Abstract
The temperature sensitivity of soil organic matter (SOM) decomposition in tropical forests will influence future climate. Studies of a 3.5-kilometer elevation gradient in the Peruvian Andes, including short-term translocation experiments and the examination of the long-term adaptation of biota to local thermal and edaphic conditions, have revealed several factors that may regulate this sensitivity. Collectively this work suggests that, in the absence of a moisture constraint, the temperature sensitivity of decomposition is regulated by the chemical composition of plant debris (litter) and both the physical and chemical composition of preexisting SOM: higher temperature sensitivities are found in litter or SOM that is more chemically complex and in SOM that is less occluded within aggregates. In addition, the temperature sensitivity of SOM in tropical montane forests may be larger than previously recognized because of the presence of “cold-adapted” and nitrogen-limited microbial decomposers and the possible future alterations in plant and microbial communities associated with warming. Studies along elevation transects, such as those reviewed here, can reveal factors that will regulate the temperature sensitivity of SOM. They can also complement and guide in situ soil-warming experiments, which will be needed to understand how this vulnerability to temperature may be mediated by altered plant productivity under future climatic change.
Keywords: decomposition, temperature sensitivity, tropical lowland forest, tropical montane forest, soil microorganisms, soil organic matter
The response of soil carbon to future temperature change will influence atmospheric composition and climate (Davidson and Janssens 2006, Conant et al. 2011), but the direction and magnitude of the resulting climate feedback remain unclear (IPCC 2013, Wieder et al. 2013). This is especially so for tropical forests, which constitute a disproportionately large component of the global carbon cycle, exchanging more carbon dioxide (CO2) with the atmosphere than any other ecosystem and accounting for over two-thirds of terrestrial plant biomass (Pan et al. 2011) and a third of global soil carbon (Jobbagy and Jackson 2000). But the future carbon cycle of tropical forests, for which in situ temperature manipulation experiments have not been conducted (Cavaleri et al. 2015), remains an important source of uncertainty in climate model projections (Cox et al. 2013).
The tropics are predicted to experience novel heat regimes with a warming of 1.8 to 5.0 degrees Celsius (°C) over this century, resulting in average temperatures under which no closed canopy forest exists today (Wright et al. 2009, IPCC 2013). Earth system models currently predict that this warming will increase microbial mineralization of stable soil organic matter (SOM), resulting in a net release of carbon from soil into the atmosphere (Davidson and Janssens 2006, Wieder et al. 2013). Even a small fractional release of total soil carbon will have a significant impact on the concentration of atmospheric CO2, because carbon storage in soils is three times greater than in the atmosphere and four times greater than in vegetation (Jobbagy and Jackson 2000).
Predictions about the future of tropical soil carbon remain uncertain, not only because no soil-warming experiments have been conducted in tropical forests (Cavaleri et al. 2015), but also because we still lack a general understanding of the complex direct and indirect factors that determine the long-term sensitivity of soil-carbon loss under elevated temperature. Short-term soil-warming experiments have consistently reported the loss of soil carbon as respired CO2 (Melillo et al. 2002, Craine et al. 2010), typically explained by kinetic theory as pertaining to increased reaction rates under warmer temperatures (box 1). Long-term warming studies, however, report more complex outcomes, including declining rates of soil-respired CO2 with time (Melillo et al. 2011). This long-term response of soil carbon to warming may be influenced by several indirect and confounding factors, including plant–soil feedback, the altered availability of nutrients and labile carbon (Melillo et al. 2011), and the thermal adaptation of microbial community composition and/or activity (Wieder et al. 2013). What is clear from these studies is that the response of soil carbon to future climate will be more complex than the response predicted by contemporary Earth system models, in which the major constraints are typically temperature and soil moisture, following first-order kinetics (Todd-Brown et al. 2012).
Box 1. The temperature sensitivity of soil organic matter cycling: “Intrinsic” and “apparent” sensitivity.
Soil organic matter (SOM) accumulates during the decomposition of recently dead plant and animal biomass, and its stability is determined by the balance between plant-derived carbon inputs (leaf litter, woody debris, root turnover, and exudation) and losses through the metabolism and respiration of soil heterotrophs and the leaching of dissolved and particulate organic matter. The biochemical processes that determine this balance are all subject to the first law of thermodynamics, which states that increases in temperature (but below the limit at which proteins denature) will result in increased reaction rates. Plants are able to “acclimate” and respond to increased temperature by adjusting their rates of biochemical reactions (Ghannoum and Way 2011). The capacity for soil microorganisms to either acclimate (a direct physiological response) or ‘adapt’ (a more integrative term to capture physiological or biochemical responses across multiple-generations) to increased temperature is poorly understood (Bradford 2013, Wieder et al. 2013), but it is widely assumed that increased temperatures will result in the accelerated microbial mineralization of SOM, resulting in a positive forcing on atmospheric carbon dioxide (CO2) and climate (Davidson and Janssens 2006).
The Q10 value is central to the study of the temperature sensitivity of SOM and is defined as the factor by which a reaction increases with a 10 degrees Celsius (°C) rise in temperature. The intrinsic Q10 values of different reactions can differ widely, according to differences in the activation energy required to trigger chemical reactions, following the Arrhenius equation: K = a exp (–Ea/KT), in which K is the reaction rate constant, a is a preexponential factor, R is the universal gas constant, Ea is the activation energy, and T is the temperature in Kelvin (K). The Arrhenius equation predicts a Q10 of 2 for intermediate activation energies of approximately 50 kilojoules per mole at typical ambient temperatures of 273–303 K (Davidson and Janssens 2006). It also makes two further important predictions, given that the activation energies of different chemical reactions and ambient temperatures may vary: First, the Q10 of chemical reactions decreases with increasing temperature, because molecules become more dispersed and the frequency of interactions between them decreases. Second, it predicts that less reactive and more chemically recalcitrant molecules with greater activation energies have greater temperature sensitivity. This kinetic theory of chemical reactions frames the study of temperature effects on soil carbon and is now supported by substantial experimental evidence (Craine et al. 2010, Conant et al. 2011).
The complex interactions that govern SOM cycling are subject to kinetic theory, but the experimentally observed Q10 values are often better defined as apparent rather than intrinsic Q10 values, because they are generally confounded by parallel temperature effects on other interacting soil processes. The temperature sensitivity of SOM breakdown is influenced by the chemical protection of the organic matter (adsorption to mineral surfaces), by the physical protection of organic matter within soil aggregates, by microbial communities (Ostle and Ward 2012), by moisture availability, and by substrate availability (Conant et al. 2011, Billings and Ballantyne 2012). Each of these factors can also be affected by temperature change, enhancing or inhibiting organic matter cycling. The determination of soil-carbon vulnerability to temperature change therefore requires an understanding of the parallel responses of these interacting factors.
Studies of elevation gradients can provide insight into the impact of temperature change on soil-carbon cycling that is unattainable by conventional manipulation experiments (box 2; González et al. 2013, Sundqvist et al. 2013). This is because translocation experiments can be used to study the direct influence of temperature on specific short-term processes, such as decomposition (Couteaux et al. 2002, Salinas et al. 2011), whereas observational studies can help researchers examine the long-term adaptation of organisms and processes, such as plant primary productivity (Kitayama and Aiba 2002, Girardin et al. 2010) and the community composition of biota (Fierer et al. 2011, Wagai et al. 2011, Rapp et al. 2012). In contrast to latitudinal gradients, wet tropical elevation gradients have the advantage of not experiencing a dormant (winter) season, which can confound studies of temperature effects. However, elevation gradients can only help us to understand the temperature responses of ecosystem processes if we carefully consider the confounding influence of other indirect factors that can covary with elevation and temperature, including rainfall, lithology, fertility, and the community composition of plant and soil biota (Körner 2007).
Box 2. Elevation gradients for studying ecosystem processes.
