Abstract
Background:
Exposure to particulate matter from burning biomass fuels is believed to affect oxidant-antioxidant balance and to induce oxidative stress.
Methods:
Fifty-nine mother-child pairs from 59 households that used firewood exclusively for cooking in three rural communities in southwest Nigeria underwent blood test for albumin, pre-albumin, retinol-binding protein (RBP), superoxide dismutase (SOD), vitamins C, vitamin E, malondialdehyde (MDA) and C-reactive protein (CRP). Spirometry was performed and indoor levels of PM2.5 were determined.
Results:
Mean age (± SD; years) of mothers and children was 43.0±11.7 and 13.6±3.2, respectively. The median indoor PM2.5 level was 1575.1 µg/m3 (IQR 943.6–2847.0, p<0.001), which is substantially higher than the World Health Organization (WHO) standard of 25 µg/m3. The mean levels of pre-albumin (0.21±0.14 g/dL) and RBP (0.03±0.03 g/dL) in women were significantly lower than their respective normal ranges (1-3 g/dL and 0.2-0.6 g/dL, respectively, p<0.05). Similarly, the mean levels of pre-albumin (0.19±0.13 g/dL) and RBP (0.01±0.01 g/dL) in children were significantly lower than the respective normal ranges (1-3 g/dL and 0.2-0.6 g/dL, respectively, p<0.05). Mean serum concentrations of MDA in children (5.44±1.88 µmol/L) was positively correlated to serum concentrations of CRP (r=0.3, p=0.04) and negatively correlated to lung function (FEV1/FVC) in both mothers and children (both r=-0.3, p<0.05). Also, regression analysis indicates that CRP and SOD are associated with lung function impairment in mothers (-2.55±1.08, p<0.05) and children (-5.96±3.05, p=0.05) respectively.
Conclusion:
Exposure to HAP from biomass fuel is associated with pulmonary dysfunction, reduced antioxidant defense and inflammation of the airways. Further studies are needed to better define causal relationships and the mechanisms involved.
Keywords: biomass fuel, rural communities, oxidants, antioxidants, lung function, oxidative stress
1. Introduction
Almost half of the world's population lives in rural areas where biomass fuels remain the main source of energy. It is now well established that burning of biomass fuels can cause household air pollution (HAP) and significantly elevate indoor exposure to particulate matter (PM) and high quantities of health-damaging pollutants, including carcinogens (Kocbach et al., 2009), that generate oxidants and free radicals (Ohyama, Otake, Adachi, Kobayashi, & Morinaga, 2007; Kampa & Castanas 2008). Chronic exposure to these compounds may elicit inflammation in the lungs, increase susceptibility to lung infection (Mott et al., 2005; Naeher et al., 2007), and induce cell membranes to undergo direct oxidant damage (lipid peroxidation), which can be measured with an assay of malondialdehyde (MDA). The adverse health effects of exposure to HAP may be explained by several mechanisms (Nel, Xia, Madler, & Li, 2006). One of the proposed mechanisms is oxidative stress resulting from one or a combination of oxidant/antioxidant imbalances, an excess of oxidants or a depletion of antioxidants (M. Cemek, Caksen, Bayiroglu, F. Cemek, & Dede, 2006), providing evidence for an etiologic role of oxidative stress in obstructive lung disease.
Women and children are particularly vulnerable to these adverse health effects because of the daily repetitive exposure to PM during cooking. Exposure to biomass smoke have been strongly linked to impaired pulmonary function (Saha, Rao, Kulkarni, Majumdar, & Saiyed, 2005; Regalado et al., 2006; Padhi & Padhy 2008; Fullerton et al., 2011), ranging from mild to moderate reductions in spirometric variables such as forced expiratory volume in one second (FEV1), peak expiratory flow (PEF), and forced expiratory flow (FEF25-75), which are measures of airways obstruction (Regalado et al., 2006; Torres-Duque, Maldonado, Perez-Padilla, Ezzati, & Viegi, 2008); as well as low antioxidants levels (Mishra & Retherford, 2007). Also a recent study linked increased exposure to outdoor air pollutants particularly PM2.5 (particles less than or equal to 2.5µm in aerodynamic diameter) with acute changes in biomarkers of inflammation such as CRP in healthy individuals (Rich et al., 2012). In addition, using MDA as marker of oxidant-antioxidant imbalance, women exposed to HAP from burning biomass fuels have significantly high levels of MDA (B. Isik, R. S. Isik, Akyildiz, & Topcu, 2005). The composition and quantity of antioxidants in the body represents an important determinant of individual susceptibility to HAP. Increased production of free radicals secondary to pollutant exposure may exceed the capacity of the antioxidant defense system, resulting in particulate-induced inflammation in the airways (Nel, 2005; Schlesinger, Kunzli, Hidy, Gotschi, & Jerrett, 2006), oxidative damage (Hiura, Kaszubowski, Li, & Nel, 1999; Mazzoli-Rocha, Fernandes, Einicker-Lamas, & Zin, 2010), and impaired lung function (Mazzoli-Rocha et al., 2008). HAP exposure has been reported to be one of the factors responsible for an estimated 2.5 million premature deaths and 3.7% of the loss of disability adjusted life years (DALY) every year in developing countries (Smith & Mehta, 2003; Emmelin & Wall, 2007).
In Nigeria, as in many other developing countries, most women and children who live in rural communities are poor and suspected to be nutritionally deficient. In addition, it has been reported that women in rural communities in Nigeria who cook regularly with biomass fuels had mean daily PM10 exposure of approximately 730 µg/m3; which is about 29-fold higher than the WHO daily limit of 25 µg/m3 (Ana, Adeniji, Ige, Oluwole, & Olopade, 2012). Since continuous exposure to high levels of HAP in the presence of poor nutrition may induce oxidative stress and trigger inflammatory responses (Mishra & Retherford, 2007; Montano et al., 2010), it is reasonable to assume that the women and children in rural communities in Nigeria who are nutritionally deficient and chronically exposed to HAP from biomass fuels are also at a considerable risk of oxidative stress. However, the level of HAP from biomass fuel use in rural households in Nigeria, its effects on pulmonary health, nutritional status and systemic inflammation is yet to be elucidated as little attention has been paid to this important public health issue. In view of this, we conducted a cross-sectional pilot study among women and children in households that cook with biomass fuels in rural communities in Nigeria. The objective was to investigate if a relationship exists between HAP from biomass smoke exposure and pulmonary health, oxidant-antioxidant imbalance and systemic inflammation. We also examined the relationship between oxidative stress (as measured by biomarkers of exposure) and lung function in this rural cohort.
