Abstract
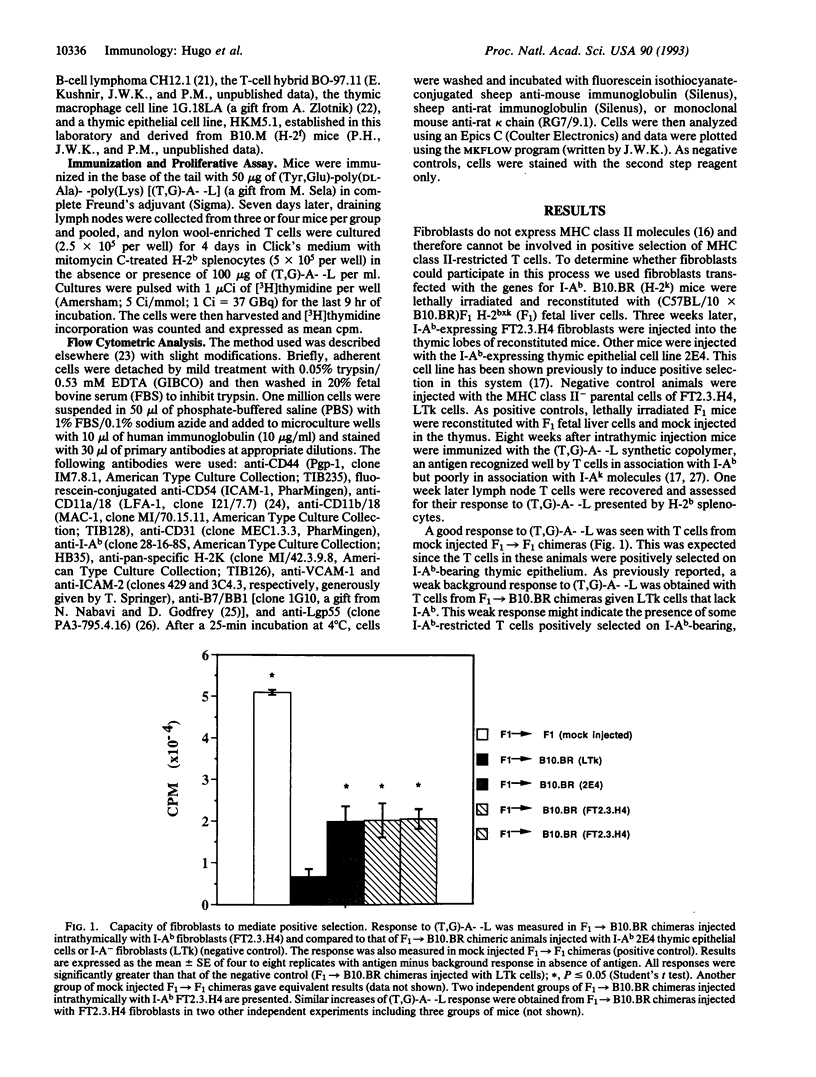
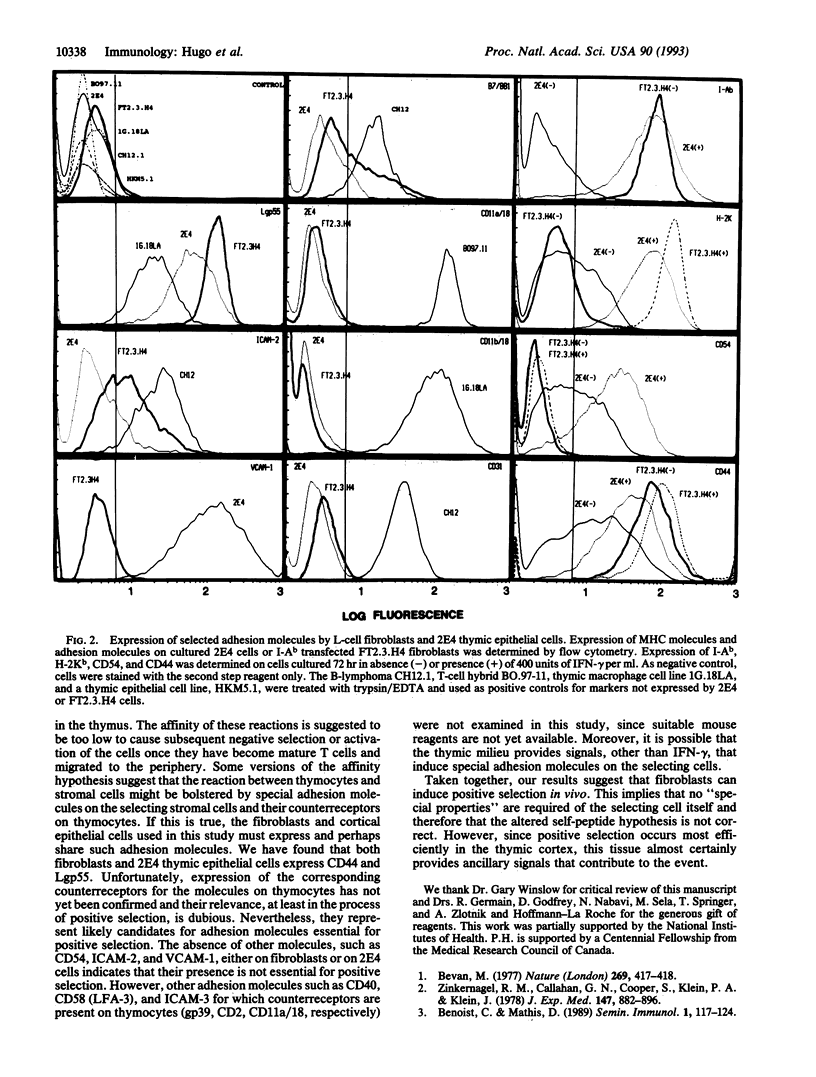
During development in the thymus, thymocytes bearing alpha beta T-cell receptors are selected to mature if the receptors they bear are able to interact in some way with major histocompatibility complex (MHC) proteins expressed on thymic stromal cells. It has been shown that thymus cortical epithelial cells are usually the cells presenting the MHC molecules involved in this process of so-called positive selection. Here we tested the ability of fibroblasts to mediate positive selection in vivo. Fibroblasts transfected with the genes for the MHC I-Ab proteins were injected intrathymically into irradiated H-2k animals reconstituted with H-2bxk F1 fetal liver cells. Eight weeks later, the recipient mice were immunized and shown to contain peptide-specific I-Ab-restricted T cells. This demonstrates the ability of I-Ab-transfected fibroblasts to participate in positive selection. Thus a cell type that is not specialized to process and present antigens in the context of MHC class II molecules can mediate positive selection when transfected with an appropriate MHC molecule. The data also support the idea that the ability to mediate positive selection may not be limited to thymic cortical epithelium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold L. W., LoCascio N. J., Lutz P. M., Pennell C. A., Klapper D., Haughton G. Antigen-induced lymphomagenesis: identification of a murine B cell lymphoma with known antigen specificity. J Immunol. 1983 Oct;131(4):2064–2068. [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Benoist C., Mathis D. Positive and negative selection of the T cell repertoire in MHC class II transgenic mice. Semin Immunol. 1989 Nov;1(2):117–124. [PubMed] [Google Scholar]
- Benoist C., Mathis D. Positive selection of the T cell repertoire: where and when does it occur? Cell. 1989 Sep 22;58(6):1027–1033. doi: 10.1016/0092-8674(89)90501-1. [DOI] [PubMed] [Google Scholar]
- Berg L. J., Pullen A. M., Fazekas de St Groth B., Mathis D., Benoist C., Davis M. M. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989 Sep 22;58(6):1035–1046. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- Bevan M. J. In a radiation chimaera, host H-2 antigens determine immune responsiveness of donor cytotoxic cells. Nature. 1977 Sep 29;269(5627):417–418. doi: 10.1038/269417a0. [DOI] [PubMed] [Google Scholar]
- Bill J., Palmer E. Positive selection of CD4+ T cells mediated by MHC class II-bearing stromal cell in the thymic cortex. Nature. 1989 Oct 19;341(6243):649–651. doi: 10.1038/341649a0. [DOI] [PubMed] [Google Scholar]