The value of elevation gradients in plant ecology and biogeography has long been recognized (von Humboldt and Bonpland 1805). Elevation gradients have traditionally been used to study the distribution of plants and animals along climatic zones, demonstrating how environmental factors drive speciation and result in patterns of plant diversity (Whittaker and Niering 1965). More recently, given the growing concerns of the effects that climatic change may have on ecosystem processes, elevation gradients have come to be used as powerful “natural experiments” to test theories on how ecosystems may respond and feedback to climatic change (Colwell et al. 2008, Malhi et al. 2010). Elevation gradients can indeed provide insight into this climate-change feedback, but only with careful consideration of other climatic and edaphic properties that often covary with elevation, including precipitation, radiation, soil parent material, fertility, and land use (Körner 2007). These properties can be summarized by the five soil forming factors plus disturbance history (table 1).
The Kosñipata elevation transect is situated in southeastern Peru, in the upper Madre de Dios/Madeira watershed (figure 1). It lies on the eastern flank of the Andes mountain range, spanning 4 kilometers in elevation, and is on the boundary of Manu National Park, a recognized global biodiversity hotspot. Research is being performed along this transect by the Andes Biodiversity and Ecosystem Group (http://andesconservation.org), with the primary goal to understand ecosystem ecology and biodiversity and their responses to past and future climate change (Malhi et al. 2010). The permanent sampling plots span a zone of continuous forest cover from the lowland Amazon rainforest to the upper montane cloud forest. The timberline ranges between 3100 and 3700 meters above sea level (m asl), above which there is grassland. The elevation gradient provides particular insight into the influence of temperature on ecosystem processes because the mean annual temperature strongly decreases with increasing elevation (from 26 degrees Celsius, °C, at 194 m asl to 6°C at 3700 m asl), but the mean annual precipitation does not vary consistently with elevation, and soil moisture content remains quite high at all elevations (table 2; Rapp and Silman 2012). The evaluation of temperature effects independent of precipitation is important, because temperature and moisture are often the two key variables in soil-carbon models (Todd-Brown et al. 2012). Soil pH does not vary along the gradient, enabling evaluation of the soil microbial community composition and resource availability independent of soil pH, which is one of the primary soil properties influencing soil microbial communities (Fierer et al. 2011). However, with increased elevation, there is a marked increase in soil organic carbon contents (figure 2a) and the depth of the organic horizon (Girardin et al. 2010, Zimmermann et al. 2012), a common observation along elevation gradients (Moser et al. 2011). In addition, there are changes in soil nutrients with elevation (figure 2a). Soils are Inceptisols throughout the gradient except for the lowest site on Ultisols and are situated predominantly on Paleozoic metasedimentary mudstones but with granite intrusions underlying the sites between 1500 and 2020 m asl. The Kosñipata transect therefore allows investigation into the role of temperature—and the interacting influence of other covarying edaphic factors (table 1)—in regulating the biogeochemical processes that determine organic matter decomposition. The differences in soil (Quesada et al. 2010, Zimmermann et al. 2012), plant communities (Girardin et al. 2010, Feeley et al. 2011), and soil microbial communities (Fierer et al. 2011, Whitaker et al. 2014b) are described in detail elsewhere.
Here, we draw on findings from a 3.5-kilometer elevation gradient of contiguous tropical forest in the Peruvian Amazon–Andes, the Kosñipata transect, to probe the complex direct and indirect effects of climate warming on soil-carbon cycling in tropical forests (box 2, figure 1). The Kosñipata transect is unusual in enabling the detailed study of multiple covarying factors, because it consists of a large network of long-term study sites of tropical forest and grassland, spanning a 20°C mean annual temperature gradient (table 1; Malhi et al. 2010). In this synthesis, we use findings from translocation experiments to examine the direct effect of warming on decomposition processes. However, the long-term response of soil carbon to warming will be constrained by several indirect factors. We consider this by examining the interacting influences of covarying factors along the Kosñipata transect, which can be summarized according to the fundamental soil forming factors, plant and microbial communities, and nutrient constraints to their metabolism (2). By examining the findings from the Kosñipata transect in the context of studies elsewhere, we identify the broad range of driving mechanisms likely to determine the tropical forest carbon balance under future warming.
Figure 1.
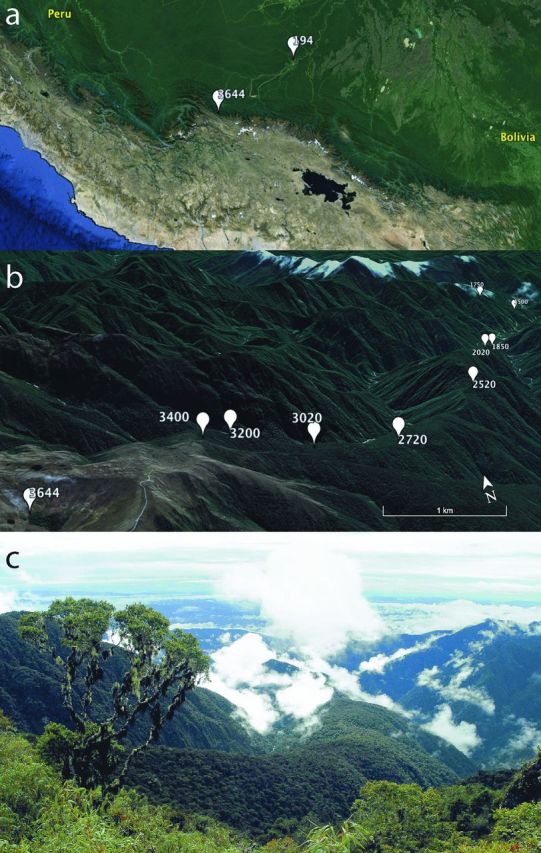
The Kosñipata elevation transect, Manu National Park, Peru. Images show (a) the highest- (3644 meters above sea level, m asl) and lowest-elevation (194 m asl) sites; (b) all sites from 3644 m asl to 1500 m asl viewed facing approximately northeast from the top of the transect; (c) a photograph of the transect of the same view as shown in 1B. Abbreviation: km, kilometers.
Table 1.
A summary of site characteristics and soil chemical and physical properties along the elevation gradient.
| Total phosphorus (mg per gram) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total nitroge n (%) | Leaf litter C:N | |||||||||||
| Vegetation type | Elevation (m asl) | Mean annual air temp (°C) | Mean annual precipitation (mm per year) | Soil organic horizon depth (cm) | Mean (M) | Standard error (SE) | M | SE | Soil pH | M | SE | Relative recalcitrance of soil C |
| Lowland rainforest | 194 | 26.4 | 2730 | 1 | 0.35 | 0.03 | 0.49 | 0.07 | 3.7 | NA | 0.91 | |
| 210 | 26.4 | 3199 | 2 | 0.23 | 0.03 | 0.18 | 0.03 | 4.0 | 29.9 | 7.7 | NA | |
| Premontane rainforest | 1000 | 20.7 | 3087 | 4 | 1.34 | 0.12 | 0.73 | 0.05 | 4.7 | 23.7 | 1.9 | 0.53 |
| Lower montane cloud-forest | 1500 | 17.4 | 2631 | 16 | 0.91 | 0.12 | 1.36 | 0.37 | 3.5 | 29.1 | 5.3 | 0.58 |
| 1750 | 15.8 | 2631 | 10 | 1.56 | 0.50 | 1.44 | 0.09 | 3.6 | NA | NA | ||
| 1850 | 16.0 | 2472 | 16 | 1.86 | 0.24 | 0.76 | 0.06 | 3.5 | NA | NA | ||
| 2020 | 14.9 | 1827 | 17 | 2.00 | 0.24 | 0.71 | 0.10 | 3.4 | NA | NA | ||
| Upper montane cloud-forest | 2520 | 12.1 | NA | 14 | 1.73 | 0.34 | 0.98 | 0.14 | 3.7 | NA | NA | |
| 2720 | 11.1 | 2318 | 21 | 1.64 | 0.25 | 0.87 | 0.19 | 3.6 | 39.6 | 2.3 | NA | |
| 3020 | 9.5 | 1776 | 17 | 1.57 | 0.21 | 0.92 | 0.13 | 3.4 | NA | NA | ||
| 3025 | 11.1 | 1706 | 23 | 2.39 | 0.12 | 1.09 | 0.08 | 3.5 | 71.5 | 19.2 | 0.52 | |
| 3200 | 8.9 | NA | 12 | 2.42 | 0.20 | 0.91 | 0.02 | 3.5 | NA | NA | ||
| 3400 | 7.7 | 2555 | 14 | 2.49 | 0.17 | 1.09 | 0.09 | 3.4 | NA | NA | ||
| Grassland | 3644 | 6.5 | NA | 4 | 1.44 | 0.07 | 0.92 | 0.13 | 3.4 | NA | NA |
Note: Leaf-litter carbon-to-nitrogen ratios (C:N) are the average of three dominant species for respective plots (Salinas et al. 2011). Total nitrogen and phosphorus were determined for surface soil (0–10 centimeters, cm, in depth). Soil pH was measured in water. The relative recalcitrance of soil carbon is the ratio of the proportion of total soil carbon in recalcitrant carbon divided by the proportion in recent plant-derived carbon within surface mineral soils as determined by nuclear magnetic resonance (percentage of carbon in groups: alkyl/O-alkyl; Zimmermann et al. 2012). n = 5. Abbreviations: °C, degrees Celsius; cm, centimeters; m asl, meters above sea level; mg, milligrams; mm, millimeters; NA, data not available (Girardin et al. 2010, Rapp and Silman 2012, Whitaker et al. 2014b).
