Abstract
Background
Disruption of epithelial tight junctions (TJ), gut barrier dysfunction and endotoxemia play crucial role in the pathogenesis of alcoholic tissue injury. Occludin, a transmembrane protein of TJ, is depleted in colon by alcohol. However, it is unknown whether occludin depletion influences alcoholic gut and liver injury.
Methods
Wild type (WT) and occludin deficient (Ocln−/−) mice were fed 1–6% ethanol in Lieber-DeCarli diet. Gut permeability was measured by vascular-to-luminal flux of FITC-inulin. Junctional integrity was analyzed by confocal microscopy. Liver injury was assessed by plasma transaminase, histopathology and triglyceride analyses. The effect of occludin depletion on acetaldehyde-induced TJ disruption was confirmed in Caco-2 cell monolayers.
Results
Ethanol feeding significantly reduced body weight gain in Ocln−/− mice. Ethanol increased inulin permeability in colon of both WT and Ocln−/− mice, but the effect was 4-fold higher in Ocln−/− mice. The gross morphology of colonic mucosa was unaltered, but ethanol disrupted the actin cytoskeleton, induced redistribution of occludin, ZO-1, E-cadherin and β-catenin from the junctions and elevated TLR4, which was more severe in Ocln−/− mice. Occludin knockdown significantly enhanced acetaldehyde-induced TJ disruption and barrier dysfunction in Caco-2 cell monolayers. Ethanol significantly increased liver weight and plasma transaminase activity in Ocln−/− mice, but not in WT mice. Histological analysis indicated more severe lesions and fat deposition in the liver of ethanol-fed Ocln−/− mice. Ethanol-induced elevation of liver triglyceride was also higher in Ocln−/− mice.
Conclusion
This study indicates that occludin deficiency increases susceptibility to ethanol-induced colonic mucosal barrier dysfunction and liver damage in mice.
Keywords: Alcohol, tight junction, claudin, Actin, fatty liver, adherens junction
Introduction
Epithelial cells lining the gut lumen provide the first line of defense by maintaining an intricate balance between absorption of nutrients and prevention of harmful substances from entering into internal organs. Epithelial tight junctions (TJ) partly impart this sieving capacity and maintain gut mucosal homeostasis. Dysregulation of gut homeostasis due to stress, infection, altered gut flora or immune response may lead to change or damage in TJ and vice versa. Such conditions are involved in the pathogenesis of various gastrointestinal and other pathologies, such as inflammatory bowel disease, fatty liver disease, viral or bacterial infection, type-1 diabetes and allergies among many others. Alcoholic liver disease is associated with disruption of TJ, increased gut permeability and endotoxemia [1, 2]. Although the disruption of and barrier dysfunction in Caco-2 cell monolayers by ethanol or its metabolic product, acetaldehyde, has been addressed by us and others [3–6], the mechanistic aspect of intestinal mucosal barrier dysfunction and increased gut permeability in mice and rats by ethanol feeding has not been defined yet [1, 2]. It is essential to understand the mechanisms involved in alcohol-mediated TJ disruption and barrier dysfunction in order to understand the pathogenesis of alcoholic diseases and design of novel therapeutics.
Occludin is one of the transmembrane proteins of TJ. A single occludin gene exhibits alternative splicing resulting in four splice variants [7] which are under tight regulation at the post transcriptional level [8]. The C-terminal domain of occludin is highly conserved, the phosphorylation status of which is important in TJ assembly and disassembly in different epithelia under varying physiologic/pathophysiologic conditions [9–13]. Occludin down regulation in keratinocytes decreases cell-cell adhesion, Ca2+ homeostasis and reduces susceptibility of these cells to apoptosis [14]. Interference with differential occludin expression during follicular development suppresses follicular growth in primates [15]. Another study using cell lines of different origin shows critical role of occludin in regulating polarized migration during wound healing [16]. Occludin is required for recruiting aPKC-Par3/PA-TJ complex to the leading edge and is crucial for activation of PI3K and lamellopodia formation during cell migration. Proteasomal degradation of occludin has been associated with the pathophysiology of irritable bowel syndrome [17]. However, surprisingly, occludin knockout mice (Ocln−/− mice) showed no apparent anomaly in the intestinal epithelial TJ [18]. These mice were not challenged, and therefore, there is no information present whether occludin deficient mice are susceptible or resistant to challenges relevant to physiologic and pathophysiologic conditions.
Ethanol and acetaldehyde treatment induces redistribution of occludin from the intercellular junctions of intestinal epithelium in vivo and in vitro leading to disruption of TJ and barrier dysfunction [3, 5, 19, 20]. Experimental studies indicate that ethanol feeding depletes occludin in colonic epithelium [21–23]. However, the significance of occludin depletion per se in alcoholic barrier dysfunction is unclear. We hypothesized that initial depletion of occludin would sensitize intestinal epithelium to ethanol-induced increase in gut permeability and liver damage. Therefore, we evaluated the susceptibility of Ocln−/− mice to ethanol-induced gut barrier dysfunction as well as liver damage. The effect on the intestinal epithelium was confirmed in Caco-2 cell monolayers, a cell culture model of the intestinal epithelium.
Materials and Methods
Chemicals
Maltose dextrin, feeding tubes and holders were purchased from Bioserv (Flemington, NJ). Lieber DeCarli liquid diet (Dyet #717780) was purchased from Dyets Inc. (Bethlehem, PA). EnzyChrom Alanine transaminase (EALT-100) assay kit was purchased from BioAssay systems (Hayward, CA). Triglyceride reagent kit set was purchased from Pointe Scientific Inc., (Canton, MI). Hoechst 33342 dye was from Life technologies (Grand Island, NY). All other chemicals were purchased from either Sigma Aldrich (St. Louis, MO) or Thermo Fisher Scientific (Tustin, CA).