2. Materials and Methods
2.1 Subjects
This cross-sectional study is part of an intervention pilot study to evaluate the extent, impact, and implication of HAP from biomass fuel use for cooking and monitored use of low-emission stoves on the health of women and children in southwest Nigeria. Fifty-nine mother-child pairs from 59 households that use biomass fuel almost exclusively for cooking in three rural communities (Ajibade, Eruwa, and Olorisaoko) near Ibadan, Nigeria, underwent detailed serum nutritional assessment and pulmonary function testing to investigate levels of serum oxidants/antioxidants following exposure to HAP. We performed spirometry in all mother-child pairs. To be eligible, subjects expressed willingness to participate in the study and were aged between 20–60 years (for mothers) and 7–17 years (for children). The lower age limit for children was set to ensure successful performance of spirometry, which requires subject cooperation.
The Institutional Review Boards at the Universities of Ibadan and Chicago gave ethical approval for the conduct of the study with approval numbers UI/EC/10/0045 and 10-263-B, respectively. All adult participants gave verbal and written consent for their participation and that of their children. Additionally, the children provided assent to participate.
2.2 Data Collection Methods
2.2.1 Indoor Air Sampling during Cooking
Indoor air sampling was conducted in 59 households. Real-time measurement of PM2.5 was conducted in cooking areas before and during cooking using the pDR 1500 personal aerosol monitor and data logger (Thermo Scientific, Franklin, Massachusetts), which uses gravimetric and optically-based methods and compensates for many environmental variables during sampling. After calibration and equilibration, the instrument was set to cycle every minute for an hour during the cooking of evening meals. The sampler was positioned at the center of the kitchen, and it was placed approximately 0.5 meters from the ground and within a 1-meter radius of the plume arising from the pollution source to capture the exposure of the women and children during cooking.
2.2.2 Pulmonary Function Tests
Pulmonary function tests were performed with the PC-based full function KoKo spirometer (nSpire Health, Inc. Longmont, Colorado) in accordance with the American Thoracic Society's (ATS) recommendations. Spirometry was performed at similar times of the day to minimize diurnal variation (Crapo, Morris, & Gardner, 1981); the spirometer was calibrated daily and operated within the ambient temperature. We measured forced vital capacity (FVC), forced expiratory volume in one second (FEV1), FEV1/FVC ratios, and peak expiratory flow rate (PEFR), which are reliable measures of airways obstruction.
2.2.3 Blood Sample Preparation and Analytical Methods
Of the 59 mother-child pairs, 55 mother-child pairs consented to blood draw. Venous blood samples were collected in 10 ml Vacutainer EDTA tubes. All blood samples were processed at the Institute for Advanced Medical Research and Training laboratory at the University College Hospital, Ibadan, Nigeria. The serum samples were frozen and stored at -80°C until analysis was performed. Serum pre-albumin, albumin, transferrin, and retinal-binding protein (RBP) were determined using single immunodiffusion methods with results expressed as g/dL (Arinola & Ezeh, 2007).
Antioxidant enzyme superoxide dismutase (SOD) was assayed by the epinephrine autoxidation inhibition method (Magwere, Naik, & Hasler, 1997), while serum lipid peroxidation was assayed by the colorimetric reaction between MDA and thiobarbituric acid for measuring MDA (Kamal et al., 1989). Determination of vitamin C was performed using colorimetric method (Halliwell & Gutteridge 1995) while vitamin E determination was performed by high-performance liquid chromatography (Lang, Gohil, & Packer, 1986). Serum C-reactive protein (CRP) levels were determined by enzyme-linked immunosorbent assay (ELISA) immunoplate as described by the manufacturer (Immuno-Biological Labs. Inc.).
2.3 Data Analysis
Analyses were performed using STATA Version 12.0. The median (25th–75th percentile) value of household PM2.5 was determined. Mean values and standard deviations were calculated for lung function and serum nutritional biomarkers. Predicted normal values for lung function variables were obtained from the ATS recommendations. Student's t-test was used to compare lung functions and nutritional biomarkers with their corresponding normal values. In both women and children, bivariate Pearson's correlation coefficients (r) were used to determine the correlation between MDA and lung function, MDA and CRP, as well as SOD and CRP. To determine predictive factors of lung function impairment, multiple regression analysis was performed for %FEV1/FVC against variables of biomarkers of oxidative stress (MDA and CRP) and antioxidant variables (SOD, vitamin C and vitamin E). A p≤0.05 is considered statistically significant.
3. Results
3.1 Household Air Pollution and Lung Function
The sample included 118 subjects (59 mothers and 59 children). The mean age (±SD) of mothers and children were 43.0±11.7 and 13.6±3.2 years, respectively. Median concentrations of PM2.5 in the selected 59 households during cooking was 1575.1 µg/m3 [IQR 943.6–2847.0 µg/m3] and was significantly higher than WHO standards of 25 µg/m3 (p<0.001). Mean FEV1 was significantly lower than mean predicted FEV1 in both mothers (1.94±0.51 vs. 2.79±0.36 L/s, p<0.05) and children (1.76±0.54 vs. 2.57±0.66 L/s, p<0.05) (Table 1).
Table 1.