Temperature as a direct driver of decomposition in tropical forests
Temperature directly affects the rate of organic matter decomposition through its influence on biochemical reaction rates and the activity of the soil microbial community. However, the nature of the relationship between climate warming and decomposition remains unresolved. Much of the research on warming effects on SOM has focused on laboratory incubations with a smaller number of field warming experiments and cross-site studies conducted in temperate forests and grasslands, in which the respiratory response of decomposers to warming was measured (Conant et al. 2011). The temperature sensitivity of decomposition processes is often quantified using “Q10” values, which are defined as the factor by which a reaction rate increases with a 10°C rise in temperature (box 1). Comparisons of temperature sensitivities (Q10 values) from these studies may be misleading, however, because the values usually define an apparent Q10 resulting from complex interacting processes that differ among studies but do not define the intrinsic Q10 of the individual processes (box 1). In order to accurately model the temperature sensitivity of decomposition in different ecosystems, we need to understand how these intrinsic Q10 values give rise to the apparent Q10 of decomposition (Billings and Ballantyne 2012). Translocation experiments and observations along the Kosñipata transect have addressed this knowledge gap, demonstrating how the intrinsic temperature sensitivities of multiple ecosystem processes collectively determine the overall apparent temperature sensitivity of organic matter decomposition.
Translocation experiments
A common approach to quantifying the thermal vulnerability of soil carbon is to determine how experimental warming alters the temperature sensitivity of gross soil processes, such as rates of decomposition and respiration. The temperature sensitivity of these processes has been determined in the Kosñipata study by using manipulation experiments (table 3), in which organic matter was translocated among sites spanning a 3400-meter (m) elevation and 20°C mean annual temperature difference and the temperature sensitivity of decomposition and respiration rates were measured. This approach has been applied to leaf litter (Salinas et al. 2011) and fine wood (Meier et al. 2010)—the major sources of plant-derived carbon input to soil—and to the major store of soil carbon held in SOM (table 3; Zimmermann et al. 2009). Plant litter was translocated within mesh bags (Salinas et al. 2011), whereas SOM was translocated as 50-centimeter–deep soil monoliths (Zimmermann et al. 2009). In the plant–litter translocation experiments, the elevation-related temperature difference was the major treatment, with an interaction with rainfall and soil nutrients (Salinas et al. 2011). For the SOM translocation experiment, the temperature difference alone was the major treatment, because rainfall inputs were controlled and new inputs of litter and root-derived organic matter were excluded using protective mesh (Zimmermann et al. 2009). These studies, which estimated the overall temperature sensitivity of major decomposition processes (mass loss, chemical alteration, and respiration rates) for the major carbon pools (litter, wood, and soil), were complemented by studies of the temperature sensitivity of fungal growth (Meier et al. 2010) and the biochemical mechanisms of decomposition (soil enzymes).
Table 3.
The temperature sensitivity (Q10 values) of decomposition processes and properties, determined by experiment and the measurement of organic matter properties from the Kosñipata transect.
| Process/property | Site | Q10 value | Reference |
|---|---|---|---|
| Wood decomposition | Average response 210–3025 meters (m) | 4.0 | |
| Fungal growth | Average response 1500–3400 m | 3.9 | Meier et al. 2010 |
| Leaf-litter decomposition | Average response 210–3025 m | 3.1 | Salinas et al. 2011 |
| Soil organic matter decomposition | soil origin 3030 m | 3.4 | Zimmermann et al. 2012 |
| soil origin 1500 m | 2.3 | ||
| soil origin 1000 m | 2.8 | ||
| soil origin 210 m | 4.9 | ||
| Heterotrophic respiration | in situ 3030 m | 2.5 | Zimmermann et al. 2009 |
| in situ 1500 m | 1.6 | ||
| in situ 1000 m | 1.5 | ||
| in situ 210 m | 1.2 | ||
| Total soil respiration | in situ 3030 m | 4.3 | Zimmermann et al. 2009 |
| in situ 1500 m | 2.1 | ||
| in situ 1000 m | 2.9 | ||
| in situ 210 m | 6.9 | ||
| Soil enzyme activities (Vmax) | Average response 194–3644 m | ||
| ®-glucosidase | 1.6 | ||
| Cellobiohydrolase | 2.0 | ||
| ®-xylanase | 1.7 | ||
| Phenol oxidase | 1.4 | ||
| N-acetyl ®-glucosaminidase | 1.7 | ||
| Phosphomonoesterase | 1.6 |
Note: The values represent the average response determined across sites along the elevation gradient, calculated by comparing responses at the different sites (decomposition rates and fungal growth), or the range of site-specific values, determined using diurnal temperature variation (respiration) or by manipulating temperature in a laboratory incubation (enzyme activities). The Q10 values for soil organic matter decomposition were determined by comparing the response for each soil type (soil origin) among the four sites according to the difference in MAT.
Temperature sensitivity of decomposition: Plant debris, soil organic matter, and enzyme activities
Organic matter translocation studies along the Kosñipata transect support predictions from kinetic theory by showing that the Q10 of decomposition is larger for organic matter compounds of greater chemical complexity, such as those containing a higher proportion of lignin, when compared with more simple compounds containing a higher proportion of cellulose (table 3; Salinas et al. 2011). Similar results have been observed in warming experiments performed outside the tropics (Hartley and Ineson 2008, Craine et al. 2010, Conant et al. 2011). In a study spanning 2800 m of the transect and focusing on 15 tree species, the average Q10 of decomposition was 3.1 for leaf litter, in contrast to 4.0 for fine wood debris, with woody matter containing a greater proportion of complex carbon compounds than leaf litter (table 3; Salinas et al. 2011). Furthermore, these mean Q10 values obscured significant interspecies variation: Sevenfold and twofold differences were observed in litter and wood decomposition rates, respectively, and this variance was not correlated with carbon to nitrogen ratios. This suggests greater chemical variation in leaf litter compared with fine wood debris, and that interspecific variation in carbon chemistry (e.g., abundance or structure of lignin) is a dominant control on decomposition rates. The Q10 for fine wood (4.0), which is predominantly composed of lignocellulose, and is decomposed by fungi (table 3) was consistent with the Q10 of fungal growth (Q10 = 3.9), which was estimated separately, on the basis of the accumulation of ergosterol (a sterol found only in fungal cell membranes) on a common wood substrate placed along the transect (Meier et al. 2010).