Antibodies
Anti-ZO-1 (rabbit polyclonal), anti-occludin (mouse monoclonal), anti-claudin-2 (mouse monoclonal) and anti-claudin-3 (rabbit polyclonal) antibodies were purchased from Invitrogen (Carlsbad, CA). Rabbit polyclonal anti-toll like receptor-4 (TLR4) antibody was from Santa Cruz Biotechnology, Inc. (Dallas, TX). Anti E-Cadherin (mouse monoclonal) and anti β-catenin (rabbit polyclonal) antibodies were purchased from BD Biosciences (Billerica, MA). Horseradish peroxidase (HRP)-conjugated anti-mouse IgG, HRP-conjugated anti-rabbit IgG and anti-β-actin (mouse monoclonal) antibodies were obtained from Sigma Aldrich. AlexaFlour-488-conjugated anti-mouse IgG and Cy3-conjugated anti-rabbit IgG were purchased from Molecular Probes (Eugene, OR).
Animals
All animal experiments were performed according to the protocol approved by the University of Tennessee Health Science Center (UTHSC) Institutional Animal Care and Use Committee (IACUC). Ocln−/− mice (mixed background), generated as described before [24], were bred and the progeny genotyped to obtain wild type and Ocln−/− mice. Animals were housed in institutional animal care facility with 12-hour light and dark cycles, and were fed regular laboratory chow until the start of experiments. Both male and female mice were used for this study. Although female mice were slightly more sensitive to ethanol effects, we had to use mice of both gender, due to limited availability of Ocln−/− mice. Due to infertility of male Ocln−/− mice heterozygote male and female mice were used for breeders. The total numbers of mice used in 3 independent experiments were 12 (6 males + 6 females), 15 (7 males + 8 females), 11 (7 males + 8 females), and 15 (5 males + 6 females) for Wild type-Pair fed, Wild type-Ethanol fed, Ocln−/−-Pair fed and Ocln−/−-Ethanol fed groups, respectively. All mice had free access to standard rodent diet and water before the study.
Ethanol feeding
Wild type and Ocln−/− mice (10–14 wk. old) were fed Lieber-DeCarli liquid diet containing ethanol (1% 2 d, 2% 2 d, 4% 1 wk., 5% 1 wk. and 6% 1 wk.). Control mice were pair fed with isocaloric maltodextrin. To ensure similar diet intake all groups were pair fed. Diet intake and body weight were recorded. In all experiments animals were maintained in pairs to facilitate body temperature maintenance. The ethanol-feeding model used in this study is the same as the one previously used by others [21] and us [25, 26]. Ethanol concentration at 6% corresponds to 32% dietary calorie, which is in the range of ethanol-derived calorie consumption by alcoholics.
Gut permeability in vivo
At the end of 4 weeks of ethanol feeding, mice were intravenously injected with FITC-inulin (50 mg/ml solution; 2 μl/g body weight) via tail vein using a restrainer. One hour after injection, blood samples were collected by cardiac puncture under isoflurane anesthesia to prepare plasma. Mice were euthanized by cervical dislocation under isoflurane anesthesia. Luminal contents of intestinal segments were flushed with 0.9% saline. Fluorescence in plasma and luminal flushing was measured using a fluorescence plate reader. Fluorescence values in the luminal flushing were normalized to fluorescence values in corresponding plasma samples.
Cell culture and transfection
Caco-2 cells (ATCC, Rockville, MD) were passaged under standard cell culture conditions as described before [27]. The shRNA specific for human occludin and control RNA were purchased from Origene (Rockville, MD; Cat#TL311039). Cells grown in 6-well plates for 24 h showing ~75% confluence were transfected using serum-free Opti-MEM®, 1.0 μg shRNA constructs and 3.15 μl of Lipofectamine® LTX with plus reagent as described previously [28]. Cells were seeded onto transwell inserts (6.5 mm diameter). Experiments were performed on day 3 after seeding. Knockdown was confirmed by immunoblot analysis as described below.
Acetaldehyde treatment
Acetaldehyde (200 μM) treatment was performed as previously described [3]. Cells were pre-incubated in PBS containing 1.2 mM CaCl2, 1 mM MgCl2, bovine serum albumin and glucose for one hour. Then cells were exposed to vapor-phase acetaldehyde to achieve 200 μM concentration in sealed culture plates (25).
Analysis of barrier function TJ integrity
Barrier function was evaluated by measuring transepithelial electrical resistance (TER) and unidirectional flux of FITC-inulin.
Transepithelial electrical resistance (TER)
TER was measured as described previously (25) by using a Millicell-ERS Electrical Resistance System (Millipore, Bedford, MA). Basal TER of supporting semi-permeable membrane of transwells was subtracted from all values (80–100 Ohms/cm2). The baseline TER on day-3 post seeding for shRNA and control RNA transfected cell monolayers were 531 ± 11 and 551 ± 13 Ohms/0.33 cm2, respectively.
Unidirectional FITC-inulin flux
Prior to acetaldehyde treatment FITC-inulin (6 kDa; 0.5 mg/ml) was administered to the buffer in the apical well. At the end of incubation with or without acetaldehyde, 100 μl of basal medium and 20 μl of apical medium were sampled, and fluorescence was measured using an Flx-800 microplate fluorescence reader (BioTEK instruments, Winooski, VT). Flux into the basal well was calculated as the percentage of total apical well fluorescence/cm2 surface area.
Tight junction integrity
Fixed cell monolayers were stained for occludin and ZO-1 by immunofluorescence methods as described above.
Immunoblot analysis
Proteins were separated by SDS-PAGE and transferred to PVDF membranes. Membranes were blotted with specific antibodies for occludin or actin followed by incubation with HRP-conjugated specific secondary antibodies. Blots were developed by chemiluminescence method.
