. Altogether, 30% of the sample had reported financial problems or severe conflicts in their family (29% of males, 32% of females).
Genotyping
Genotyping procedures are described in Supplementary Information.
RESULTS
Candidate gene analysis
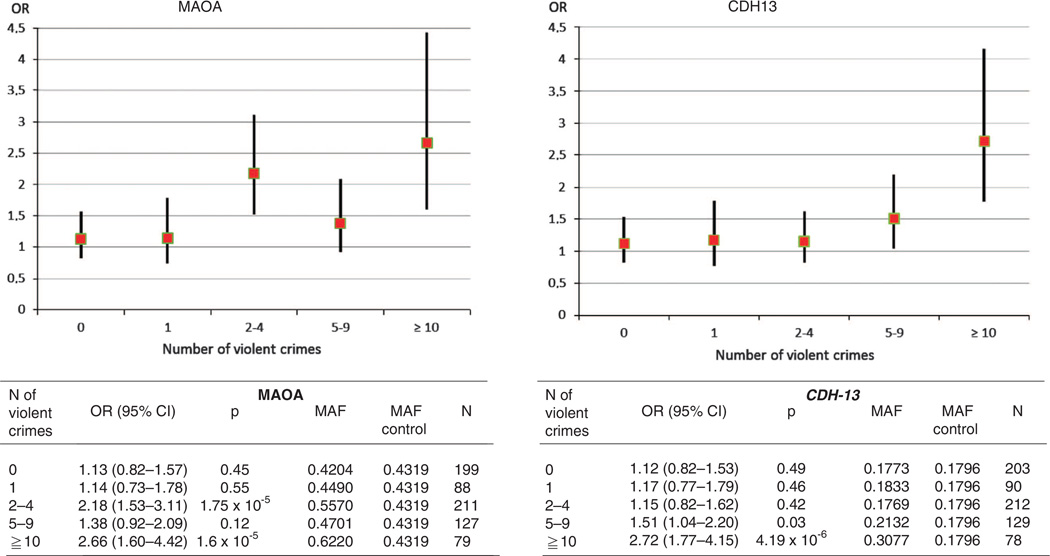
The low-activity MAOA genotype was associated with violent offending in the crime cohort (odds ratio (OR) 1.71, P = 2.9 × 10−5; attributable risk 9%, 95% confidence interval (CI) 4–15%). This finding did not differ between males and females, and childhood maltreatment did not modify the risk (OR 1.62; Supplementary Tables 1a and 1b). The association was even stronger among extremely violent offenders (OR 2.66, P = 1.6 × 10−4, attributable fraction 16%, 95% CI 8–24%; Figure 1). In the cohort of homicide offenders (N = 96), the OR was slightly diluted (1.50, 95% CI 0.82–2.72). No signal was detected from the previously reported HTR2B stop codon SNP (rs798745406) (allele frequency 1.5% among violent offenders vs 0.6% among extremely violent offenders vs 1.6% among controls, P > 0.5).
Figure 1.
Age- and gender-adjusted odds ratios for low-activity MAOA genotype and CDH13 (rs11649622) as a function of the number of committed violent crimes. The number of individuals, allele frequencies and odds ratios are shown below. The number of control subjects was 1946 for MAOA and 1877 for CDH13 analysis.
Genome-wide association analysis
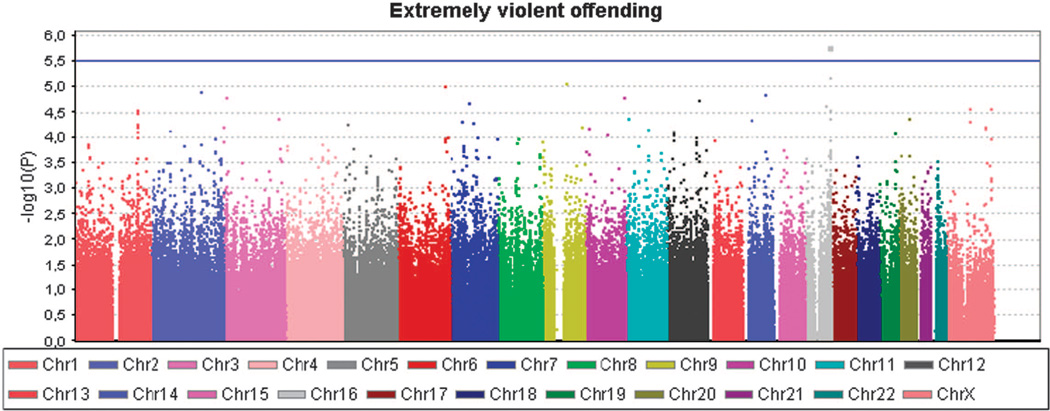
We first performed a genome-wide association analysis of violent criminal behavior as two different traits: Violent offending (at least one conviction for violent crime, N = 360 for GWAS) and extremely violent offending (10 or more homicides and/or batteries, N = 58). In the Supplementary Material, the most significant 50 associations are presented with violent offending and extremely violent offending, respectively (Supplementary Tables 2a and 2b). The genomic inflation factor lambda (λ) for violent offending was 1.0081, and 0.9966 for extremely violent offending, reflecting no significant genomic inflation/deflation in either analysis. The quantile-quantile plot for the observed vs expected results (−2log(P-value)) for the analysis of violent offending resulted in more significant associations than expected by chance, but none of these reached the genome-wide significance threshold of P < 5 × 10−8 (Supplementary Figure 2a). In the quantile-quantile plot of the analysis for extremely violent offending, only one data point was observed above the lift off line (Supplementary Figure 2b).