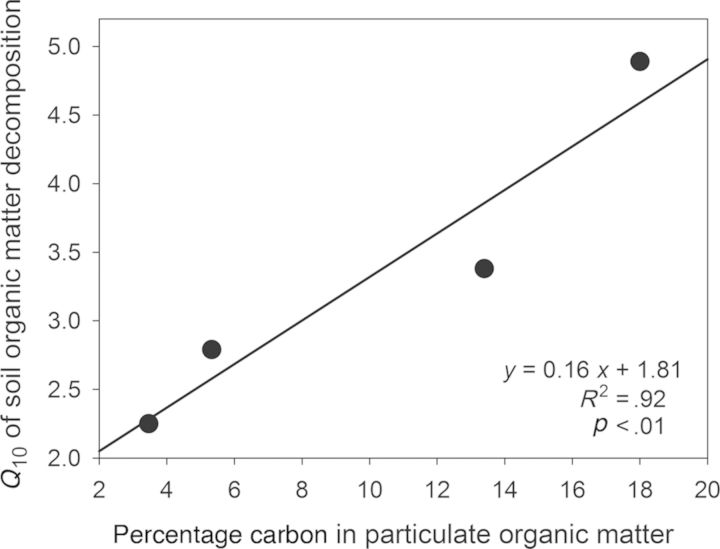
The SOM translocation studies along the transect have revealed patterns consistent with kinetic theory and have also indicated a role of physical soil properties in constraining the temperature sensitivity of SOM decomposition. Significant warming-induced losses of soil carbon were observed in this experiment: Following 2 years of warming by approximately 5.5°C (translocation of soil from 1000 m to 210 m), the rates of carbon loss as respired CO2 increased by 31.5% (Zimmermann et al. 2010, 2012). These losses were found to originate from more labile carbon stores, because the relative abundance of recent plant-derived compounds (e.g., O-alkyl) decreased and the relative abundance of more recalcitrant compounds (e.g., alkyl) increased over time (Zimmermann et al. 2012). Concurrent with this was an increase in the Q10 values of SOM decomposition (Zimmermann et al. 2012), further supporting the prediction from kinetic theory of higher Q10 values for more chemically complex carbon. Similar increases with time for Q10 values of SOM decomposition have been reported for soil incubation experiments in temperate grassland (Conant et al. 2008), elevation gradients in Australian tropical forest (Zimmermann and Bird 2012), and Chinese subtropical forest (Xu et al. 2010). Although the Q10 of respiration increased at each site following extended periods of incubation (consistent with the decreasing availability of simple carbon substrates), the Q10 of SOM decomposition over 2 years following translocation was not correlated with the molecular composition of residual SOM (determined using 13C nuclear magnetic resonance) but it was strongly correlated with the relative abundance of carbon in physically unprotected particulate SOM (figure 2; Zimmermann et al. 2012). Therefore, although short-term soil respiration responses were consistent with kinetic theory, in the longer term, soil aggregation was shown to be an overriding constraint on SOM decomposition. These results demonstrate how a simple application of kinetic theory to SOM decomposition is complicated by soil physical properties.
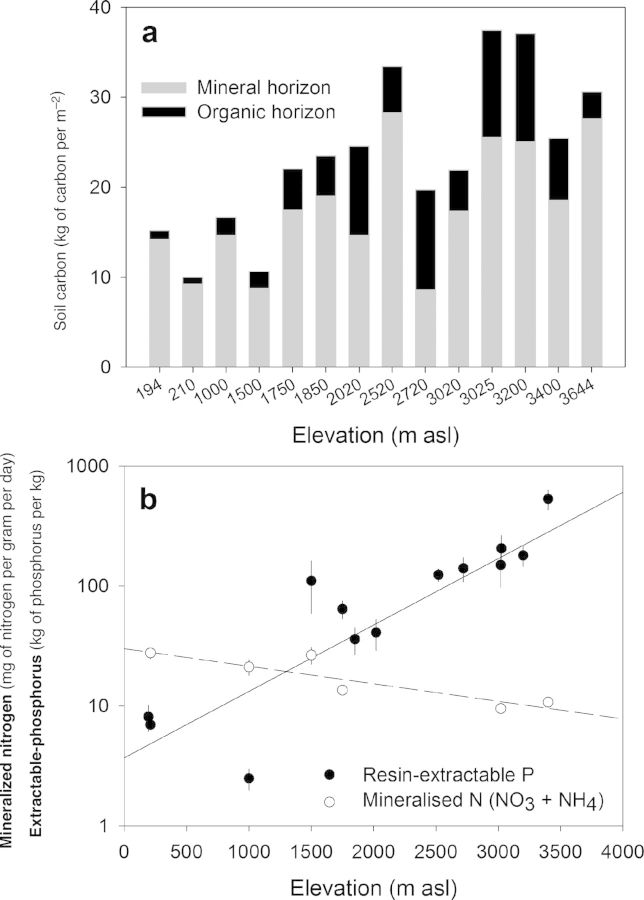
Figure 2.
Carbon stocks (a) and available nutrients (b) in tropical forest soils along the Kosñipata transect. Data were determined from five samples within 1-hectare plots at each elevation. Carbon in the organic horizon (ranging from 1 to 23 centimeters in depth) and mineral horizons was determined to a 50-cm depth from the soil surface and is presented on an area basis. Mineralized nitrogen and resin-extractable phosphorus were determined to a 10-cm depth from the soil surface using in situ resin bags. Nutrient data are log-transformed to more clearly show elevation transitions (Nottingham et al. 2015b). The error bars represent one standard error. Abbreviations: kg, kilograms; m2, square meter; m asl, meters above sea level; mg, miligram.
Microorganisms produce extracellular enzymes to catalyze the depolymerization of organic matter in soil, so the study of enzymatic activity provides insight into the processes of decomposition that together contribute substantially to net soil respiration fluxes and their responses to changes in temperature or substrate availability (Billings and Ballantyne 2012). As with the gross processes of decomposition and respiration, kinetic theory predicts that enzymes may have intrinsic temperature sensitivities that increase with the complexity of the target substrate, given that the activation energy required in degrading an organic molecule applies to both the depolymerization reaction and the enzyme that catalyzes it (box 1). Few measurements of soil enzyme activity have been reported for tropical forests to test this theoretical prediction. For the Kosnipata transect, most but not all of the extracellular enzymes showed temperature-response behavior consistent with theory (table 3). Although a relatively narrow range of values for Q10 of enzyme activities was observed among enzyme classes and elevations (Q10 = 1.4–2.6), which are similar to values for other ecosystems (Wallenstein et al. 2011), there were notable differences among enzyme classes. Greater temperature sensitivities were recorded for cellobiohydrolase and xylanase (which catalyze the hydrolysis of intermediate-complexity carbohydrates such as cellulose and hemicellulose, respectively), compared with β-glucosidase (which catalyzes the hydrolysis of simple bonds between glucose molecules). However, the activity of phenol oxidase, which catalyzes the oxidation of complex phenolic compounds, had the lowest temperature sensitivity of all enzymes tested (Q10 = 1.4). Therefore, although the majority of enzymes tested had Q10 values consistent with kinetic theory, it seems that chemical or physical stabilization of the relatively large polymers abundant in phenolic compounds, such as in lignin, prevents a simple application of this rule, even when decomposition processes are considered at the biochemical level. Indeed, this same caveat of protection was also found to apply when interpreting the temperature response of gross respiration fluxes from the translocated SOM monoliths at different elevations (Zimmermann et al. 2012).