Immunofluorescence microscopy
Caco-2 cell monolayers and cryosections (12 μm thick) of distal colon were fixed in acetone: methanol (1:1) at −20ºC for 2 min and blocked with 4% milk in Tris/Tween buffer after permeabilizing with 0.05% Triton-X 100 in PBS; for F-actin staining, cell monolayers were fixed in 3% paraformaldehyde in PBS. Sections were then stained with a mixture of anti-occludin (mouse monoclonal) and anti-ZO-1 (rabbit polyclonal) antibodies, anti-E-cadherin and anti-β-catenin antibodies or anti-Cldn-2 (mouse monoclonal) and anti-Cldn-3 (rabbit polyclonal) or anti-TLR4 antibodies. Appropriate secondary antibodies were used for primary antibody detection. Nuclear staining was done using Hoechst 33342. F-actin was stained with AlexaFluor-488 conjugated phalloidin. Sections were then mounted and images collected using confocal microscope. Fluorescence density at the epithelium was evaluated by using Image J software (NIH, Bethesda, MD). Average of fluorescence from 8–10 spots was measured for epithelial section from each animal. Fluorescence measured at the lumen of the colonic tissue was used as background fluorescence to subtract from the epithelial fluorescence values. This measurement included fluorescence from both the junctions and the intracellular compartment. Therefore, fluorescence values represent the total protein in the epithelial cells rather than junctional fluorescence alone.
Histopathology
Segments of colon and liver were fixed in buffered formalin overnight, dehydrated in graded ethanol solutions and embedded in paraffin. Paraffin sections (7 μm thick) were deparaffinized in Citrosolv for 10 minutes (2 times) and rehydrated in ethanol solution (graded decrease in ethanol). Sections were stained in hematoxylin for 3 minutes and decolorized in acid water, followed by Eosin counterstaining. For Picro-Sirius Red staining, paraffin sections were de-waxed and hydrated with xylene and ethanol, respectively. Nuclei were stained with Weigert's hematoxylin for 5–8 minutes. Collagen staining was done by exposure to Picro-Sirius Red for one hour.
Plasma transaminase assay
Plasma alanine transaminase (ALT) activity was measured by colorimetric assay using EnzyChrom ALT/AST assay kits (Bioassay Systems, Hayward, CA) according to vendor’s instructions.
Oil Red-O staining
To detect fat deposition in the liver, frozen liver sections were fixed in 4% paraformaldehyde for 10 min, stained with Oil Red O (Sigma-Aldrich, St. Louis, MO, USA). Nuclei were lightly stained with hematoxylin stain and images collected in a Nikon 80Ti microscope using 10x objective lens and a color camera.
Triglyceride assay
Liver triglyceride levels were determined by GPO method using an assay kit from Beckman Coulter (Brea, CA). Lipids were extracted by digesting the liver tissue with 3 M potassium hydroxide (in 65% ethanol) for 1 hour at 70°C followed by incubation at room temperature for 24 hours. Triglycerides were measured by enzymatic hydrolysis of triglycerides to glycerol and free fatty acids followed by colorimetric measurement (at 540 nm wavelength) of glycerol. Values for hepatic triglycerides were expressed as mg triglyceride/g liver tissue.
Statistical analyses
All data are expressed as Mean ± SEM. The differences among multiple groups were first analyzed by ANOVA. When a statistical significance was detected, Tukey's t test was used to determine the statistical significance between multiple testing groups and the corresponding control. Statistical significance was established at 95%.
Results
Occludin deficiency exacerbates chronic ethanol-induced increase in colonic mucosal permeability
To determine the effect of occludin deficiency on alcohol induced increase in gut permeability we evaluated the effect of chronic ethanol feeding on mucosal inulin permeability in wild type and Ocln−/− mice in vivo. Since ethanol at 4% and higher concentrations resulted in a significant reduction in the diet intake, animals were pair fed in different groups. Therefore, the diet intake was similar in different groups of wild type and Ocln−/− mice (Fig. 1A and 1B). The changes in body weights during the experimental period were not different between ethanol fed and pair fed groups of wild type mice (Fig. 1C), whereas ethanol feeding significantly reduced body weight gain in Ocln−/− mice (Fig. 1D).
Figure 1. Effect of chronic ethanol feeding on diet intake and body weight of occludin deficient mice.
Adult wild type and occludin knockout (Ocln−/−) mice were fed ethanol in Lieber DeCarli liquid diet (1–6%) (●) over 4-week period. Control mice were pair fed with isocaloric ethanol-free diet (○). Diet intake (A & B) and body weights (C & D) were recorded. Values are mean ± SEM (n = 11–15 as indicated in Methods section). Asterisks indicate the values for ethanol fed groups are significantly (p<0.05) different from the corresponding pair fed group values.
Chronic ethanol feeding significantly increased mucosal inulin permeability in distal colon of both wild type and Ocln−/− mice (Fig. 2A), but the increase in inulin permeability in distal colon was nearly 4-fold higher in Ocln−−/− mice compared to that in wild type mice. Similarly, in the proximal colon, ethanol-induced increase in inulin permeability tends to be higher in Ocln−−/−mice, although it was statistically insignificant (Fig. 2B). Interestingly, in both distal and proximal colon, mucosal permeability in the pair fed Ocln−−/− mice was slightly, but significantly, greater than that in pair fed wild type mice. Ethanol feeding showed no significant effect on inulin permeability in the ileum (Fig. 2C). Despite these changes in the permeability blood alcohol levels in wild type and Ocln−/− mice are not different from each other. However, immunofluorescence images for toll like receptor-4 (TLR4) in colon (Fig. 2D) and fluorescence densitometry (Fig. 2E) showed that ethanol feeding significantly increased TLR4 levels in the colon of Ocln−−/− mice.
Figure 2. Chronic ethanol feeding induces colonic mucosal barrier dysfunction more severely in occludin deficient mice.