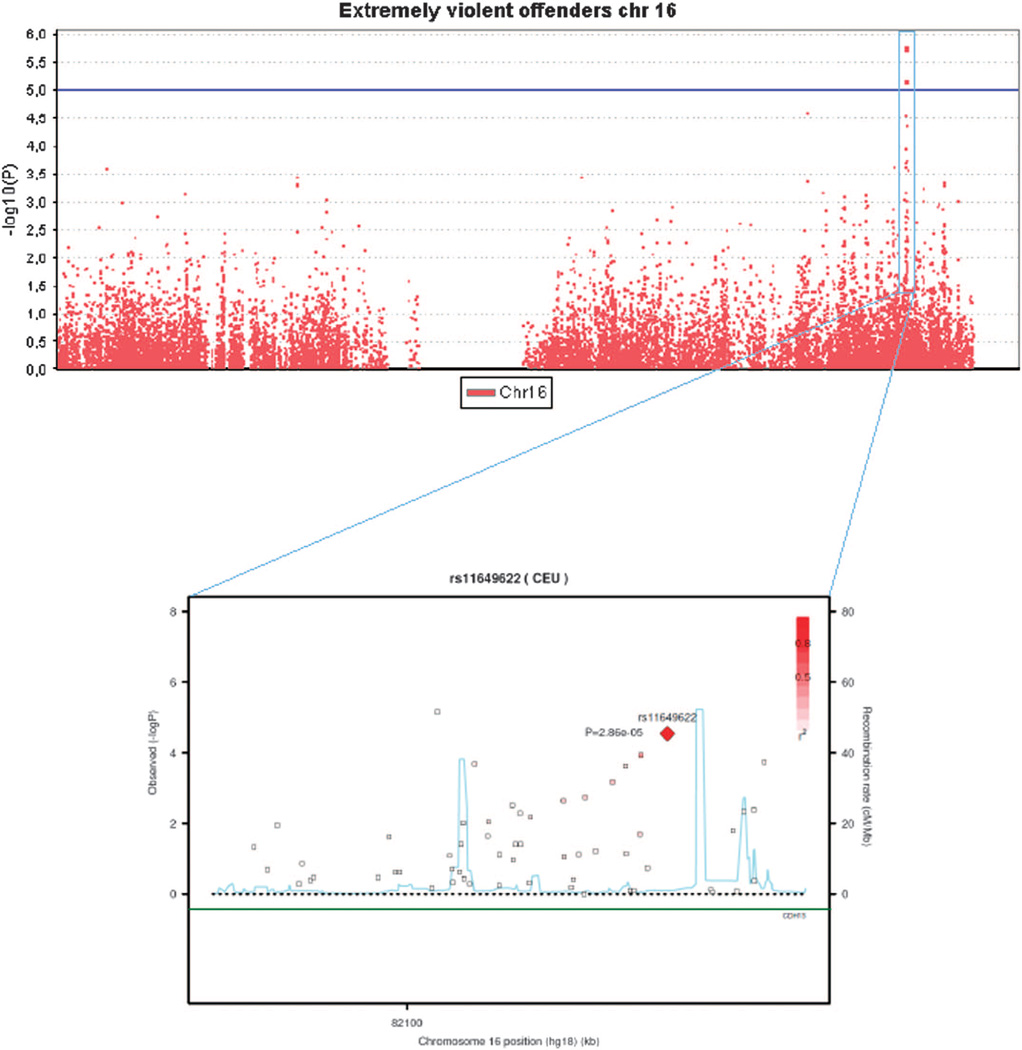
The strongest association signals (P < 1.0 × 10−5) were observed for the violent offenders for two variants located on chromosome 9q22.1 at the SPIN1 gene (P = 3.3 − 10−6 for rs10123897 and 3.4 × 10−6 for rs11142288), and for the extremely violent offenders for two markers on chromosome 16q23.3 at the CDH13 gene (P = 1.7 × 10−6 for rs12919501 and 6.8 × 10−6 for rs4075942), and for single marker at chromosome 9q21.2 at PRUNE2 gene (P = 8.5 × 10−6 for rs17787449). These five markers were selected for subsequent genotyping in the extended discovery cohort to increase the sample size and to verify that genotyping with different method had not caused batch effects between the case and the control sample in the discovery (CRIME) cohort. In addition, two additional markers from the CDH13 gene that were among the 25 best genome-wide signals in the extremely violent offenders' group were genotyped in the discovery cohort. Analysis with the regenotyped sample produced concordant genotypes with the original genotyping. Figure 2 shows Manhattan plot in whole genome, and Figure 3 in chromosome 16, with a more detailed view of the SNPs in the region in association with extremely violent offending. The most consistent signal is seen in locus 16q23. The results for six variants within the MAOA gene are shown in Supplementary Table 3. The ORs for the five common variants were 1.1 among the entire violent offender groups, and from 1.4 to 1.7 among the extremely violent offenders (P > 0.06).
Figure 2.
Manhattan plot of extremely violent offenders GWAS. The highest cluster is seen in locus 16q23.3.
Figure 3.
Manhattan plot in chromosome 16 (generated by Haploview) for the GWAS of extremely violent offenders. The insert shows a detailed view (generated by SNAP) of the CDH13 region in association with extremely violent offending.
Analysis of single variants for the CRIME cohort and in the replication sample
Altogether seven variants were genotyped in the CRIME cohort (N = 509) and in 6600 individuals from the Health 2000 sample. Neither of the regenotyping analyses revealed any genome-wide significant signals (P < 5 × 10−8), and no suggestive findings (P < 1 × 10−5) were observed for an increased risk of violent offending, regardless of whether the individuals had