Lung function parameters in exposed mothers and children
| Mothers (N=59) | Children (N=59) | |||||
|---|---|---|---|---|---|---|
| Predicted Normal Value (mean±SD) | Best Effort (mean±SD) | % Predicted Normal Value† | Predicted Normal Value (mean±SD) | Best Effort (mean±SD) | % Predicted Normal Value† | |
| FVC (L/s) | 3.32±0.40 | 2.60±0.45 | 78.63±10.99 | 2.77±0.72 | 2.25±0.68 | 80.09±28.65 |
| FEV1 (L/s) | 2.79±0.36 | 1.94±0.51 | 69.78±16.58 | 2.57±0.66 | 1.76±0.54 | 69.29±15.01 |
| FEV1/FVC (%) | 0.84±0.29 | 0.74±0.13 | 0.93±0.24 | 0.81±0.12 | ||
| FEF25-75 (L/s) | 3.08±0.71 | 1.87±0.81 | 59.32±25.21 | 3.11±1.03 | 1.95±0.99 | 64.10±30.28 |
| PEFR (L/s) | 6.11±0.31 | 3.52±1.45 | 58.47±23.21 | 5.78±1.17 | 3.25±1.48 | 57.97±24.59 |
p-values <0.05 for all variables when compared with the predicted normal values
Abbreviation: FVC–Forced vital capacity; FEV1–Forced expiratory volume in one second; FEF–Forced expiratory flow; PEFR–Peak expiratory flow rate
3.2 Serum Biomarkers
Table 2 shows the summary of serum biomarkers of nutrition and antioxidants. Mean serum concentration levels of pre-albumin and RBP were below the lower limit of normal ranges, while concentration of albumin, transferrin, vitamin C, and vitamin E were all within the normal ranges in both mothers and children. Additionally, mean serum CRP concentrations in mothers (3.79±1.89 µg/ml) and children (3.56±1.54 µg/ml) were higher than normal range (1-3 µg/ml, p=0.39 and p<0.05, respectively). Mean serum MDA concentrations in mothers (5.90±2.40 µmol/L) and children (5.44±1.88 µmol/L) were also higher than normal range (<4.5 µmol/L, p<0.05 for both).
Table 2.
Nutritional biomarkers in mothers and children
| Mothers | Children | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ref. range | N | Mean (±SD) | Min-Max | %Def. | N | Mean (±SD) | Min-Max | %Def. | ||
| Albumin (g/dL) | 34–54 | 55 | 43.07±5.99 | 30–60 | 1.8 | 55 | 43.41±7.24 | 37–68 | 0 | |
| Transferrin (mg/dL) | 1.2–2.6 | 55 | 1.74±0.53 | 0.92–2.8 | 5.5 | 54 | 1.61±0.56 | 0.7–2.8 | 11.1 | |
| Pre-albumin (g/dL) | 1–3 | 55 | 0.21±0.14* | 0.04–0.60 | 100 | 54 | 0.19±0.13* | 0.04–0.60 | 100 | |
| RBP (g/dL) | 0.2–0.64 | 55 | 0.03±0.03* | 0.00–0.17 | 100 | 54 | 0.01±0.01* | 0.00–0.05 | 100 | |
| SOD (Unit/L) | 2.9–6.7 | 55 | 2.53±0.99 | 1–5.8 | 41.8 | 54 | 2.39±0.99 | 1–5.9 | 50.9 | |
| Vitamin C (mg/dL) | 0.4–1.5 | 42 | 1.26±0.95 | 0.68–7.13 | 0 | 40 | 1.04±0.30 | 0.58–2.11 | 0 | |
| Vitamin E (mg/dL) | 5–18 | 42 | 13.64±2.34 | 9.11–17.32 | 0 | 40 | 12.46±2.79 | 7.24–17.98 | 0 | |
%Def. = % of subjects with values lower than the lower limit of normal range
Significantly lower when compared to the lower limit of normal range
3.3 Relationship between Particulate Matter, Antioxidants, Oxidants, Lung Function, and Inflammation
Correlation analysis showed no significant association between PM2.5 concentration levels and serum oxidant parameters. Also, no significant association was observed between PM2.5 concentration levels and FEV1 in mothers and children (r=0.08; p=0.57 and r=0.60; p=0.65, respectively). However, MDA concentration was negatively associated with FEV1/FVC in mothers and children (both r=-0.3, p=0.05; Figure 1). Assessing relationships between lipid peroxidation and propensity for inflammation, MDA showed a significant positive correlation with CRP only in children (r=0.3, p<0.05) but no significant association was observed in mothers (r=0.1, p=0.64; Figure 2). The predictor variables obtained for lung function impairment are presented in Table 3. Regression analysis indicates that serum CRP and SOD concentration levels were strong predictors of impaired lung function in mothers and children, respectively.
Figure 1.
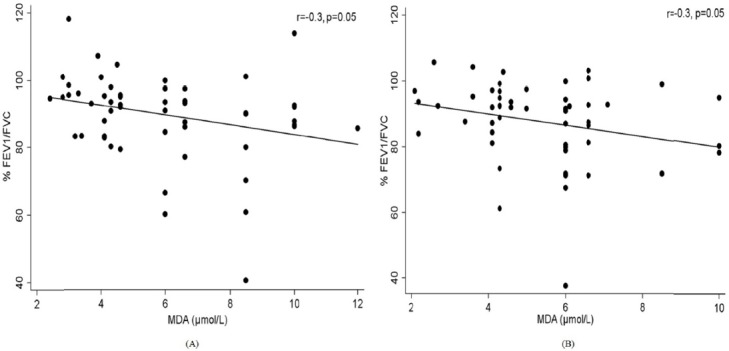
Association between lung function and lipid peroxidation (MDA) in mothers (A) and children (B)
Figure 2.
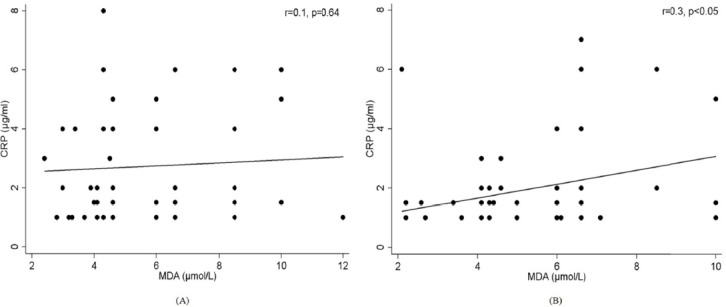
Association between MDA and marker of inflammation (CRP) in mothers (A) and children (B)
Table 3.