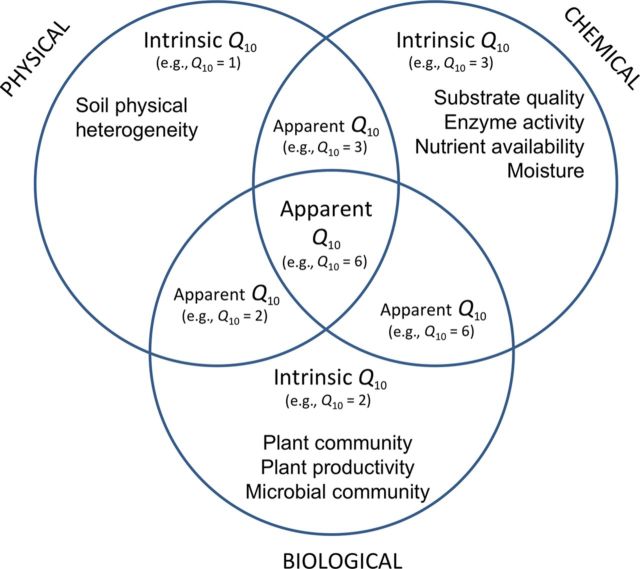
The SOM translocation studies also demonstrated the potential for estimates of apparent Q10 values to be confounded by substrate availability (Davidson et al. 2006). The apparent Q10 values of total soil respiration, in which substrate supply was uncontrolled, were high and variable (2.1 to 6.9). In contrast, the Q10 values of heterotrophic respiration alone, in which substrate supply was controlled by excluding litter inputs, were relatively low (1.2 to 2.5; Zimmermann et al. 2010) and similar to the Q10 values of enzymatic activity (1.4 to 2.6; table 3). The Kosñipata transect therefore demonstrates how apparent Q10 values of SOM decomposition in natural ecosystems are the sum of multiple interacting properties, including an important role for substrate availability as determined by the activity and composition of plant and microbial communities, each with their own intrinsic temperature sensitivity (figure 3).
Figure 3.
Ecosystem properties and processes—each of which has its own intrinsic temperature sensitivity (Q10)—that may interact to determine the overall apparent Q10 of soil-carbon degradation.
In summary, the experiments and observational studies from the Kosñipata transect broadly support the kinetic theory–based prediction of higher temperature sensitivity for more chemically complex soil-carbon compounds, with consistency in data sets spanning different types of litter and SOM and a range of extracellular enzymes. This overall finding indicates a higher temperature sensitivity for the more chemically complex soil carbon found in lowland forest soils (table 1), although the absolute amount of soil carbon is greater at higher elevation (figure 4a). However, studies from the Kosñipata transect also identified important exceptions to simple theory-based predictions, because of the protection of soil carbon from microbial and enzymatic access in soil aggregates. We note that similar effects on Q10 from chemical or physical protection of SOM may also occur in mineral soils in the tropics given the recently observed correlation of carbon stabilization with aluminium minerals along an elevation gradient in Hawaii (Giardina et al. 2014). These factors are likely to influence carbon losses under warming in lowland tropical soils that are strongly aggregated and abundant in stabilizing minerals (Quesada et al. 2010).
Figure 4.
The relationship between the Q10 values of soil organic carbon degradation during the first 2 years following translocation and the relative portions of carbon stored in particulate organic matter (physically unprotected; r = –.96, p < .01). The four points represent the four sites included in the soil translocation experiment (situated at 210, 1000, 1500, and 3030 meters above sea level), where Q10 values were determined by respiration responses following translocation among sites and soil physical fractions were determined for soil from each site (Zimmermann et al. 2012).
Temperature as an indirect driver of decomposition: Confounding interactions
Notwithstanding the uncertainties discussed above, kinetic theory does provide reasonably accurate predictions of the short-term response of soil carbon to warming (Craine et al. 2010, Zimmermann et al. 2012). However, the long-term response is also influenced by several indirect and confounding factors, which differ across landscapes and may change under warming, such as the metabolism and community composition of plants and soil microorganisms (Wright et al. 2009, Bradford 2013) and abiotic variables including atmospheric CO2 concentration, soil moisture, radiation loads, and soil-nutrient availability (Melillo et al. 2011, Wood et al. 2012). These indirect factors will result in more complex responses of soil carbon to warming than can be predicted by its chemical composition alone. To fully understand the consequence of these interacting factors requires long-term, multiple-factor experiments (Cavaleri et al. 2015), but we can also learn from studying gradients of these factors within natural ecosystems (Sundqvist et al. 2013).
The Kosñipata transect has provided an opportunity to investigate the influence of some of these interacting factors in constraining the response of soil carbon to temperature change, in particular the role of plant and soil microbial community composition and soil nutrients (table 2). Other potentially important factors that will covary with future temperature change but do not vary strongly along the Kosñipata transect include precipitation patterns and atmospheric CO2 concentrations. The lack of linear variation in precipitation and absence of seasonal soil moisture constraints (Zimmermann et al. 2009) along the Kosñipata transect is an advantage of studying this gradient, because it reduces the number of confounding factors. Changes in precipitation patterns, relative humidity, and evaporation can influence soil carbon by modulating soil moisture and microbial processes (Wood et al. 2012). Increased atmospheric CO2 concentrations are predicted to affect plant production and the magnitude of plant-derived carbon inputs to soils (Lloyd and Farquhar 2008). However, we cannot evaluate the impact of raised atmospheric CO2 by using natural gradients, and our knowledge is limited by the lack of elevated-CO2 experiments in tropical forests.
Table 2.
How the five soil-forming factors (climate, vegetation, topography, parent material, and time) and disturbance history vary along the Kosñipata transect and how they are hypothesized to interact with warming to influence soil-carbon (C) storage.
| Factor | Variation with increasing elevation along the Kosñipata transect | Reference | Warming Interaction | Hypothesised SOM interactions |
|---|---|---|---|---|
| Climate | ||||
| Temperature | Linear decrease (table 2) | Rapp and Silman 2012 | Direct | Losses following kinetic theory but confounding interactions with soil properties, vegetation, rainfall (see below) and potential for microbial adaptation to temperature change |
| Rainfall | Nonlinear (table 2) | Girardin et al. 2010; Rapp and Silman 2012 | Strong effect, non-linear and regional | Effects on soil oxygen, substrate availability to microbes and plant production. Effects on nutrient status through soil weathering and leaching. |
| Vegetation | Transition in plant communities Productivity decreases | |||
| Diversity decreases | ||||
| Associated transition in soil microbial communities (especially rhizosphere and potentially mycorrhizal) | Girardin et al. 2010 Rapp et al. 2012 | |||
| Whitaker et al. 2014b Fierer et al. 2011 | Strong effect on productivity and community composition | Stimulated productivity (while below thermal limit) and up-slope migration. Up-slope movement of cloud base may increase radiation and plant productivity. Associated shift in belowground communities. Increased quantity but lower quality of C input to soil. | ||
| Topography | Steeper slopes at mid–high elevation | Whitaker et al. 2014b | Weak effect via altered rainfall | Climate changes may affect soil moisture and landslide activity (affecting exposure and weathering of bedrock). |
| Parent material | Paleozoic metasedimentary mudstones except for granite intrusion between 1500 and 2020 meters above sea level (box 2) | Quesada et al. 2010 | Weak effect via altered rainfall | Parent material and its weathering state will constrain impacts of warming on biotic responses by influencing availability of rock-derived nutrients, such as phosphorus. Greater losses for soils with low available nitrogen, lower protection in aggregates and minerals. |
| Time | Less weathered (“younger”) soil at higher elevation | Nottingham et al. 2015b | Weak effect via altered rainfall | Increased rainfall can accelerate soil weathering and affect soil nutrient status. Soil nutrient status will constrain warming impacts on soil C, with higher C losses in “younger,” low nitrogen and C rich, soils. |
| Disturbance | Recent (< 50 years) low intensity cattle grazing at highest site (3644 meters), but no documented evidence for other sites | NA | Unknown effect | Climate-driven human migration and land-use change. Effect on soil C by altered plant communities, production and soil nutrient status. |
Changing plant communities and their inputs to soil
To understand the impacts of climate warming on soil carbon, we need also to evaluate parallel changes in plant-derived organic matter inputs to soil, which affect soil-carbon cycling and its temperature sensitivity by altering substrate and nutrient availability to soil heterotrophs (Hartley and Ineson 2008, Conant et al. 2011). Changes in leaf litter quantity and quality (generally defined by its chemical complexity or carbon-to-nitrogen, or C:N, ratio) may occur should climatic or atmospheric change alter the physiology of individual plants or change the species composition of plant communities. Overall C:N ratios in plant tissue may vary as a result of thermal or other physiological acclimation processes (Ghannoum and Way 2011). Larger changes in the quality and quantity of litterfall in montane forests may occur if there are climate-related shifts in species ranges and plant community composition (Chen et al. 2011). On the basis of current characteristics and recent changes in plant communities along the Kosñipata transect, we can estimate how plant–soil feedback influences soil-carbon dynamics in montane forests, but the feedback arising from warming in lowland forest remains difficult to assess without in situ experimentation.
