Abstract
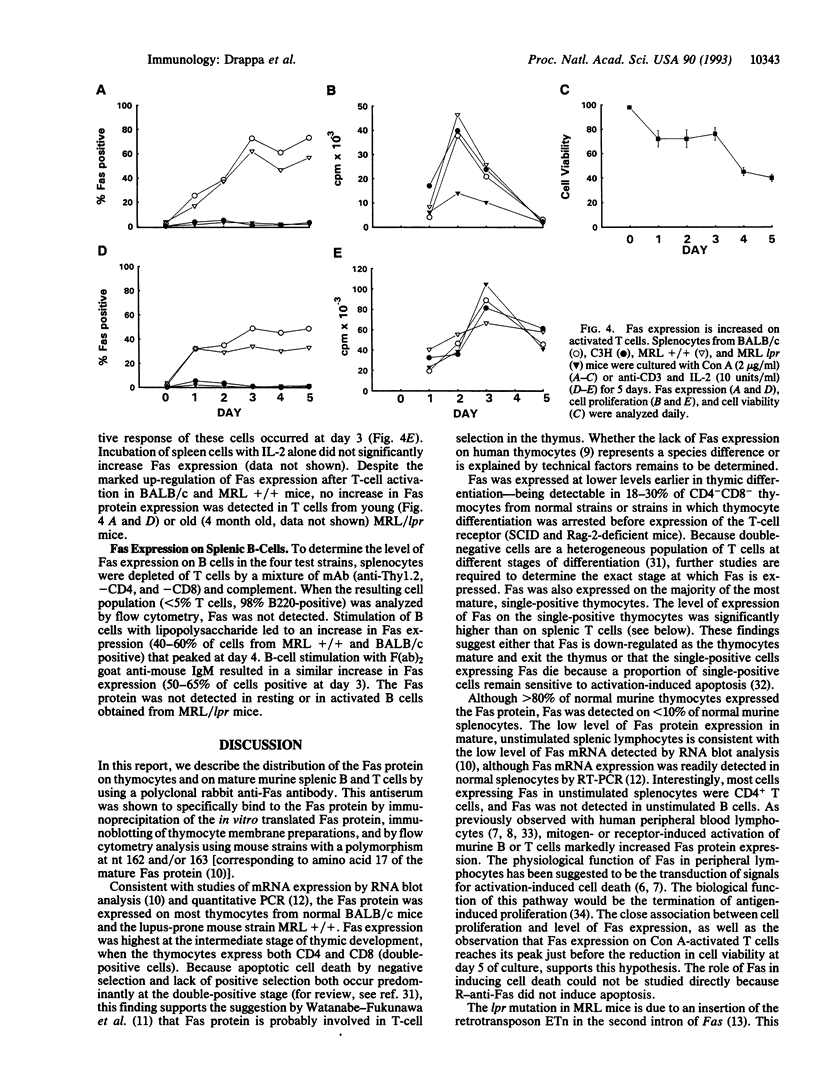
The lymphoproliferation (lpr) mutation in the MRL strain of mice is caused by the insertion of the early transposable element ETn in the Fas gene. The insertion causes a striking decrease in Fas mRNA expression and is associated clinically with marked acceleration of the lupus-like disease. To further explore the role of the Fas protein in T-cell selection in the thymus and tolerance in the peripheral immune system, we produced a monospecific polyclonal anti-murine Fas antibody that binds to a polymorphic region of the protein. Fas protein expression was detected on approximately 90% of BALB/c and MRL +/+ thymocytes, and the expression was highest on CD4+CD8+ thymocytes, the stage at which most thymocytes die by apoptosis. In contrast to the high level of expression of Fas on thymocytes, Fas was detected on < 10% of normal splenic T cells. After activation of splenic T cells with Con A or anti-CD3 and interleukin 2, Fas expression increased approximately 10-fold. Fas expression on splenic B cells was also markedly up-regulated after activation with lipopolysaccharide or anti-mu antibodies. The Fas protein was not detected on resting or activated lymphocytes obtained from MRL lpr/lpr mice. Together, these findings suggest that Fas plays a role in both thymic selection and T-cell survival in the periphery and that the accelerated autoimmunity in MRL lpr/lpr mice results from a defect in both of these pathways.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Watanabe-Fukunaga R., Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashany D., Hines J. J., Gharavi A. E., Mouradian J., Drappa J., Elkon K. B. MRL/lpr-->severe combined immunodeficiency mouse allografts produce autoantibodies, acute graft-versus-host disease or a wasting syndrome depending on the source of cells. Clin Exp Immunol. 1992 Dec;90(3):466–475. doi: 10.1111/j.1365-2249.1992.tb05869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J. L., Brot N., Weissbach H., Elkon K. Lupus antiribosomal P antisera contain antibodies to a small fragment of 28S rRNA located in the proposed ribosomal GTPase center. J Exp Med. 1991 Sep 1;174(3):507–514. doi: 10.1084/jem.174.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J. L., Brot N., Weissbach H., Elkon K. Lupus antiribosomal P antisera contain antibodies to a small fragment of 28S rRNA located in the proposed ribosomal GTPase center. J Exp Med. 1991 Sep 1;174(3):507–514. doi: 10.1084/jem.174.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon P. J., Morrissey P. J., Nordan R. P., Grabstein K. H., Prickett K. S., Reed S. G., Goodwin R., Cosman D., Namen A. E. Murine thymocytes proliferate in direct response to interleukin-7. Blood. 1989 Sep;74(4):1368–1373. [PubMed] [Google Scholar]
- Crispe I. N., Bevan M. J. Expression and functional significance of the J11d marker on mouse thymocytes. J Immunol. 1987 Apr 1;138(7):2013–2018. [PubMed] [Google Scholar]
- Duke R. C., Cohen J. J. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986 Fall;5(4):289–299. [PubMed] [Google Scholar]
- Elkon K. B., Gharavi A. E., Patel B. M., Hughes G. R., Frankel A. IgA and IgM rheumatoid factors in serum, saliva and other secretions: relationship to immunoglobulin ratios in systemic sicca syndrome and rheumatoid arthritis. Clin Exp Immunol. 1983 Apr;52(1):75–84. [PMC free article] [PubMed] [Google Scholar]
- Elkon K. B., Hines J. J., Chu J. L., Parnassa A. Epitope mapping of recombinant HeLa SmB and B' peptides obtained by the polymerase chain reaction. J Immunol. 1990 Jul 15;145(2):636–643. [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Groux H., Torpier G., Monté D., Mouton Y., Capron A., Ameisen J. C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992 Feb 1;175(2):331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Ramos J. C., Andreu J. L., Revilla Y., Viñuela E., Martinez C. Recovery from autoimmunity of MRL/lpr mice after infection with an interleukin-2/vaccinia recombinant virus. Nature. 1990 Jul 19;346(6281):271–274. doi: 10.1038/346271a0. [DOI] [PubMed] [Google Scholar]