Regression analysis of lung function (FEV1/FVC) against serum oxidative biomarkers and antioxidants
| Mothers | Children | |||
|---|---|---|---|---|
| Regression coefficient (±SE) | p value* | Regression coefficient (±SE) | p value* | |
| MDA (µmol/L) | -0.65±0.91 | 0.48 | 0.39±1.39 | 0.78 |
| CRP (µg/ml) | -2.55±1.08 | 0.02 | 0.07±1.38 | 0.44 |
| SOD (Unit/L) | 4.20±3.18 | 0.12 | -5.96±3.05 | 0.05 |
| Vit C (mg/dL) | 0.69±2.10 | 0.74 | -20.50±19.89 | 0.31 |
| Vit E (mg/dL) | 1.18±0.85 | 0.17 | 0.17±1.97 | 0.93 |
p-values after adjusting for age, height and exposure to tobacco smoke
4. Discussion
Exposure to HAP from biomass smoke has been implicated as a significant risk factor for the development of inflammation and respiratory diseases (Ghaffari & Taghizadieh, 2010; Hu et al., 2010). Although the underlying mechanisms for exposure-related injury are still being investigated, exposure induced-oxidative damage has been alleged to play an important role (Schlesinger et al., 2006; Romieu, Castro-Giner, Kunzli, & Sunyer, 2008). In this study, the median PM2.5 concentration levels were 1575.1 µg/m3, more than 60 times higher than the WHO 24-hour acceptable levels of 25 µg/m3. Also, lung function, pre-albumin and RBP were significantly lower than their respective normal values among exposed mothers and children. Similar to previous studies which showed a relationship between biomass smoke exposure and adverse health outcomes in developing countries (Barnes, Mathee, Thomas, & Bruce, 2009; Fatmi, Rahman, Kazi, Kadir, & Sathiakumar, 2010), our findings suggest that exposure to HAP from biomass fuels may have significant effects on lung function and nutritional status. However, long term studies with a control arm of individuals who cook with cleaner fuel are needed to confirm this relationship.
Exposure to biomass fuels is recognized as an important cause of impaired pulmonary function (Saha et al., 2005; Regalado et al., 2006; Padhi & Padhy, 2008). In the present study, we observed impaired lung function in both non-smoking women and children (Table 1). Several studies have also reported reduction in lung function, characterized by FVC <80% of predicted value, in women chronically exposed to biomass smoke (Sumer, Turaclar, Onarlioglu, Ozdemir, & Zwahlen, 2004; Regalado et al., 2006; Revathi, Kutty, & Annamalai, 2012; Kurmi et al., 2013). Similar reductions in FVC and FEV1 have been observed in children living in homes that used biomass fuel for cooking (Rinnie et al., 2006). The impaired lung function that was observed in both mothers and children in this study may be due to PM2.5 that is present in biomass smoke, which is believed to induce oxidative injury to the lungs through their ability to form free radicals and cause airway inflammation (Romieu et al., 2008).
The study also demonstrates pro-oxidative potential effects of exposure to biomass smoke as indicated by elevated levels of MDA—a serum marker of systemic oxidative stress—and CRP in women and children. CRP is an indicator of generalized inflammation and most epidemiological studies have found that CRP level is significantly increased following exposure to cigarette smoke (Bazzano, He, Muntner, Vupputuri, & Whelton, 2003; Frohlich et al., 2003). Similarly, the positive association observed between MDA and CRP in our study suggests that continuous exposure to biomass smoke may also be inducing systemic oxidative stress in this rural population. This is consistent with other studies which found significantly increased levels of CRP following exposure to air pollution from particulate matter (PM) (Peters et al., 2001; Pope et al., 2004). This may be as a result of increased production of free radicals such as reactive oxygen species (ROS), which is exacerbated by the depletion of plasma antioxidants that protect against oxidative damage (Cemek et al., 2006; Pereira, Heck, Saldiva, & Rhoden, 2007).
The importance of antioxidant status in protecting against oxidative stress has been demonstrated in intervention studies linking supplementation of antioxidants to lower lipid peroxidation, especially among smokers (Block et al., 2002; Dietrich et al., 2002). Similar studies have also demonstrated that depletion of body antioxidant capacity either through low levels of non-enzymatic or enzymatic antioxidants rendered cells vulnerable to oxidative attack (Raijmakers, Peters, Steegers, & Poston, 2005; Forman, Maiorino, & Urseni 2010). Since the composition and quantity of antioxidants in the body represents an important determinant of individual responsiveness to toxic air pollutants (Romieu et al., 2008), we suggest that low levels of serum nutritional biomarkers and antioxidants in our study may have contributed to the systemic inflammatory responses and oxidative stress observed in our subjects. Serum pre-albumin and RBP concentrations were significantly lower than normal values. This is consistent with earlier reports suggesting that chronic exposure to biomass smoke may significantly lower antioxidant levels and contribute to nutrient deficiency and stunted growth in children (Mishra & Retherford 2007; Padhy & Padhi 2009).
Depletion of pre-albumin is an indicator of poor nutritional status and has been showed to be associated with COPD, especially in heavy cigarette smokers (Gocmen et al., 2010; Obase et al., 2011). We also observed low levels of pre-albumin, which was not independently associated with reduced lung function. However, since nutritional and antioxidant availability in the body have impacts on individual levels of oxidative stress and lung function (Larcombe et al., 2008), we posit that the low level of pre-albumin in combination with other nutrient deficiencies may have indirectly contributed to the increased levels of CRP. Our results also confirmed the reports of studies that showed oxidative stress to be negatively correlated with lung function and play a role in enhancing systemic inflammation (Ichinose, Sugiura, Yamagata, Koarai, & Shirato, 2000; Rahman & Adcock, 2006), as we also observed with CRP. Additionally, low serum RBP concentration is an indicator of vitamin A deficiency (Reifen, 2002; Ambalavanan, Ross, & Carlo, 2005). This deficiency, which may be exacerbated by inadequate nutritional intake has been shown to induce systemic inflammation and aggravates existing inflammatory conditions (Reifen, 2002). Also, low serum vitamin A is an independent risk factor for inflammation in children (Gamble et al., 2004). Nearly all mothers and children in our study showed evidence of inflammation, as indicated by elevated CRP levels. Although we did not assess vitamin A status in our subjects, the low levels of RBP, a carrier for vitamin A in the blood (Baeten et al., 2004), suggests significant vitamin A deficiency and may have contributed to the inflammation and oxidative stress.