Temperature is a fundamental constraint on plant productivity (van de Weg et al. 2014) and community composition (Rapp et al. 2012) along the Kosñipata transect. Aboveground forest biomass is twofold smaller at the highest relative to the lowest elevations—and aboveground productivity is fourfold smaller in montane forest relative to lowland forest—as a result of the limitations to productivity by temperature and nitrogen at higher elevation and also by light availability in the cloud forest zone (Girardin et al. 2010, van de Weg et al. 2014). Recent multiyear recensus data have also reported the up-slope warming-related migration of tree species ranges at a rate of 2.5–3.5 vertical meters per year (Feeley et al. 2011), a pattern which appears to be common globally (Chen et al. 2011). This indicates that the plant community composition of tropical montane forests will undergo large changes under climate warming, because tree species are migrating at different rates and some may not be migrating fast enough to avoid extinction (Feeley et al. 2011). These observations suggest that under climate warming, montane forests on the Kosñipata transect will be more productive and contain more of the tree species that currently occupy lower elevations. Furthermore, these changes will likely increase the quantity and quality of litter fall because, on average, lower-elevation tree species on this transect have higher net primary production and litter quality (lower foliar C:N ratios; table 2; van de Weg et al. 2009, Girardin et al. 2010), as was also observed in other tropical montane ecosystems (Vitousek et al. 1992, Kitayama and Aiba 2002). The consequence of these changes in aboveground vegetation properties may be a shift toward higher rates of decomposition and nutrient mineralization and declines in soil-carbon content (Wardle et al. 2004).
An additional but particularly poorly understood impact of large-scale alterations in plant community properties is the effect on rhizosphere-derived carbon inputs to soil. The observed increase in soil carbon with elevation (figure 4a) may result partly from differences in root dynamics and mycorrhizal communities. The mean annual residence time of fine roots increases by an order of magnitude with increased elevation on this transect, although the proportion of primary productivity allocated belowground does not (Girardin et al. 2010). This suggests that root-derived carbon, which has a greater mean annual residence time compared with that of leaf-litter carbon (Rasse et al. 2005), contributes a larger portion of soil carbon at a higher elevation. Elevation-related differences in the relative importance of arbuscular (AM) and ectomycorrhizal (EM) associations may also have large consequences for soil-carbon dynamics. Large soil carbon stores have been associated with EM-dominated systems, because EM fungi are saprophytic and compete directly with other free-living microorganisms for organic nitrogen, which promotes nitrogen limitation and reduces rates of decomposition (Clemmensen et al. 2013, Averill et al. 2014). Mycorrhizal communities are yet to be investigated along the Kosñipata transect, but if the relative importance of AM to EM associations is related to a transition from mineral to organic soil (Read and Perez-Moreno 2003), an increased dominance of EM associations at higher elevations may be found (table 1, figure 4a). The impacts of these root and mycorrhizal community changes on soil carbon under climate warming are therefore potentially significant but remain poorly resolved.
Nutrient constraints to heterotrophic metabolism
The response of plant and microbial metabolism to warming will be constrained by nutrient availability. This includes the plant physiological responses to warming that determine the quality and quantity of plant-carbon inputs to soil, in addition to the soil microbial responses that directly affect soil-carbon storage (Wood et al. 2012). Soil-nutrient availability itself may change under warming, largely as a result of these plant and microbial responses, through changes in plant inputs, nitrogen fixation, nitrification, denitrification, enzymatic activity, and sorption and desorption reactions. Seven years of experimental soil warming in temperate forest resulted in the stimulated mineralization of soil organic carbon and nitrogen (Melillo et al. 2011), indicating that one of the largest perturbations of soil carbon cycling may arise through the accelerated decomposition of SOM to liberate organic nitrogen (Chena et al. 2013). Whether phosphorus constraints on decomposers will have similar impacts on soil carbon under warming is less clear. Microbial phosphorus acquisition can be decoupled from organic carbon degradation, partly because of the biochemical mineralization of organic phosphorus esters and the abundance of inorganic phosphate (Nottingham et al. 2015a). The vulnerability of SOM to these “nutrient-mining” effects (Melillo et al. 2011) may therefore depend on the degree of nitrogen relative to phosphorus constraints on the soil microbial community.
Tropical mountains have been characterized as gradients of nutrient limitation to plant and microbial metabolism, with a shift from predominant phosphorus to nitrogen constraints with increasing elevation. This gradient arises because soil nitrogen inputs are primarily atmospherically derived, whereas soil phosphorus inputs are primarily rock derived. In lowland tropical forests, strongly weathered soils—with a consequent scarcity of rock-derived nutrients—are widespread (Vitousek 1984). In montane tropical forests, lower temperatures result in less biological nitrogen fixation, slower rates of decomposition (Salinas et al. 2011), and bedrock rejuvenation because of greater rates of soil erosion and landslide activity (Porder and Hilley 2010). Multiscale data sets from the Kosñipata transect, including soil-nutrient concentrations, enzyme activities, and soil CO2 fluxes, are consistent with this paradigm. At higher elevations, soils contain more available phosphorus, less total mineral nitrogen (figure 4b), and low ratios of nitrogen-degrading to phosphorus-degrading enzymes (Nottingham et al. 2015b), implying nitrogen limitation. In contrast, soils at low elevation contain less available phosphorus and more total mineral nitrogen (figure 4b) and high ratios of nitrogen-degrading to phosphorus-degrading enzymes, implying phosphorus limitation. Fertilization at different elevations on the transect influenced respiration fluxes consistent with this finding: the addition of nitrogen increased microbial respiration at a high elevation (3000 m above sea level, asl), whereas the addition of nitrogen and phosphorus together increased microbial respiration at a low elevation (200 m asl; Fisher et al. 2013). Although few data are available for tropical forest montane-to-lowland comparisons, we note consistent results from elsewhere showing low bioavailable soil nitrogen (Corre et al. 2010, Wolf et al. 2011) together with nitrogen-limited microbial growth (Corre et al. 2010, Cusack et al. 2011) in other montane forests and phosphorus-limited microbial growth and carbon mineralization in lowland forests of Panama and Costa Rica (Cleveland et al. 2006, Turner and Wright 2014).
The likely transition from phosphorus to nitrogen constraints on the soil microbial biomass with increased elevation on tropical mountains suggests a transition in the magnitude of nutrient-mining impacts on decomposition rates under warming, with greater impacts in montane forest where nitrogen is the predominant constraint on decomposers and where aboveground production is more likely to be stimulated by warming (either directly or through species composition shifts). Therefore, evidence from the Kosñipata transect and elsewhere points toward an analogous response of montane forest to warming as observed in temperate forest (Melillo et al. 2011), with an accelerated decomposition of SOM to liberate organic nitrogen by nitrogen-limited decomposers (Chena et al. 2013). However, because tropical forests span 10 soil orders (Quesada et al. 2010), there is likely to be a great deal of variation in the extent of this microbial–nutrient feedback under warming among lowland and montane sites on different parent material.
Do all microbial communities function similarly?
Soil microorganisms drive soil biogeochemical processes, so it has been hypothesized that the composition, or “functional dissimilarity,” of the soil microbial community directly affects organic matter degradation and its response to temperature change (Ostle and Ward 2012). This hypothesis is based on recent studies showing that microbial community composition shifts in response to soil treatments are related to soil processes such as mineralization rates (Bardgett and van der Putten 2014). An important difference in determining how warming will constrain rates of organic matter decomposition is the relative dominance of the soil microbial community by bacteria and fungi. Fungi are associated with a slow energy channel (the slow turnover of more recalcitrant and nitrogen-poor substrates, leading to high soil-carbon accumulation), whereas bacteria are associated with a fast energy channel (the fast turnover of labile and nitrogen-rich substrates, leading to low soil-carbon accumulation; Clemmensen et al. 2013, Bardgett and van der Putten 2014).