- Bix M., Raulet D. Inefficient positive selection of T cells directed by haematopoietic cells. Nature. 1992 Sep 24;359(6393):330–333. doi: 10.1038/359330a0. [DOI] [PubMed] [Google Scholar]
- Braunstein N. S., Germain R. N. Allele-specific control of Ia molecule surface expression and conformation: implications for a general model of Ia structure-function relationships. Proc Natl Acad Sci U S A. 1987 May;84(9):2921–2925. doi: 10.1073/pnas.84.9.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D., Chan S. H., Waltzinger C., Benoist C., Mathis D. The thymic compartment responsible for positive selection of CD4+ T cells. Int Immunol. 1992 Jun;4(6):707–710. doi: 10.1093/intimm/4.6.707. [DOI] [PubMed] [Google Scholar]
- Fine J. S., Kruisbeek A. M. The role of LFA-1/ICAM-1 interactions during murine T lymphocyte development. J Immunol. 1991 Nov 1;147(9):2852–2859. [PubMed] [Google Scholar]
- Golde W. T., McDuffie M., Kappler J., Marrack P. Identification of a new cell surface glycoprotein with accessory function in murine T cell responses. J Immunol. 1990 Feb 1;144(3):804–810. [PubMed] [Google Scholar]
- Hugo P., Kappler J. W., Godfrey D. I., Marrack P. C. A cell line that can induce thymocyte positive selection. Nature. 1992 Dec 17;360(6405):679–682. doi: 10.1038/360679a0. [DOI] [PubMed] [Google Scholar]
- Hugo P., Waanders G. A., Scollay R., Shortman K., Boyd R. L. Ontogeny of a novel CD4+CD8-CD3- thymocyte subpopulation: a comparison with CD4- CD8+ CD3- thymocytes. Int Immunol. 1990;2(3):209–218. doi: 10.1093/intimm/2.3.209. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Lo D., Ron Y., Sprent J. Induction of MHC-restricted specificity and tolerance in the thymus. Immunol Res. 1986;5(3):221–232. doi: 10.1007/BF02919203. [DOI] [PubMed] [Google Scholar]
- Lo D., Sprent J. Exogenous control of I-A expression in fetal thymus explants. J Immunol. 1986 Sep 15;137(6):1772–1775. [PubMed] [Google Scholar]
- MacDonald H. R., Casanova J. L., Maryanski J. L., Cerottini J. C. Oligoclonal expansion of major histocompatibility complex class I-restricted cytolytic T lymphocytes during a primary immune response in vivo: direct monitoring by flow cytometry and polymerase chain reaction. J Exp Med. 1993 May 1;177(5):1487–1492. doi: 10.1084/jem.177.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz J. S., Auchincloss H., Jr, Grusby M. J., Glimcher L. H. Class II-positive hematopoietic cells cannot mediate positive selection of CD4+ T lymphocytes in class II-deficient mice. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2779–2783. doi: 10.1073/pnas.90.7.