Chronic exposure to biomass smoke generally causes cellular injury via oxidative stress; and to combat oxidative stress, the body has to develop a strong antioxidant defense system (Romieu et al., 2008). An important member of this defense system is the enzyme SOD. In the present study, SOD levels in both mothers and children were below the lower limit of normal range with 42% of mothers and 51% of children falling below this limit (Table 2). SOD scavenges free radicals and can prevent smoke-induced inflammatory responses (Hoshino et al., 1990; Foronjy et al., 2006). However, epidemiological studies examining the relationship between serum SOD levels and exposure to smoke have showed conflicting results. Two such studies observed significantly low SOD activity in population exposed to biomass smoke (Padhy & Padhi, 2009; Montano et al., 2010). In other studies, SOD levels were observed to be higher in cigarette smokers when compared to controls (Yokus, Mete, Mete, Cakir, & Toprak, 2005; Baharvand et al., 2010). Our result also showed generation of oxidative stress as SOD showed positive correlation with CRP in mothers and also appeared to be a strong predictor of lung function in children (Table 3). This indicates that SOD may have relevance as a potential biomarker of systemic inflammation. Also, given the evidence of systemic oxidative stress in individuals with chronic exposure to biomass smoke (Barreiro et al., 2005), we believe that the decrease in SOD levels in our subjects may be due to either increased oxidative stress from HAP and/or nutritional deficiency, which collectively limits antioxidant defense.
One of the major limitations of this study is the lack of information on the dietary intake among the population sampled. Many epidemiological studies have suggested that consumption of fruits and vegetables may play important roles in neutralizing free radicals and in protecting against oxidative damage. The low levels of nutritional biomarkers below the lower limit of normal are highly suggestive of poor nutrition and antioxidant deficiency. Another limitation is the lack of control households where cleaner fuels are used for cooking in the community where the study was performed. This made it impossible for us to be certain if the adverse health effects, oxidative stress and inflammation we observed is related to exposure to biomass smoke. It is also likely that this malnourished study population has elevated CRP and MDA due to nutritional deficiency or unrecognized lung infection and not because of exposure to biomass smoke. However, our data confirm previous reports concerning the relationship between HAP exposure and systemic oxidative stress. While exposure to biomass smoke may be a plausible explanation for the observed impaired pulmonary function (predominantly obstructive lung disease) and oxidative stress, we believe a more detailed, large-sample-size case-control study involving biomass users and clean fuel users (e.g. liquefied petroleum gas or electricity) would be necessary to accurately establish the causal relationships and to control for other confounding variables.
Despite these limitations, our results showed that exposure to HAP from biomass smoke in the setting of poor nutrition and antioxidant deficiency may have significant health implications. Exposed women and children had reduced lung function and inflammation of the airways. Results from our study also underscore the role of imbalance between oxidants and antioxidants mechanisms in the development of oxidative stress and impairment of lung function.
In conclusion, the observed associations between HAP exposure, oxidative stress and decrease in pulmonary function suggest that HAP from biomass fuel has detectable adverse effects in exposed mothers and children, which may be worsened by inadequate antioxidant capacity. Since biomass fuel remains the main source of domestic energy in many rural communities in Nigeria, health impairments observed among exposed mothers and children in this study may be representative of the health conditions of women and children in rural communities, who are similarly exposed to biomass smoke. Thus the magnitude of this public health problem calls for immediate attention to mitigate this problem. While the best intervention for mothers and children in rural communities is to avoid or minimize exposure to HAP from biomass fuels, the present data suggest that they may also benefit from improved nutrition. Additional studies with a control group that relate dietary intake to antioxidant levels are needed to better understand the role of antioxidant defense system in protection against the damaging effects of exposure to HAP.
Acknowledgements
The Falk Medical Trust Foundation, USA, and the Chest Foundation of the American College of Chest Physicians funded the project. The authors are also grateful to Healthy Life for All Foundation (HLF), Ibadan, Nigeria, for assistance with project execution and to Rebecca Incledon for editorial assistance.
Footnotes
Conflict of Interest: None of the authors have a conflict of interest to declare on this project
References
- Ambalavanan N, Ross A. C., Carlo W. A. Retinol-binding protein, transthyretin, and C-reactive protein in extremely low birth weight (ELBW) infants. JPerinatol. 2005;25(11):714–719. doi: 10.1038/sj.jp.7211398. http://dx.doi.org/10.1038/sj.jp.7211398 . [DOI] [PubMed] [Google Scholar]
- Ana G, Adeniji B, Ige O, Oluwole O, Olopade C. O. Exposure to emissions from firewood cooking stove and the pulmonary health of women in Olorunda community, Ibadan, Nigeria. Air Qual Atmos Health (online version) 2012. http://dx.doi.org/10.1007/s11869-012-0183-6 .