- Honjo T. Seppuku and autoimmunity. Science. 1992 Oct 23;258(5082):591–592. doi: 10.1126/science.1384132. [DOI] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klas C., Debatin K. M., Jonker R. R., Krammer P. H. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993 Jun;5(6):625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- Kotzin B. L., Babcock S. K., Herron L. R. Deletion of potentially self-reactive T cell receptor specificities in L3T4-, Lyt-2- T cells of lpr mice. J Exp Med. 1988 Dec 1;168(6):2221–2229. doi: 10.1084/jem.168.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T., Uehara T., Nibu R., Tsuji T., Yachie A., Yonehara S., Taniguchi N. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992 Dec 1;149(11):3753–3758. [PubMed] [Google Scholar]
- Murphy K. M., Heimberger A. B., Loh D. Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990 Dec 21;250(4988):1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- Murray R., Suda T., Wrighton N., Lee F., Zlotnik A. IL-7 is a growth and maintenance factor for mature and immature thymocyte subsets. Int Immunol. 1989;1(5):526–531. doi: 10.1093/intimm/1.5.526. [DOI] [PubMed] [Google Scholar]
- Newell M. K., Haughn L. J., Maroun C. R., Julius M. H. Death of mature T cells by separate ligation of CD4 and the T-cell receptor for antigen. Nature. 1990 Sep 20;347(6290):286–289. doi: 10.1038/347286a0. [DOI] [PubMed] [Google Scholar]
- Nikolić-Zugić J. Phenotypic and functional stages in the intrathymic development of alpha beta T cells. Immunol Today. 1991 Feb;12(2):65–70. doi: 10.1016/0167-5699(91)90160-u. [DOI] [PubMed] [Google Scholar]
- Oehm A., Behrmann I., Falk W., Pawlita M., Maier G., Klas C., Li-Weber M., Richards S., Dhein J., Trauth B. C. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992 May 25;267(15):10709–10715. [PubMed] [Google Scholar]
- Owen-Schaub L. B., Yonehara S., Crump W. L., 3rd, Grimm E. A. DNA fragmentation and cell death is selectively triggered in activated human lymphocytes by Fas antigen engagement. Cell Immunol. 1992 Mar;140(1):197–205. doi: 10.1016/0008-8749(92)90187-t. [DOI] [PubMed] [Google Scholar]
- Russell J. H., Rush B., Weaver C., Wang R. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4409–4413. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P. A., Balderas R. S., McEvilly R. J., Bobardt M., Theofilopoulos A. N. Tolerance-related V beta clonal deletions in normal CD4-8-, TCR-alpha/beta + and abnormal lpr and gld cell populations. J Exp Med. 1989 Dec 1;170(6):1869–1877. doi: 10.1084/jem.170.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom D., Powell A. J., Lloyd C. W., Rees D. A. Rapid isolation of plasma membranes in high yield from cultured fibroblasts. Biochem J. 1977 Nov 15;168(2):187–194. doi: 10.1042/bj1680187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Itoh N., Yonehara S., Copeland N. G., Jenkins N. A., Nagata S. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J Immunol. 1992 Feb 15;148(4):1274–1279. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharchuk C. M., Merćep M., June C. H., Weissman A. M., Ashwell J. D. Variations in thymocyte susceptibility to clonal deletion during ontogeny. Implications for neonatal tolerance. J Immunol. 1991 Jul 15;147(2):460–465. [PubMed] [Google Scholar]
- Zhou T., Bluethmann H., Eldridge J., Berry K., Mountz J. D. Origin of CD4-CD8-B220+ T cells in MRL-lpr/lpr mice. Clues from a T cell receptor beta transgenic mouse. J Immunol. 1993 Apr 15;150(8 Pt 1):3651–3667. [PubMed] [Google Scholar]