Adult wild type (WT) and occludin knockout (Ocln−/−) mice were fed ethanol (1–6%) (EF) in Lieber DeCarli liquid diet for 4 weeks. Control mice were pair fed with isocaloric ethanol-free diet (PF). Mucosal permeability of colon and ileum was evaluated by measuring vascular-to-luminal flux of FITC-inulin in vivo. Values for distal colon (A), proximal colon (B) and ileum (C) are mean ± SEM (n as indicated in the figure). Colonic sections were stained for TLR4 by immunofluorescence method (D). Fluorescence density was evaluated (E); values are mean ± SEM (n = 4). Asterisks in all graphs indicate the values that are significantly (p<0.05) different from the corresponding values for PF group. Hash tags indicate the values that are significantly different from corresponding values for WT group.
Since the permeability through epithelial is a matter of simple diffusion, vascular-to-lumen and lumen-to-vascular permeability should be comparable. Vascular-to-luminal flux of inulin, that involves no physical manipulation of the intestinal segment, was used to assess the intestinal mucosal permeability in vivo. Inulin is a non-toxic, extracellular marker that is extensively used in the clinic due to its metabolically inert nature. It is also evident from our past studies that inulin permeability and TJ disruption, as assessed by confocal microscopy, corroborate well with each another [3, 6, 25, 26, 29].
Ethanol-induced disruption of colonic epithelial TJ is more severe in occludin deficient mice
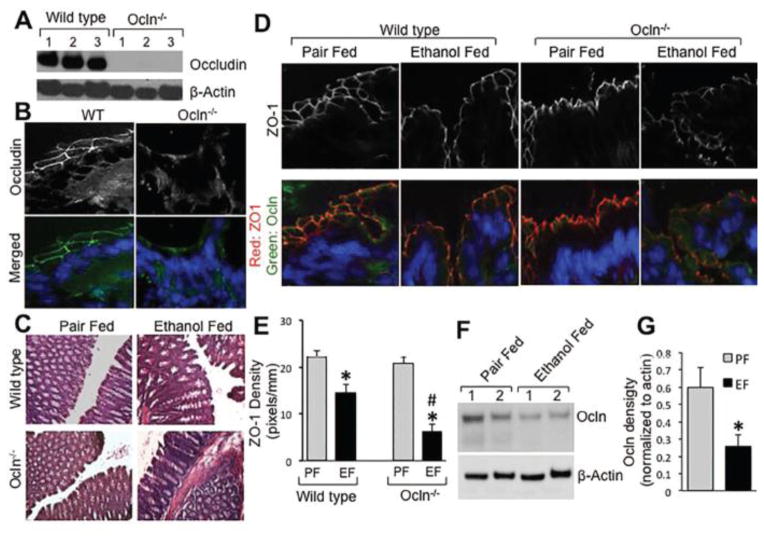
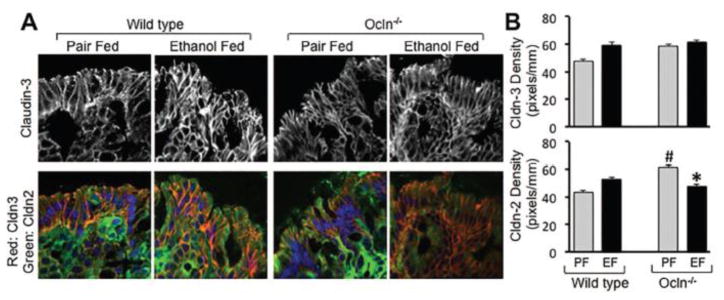
Immunoblot analysis (Fig. 3A) and immunofluorescence localization (Fig. 3B) confirmed the lack of occludin in the colon of Ocln−/− mice. The gross mucosal morphology of colon was unaffected by chronic ethanol feeding (Fig. 3C). Immunofluorescence imaging showed that occludin and ZO-1 are co-localized at the intercellular junctions of colonic epithelium, and that ethanol feeding induced a redistribution of these proteins from the junctions in wild type mice (Fig. 3D). Ethanol-induced ZO-1 redistribution was more severe in Ocln−/− mice, which was confirmed by densitometric analysis of ZO-1 fluorescence (Fig. 3D & 3E). In wild type mice, ethanol feeding partially, but significantly reduced the level of occludin in the colonic mucosa (Fig. 3F & 3G). Cldn-3 was also localized at the intercellular junctions of colonic epithelium in pair fed mice, whereas Cldn-2 distribution was mostly intracellular (Fig. 4A). Interestingly, epithelial distribution of Cldn-3 was unaffected by ethanol feeding in both wild type and Ocln−/− mice (Fig. 4A & 4B). Densitometric analysis of fluorescence indicated that Cldn-2 level is significantly higher in the colonic epithelium of Ocln−/− mice, which was reduced by ethanol feeding (Fig. 4B).
Figure 3. Chronic ethanol feeding disrupts colonic epithelial TJ more severely in occludin deficient mice.
Adult wild type and occludin knockout (Ocln−/−) mice were fed ethanol (1–6%) (EF) in Lieber DeCarli liquid diet for 4 weeks. Control mice were pair fed with isocaloric ethanol-free diet (PF). Absence of occludin in Ocln−/− mice was confirmed by immunoblot analysis (A) and immunofluorescence staining (B). Gross mucosal morphology was examined by light microscopy of H & E stained colonic sections (C). Cryosections of distal colon was stained for occludin and ZO-1 by immunofluorescence method and confocal microscopy (D). The merged images represent overlay of occludin (green), ZO-1 (red) and nucleus (blue). ZO-1 fluorescence in the epithelial cells was evaluated by densitometry using Image J (E). Densitometric values are mean ± SEM (n = 4). Asterisks indicate the values for ethanol fed groups are significantly different from the corresponding values for pair fed group, and the pound symbol indicates the value that is significantly (p<0.05) different from corresponding value for wild type mice. Colonic mucosal extracts from pair fed and ethanol fed wild type mice were immunoblotted for occludin and β-actin (F). Occludin band density was measured and normalized to the density of corresponding band for β-actin (G). Values are mean ± SEM (n = 5). Asterisks indicate the value for ethanol fed group is significantly (p<0.05) different from the corresponding value for pair fed group.