been maltreated or not. The association signal for SPIN1 variants with violent offenders originated from the major alleles and were somewhat diluted with the complete data from the CRIME cohort (P = 2.4 × 10−5 for rs10123897 and 1.7 × 10−5 for rs11142288). Analysis of the extremely violent offender data with the complete dataset (n = 78 cases) (Supplementary Table 4) shows a suggestive association of P < 1.0 × 10−5 and a strengthening of the association signal only for rs11649622, which is located in the intronic region of the CDH13 gene (OR = 2.7, P = 4.19 × 10−6). Two other CDH13 variants (rs4075942 and rs12919501), located within 100 kb of the most significant variant, had P-values < 5 × 10−4. Haplotype analysis of the CDH13 variants provided the strongest evidence for an association of a relatively rare haplotype (f = 0.092) of minor alleles A-A from rs11649622 and rs7190768 (P = 6.2 × 10−7, OR = 4.32; Supplementary Figure 3). The haplotype G-G-A-A covering all the genotyped CDH13 variants (rs12919501, rs4075942, rs11649622 and rs7190768) yielded a P-value of 7.0 × 10−6 (OR = 7.21, f = 0.030) for extreme violent offending.
The variants from CDH13 were genotyped in an independent replication sample of 103 homicide offenders to test for a possible replication association of rs11649622 (as a single variant) and of the variants rs12919501, rs4075942, rs11649622 and rs7190768 (as 2- and 4-nt haplotypes; Table 2). Rs11649622 was associated with homicide offending with P < 0.05 (P = 0.013; OR 1.73, corresponding to attributable risk of 4%). The haplotypes A-A from rs11649622 and rs7190768, and G-G-A-A from rs12919501, rs4075942, rs11649622 and rs7190768 gave signals of P = 0.019 (OR = 2.05) and P = 0.023 (OR = 3.1), respectively, in the replication sample. Thus, the findings for an association of both rs11649622 and CDH13 haplotypes were replicated in the sample of homicide offenders. Post hoc analysis of other single CDH13 variants showed no signal of association (P > 0.2) in the replication sample, but there was a signal of association of the haplotype G-G-A from rs11649622, rs12919501 and rs4075942 to homicide offending (P = 0.013, OR 2.44). When discovery and replication cohorts were pooled in the meta-analysis, a P-value of 5.3 × 10−7 (OR = 2.17) was achieved for rs11649622.
Table 2.