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The T cell receptor. Science. 1987 Nov 20;238(4830):1073–1079. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Deak B. D., Shreffler D. C., Klein J., Stimpfling J. H., Snell G. D. Genetic control of the immune response. Mapping of the Ir-1 locus. J Exp Med. 1972 Jun 1;135(6):1259–1278. doi: 10.1084/jem.135.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi N., Freeman G. J., Gault A., Godfrey D., Nadler L. M., Glimcher L. H. Signalling through the MHC class II cytoplasmic domain is required for antigen presentation and induces B7 expression. Nature. 1992 Nov 19;360(6401):266–268. doi: 10.1038/360266a0. [DOI] [PubMed] [Google Scholar]
- Pardoll D., Carrera A. Thymic selection. Curr Opin Immunol. 1992 Apr;4(2):162–165. doi: 10.1016/0952-7915(92)90006-z. [DOI] [PubMed] [Google Scholar]
- Ransom J., Wu R., Fischer M., Zlotnik A. Antigen presenting ability of thymic macrophages and epithelial cells: evidence for defects in the antigen processing function of thymic epithelial cells. Cell Immunol. 1991 Apr 15;134(1):180–190. doi: 10.1016/0008-8749(91)90341-8. [DOI] [PubMed] [Google Scholar]
- Sprent J., Lo D., Gao E. K., Ron Y. T cell selection in the thymus. Immunol Rev. 1988 Jan;101:173–190. doi: 10.1111/j.1600-065x.1988.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Molecular complexity of leukocyte surface glycoproteins related to the macrophage differentiation antigen Mac-1. J Exp Med. 1981 Nov 1;154(5):1517–1524. doi: 10.1084/jem.154.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanović S., Grandea A. G., 3rd, Faas S. J., Knowles B. B., Bevan M. J. Positive selection of T-lymphocytes induced by intrathymic injection of a thymic epithelial cell line. Nature. 1992 Oct 22;359(6397):729–732. doi: 10.1038/359729a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Callahan G. N., Althage A., Cooper S., Klein P. A., Klein J. On the thymus in the differentiation of "H-2 self-recognition" by T cells: evidence for dual recognition? J Exp Med. 1978 Mar 1;147(3):882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk W., Ron Y., Monaco J., Kappler J., Marrack P., Le Meur M., Gerlinger P., Durand B., Benoist C., Mathis D. Compartmentalization of MHC class II gene expression in transgenic mice. Cell. 1988 May 6;53(3):357–370. doi: 10.1016/0092-8674(88)90156-0. [DOI] [PubMed] [Google Scholar]
- van Ewijk W. T-cell differentiation is influenced by thymic microenvironments. Annu Rev Immunol. 1991;9:591–615. doi: 10.1146/annurev.iy.09.040191.003111. [DOI] [PubMed] [Google Scholar]