Figure 4. Effect of ethanol feeding on Cldn-2 and Cldn-3 in colonic epithelium.
Adult wild type and Ocln−/− mice were fed ethanol (1–6%) (EF) in Lieber DeCarli liquid diet for 4 weeks. Control mice were pair fed with isocaloric ethanol-free diet (PF). Cryosections of distal colon were stained for Cldn-2 and Cldn-3 by immunofluorescence method and confocal images were captured (A). The merged images represent overlay of Cldn-2 (green), Cldn-3 (red) and nucleus (blue). Fluorescence for Cldn-2 and Cldn-3 was evaluated by densitometry using Image J (B). Densitometric values are mean ± SEM (n = 5). Asterisk indicates the value for ethanol fed group is significantly different from the corresponding value for pair fed group, and the pound symbol indicates the value that is significantly (p<0.05) different from corresponding value for wild type mice.
Ethanol-induced disruption of colonic epithelial adherens junction and actin cytoskeleton is more severe in occludin deficient mice
Previous studies showed that acetaldehyde induced redistribution of E-cadherin and β-catenin, the adherens junction proteins, from the intercellular junctions in Caco-2 cell monolayers [5]. In the present study, E-cadherin and β-catenin were found to co-localize at the intercellular junctions of the colonic epithelium in pair fed mice (Fig. 5A). In wild type mice, ethanol feeding caused no obvious effect on the junctional organization of E-cadherin and β-catenin, but ethanol feeding significantly reduced junctional distribution of these adherens junction in the colon of Ocln−/− mice (Fig. 5A & 5B). Both TJ and adherens junction protein complexes are anchored to the actin cytoskeleton in the intact epithelium, and disruption of actin cytoskeleton has been shown to disrupt TJ and adherens junctions [30]. Here we show that F-actin is organized at the microvilli and the lateral membrane of colonic epithelium in pair fed wild type and Ocln−/− mice (Fig. 6A). Ethanol feeding reduced the F-actin fluorescence without significance difference between wild type and Ocln−−/− mice (Fig. 6A & 6B).
Figure 5. Ethanol-induced disruption of colonic epithelial adherens junction is more severe in occludin deficient mice.
Adult wild type and Ocln−/− mice were fed ethanol (1–6%) (EF) in Lieber DeCarli liquid diet for 4 weeks. Control mice were pair fed with isocaloric ethanol-free diet (PF). Cryosections of distal colon were stained for E-cadherin and β-catenin by immunofluorescence method and confocal images were captured (A). The merged images represent overlay of E-cadherin (green), β-catenin (red) and nucleus (blue). Fluorescence for β-catenin was evaluated by densitometry using Image J (B). Densitometric values are mean ± SEM (n = 5). Asterisk indicates the value for ethanol fed group that is significantly (p<0.05) different from the corresponding value for pair fed group.
Figure 6. Effect of ethanol feeding on colonic epithelial actin cytoskeleton.
Adult wild type and Ocln−/− mice were fed ethanol (1–6%) (EF) in Lieber DeCarli liquid diet for 4 weeks. Control mice were pair fed with isocaloric ethanol-free diet (PF). Cryosections of distal colon were stained for F-actin by immunofluorescence method and confocal images were captured (A). The merged images represent overlay of F-actin (green) and nucleus (blue). Fluorescence for F-actin was evaluated by densitometry using Image J (B). Densitometric values are mean ± SEM (n = 5). Asterisks indicate the values for ethanol fed group that are significantly (p<0.05) different from the corresponding values for pair fed group.
Knockdown of occludin in Caco-2 cells potentiates acetaldehyde-induced disruption of and barrier dysfunction
Our previous studies showed that acetaldehyde, the metabolic product of ethanol, directly affects the TJ of intestinal epithelium [3, 31]. To determine a direct influence of occludin depletion on acetaldehyde-induced TJ disruption, we knocked down occludin in Caco-2 cells by shRNA transfection. Immunoblot analysis confirmed knockdown of occludin expression by shRNA (Fig. 7A). Occludin knockdown showed slight, but significant reduction of TER (Fig. 7B) and elevation of inulin flux (Fig. 7C) in the absence of acetaldehyde. Acetaldehyde treatment reduced TER and elevated inulin permeability in both control RNA and shRNA-transfected cell monolayers, but these effects were significantly greater in shRNA-transfected cell monolayers compared to that in control cell monolayers. Similarly, acetaldehyde-induced redistribution of occludin and ZO1 was greater in shRNA-transfected cell monolayers (Fig. 7D).
Figure 7. Effect of occludin knockdown on ethanol-induced TJ disruption in Caco-2 cells.
Caco-2 cells were transfected with occludin-specific shRNA or control RNA. Control RNA and shRNA-transfected cells on transwells were treated with 200 μM acetaldehyde for 4 hours. Cell extracts were immunoblotted for occludin and β-actin (A). TER (B) and inulin permeability (C) were measured, and fixed cell monolayers were stained for occludin and ZO-1 (D). Values are mean ± SEM (n = 6). Asterisks indicate the values that are significantly (p<0.05) different from corresponding value for control RNA-transfected cell monolayers. Hash tags indicate the values that are significantly (p<0.05) different from corresponding values for sham-treated control cell monolayers.
Occludin deficiency promotes ethanol-induced liver damage
A significant body of evidence indicates that gut barrier dysfunction and endotoxin absorption play a crucial role in the pathogenesis of alcoholic liver disease and likely other alcoholic tissue injury [1, 2]. Promotion of gut barrier dysfunction and disruption of epithelial junctions by occludin deficiency suggested its potential influence on alcoholic liver injury. There was no visual difference between the liver from ethanol fed wild type and Ocln−/− mice, however ethanol fed Ocln−/− mice showed significantly higher liver weight (Fig. 8A). Plasma ALT level was significantly increased by ethanol feeding in wild type and Ocln−/− mice (Fig. 8B), but the effect was significantly greater in Ocln−/− mice compared to that in wild type mice. Histopathological analysis indicated that liver was unaffected by ethanol feeding in the wild type mice, but significant lesions were present in the liver of ethanol-fed Ocln−/−mice (Fig. 8C).