Results for CDH13 SNPs from the analysis of extremely violent offenders, replication and meta-analysis
| Discovery sample | Replication sample | Meta-analysis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHR | SNP | BP | A1 | A2 | P | OR | MAF Cases |
MAF Controls |
P | OR | MAF Cases |
MAF Controls |
P | P(R) | OR | OR(R) |
| 16 | rs11649622 | 83605265 | A | G | 4.19E-06 | 2.715 | 0.3077 | 0.1796 | 0.01333 | 1.725 | 0.2184 | 0.1751 | 5.3E-07 | 0.000647 | 2.172 | 2.168 |
| 16 | rs4075942 | 83549820 | G | A | 0.00023 | 2.502 | 0.1859 | 0.0936 | 0.2368 | 1.445 | 0.1019 | 0.0862 | 0.0003 | 0.01342 | 2.019 | 1.962 |
| 16 | rs12919501 | 83511016 | G | A | 0.00049 | 2.215 | 0.2372 | 0.1261 | 0.2642 | 1.319 | 0.1553 | 0.1202 | 0.0009 | 0.03532 | 1.746 | 1.725 |
| 16 | rs7190768 | 83741891 | A | G | 0.00181 | 1.779 | 0.4936 | 0.3471 | 0.2892 | 1.207 | 0.3932 | 0.3709 | 0.00342 | 0.05074 | 1.455 | 1.461 |
Abbreviations: BP, basepair; MAF, minor allele frequency; OR, odds ratio; SNP, single nucleotide polymorphism.
Note; build GRCh37/hg19.
Figure 1 shows ORs for rs11649622 among subpopulations of the offenders. Although the risk was not substantially elevated (OR < 1.2) among those with 0, 1 or 2–4 violent crimes, it was significantly increased among offenders who had committed 5–9 violent crimes (OR 1.51, 95% CI 1.04, 2.20) or 10 or more crimes (OR 2.72, 95% CI 1.77, 4.15).
The overlap between MAOA and CDH13 genotypes among the extremely violent offenders are shown in Supplementary Table 5. The OR for combined homozygosity for low-activity MAOA and rs11649622 minor allele was 13.45 (95% CI 3.86–46.94).
Results from the population-based sample
Thus, only rs11649622 showed replicable GWAS-based findings in the two cohorts for extremely violent offenders. As the risk allele (A) is relatively frequent in the general population (f = 0.178 in Health 2000 sample and 0.174 in HapMap-CEU European population), we examined its possible contribution to alcoholism, a trait strongly associated with violent crimes in epidemiological studies. We hypothesized that the risk variant would exert a genetic risk in cases where individuals encountered difficulties or maltreatment during their early lives, for example, economic difficulties or other childhood family trauma. The variant associated to alcoholism among cases with the childhood risk environment (P = 0.024, OR = 1.36), but not among those without this environmental risk (P = 0.825, OR = 1.02). Furthermore, the logistic regression model included an interaction term that showed a significant gene–environment interaction (P = 5.7 × 10−4) for the childhood risk environment and alcoholism in the Health 2000 sample.
DISCUSSION
To our knowledge, this is the first study to investigate the genetic background of severe recidivistic violent behavior. Our candidate gene study yielded a highly statistical significance for low-activity MAOA genotype, but not for HTR2B. Caspi et al.,7 observed among about 50 violent offenders that the low-activity genotype was associated with offending, but only when concomitant childhood maltreatment was present. Our results, from over 500 offenders, showed a strong main effect for this genotype, but maltreatment did not modify the risk in any way. This may be explained by difference between study populations: the offenders in the Dunedin cohort were young individuals with any type of conviction for a violent crime, whereas our study subjects had committed at least one severe violent crime that led to a prison sentence. Even in this prison population, the main effect for MAOA was only seen among those offenders who had committed at least two severe violent crimes. A majority of all severe violent crimes in Finland are committed under the influence of alcohol or amphetamine,14 both of which induce a transient increase in the dopamine levels in the brain. Therefore, it is logical to assume that a low dopamine metabolism rate due to low-activity MAOA genotype may result in higher level of aggression during alcohol or stimulant intoxication. The low-activity MAOA genotype also affects the metabolism of serotonin and serotonin signaling within the corticolimbic circuitry, leading to increased impulsive aggression.15 The serotonin re-uptake inhibitor fluoxetine has been shown to decrease aggressive behavior among low-activity MAOA genotype mice,16 and serotonin re-uptake inhibitors have proven effective in placebo-controlled randomized trials in humans.17
The high proportion of intoxicated offenders in our cohort is well in line with a comprehensive review on the toxicology of homicidal offenders in developed countries, which concluded that the majority of offenders are intoxicated by a psychoactive substance at the time of homicide, with alcohol being the most commonly reported substance.18 Our results indicate that about 9% of severe violent crime in Finland is attributable to the low-activity MAOA genotype, which is very close to the results of Caspi et al.,7 who estimated that about 11% of any violent crime in the New Zealand cohort was attributable to this low-activity genotype and childhood maltreatment. However, it is essential to emphasize that the sensitivity and specificity of the genotype findings are much too low for any screening purposes, either for primary or secondary prevention of violent offending. It is equally important to realize that, according to the basic principles of forensic psychiatry, only the actual mental capability (phenotype) of the offender matters when punishment or legal responsibility is considered, and the putative risk factors per se (such as genotype) have no legal role in the resulting judgment. Concerning the secondary prevention of violent crime in general, it is obvious that the use of alcohol, amphetamine and other substances that induce a transient dopamine burst, and subsequent aggressive behavior,19 should be minimized after release from prison. This could be carried out by implementing obligatory supervised treatment with disulfiram20 or long-acting naltrexone21,22 as part of probation surveillance.