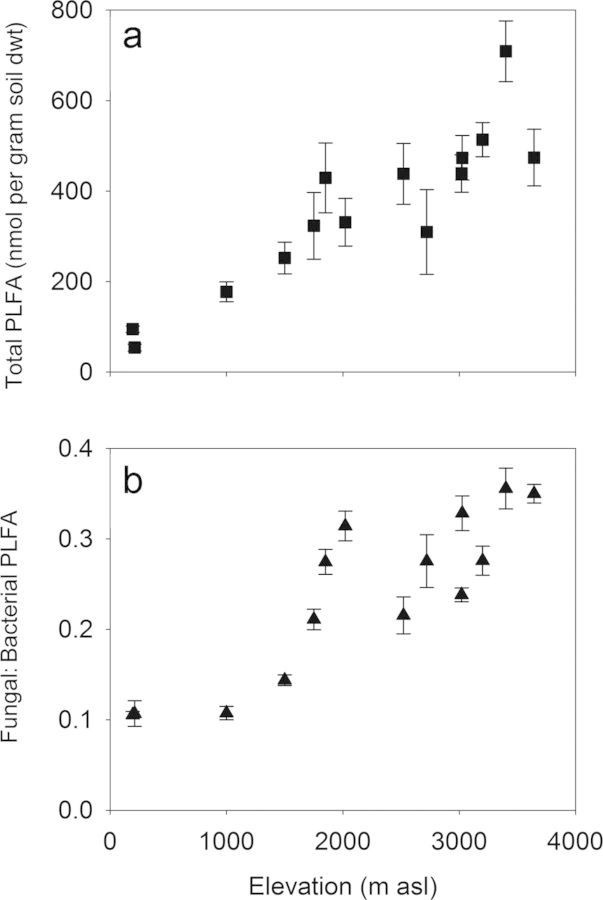
A functional dissimilarity of tropical forest soil microbial communities during carbon metabolism has recently been reported for the Kosñipata transect (Whitaker et al. 2014b). Carbon substrates of varying complexity were added to soil from 14 sites, and the variance in the respiration response was best described by the relative abundance of functional groups of microorganisms, in particular the relative abundance of bacteria and fungi. This dissimilarity in microbial community functioning along the transect has been attributed to differences in the functional capacity of specific microbial groups (gram-positive and gram-negative bacteria and fungi) to mineralize labile and recalcitrant carbon sources, demonstrated through assimilation of isotope-labeled substrates (Whitaker et al. 2014a). The extent of this functional dissimilarity appears to vary with temperature or elevation, because soil microbial processes (Zimmermann et al. 2010, Nottingham et al. 2015b), soil microbial biomass, and community composition vary with elevation, as was indicated by the large contrast in bacterial and fungal dominance of the microbial community at low and high elevation, respectively (figure 5; Fierer et al. 2011, Whitaker et al. 2014b). These findings from the Kosñipata transect support the hypothesis that the temperature response of soil-carbon cycling will be strongly influenced by the composition of these communities (Ostle and Ward 2012). Furthermore, they imply that a warming-related shift from fungal- to bacterial-dominated microbial communities in montane forests may be associated with a reduction in SOM (Clemmensen et al. 2013, Bardgett and van der Putten 2014). This functional dissimilarity may also apply to soil macrofauna. For example, termites are important decomposers in lowland sites, but they are largely absent from sites above 1500 m asl in elevation (Palin et al. 2011), and their up-slope migration under warming may similarly be associated with carbon losses in montane forest ecosystems.
Figure 5.
(a) Total abundance of phospholipid fatty acids (PLFA) and (b) the ratio of bacterial to fungal PLFA in soils across the Kosñipata elevation transect. Trends indicate the shift in the relative importance of fungal versus bacterial biomass in the microbial decomposer community along the transect (Whitaker et al. 2014b). The error bars represent one standard error. Abbreviations: dwt, dry weight; g, grams; m asl, meters at sea level; nmol, nanomoles.
Does thermal adaptation alter temperature sensitivity?
In addition to the physicochemical constraints on the temperature sensitivity of decomposition discussed above, the thermal adaptation of soil microorganisms or biochemical processes may also complicate the simple application of kinetic theory in a warming scenario (Billings and Ballantyne 2012, Bradford 2013). Warming experiments have demonstrated soil-carbon losses following short-term increases in soil heterotrophic respiration, which decline in the longer term (Melillo et al. 2011). One explanation for these longer-term declines in soil-carbon losses is acclimation or adaptation of microbial communities or processes (Bradford et al. 2009), which is suggested to occur via changes in microbial growth efficiency and the production of extracellular enzymes that have lower catalytic rates and are more stable at warmer temperatures (Bradford et al. 2010). However, evidence for acclimation or adaptation in response to warming is not unanimous (Hartley and Ineson 2008). In a 90-day incubation experiment using soils collected globally and with tropical soil from the Kosñipata transect, more soils showed enhanced rather than reduced (or adaptive) respiration responses, and this result was more pronounced for soils from cold climates and, among them, with high carbon-to-nitrogen ratios (Karhu et al. 2014). Therefore, cold-adapted communities and substrate (e.g., carbon and nitrogen) availability may constrain the overall response to warming, although more data are needed to confirm this first survey, particularly for tropical forests.
Complementing this incubation experiment, the large natural temperature gradient along the Kosñipata transect has facilitated further investigation of the temperature adaptation of microbial communities. In a study of the temperature responses of extracellular enzymes, the temperature sensitivities of two important carbon-degrading enzymes (β-glucosidase and β-xylanase) were greater in higher elevation sites. This pattern could be explained by the presence of “cold-adapted” isoenzymes at higher elevation sites (Bradford et al. 2010), which catalyze the same biochemical reaction but differ in terms of protein structure and conformational flexibility, exhibiting greater flexibility at lower temperatures (Wallenstein et al. 2011). Temperature adaptation of microbial communities along the Kosñipata transect was also suggested by the higher soil microbial carbon use efficiency in colder sites (Whitaker et al. 2014a), which is a common outcome in models of microbial responses to temperature (Allison 2014). Therefore, data from the Kosñipata transect are also consistent with evidence for the higher temperature sensitivity of cold-adapted microbial communities (Karhu et al. 2014). The combination of enhanced respiration responses and the presence of cold-adapted enzymes suggest the high vulnerability of montane forest carbon stocks to future warming.
Synthesis
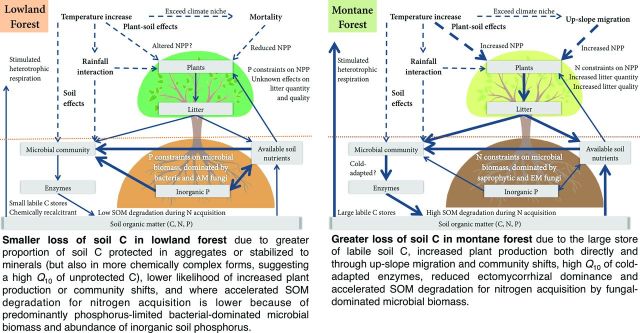
The Kosñipata studies collectively show the importance of defining the component processes and properties that determine the overall Q10 of soil organic matter decomposition (table 3, figure 3). Furthermore, by examining how these component processes and properties vary along the Kosñipata transect, we can make some predictions of the responses of soil carbon to future warming in lowland and montane tropical forests (figure 6).
Figure 6.
The complexity of proposed climate-warming effects on soil carbon (C) in lowland and montane tropical forests. The thermal adaptation of soil microorganisms and changes in plant productivity, rainfall, and atmospheric carbon dioxide (CO2) will modulate these responses, but the mode of adaptation is uncertain. To understand the impacts of warming in lowland tropical forest, we need in situ experiments to simulate warming. Driving processes are represented by the dashed arrows; fluxes of energy or nutrients are represented by solid arrows (weighted by their relative importance in montane and lowland tropical forests); the boxes are organisms or resource pools. Abbreviations: N, nitrogen; NPP, net primary production; P, phosphorus; SOM, soil organic matter.