Figure 8. Effect of chronic ethanol feeding on liver.
Adult wild type and Ocln−/− mice were fed ethanol (1–6%) (EF) in Lieber DeCarli liquid diet for 4 weeks. Control mice were pair fed with isocaloric ethanol-free diet (PF). Liver weights were recorded (A) and plasma ALT activity was measured (B). Values are mean ± SEM (n as indicated in the figure). Asterisks indicate the values for ethanol fed groups that are significantly (p<0.05) different from the corresponding values for pair fed group. Paraffin sections of liver were stained with H & E (C).
Ethanol-induced fatty liver is more severe in occludin deficient mice
Fatty liver is an initial event in the pathogenesis of alcoholic liver damage. Oil Red-O staining of liver sections indicated that ethanol-induced accumulation of fat droplets is more severe in Ocln−/− mice, compared to that in wild type mice (Fig. 9A). Higher fat deposition the live of Ocln−/− mice was further supported by higher triglyceride levels in the liver of ethanol fed Ocln−/− mice compared to that in ethanol fed wild type mice (Fig. 9B). Staining liver sections with Serius Red dye for fibrosis showed no considerable accumulation of collagen in this model of alcoholic liver damage in both wild type and Ocln−/− mice (Fig. 9C), although chicken wire type of lesions were present in the liver of ethanol fed Ocln−/− mice, but not in the liver of ethanol fed wild type mice.
Figure 9. Ethanol-induced fat deposition in liver is more sever in occludin deficient mice.
Adult wild type and Ocln−/− mice were fed ethanol (1–6%) (EF) in Lieber DeCarli liquid diet for 4 weeks. Control mice were pair fed with isocaloric ethanol-free diet (PF). Cryo-sections of liver were stained with Oil Red-O (A), and triglyceride contents in liver extracts were measured by spectrophotometric assay (B). Values are mean ± SEM (n as indicated in the figure). Paraffin sections of liver were stained with Picro-Serius Red (C).
Discussion
Increased gut permeability and the resulting endotoxemia are well-established factors in the pathogenesis of alcoholic liver disease [1, 2]. Evidence indicates that ethanol depletes occludin in the intestinal epithelium, however the potential effect of this occludin depletion on alcoholic gut barrier dysfunction is unknown. In the present study, we provide evidence that occludin deficiency sensitizes mice for alcohol-induced gut barrier dysfunction and liver damage, suggesting that occludin depletion in the intestinal epithelium by a potential second hit might promote the pathogenesis of alcoholic gut and liver injury.
Chronic ethanol feeding did not significantly affect body weight gain during the experimental period in wild type mice, which is different from previous studies showing a decline in body weight gain by ethanol feeding in mice [25]. Previous studies were performed in mice of C57BL/6 background. The Ocln−/− mice were derived as 129/sv and C57BL/6 with agouti knockout offspring [18]. Mice of 129sv background were previously found to be relatively less sensitive to ethanol [32]. Therefore, it is likely that the mice used in this study are slightly resistant to ethanol effect, but occludin deficiency in these mice sensitizes them for ethanol-induced reduction in weight gain.
Ethanol feeding significantly increased mucosal permeability to inulin in the colon. In both proximal and distal colon, ethanol-induced increase in mucosal permeability was significantly greater in Ocln−/− mice compared to that in wild type mice. These findings suggest that occludin deficiency elevates susceptibility of mice for alcohol-induced gut barrier dysfunction. Consistent with our recent studies [25, 26] colon is the primary target of ethanol-induced barrier dysfunction, while ileum is resistant to ethanol. However, studies by other laboratories have shown that chronic ethanol feeding increases permeability in the ileum [21, 33]. As discussed in our previous study [25] multiple factors may be involved in this discrepancy between results from similar experiments in different laboratories.
Previous studies have demonstrated that ethanol consumption leads to disruption of intestinal epithelial TJ [1, 2, 25, 26]. Present study shows that ethanol feeding induces redistribution of occludin and ZO-1 from the epithelial junctions in the colon of both wild type and Ocln−/− mice, indicating the ethanol-induced TJ disruption. The present study also shows that ethanol-induced TJ disruption was more severe in the colon of Ocln−/− mice. Although occludin is the integral part of the TJ structure Ocln−/− mice show a normal assembly of TJ and barrier function in the intestine [18], and therefore, the precise function of occludin in TJ remains to be understood. Our data show that mucosal permeability to inulin is slightly, but significantly greater in the colon of Ocln−/− mice in the absence of ethanol feeding, indicating that the barrier function may be slightly compromised under resting conditions in Ocln−/− mice. Our data in this study suggest that lack of occludin may increase the susceptibility of intestinal epithelial TJ for damage by injurious factors, such as ethanol.
Disruption of colonic mucosal barrier dysfunction leads to leakage of bacterial toxins from the colonic lumen into mucosa and systemic circulation. Assay for lipopolysaccharide (LPS) level in plasma has been difficult in our model of alcohol feeding due to only a modest increase in plasma LPS in this model of ethanol feeding as well as high variability in values. Therefore we assessed TLR4 by immunofluorescence staining of colonic sections for TLR4 expression. A previous study indicated that exposure of intestinal epithelial cells to LPS on the basolateral surface increased TLR4 expression [34]. Fluorescence images and the densitometric analysis indicate that ethanol-induced increase in TLR4 level in the colon was higher in Ocln−/− mice, compared to that in wild type mice.