- Arinola O. G., Ezeh C. C. C1 inhibitor, C3 activator and IgA, IgG and IgM titers in Nigerian HbSS patients with Plasmodium falciparum. Iranian JImmunol. 2007;4:44–49. [PubMed] [Google Scholar]
- Baeten J. M., Richardson B. A., Bankson D. D., Wener M. H., Kreiss J. K, Lavreys L, Mandaliya K. Use of serum retinol-binding protein for prediction of vitamin A deficiency: effects of HIV-1 infection, protein malnutrition, and the acute phase response. Am JClin Nutr. 2004;79(2):218–225. doi: 10.1093/ajcn/79.2.218. [DOI] [PubMed] [Google Scholar]
- Baharvand M, Maghami A. G., Azimi S, Bastani H, Ahmadieh A, Taghibakhsh M. Comparison of superoxide dismutase activity in saliva of smokers and nonsmokers. South Med J. 2010;103(5):425–427. doi: 10.1097/SMJ.0b013e3181d7e0d8. http://dx.doi.org/10.1097/SMJ.0b013e3181d7e0d8 . [DOI] [PubMed] [Google Scholar]
- Barnes B, Mathee A, Thomas E, Bruce N. Household energy, indoor air pollution and child respiratory health in South Africa. Journal of Energy in Southern Africa. 2009;20(1):4–13. [Google Scholar]
- Barreiro E, de la Puente B, Minguella J, Corominas J. M., Serrano S, Hussain S. N., Gea J. Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am JRespir Crit Care Med. 2005;171(10):1116–1124. doi: 10.1164/rccm.200407-887OC. http://dx.doi.org/10.1164/rccm.200407-887OC . [DOI] [PubMed] [Google Scholar]
- Bazzano L. A., He J, Muntner P, Vupputuri S, Whelton P. K. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann Intern Med. 2003;138(11):891–897. doi: 10.7326/0003-4819-138-11-200306030-00010. [DOI] [PubMed] [Google Scholar]
- Block G, Dietrich M, Norkus E. P., Morrow J. D., Hudes M, Caan B, Packer L. Factors associated with oxidative stress in human populations. Am JEpidemiol. 2002;156(3):274–285. doi: 10.1093/aje/kwf029. http://dx.doi.org/10.1093/aje/kwf029 . [DOI] [PubMed] [Google Scholar]
- Cemek M, Caksen H, Bayiroglu F, Cemek F, Dede S. Oxidative stress and enzymic-non-enzymic antioxidant responses in children with acute pneumonia. Cell Biochem Funct. 2006;24(3):269–273. doi: 10.1002/cbf.1220. http://dx.doi.org/10.1002/cbf.1220 . [DOI] [PubMed] [Google Scholar]
- Crapo R. O., Morris A. H., Gardner R. M. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123(6):659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- Dietrich M, Block G, Hudes M, Morrow J. D., Norkus E. P., Traber M. G., Cross C. E. Antioxidant supplementation decreases lipid peroxidation biomarker F(2)-isoprostanes in plasma of smokers. Cancer Epidemiol Biomarkers Prev. 2002;11(1):7–13. [PubMed] [Google Scholar]
- Emmelin A, Wall S. Indoor air pollution: a poverty-related cause of mortality among the children of the world. Chest. 2007;132(5):1615–1623. doi: 10.1378/chest.07-1398. http://dx.doi.org/10.1378/chest.07-1398 . [DOI] [PubMed] [Google Scholar]
- Fatmi Z, Rahman A, Kazi A, Kadir M. M., Sathiakumar N. Situational analysis of household energy and biomass use and associated health burden of indoor air pollution and mitigation efforts in Pakistan. Int JEnviron Res Public Health. 2010;7(7):2940–2952. doi: 10.3390/ijerph7072940. http://dx.doi.org/10.3390/ijerph7072940 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman H. J., Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49(5):835–842. doi: 10.1021/bi9020378. http://dx.doi.org/10.1021/bi9020378 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foronjy R. F., Mirochnitchenko O, Propokenko O, Lemaitre V, Jia Y, Inouye M, Okada Y. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am JRespir Crit Care Med. 2006;173(6):623–631. doi: 10.1164/rccm.200506-850OC. http://dx.doi.org/10.1164/rccm.200506-850OC . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich M, Sund M, Lowel H, Imhof A, Hoffmeister A, Koenig W. Independent association of various smoking characteristics with markers of systemic inflammation in men. Results from a representative sample of the general population (MONICA Augsburg Survey 1994/95) Eur Heart J. 2003;24(14):1365–1372. doi: 10.1016/s0195-668x(03)00260-4. http://dx.doi.org/10.1016/S0195-668X(03)00260-4 . [DOI] [PubMed] [Google Scholar]
- Gamble M. V., Palafox N. A., Dancheck B, Ricks M. O., Briand K, Semba R. D. Relationship of vitamin A deficiency, iron deficiency, and inflammation to anemia among preschool children in the Republic of the Marshall Islands. Eur JClin Nutr. 2004;58(10):1396–1401. doi: 10.1038/sj.ejcn.1601982. http://dx.doi.org/10.1038/sj.ejcn.1601982 . [DOI] [PubMed] [Google Scholar]
- Ghaffari M. R., Taghizadieh A. ADescription of the Lung Disease Found in IranianWomen Exposed to Dung Smoke. JCardiovasc Thorac Res. 2010;2(2):29–32. [Google Scholar]
- Gocmen H, Ediger D, Uzaslan E, Doganay S, Guney N. A., Ege E. The Relationships of Serum Prealbumin Levels With Parameters That Indicate Severity of Disease and Emphysema Pattern in Patients with Stable Chronic Obstructive Pulmonary Disease. EAJM. 2010;42:105–110. doi: 10.5152/eajm.2010.31. http://dx.doi.org/10.5152/eajm.2010.31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J. M. The definition and measurement of antioxidants in biological systems. Free Radic Biol Med. 1995;18(1):125–126. doi: 10.1016/0891-5849(95)91457-3. http://dx.doi.org/10.1016/0891-5849(95)91457-3 . [DOI] [PubMed] [Google Scholar]
- Hiura T. S., Kaszubowski M. P., Li N, Nel A. E. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. JImmunol. 1999;163(10):5582–5591. [PubMed] [Google Scholar]
- Hoshino E, Shariff R, Van Gossum A, Allard J. P., Pichard C, Kurian R, Jeejeebhoy K. N. Vitamin E suppresses increased lipid peroxidation in cigarette smokers. JPEN JParenter Enteral Nutr. 1990;14(3):300–305. doi: 10.1177/0148607190014003300. http://dx.doi.org/10.1177/014↟190014003300 . [DOI] [PubMed] [Google Scholar]