In a previous study by Bevilacqua et al.,8 it was observed that a stop codon in HTR2B was associated with substance abuse and impulsive crimes, such as homicides, batteries and arsons, but it was not possible to demonstrate that the genotype would be specific to violent behavior. In the present study, no association was detected between the HTR2B genotype and violent offending. The current sample differs from the previous by not including the arsonists, which comprised a large proportion of offenders in the previous study. This suggests that a stop codon genotype may be associated with impulsivity but not with severe or recidivistic violent offending per se.
GWAS results revealed one suggestive polymorphism that was specifically associated with an extremely violent phenotype, that is, individuals who had been convicted of at least 10 aggravated violent crimes. The locus at chromosome 16q23.3 was inside the intronic region of a gene that codes for neural adhesion protein (CDH13), where three hits were observed within a span of 100 kb. The best hit was replicated in an independent cohort of prisoners who had been convicted of at least one homicide. Thus far, more than 20 studies have found associations between CDH13 and attention deficit/hyperactivity disorder (ADHD), autism, schizophrenia, substance abuse or bipolar disorder.23–33 CDH13 was identified as a ADHD-related gene in 2008, and it is one of the most important genes associated with ADHD,26 which is a disorder strongly associated with violent criminality.34,35 Our best hit, the rs11649622 genotype, has been observed to be linked to CDH13 expression especially in amygdala,36 and other CDH13 variants have been observed to be associated with working memory24 and cortical thickness in dorsolateral prefrontal cortex of patients with ADHD.37 CDH13 codes for T-cadherin, a GPI-anchored protein, which has an important role in the proliferation, migration and connectivity of neurons,30,32 and disturbed neural connectivity has been proposed as the main pathophysiological mechanism underlying behavioral problems in ADHD.30 A recent meta-analysis concluded that about 25–30% of prisoners fulfill criteria for ADHD.38 Our study did not include a diagnostic interview for ADHD, but it is likely that many of the participants had ADHD symptoms. As CDH13 is currently considered the most important gene in ADHD, it is logical to assume that the CDH13 genotype contributes to deficit in impulse control, which is one of the core symptoms of ADHD. About 80% of Finnish homicides are impulsive, non-meditated crimes,14 and it is plausible that an impulse control deficit is a link between the CDH13 genotype and impulsive violent behavior. On the basis of the replication cohort results, up to several per cent of violent crimes in Finland may be attributable to this CDH13 genotype.
It has been concluded that the role of cadherin-13 as a signaling molecule may be more important than its role as a cell adhesion molecule, and that it regulates several key signaling pathways, such as the P13K-AKT pathway and small RhoGTPases signaling.30 CDH13 is expressed especially in 5-HT neurons, and RNAi of CDH13 produces a decrease in the density of both GABAergic and glutamatergic synapses.30 In addition to its role in neuronal development, CDH13 is also believed to contribute in the maintenance of excitatory and inhibitory synapses.39 As our best SNP results were located within 100 kb of the intronic region, sequencing of this locus is needed to find the exact functional variant at this locus.