Claudins are required for the assembly and maintenance of TJ in different epithelia [35]. Two major types of claudins are barrier-forming claudins such as Cldn-3 that prevent diffusion of macromolecules and pore-forming claudins such as Cldn-2 that selectively allow transport of specific ions [35, 36]. We examined the effect of ethanol feeding on distribution of Cldn-2 and Cldn-3 in the colonic epithelium. Interestingly, distribution or the level of Cldn-3 in distal colon was unaffected by ethanol feeding under the present experimental conditions. Immunofluorescence localization showed that Cldn-2 is predominantly localized in the cytosol in the distal colon, and ethanol feeding did not alter the cellular localization of Cldn-2. The amount of Cldn-2 protein in the distal colon was higher in pair fed Ocln−/− mice compared to that in pair fed wild type mice. Cldn-2 level was reduced by ethanol feeding in Ocln−/− mice. The significance of this observation is unclear at this time.
Adherens junction is not a physical barrier for diffusion of macromolecules across the epithelium, but it indirectly regulates the integrity of tight junction [37]. Redistribution of E-cadherin and β-catenin, the major adherens junction proteins, from the epithelial junctions of colon of ethanol fed Ocln−/− mice indicates that ethanol disrupts colonic epithelial adherens junctions in these mice. Interestingly, ethanol feeding caused no considerable effect on adherens junction in the colon of wild type mice. Our previous studies showed a disruption of adherens junction by ethanol feeding in wild type mice of C57BL/6 background [25, 26]. The present observation of resistance to ethanol in wild type mice is likely a strain-dependent difference. But, a disruption of adherens junctions in ethanol-fed Ocln−/− mice support the view that occludin depletion increases susceptibility to ethanol-induced disruption of epithelial junctions and barrier dysfunction.
A decrease in F-actin stain in the colon by ethanol feeding indicates that ethanol induces disruption of actin cytoskeleton in the colonic epithelium. Actin cytoskeleton was disrupted in both wild type and Ocln−/− mice; densitometric analysis of F-actin fluorescence showed a similar loss of F-actin in wild type and Ocln−/− mice. TJ and adherens junction protein complexes are known to interact with the actin cytoskeleton, and this interaction is essential for the assembly and maintenance of TJ and adherens junctions. Loss of F-actin levels suggests that disruption of actin cytoskeleton may be involved in the mechanism of ethanol-induced disruption of TJ and adherens junctions, and therefore the barrier dysfunction.
Studies performed in Caco-2 cell monolayers in vitro confirm that occludin depletion leads to disruption of intestinal epithelial TJ and results in barrier dysfunction. Increased sensitivity to acetaldehyde by knockdown of occludin in Caco-2 cells also suggests that absence of occludin may have a direct impact on the colonic epithelium. Previous studies showed that acetaldehyde, the toxic metabolite of ethanol metabolism, disrupt intestinal epithelial TJ and plays a crucial role in the mechanism of alcoholic gut permeability [3, 5, 6, 31]. The present study suggests that occludin depletion by a second hit may potentiate the effect of alcohol consumption on gut permeability.
Disruption of TJ and barrier dysfunction in Ocln−/− mice is likely to have an impact on alcoholic injury to systemic organs, including the liver. Elevated ALT levels, histopathologic lesions and fatty liver demonstrated that ethanol feeding did cause liver damage in Ocln−/− mice. The liver damage in Ocln−/− mice demonstrated that occludin deficiency increases susceptibility of mice for alcoholic liver damage. Significant body of evidence indicates that increased gut permeability and endotoxin flux into liver are important factors in the pathogenesis of alcoholic liver disease. A correlation of higher increases in gut permeability with the liver damage in Ocln−/− mice further supports this view. The alcohol-feeding model used in this study is not known to induce liver fibrosis.
In summary, this study shows that Ocln−/− mice are more susceptible for chronic ethanol-induced disruption of colonic epithelial TJ, adherens junction and actin cytoskeleton, mucosal barrier dysfunction and liver damage. The outcome of this study indicates that depletion of occludin in the intestinal epithelium might sensitize mice for alcoholic gut permeability and liver damage.
Highlights.
Occludin deficiency enhances chronic ethanol-induced increase in mucosal permeability in mouse colon.
Ethanol-induced disruption of tight junctions, adherens junctions and the actin cytoskeleton in colonic epithelium is more severe in occludin deficient mice.
Occludin deficiency promotes ethanol-induced liver damage.
Ethanol-induced fatty liver is more severe in occludin deficient mice.
Acknowledgments
This study was supported by NIH grants, AA12307 and DK55532. Authors acknowledge Ms. Ernestine Hayes at the department of Comparative Medicine, UTHSC for her help with tail vein injections and cardiac puncture for collection of blood.