- Hu G, Zhou Y, Tian J, Yao W, Li J, Li B, Ran P. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010;138(1):20–31. doi: 10.1378/chest.08-2114. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Sugiura H, Yamagata S, Koarai A, Shirato K. Increase in reactive nitrogen species production in chronic obstructive pulmonary disease airways. Am JRespir Crit Care Med. 2000;162(2 Pt 1):701–706. doi: 10.1164/ajrccm.162.2.9908132. [DOI] [PubMed] [Google Scholar]
- Isik B, Isik R. S., Akyildiz L, Topcu F. Does Biomass Exposure Affect Serum MDA Levels In Women? Inhal Toxicol. 2005;17(2):695–697. doi: 10.1080/08958370500189883. http://dx.doi.org/10.1080/08958370500189883 . [DOI] [PubMed] [Google Scholar]
- Kamal A. A., Gomaa A, el Khafif M, Hammad A. S. Plasma lipid peroxides among workers exposed to silica or asbestos dusts. Environ Res. 1989;49(2):173–180. doi: 10.1016/s0013-9351(89)80062-3. http://dx.doi.org/10.1016/S0013-9351(89)80062-3 . [DOI] [PubMed] [Google Scholar]
- Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151(2):362–367. doi: 10.1016/j.envpol.2007.06.012. http://dx.doi.org/10.1016/j.envpol.2007.06.012 . [DOI] [PubMed] [Google Scholar]
- Kocbach B. A., Pagels J, Yttri K. E., Barregard L, Sallsten G, Schwarze P. E., Boman C. Health effects of residential wood smoke particles: the importance of combustion conditions and physicochemical particle properties. Part Fibre Toxicol. 2009;6:29. doi: 10.1186/1743-8977-6-29. http://dx.doi.org/10.1186/1743-8977-6-29 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurmi O. P., Devereux G. S., Smith W. C., Semple S, Steiner M. F., Simkhada P, Lam K. B. Reduced lung function due to biomass smoke exposure in young adults in rural Nepal. Eur Respir J. 2013;41(1):25–30. doi: 10.1183/09031936.00220511. http://dx.doi.org/10.1183/09031936.00220511 . [DOI] [PubMed] [Google Scholar]
- Lang J. K., Gohil K, Packer L. Simultaneous determination of tocopherols, ubiquinols, and ubiquinones in blood, plasma, tissue homogenates, and subcellular fractions. Anal Biochem. 1986;157(1):106–116. doi: 10.1016/0003-2697(86)90203-4. http://dx.doi.org/10.1016/0003-2697(86)90203-4 . [DOI] [PubMed] [Google Scholar]
- Larcombe S. D., Tregaskes C. A., Coffey J. S., Stevenson A. E., Alexander L, Arnold K. E. The effects of short-term antioxidant supplementation on oxidative stress and flight performance in adult budgerigars Melopsittacus undulatus. JExp Biol. 2008;211(Pt 17):2859–2864. doi: 10.1242/jeb.017970. http://dx.doi.org/10.1242/jeb.017970 . [DOI] [PubMed] [Google Scholar]
- Magwere T, Naik Y. S., Hasler J. A. Effects of chloroquine treatment on antioxidant enzymes in rat liver and kidney. Free Radic Biol Med. 1997;22(1-2):321–327. doi: 10.1016/s0891-5849(96)00285-7. http://dx.doi.org/10.1016/S0891-5849(96)00285-7 . [DOI] [PubMed] [Google Scholar]
- Mazzoli-Rocha F, Fernandes S, Einicker-Lamas M, Zin W. A. Roles of oxidative stress in signaling and inflammation induced by particulate matter. Cell Biol Toxicol. 2010;26(5):481–498. doi: 10.1007/s10565-010-9158-2. http://dx.doi.org/10.1007/s10565-010-9158-2 . [DOI] [PubMed] [Google Scholar]
- Mazzoli-Rocha F, Magalhaes C. B., Malm O, Saldiva P. H., Zin W. A., Faffe D. S. Comparative respiratory toxicity of particles produced by traffic and sugar cane burning. Environ Res. 2008;108(1):35–41. doi: 10.1016/j.envres.2008.05.004. http://dx.doi.org/10.1007/s10565-010-9158-2 . [DOI] [PubMed] [Google Scholar]
- Mishra V, Retherford R. D. Does biofuel smoke contribute to anaemia and stunting in early childhood? Int JEpidemiol. 2007;36(1):117–129. doi: 10.1093/ije/dyl234. http://dx.doi.org/10.1093/ije/dyl234 . [DOI] [PubMed] [Google Scholar]
- Montano M, Cisneros J, Ramirez-Venegas A, Pedraza-Chaverri J, Mercado D, Ramos C, Sansores R. H. Malondialdehyde and superoxide dismutase correlate with FEV(1) in patients with COPD associated with wood smoke exposure and tobacco smoking. Inhal Toxicol. 2010;22(10):868–874. doi: 10.3109/08958378.2010.491840. http://dx.doi.org/10.3109/08958378.2010.491840 . [DOI] [PubMed] [Google Scholar]
- Mott J. A., Mannino D. M., Alverson C. J., Kiyu A, Hashim J, Lee T, Redd S. C. Cardiorespiratory hospitalizations associated with smoke exposure during the 1997, Southeast Asian forest fires. jInt JHyg Environ Health. 2005;208(1-2):75–85. doi: 10.1016/j.ijheh.2005.01.018. http://dx.doi.org/10.1016/j.ijheh.2005.01.018 . [DOI] [PubMed] [Google Scholar]
- Naeher L. P., Brauer M, Lipsett M, Zelikoff J. T, Simpson C. D., Koenig J. Q., Smith K. R. Woodsmoke health effects: a review. Inhal Toxicol. 2007;19(1):67–106. doi: 10.1080/08958370600985875. http://dx.doi.org/10.1080/08958370600985875 . [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. http://dx.doi.org/10.1126/science.1114397 . [DOI] [PubMed] [Google Scholar]
- Nel A. Atmosphere. Air pollution-related illness: effects of particles. Science. 2005;308(5723):804–806. doi: 10.1126/science.1108752. http://dx.doi.org/10.1126/science.110∰ . [DOI] [PubMed] [Google Scholar]
- Obase Y, Mouri K, Shimizu H, Ohue Y, Kobashi Y, Kawahara K, Oka M. Nutritional deficits in elderly smokers with respiratory symptoms that do not fulfill the criteria for COPD. Int JChron Obstruct Pulmon Dis. 2011;6:679–683. doi: 10.2147/COPD.S25293. http://dx.doi.org/10.2147/COPD.S25293 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama M, Otake T, Adachi S, Kobayashi T, Morinaga K. A comparison of the production of reactive oxygen species by suspended particulate matter and diesel exhaust particles with macrophages. Inhal Toxicol. 2007;19(Suppl 1):157–160. doi: 10.1080/08958370701496103. http://dx.doi.org/10.1080/08958370701496103 . [DOI] [PubMed] [Google Scholar]