Criminal behavior is a complex phenomenon, and the outcome is shaped by both genetic and environmental factors.40,41 Although the majority of individuals who commit petty crimes, such as minor traffic offences, are not mentally disturbed, the proportion of mentally disturbed individuals is high among those who have committed severe crimes, such as multiple homicides.42 Therefore, it is plausible that while research of the genetic background of criminal or violent behavior is hampered by many confounding factors, focusing on extreme phenotypes might yield more robust results. This was demonstrated in our analysis on the association between rs11649622 and MAOA genotypes vs the number of committed violent crimes, showing clear dose–response effects. However, collecting data from extreme phenotypes is difficult. For this reason, our number of study subjects was relatively small, which resulted in a rather low statistical power in the GWAS. However, the GWAS results provided a useful screening mechanism for a candidate locus, and replication of the best hit (CDH13) in the independent cohort was one of the two main results from our study. Concerning statistical significance, the P-value of 2.9 × 10−5 for the low-activity MAOA variant can be considered as extremely significant, as the Bonferroni-corrected level of significance in the candidate gene study was 0.025. Concerning sensitivity, there may be many false-negative associations, but that is unavoidable because of the size of the index cohort and the stringent validation criteria applied to avoid any false-positive findings.
To our knowledge, no previous studies have controlled the effect of co-existing substance abuse and antisocial personality disorder when studying the genetic background of severe violent behavior. Results from our population-based sample showed a modestly increased risk for alcoholism with rs11649622, which was especially attributable to those who had severe problems in childhood. However, our results from the present study indicate that CDH13 and low-activity MAOA are quite specific to violent crime, as the ORs of the SNPs for offenders having committed only non-violent crimes (predominantly antisocial individuals with substance abuse) were below 1.2, indicating only a slight association with substance abuse and a criminal lifestyle per se. The results indicate both low monoamine metabolism and neuronal membrane dysfunction as plausible factors in the etiology of extreme criminal violent behavior, and a conservative estimate implies that 5–10% of all severe violent crime in Finland is attributable to specific MAOA and CDH13 genotypes.
Supplementary Material
ACKNOWLEDGMENTS
We thank Auli Toivola for her contribution in the Sequenom Mass Array and MAOA VNTR genotyping and Aija Räsänen for secretarial assistance. The study was funded by the Finnish Ministry of Health and Social Affairs through the development fund for Niuvanniemi Hospital, Finland. Hanna M Ollila has received funds from Instrumentarium Science Foundation and Orion-Farmos Research Foundation. Kati Kristiansson has received grant from Orion-Farmos Research Foundation and Academy of Finland (grant number 250207).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
REFERENCES
- 1.Hamparin DM, Schuster R, Dinstz S, Conrad JP. The Violent Few: A Study of Violent Offenders. Lexington, MA: Lexington Books; 1978. [Google Scholar]
- 2.Tracy PE, Wolfgang ME, Figlio RM. Delinquency in Two Birth Cohorts. New York: Plenum Press; 1990. [Google Scholar]
- 3.Ferguson CJ. Genetic contributions to antisocial personality and behavior: a meta-analytic review from an evolutionary perspective. J Soc Psychol. 2010;150:160–180. doi: 10.1080/00224540903366503. [DOI] [PubMed] [Google Scholar]
- 4.Mednick SA, Gabrielli WH, Hutchings B. Genetic influences in crime convictions: evidence from and adoption cohort. Science. 1984;224:891–894. doi: 10.1126/science.6719119. [DOI] [PubMed] [Google Scholar]
- 5.Hjalmarsson R, Lindquist MJ. The origins of intergenerational associations in crime: Lessons from Swedish adoption data. Labour Econom. 2013;20:68–81. [Google Scholar]
- 6.Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- 7.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 8.Bevilacqua L, Doly S, Kaprio J, Yuan Q, Tikkanen R, Paunio T, et al. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468:1061–1066. doi: 10.1038/nature09629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aspinwall LG, Brown TR, Tabery J. The double-edged sword: does biomechanism increase or decrease judges' sentencing of psychopaths? Science. 2012;337:846–849. doi: 10.1126/science.1219569. [DOI] [PubMed] [Google Scholar]