Abbreviations
- ALT
alanine aminotransferase
- Cldn
claudin
- ECL
enhanced chemiluminescent
- EF
ethanol fed
- FITC
fluorescein isothiocyanate
- HRP
horseradish peroxidase
- LPS
lipopolysaccharide
- Ocln−/−
occludin gene knockout
- PF
pair fed
- shRNA
short hairpin RNA
- TER
transepithelial electrical resistance
- WT
wild type
- ZO-1
zonula occludens-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1280–1288. doi: 10.1152/ajpgi.2001.280.6.G1280. [DOI] [PubMed] [Google Scholar]
- 4.Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol. 1999;276:G965–974. doi: 10.1152/ajpgi.1999.276.4.G965. [DOI] [PubMed] [Google Scholar]
- 5.Sheth P, Seth A, Atkinson KJ, Gheyi T, Kale G, Giorgianni F, Desiderio DM, Li C, Naren A, Rao R. Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem J. 2007;402:291–300. doi: 10.1042/BJ20060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki T, Seth A, Rao R. Role of phospholipase Cgamma-induced activation of protein kinase Cepsilon (PKCepsilon) and PKCbetaI in epidermal growth factor-mediated protection of from acetaldehyde in Caco-2 cell monolayers. The Journal of biological chemistry. 2008;283:3574–3583. doi: 10.1074/jbc.M709141200. [DOI] [PubMed] [Google Scholar]
- 7.Mankertz J, Waller JS, Hillenbrand B, Tavalali S, Florian P, Schoneberg T, Fromm M, Schulzke JD. Gene expression of the tight junction protein occludin includes differential splicing and alternative promoter usage. Biochemical and biophysical research communications. 2002;298:657–666. doi: 10.1016/s0006-291x(02)02487-7. [DOI] [PubMed] [Google Scholar]
- 8.Cummins PM. Occludin: one protein, many forms. Molecular and cellular biology. 2012;32:242–250. doi: 10.1128/MCB.06029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain S, Suzuki T, Seth A, Samak G, Rao R. Protein kinase Czeta phosphorylates occludin and promotes assembly of epithelial. The Biochemical journal. 2011;437:289–299. doi: 10.1042/BJ20110587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao R. Occludin phosphorylation in regulation of epithelial. Annals of the New York Academy of Sciences. 2009;1165:62–68. doi: 10.1111/j.1749-6632.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci U S A. 2009;106:61–66. doi: 10.1073/pnas.0802741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao R. Oxidative stress-induced disruption of epithelial and endothelial. Front Biosci. 2008;13:7210–7226. doi: 10.2741/3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, Rao R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the. J Biol Chem. 2009;284:1559–1569. doi: 10.1074/jbc.M804783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rachow S, Zorn-Kruppa M, Ohnemus U, Kirschner N, Vidal-y-Sy S, von den Driesch P, Bornchen C, Eberle J, Mildner M, Vettorazzi E, Rosenthal R, Moll I, Brandner JM. Occludin is involved in adhesion, apoptosis, differentiation and Ca2+-homeostasis of human keratinocytes: implications for tumorigenesis. PLoS One. 2013;8:e55116. doi: 10.1371/journal.pone.0055116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodewald M, Herr D, Fraser HM, Hack G, Kreienberg R, Wulff C. Regulation of tight junction proteins occludin and claudin 5 in the primate ovary during the ovulatory cycle and after inhibition of vascular endothelial growth factor. Molecular human reproduction. 2007;13:781–789. doi: 10.1093/molehr/gam066. [DOI] [PubMed] [Google Scholar]
- 16.Du D, Xu F, Yu L, Zhang C, Lu X, Yuan H, Huang Q, Zhang F, Bao H, Jia L, Wu X, Zhu X, Zhang X, Zhang Z, Chen Z. The tight junction protein, occludin, regulates the directional migration of epithelial cells. Developmental cell. 2010;18:52–63. doi: 10.1016/j.devcel.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Coeffier M, Gloro R, Boukhettala N, Aziz M, Lecleire S, Vandaele N, Antonietti M, Savoye G, Bole-Feysot C, Dechelotte P, Reimund JM, Ducrotte P. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. The American journal of gastroenterology. 2010;105:1181–1188. doi: 10.1038/ajg.2009.700. [DOI] [PubMed] [Google Scholar]
- 18.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao RK, Baker RD, Baker SS, Gupta A, Holycross M. Oxidant-induced disruption of intestinal epithelial barrier function: role of protein tyrosine phosphorylation. Am J Physiol. 1997;273:G812–823. doi: 10.1152/ajpgi.1997.273.4.G812. [DOI] [PubMed] [Google Scholar]
- 21.Cresci GA, Bush K, Nagy LE. Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcohol Clin Exp Res. 2014;38:1489–1501. doi: 10.1111/acer.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Akhtar S, Choudhry MA. Alteration in intestine tight junction protein phosphorylation and apoptosis is associated with increase in IL-18 levels following alcohol intoxication and burn injury. Biochim Biophys Acta. 2012;1822:196–203. doi: 10.1016/j.bbadis.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao H, Zhao C, Dong Y, Zhang M, Wang Y, Li F, Li X, McClain C, Yang S, Feng W. Inhibition of miR122a by Lactobacillus rhamnosus GG culture supernatant increases intestinal occludin expression and protects mice from alcoholic liver disease. Toxicol Lett. 2015;234:194–200. doi: 10.1016/j.toxlet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Isse T, Oyama T, Kitagawa K, Matsuno K, Matsumoto A, Yoshida A, Nakayama K, Kawamoto T. Diminished alcohol preference in transgenic mice lacking aldehyde dehydrogenase activity. Pharmacogenetics. 2002;12:621–626. doi: 10.1097/00008571-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhry KK, Samak G, Shukla PK, Mir H, Gangwar R, Manda B, Isse T, Kawamoto T, Salaspuro M, Kaihovaara P, Dietrich P, Dragatsis I, Nagy LE, Rao RK. ALDH2 Deficiency Promotes Ethanol-Induced Gut Barrier Dysfunction and Fatty Liver in Mice. Alcohol Clin Exp Res. 2015;39:1465–1475. doi: 10.1111/acer.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhry KK, Shukla PK, Mir H, Manda B, Gangwar R, Yadav N, McMullen M, Nagy LE, Rao R. Glutamine supplementation attenuates ethanol-induced disruption of apical junctional complexes in colonic epithelium and ameliorates gut barrier dysfunction and fatty liver in mice. J Nutr Biochem. 2015 doi: 10.1016/j.jnutbio.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samak G, Suzuki T, Bhargava A, Rao RK. c-Jun NH2-terminal kinase-2 mediates osmotic stress-induced tight junction disruption in the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2010;299:G572–584. doi: 10.1152/ajpgi.00265.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G367–375. doi: 10.1152/ajpgi.00464.2004. [DOI] [PubMed] [Google Scholar]
- 30.Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem. 1999;274:35179–35185. doi: 10.1074/jbc.274.49.35179. [DOI] [PubMed] [Google Scholar]
- 31.Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1356–1364. doi: 10.1152/ajpgi.00526.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 33.Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, McClain CJ. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;36:835–846. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182:375–387. doi: 10.1016/j.ajpath.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Itallie CM, Anderson JM. Claudin interactions in and out of the tight junction. Tissue Barriers. 2013;1:e25247. doi: 10.4161/tisb.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 37.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]