- Padhi B. K., Padhy P. K. Domestic fuels, indoor air pollution, and children's health. Ann NY Acad Sci. 2008;1140:209–217. doi: 10.1196/annals.1454.015. http://dx.doi.org/10.1196/annals.1454.015 . [DOI] [PubMed] [Google Scholar]
- Padhy P. K., Padhi B. K. Effects of biomass combustion smoke on hematological and antioxidant profile among children (8-13 years) in India. Inhal Toxicol. 2009;21(8):705–711. doi: 10.1080/08958370802448961. http://dx.doi.org/10.1080/08958370802448961 . [DOI] [PubMed] [Google Scholar]
- Pereira C. E., Heck T. G., Saldiva P. H., Rhoden C. R. Ambient particulate air pollution from vehicles promotes lipid peroxidation and inflammatory responses in rat lung. Braz JMed Biol Res. 2007;40(10):1353–1359. doi: 10.1590/s0100-879x2006005000164. http://dx.doi.org/10.1590/S0100-879X2006005000164 . [DOI] [PubMed] [Google Scholar]
- Peters A, Frohlich M, Doring A, Immervoll T, Wichmann H. E., Hutchinson W. L., Pepys M. B. Particulate air pollution is associated with an acute phase response in men;results from the MONICA-Augsburg Study. Eur Heart J. 2001;22(14):1198–1204. doi: 10.1053/euhj.2000.2483. http://dx.doi.org/10.1053/euhj.2000.2483 . [DOI] [PubMed] [Google Scholar]
- Pope C. A., 3rd, Hansen M. L., Long R. W., Nielsen K. R., Eatough N. L., Wilson W. E., Eatough D. J. Particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112(3):339–345. doi: 10.1289/ehp.6588. http://dx.doi.org/10.1289/ehp.6588 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Adcock I. M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28(1):219–242. doi: 10.1183/09031936.06.00053805. http://dx.doi.org/10.1183/09031936.06.00053805 . [DOI] [PubMed] [Google Scholar]
- Raijmakers M. T., Peters W. H., Steegers E. A., Poston L. Amino thiols, detoxification and oxidative stress in pre-eclampsia and other disorders of pregnancy. Curr Pharm Des. 2005;11(6):711–734. doi: 10.2174/1381612053381837. http://dx.doi.org/10.2174/1381612053381837 . [DOI] [PubMed] [Google Scholar]
- Regalado J, Perez-Padilla R, Sansores R, Paramo R. J. I, Brauer M, Pare P, Vedal S. The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am JRespir Crit Med. 2006;174(8):901–905. doi: 10.1164/rccm.200503-479OC. http://dx.doi.org/10.1164/rccm.200503-479OC . [DOI] [PubMed] [Google Scholar]
- Reifen R. Vitamin A as an anti-inflammatory agent. Proceedings of the Nutrition Society. 2002;61:397–400. doi: 10.1079/PNS2002172. http://dx.doi.org/10.1079/PNS2002172 . [DOI] [PubMed] [Google Scholar]
- Revathi M, Kutty T. K., Annamalai N. Pulmonary Function in Rural Women Exposed to Biomass Fuel. JPulmon Resp Med. 2012;2(133):1–4. [Google Scholar]
- Rich D. Q., Kipen H. M., Huang W, Wang G, Wang Y, Zhu P, Zhang J. J. Association Between Changes in Air Pollution Levels During the Beijing Olympics and Biomarkers of Inflammation and Thrombosis in Healthy Young Adults. JAMA. 2012;307(19):2068–2078. doi: 10.1001/jama.2012.3488. http://dx.doi.org/10.1001/jama.2012.3488 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne S. T., Rodas T. J., Bender B. S., Simpson J. M., Galer-Unti R, Glickman L. T. Relationship of pulmonary function among women and children to indoor air pollution from biomass use in rural Ecquador. Respir Med. 2006;100:1208–1215. doi: 10.1016/j.rmed.2005.10.020. http://dx.doi.org/10.1016/j.rmed.2005.10.020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu I, Castro-Giner F, Kunzli N, Sunyer J. Air pollution, oxidative stress and dietary supplementation: a review. Eur Respir J. 2008;31(1):179–197. doi: 10.1183/09031936.00128106. http://dx.doi.org/10.1183/09031936.00128106 . [DOI] [PubMed] [Google Scholar]
- Saha A, Rao N. M., Kulkarni P. K., Majumdar P. K, Saiyed H. N. Pulmonary function and fuel use: a population survey. Respir Res. 2005;6:127. doi: 10.1186/1465-9921-6-127. http://dx.doi.org/10.1186/1465-9921-6-127 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger R. B., Kunzli N, Hidy G. M., Gotschi T, Jerrett M. The health relevance ambient particulate matter characteristics: coherence of toxicological and epidemiological inferences. Inhal Toxicol. 2006;18(2):95–125. doi: 10.1080/08958370500306016. http://dx.doi.org/10.1080/08958370500306016 . [DOI] [PubMed] [Google Scholar]
- Smith K. R., Mehta S. The burden of disease from indoor air pollution in developing countries: comparison of estimates. Int JHyg Environ Health. 2003;206(4-5):279–289. doi: 10.1078/1438-4639-00224. http://dx.doi.org/10.1078/1438-4639-00224 . [DOI] [PubMed] [Google Scholar]
- Sumer H, Turaclar U. T., Onarlioglu T, Ozdemir L, Zwahlen M. The association of biomass fuel combustion on pulmonary function tests in the adult population of Mid-Anatolia. Soz Praventivmed. 2004;49(4):247–253. doi: 10.1007/s00038-004-3038-6. http://dx.doi.org/10.1007/s00038-004-3038-6 . [DOI] [PubMed] [Google Scholar]
- Torres-Duque C, Maldonado D, Perez-Padilla R, Ezzati M, Viegi G. Forum of International Respiratory Studies (FIRS) Task Force on Health Effects of Biomass Exposure. Biomass fuels and respiratory diseases: a review of evidence. Proc Am Thorac Soc. 2008;5:577–590. doi: 10.1513/pats.200707-100RP. http://dx.doi.org/10.1513/pats.200707-100RP . [DOI] [PubMed] [Google Scholar]
- Yokus B, Mete N, Cakir U. D., Toprak G. Effects of active and passive smoking on antioxidant enzymes and antioxidant micronutrients. Biotechnol Biotechnol Eq. 2005;19(3):117–123. [Google Scholar]