- 10.Byrd AL, Manuck SB. MAOA, childhood maltreatment, and antisocial behavior: meta-analysis of a gene-environment interaction. Biol Psychiatry. 2014;75:9–17. doi: 10.1016/j.biopsych.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haberstick BC, Lessema JM, Hewitta JK, Smolena A, Hopferb CJ, Halpernc CT, et al. MAOA genotype, childhood maltreatment, and their interaction in the etiology of adult antisocial behaviors. Biol Psychiatry. 2014;75:25–30. doi: 10.1016/j.biopsych.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristiansson K, Perola M, Tikkanen E, Kettunen J, Surakka I, Havulinna AS, et al. Genome-wide screen for metabolic syndrome susceptibility Loci reveals strong lipid gene contribution but no evidence for common genetic basis for clustering of metabolic syndrome traits. Circ Cardiovasc Genet. 2012;5:242–249. doi: 10.1161/CIRCGENETICS.111.961482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaara S, Nieminen MS, Lokki ML, Perola M, Pussinen PJ, Allonen J, et al. Cohort Profile: The Corogene study. Int J Epidemiol. 2012;41:1265–1271. doi: 10.1093/ije/dyr090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehti M. Henkirikoskatsaus 2013. OPTL Verkkokatsauksia 29/2013. Helsinki: Oikeuspoliittinen tutkimuslaitos. 2013 (in Finnish). [Google Scholar]
- 15.Dorfman HM, Meyer-Lindenberg A, Buckholtz JW. Neurobiological mechanisms for impulsive-aggression: The role of MAOA. Curr Top Behav Neurosci. 2014;17:297–313. doi: 10.1007/7854_2013_272. [DOI] [PubMed] [Google Scholar]
- 16.Godar SC, Bortolato M, Castelli MP, Casti A, Casu A, Chen K, et al. The aggression and behavioral abnormalities associated with monoamine oxidase A deficiency are rescued by acute inhibition of serotonin reuptake. J Psychiatr Res. 2014;56:1–9. doi: 10.1016/j.jpsychires.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vartiainen H, Tiihonen J, Putkonen A, Koponen H, Virkkunen M, Hakola P, Lehto H. Citalopram, a selective serotonin reuptake inhibitor, in the treatment of aggression in schizophrenia. Acta Psychiatr Scand. 1995;91:348–351. doi: 10.1111/j.1600-0447.1995.tb09793.x. [DOI] [PubMed] [Google Scholar]
- 18.Darke S. The toxicology of homicide offenders and victims: a review. Drug Alcohol Rev. 2010;29:202–215. doi: 10.1111/j.1465-3362.2009.00099.x. [DOI] [PubMed] [Google Scholar]
- 19.Yanowitch R, Coccaro EF. The neurochemistry of human aggression. Adv Genet. 2011;75:151–169. doi: 10.1016/B978-0-12-380858-5.00005-8. [DOI] [PubMed] [Google Scholar]
- 20.Laaksonen E, Koski-Jännes A, Salaspuro M, Ahtinen H, Alho H. A randomized, multicentre, open-label, comparative trial of disulfiram, naltrexone and acamprosate in the treatment of alcohol dependence. Alcohol Alcohol. 2008;43:53–61. doi: 10.1093/alcalc/agm136. [DOI] [PubMed] [Google Scholar]
- 21.Tiihonen J, Krupitsky E, Verbitskaya E, Blokhina E, Mamontova O, Föhr J, et al. Naltrexone implant for the treatment of polydrug dependence: a randomized controlled trial. Am J Psychiatry. 2012;169:531–536. doi: 10.1176/appi.ajp.2011.11071121. [DOI] [PubMed] [Google Scholar]
- 22.Neale BM, Medland S, Ripke S, Anney RJL, Asherson P, Buitelaar J, et al. Case-control genome-wide association study of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:906–920. doi: 10.1016/j.jaac.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sizoo B, van den Brink W, Franke B, Vasquez AA, van Wijngaarden-Cremers P, van der Gaag RJ. Do candidate genes discriminate patients with an autism spectrum disorder from those with attention deficit/hyperactivity disorder and is there an effect of lifetime substance use disorders? World J Biol Psychiatry. 2010;11:699–708. doi: 10.3109/15622975.2010.480985. [DOI] [PubMed] [Google Scholar]
- 24.Arias-Vásquez A, Altink ME, Rommelse NN, Slaats-Willemse DI, Buschgens CJ, Fliers EA, et al. CDH13 is associated with working memory performance in attention deficit/hyperactivity disorder. Genes Brain Behav. 2011;10:844–851. doi: 10.1111/j.1601-183X.2011.00724.x. [DOI] [PubMed] [Google Scholar]
- 25.Edwards AC, Aliev F, Bierut LJ, Bucholz KK, Edenberg H, Hesselbrock V, et al. Genome-wide association study of comorbid depressive syndrome and alcohol dependence. Psychiatr Genet. 2012;22:31–41. doi: 10.1097/YPG.0b013e32834acd07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohannim O, Hibar DP, Stein JL, Jahanshad N, Hua X, Rajagopalan P, et al. Discovery and replication of gene influences on brain structure using LASSO regression. Front Neurosci. 2012;6:115. doi: 10.3389/fnins.2012.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart AB, Engelhardt BE, Wardle MC, Sokoloff G, Stephens M, Harriet de Wit, et al. Genome-wide association study of d-amphetamine response in healthy volunteers identifies putative associations, including cadherin 13 (CDH13) PLoS One. 2012;7:e42646. doi: 10.1371/journal.pone.0042646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesca G, Rudolf G, Labalme A, Hirsch E, Arzimanoglou A, Genton P, et al. Epileptic encephalopathies of the Landau-Kleffner and continuous spike and waves during slow-wave sleep types: genomic dissection makes the link with autism. Epilepsia. 2012;53:1526–1538. doi: 10.1111/j.1528-1167.2012.03559.x. [DOI] [PubMed] [Google Scholar]
- 29.Mavroconstanti T, Johansson S, Winge I, Knappskog PM, Haavik J. Functional properties of rare missense variants of human CDH13 found in adult attention deficit/hyperactivity disorder (ADHD) patients. PLoS One. 2013;8:e71445. doi: 10.1371/journal.pone.0071445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivero O, Sich S, Popp S, Schmitt A, Franke B, Lesch KP. Impact of the ADHD-susceptibility gene CDH13 on development and function of brain networks. Eur Neuropsychopharmacol. 2013;23:492–507. doi: 10.1016/j.euroneuro.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Børglum AD, Demontis D, Grove J, Pallesen J, Hollegaard MV, Pedersen CB, et al. GROUP investigators Genome-wide study of association and interaction with maternal cytomegalovirus infection suggests new schizophrenia loci. Mol Psychiatry. 2014;19:325–333. doi: 10.1038/mp.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mavroconstanti T, Halmøy A, Haavik J. Decreased serum levels of adiponectin in adult attention deficit hyperactivity disorder. Psychiatry Res. 2014;216:123–130. doi: 10.1016/j.psychres.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Xu W, Cohen-Woods S, Chen Q, Noor A, Knight J, Hosang G, et al. Genome-wide association study of bipolar disorder in Canadian and UK populations corroborates disease loci including SYNE1 and CSMD1. BMC Med Genet. 2014;15:2. doi: 10.1186/1471-2350-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundström S, Forsman M, Larsson H, Kerekes N, Serlachius E, Långström N, et al. Childhood neurodevelopmental disorders and violent criminality: A sibling control study. J Autism Dev Disord. 2013 Jun 27; doi: 10.1007/s10803-013-1873-0. advance online publication, (e-pub ahead of print). [DOI] [PubMed] [Google Scholar]
- 35.Satterfield JH, Faller KJ, Crinella FM, Schell AM, Swanson JM, Homer LD. A 30-year prospective follow-up study of hyperactive boys with conduct problems: adult criminality. J Am Acad Child Adolesc Psychiatry. 2007;46:601–610. doi: 10.1097/chi.0b013e318033ff59. [DOI] [PubMed] [Google Scholar]
- 36.GTEx. Genotype-Tissue Expression Portal. [accessed May 27, 2014]; http://www.gtexportal.org/home/searchEqtls. [Google Scholar]
- 37.Doyle AE, Faraone SV, McGrath L, Thermenos H, Juelich R, Chaponis J, et al. Multivariate association of CDH13 variants and cortical thickness in ADHD. Society of Biological Psychiatry 2014 Annual Meeting, New York, 2014. Biol Psychiatry. 2014;75:347S. [Google Scholar]
- 38.Young S, Moss D, Sedgwick O, Fridman M, Hodgkins P. A meta-analysis of the prevalence of attention deficit hyperactivity disorder in incarcerated populations. Psychol Med. 2014;7:1–12. doi: 10.1017/S0033291714000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paradis S, Harrar DB, Lin Y, Koon AC, Hauser JL, Griffith EC, et al. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron. 2007;53:217–232. doi: 10.1016/j.neuron.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cloninger CR, Christiansen KO, Reich T, Gottesman II. Implications of sex differences in the prevalences of antisocial personality, alcoholism, and criminality for familial transmission. Arch Gen Psychiatry. 1978;35:941–951. doi: 10.1001/archpsyc.1978.01770320035002. [DOI] [PubMed] [Google Scholar]
- 41.Carey G, Gottesman II. Genes and antisocial behavior: perceived versus real threats to jurisprudence. J Law Med Ethics. 2006;34:342–351. doi: 10.1111/j.1748-720X.2006.00039.x. [DOI] [PubMed] [Google Scholar]
- 42.Tiihonen J, Hakola P. Psychiatric disorders and homicide recidivism. Am J Psychiatry. 1994;151:436–438. doi: 10.1176/ajp.151.3.436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.