The importance of intrinsic and apparent temperature sensitivity
To understand the apparent sensitivity of soil-carbon cycling to warming, we need to understand how this sensitivity arises from the temperature responses of its various subcomponents, each with its own intrinsic temperature sensitivity (figure 3). Of these ecosystem components, perhaps the best defined is the chemical recalcitrance of organic carbon compounds. The prediction from kinetic theory of higher temperature sensitivity for more chemically recalcitrant carbon compounds has been demonstrated by numerous studies in different ecosystems (Hartley and Ineson 2008, Craine et al. 2010, Conant et al. 2011) and is further supported by studies of the temperature sensitivity of decomposition and heterotrophic respiration along the Kosñipata transect (table 3; Salinas et al. 2011, Zimmermann et al. 2012).
The intrinsic temperature sensitivity of soil-carbon decomposition may be obscured by other ecosystem properties that have their own intrinsic temperature sensitivity, including substrate supply, nutrient availability, the physical and chemical protection of organic matter, and the composition and activity of the microbial community (figure 3). For example, the temperature sensitivity of soil-CO2 efflux is known to increase under high substrate supply (Davidson et al. 2006) and can therefore vary with different plant communities and soil types. It is also known to be dependent on the composition and temperature-adaptation of the microbial community (Ostle and Ward 2012, Bradford 2013). These interactions may be responsible for the higher experimentally derived apparent Q10 values (up to 6.9) when compared with the lower laboratory-derived intrinsic Q10 values for enzymatic activity in the Kosñipata studies (between 1.4 and 2.0; table 3). Evaluation of the factors that determine these interactions along this transect—and for other elevation gradients (table 2)—should ultimately pinpoint the crucial factors that will regulate the response of soil-carbon cycling to future warming in lowland and montane tropical forests (figure 6).
The stability of lowland and montane carbon stores under future climate warming
Our analysis suggests that the vulnerability of soil carbon to elevated temperature along the Kosñipata transect—and other tropical forest elevation gradients—is likely to be regulated by different principal mechanisms at different elevations. In lowland tropical forests, kinetic theory and the greater temperature sensitivity of more chemically complex carbon is likely to play a dominant role but may be constrained by the physical or chemical protection of carbon in these soils (Zimmermann et al. 2012, Giardina et al. 2014). This conclusion is based on our prediction of smaller effects of indirect factors in lowland forest relative to montane forest (table 1, figure 6), but we note that the plant–soil feedback in lowland tropical forest under warming is poorly known (Wood et al. 2012).
In contrast, the very significant carbon stocks in tropical montane forest in the upper half of the Kosñipata transect (figure 4a; Zimmermann et al. 2010) may have a high apparent temperature sensitivity because of indirect feedback (figure 6). This is indicated by the greater temperature sensitivity of cold-adapted enzymes at the higher elevation sites and by the presence of fungal-dominated microbial communities at higher elevations (figure 5), which can respond to warming by increasing organic matter mineralization to acquire, or “mine,” organic nitrogen that would otherwise be inaccessible at lower temperatures (Melillo et al. 2011, Chena et al. 2013). Recent experimental evidence from another tropical elevation gradient in Hawaii is consistent with the conclusions we draw here for the Kosñipata transect, with higher Q10 values of litter decomposition associated with increased nitrogen release at higher elevations (Bothwell et al. 2014). Further indirect positive feedback may result from an upward shift of plant species ranges (Feeley et al. 2011), whereby the associated changes in leaf litter, rhizosphere carbon inputs, and dominant mycorrhizal associations further stimulate microbial carbon use and organic matter mineralization (figure 6). However, it is also possible that the predicted losses of soil carbon to the atmosphere under climatic warming will be countered by increased carbon inputs from warming and CO2-stimulated plant productivity (Wood et al. 2012). This soil–climate feedback may be further constrained by the potential for an adaptive response of the soil microbial community and activity (Wieder et al. 2013); which continues to be debated (Bradford 2013, Karhu et al. 2014) and is a priority for future research. For now, we lack the data and necessary in situ experiments to fully understand the net effect of warming on soil carbon inputs and outputs, but with emerging new manipulation experiments, this may change in the near future.
Conclusions
Tropical elevation gradients are valuable in enabling combined studies of temperature effects on SOM cycling over the short term, through translocation experiments, and over the long term, through the observation of temperature-adapted ecosystem properties (Sundqvist et al. 2013). They are especially valuable in tropical forest ecosystems, which are a major component of the terrestrial carbon cycle but where temperature manipulation experiments have not yet been implemented (Cavaleri et al. 2015). These approaches have yielded insights into the factors that regulate both the intrinsic and apparent temperature sensitivities of soil-carbon cycling in tropical forests, a significant knowledge gap in making projections of global carbon emissions under future climate change (Cox et al. 2013). The observational and experimental studies along the Kosñipata transect have revealed large indirect influences of warming on soil-carbon stocks in both montane and lowland tropical forests through the physical protection of organic matter in soil aggregates, through shifts in microbial community composition, and through the consequences of nutrient limitation. These mechanisms are not yet considered in Earth system models but could substantially inform our view of the vulnerability of the world's significant soil-carbon stocks that are located in tropical forests (Todd-Brown et al. 2012). The study of different elevation gradients with contrasting climatic and edaphic properties (table 1) will undoubtedly enable the generalization of these findings. We suggest that they can also guide and complement long-term in situ warming experiments, which are required to simulate the projected effects of warming in tropical forests but whose implementation is only just beginning.
Acknowledgments
This study is a product of the Andes Biodiversity and Ecosystem Research Group consortium (www.andesconservation.org) and was supported by the UK Natural Environment Research Council (NERC), grants no. NE/G018278/1 and no. NE/K01627X/1; a European Union Marie Curie Fellowship FP7–2012–329360 to ATN; and an Australian Research Council Fellowship to PM (FT110100457). We thank the Asociacion para la Conservacion de la Cuenca Amazonica (ACCA) in Cusco and the Instituto Nacional de Recursos Naturales (INRENA) in Lima for access to the study sites. For their support in Peru, we especially thank Dr. Eric Cosio and Eliana Esparza Ballón at Pontificia Universidad Católica del Perú (PUCP) and Adan J. Q. Ccahuana, Walter H. Huasco, Javier E. S. Espejo, and many others too numerous to mention here at Universidad Nacional de San Antonio Abad del Cusco (UNSAAC).
References cited
- Averill C, Turner BL, Finzi AC. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature. 2014;505:543–545. doi: 10.1038/nature12901. [DOI] [PubMed] [Google Scholar]
- Bardgett RD, van der Putten WH. Belowground biodiversity and ecosystem functioning. Nature. 2014;515:505–511. doi: 10.1038/nature13855. [DOI] [PubMed] [Google Scholar]
- Billings SA, Ballantyne F. How interactions between microbial resource demands, soil organic matter stoichiometry, and substrate reactivity determine the direction and magnitude of soil respiratory responses to warming. Global Change Biology. 2012;19:90–102. doi: 10.1111/gcb.12029. [DOI] [PubMed] [Google Scholar]
- Bothwell LD, Selmants PC, Giardina CP, Litton CM. Leaf litter decomposition rates increase with rising mean annual temperature in Hawaiian tropical montane wet forests. PeerJ. 2014;2 doi: 10.7717/peerj.685. art. e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MA. Thermal adaptation of decomposer communities in warming soils. Frontiers in Microbiology. 2013;4 doi: 10.3389/fmicb.2013.00333. art. 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MA, et al. Decreased mass specific respiration under experimental warming is robust to the microbial biomass method employed. Ecology Letters. 2009;12:E15–E18. [Google Scholar]
- Bradford MA, Watts BW, Davies CA. Thermal adaptation of heterotrophic soil respiration in laboratory microcosms. Global Change Biology. 2010;16:1576–1588. [Google Scholar]
- Cavaleri MA, Reed SC, Smith WK, Wood TE. Urgent need for warming experiments in tropical forests. Global Change Biology. 2015;21:2111–2121. doi: 10.1111/gcb.12860. doi:10.1111/gcb.12860. [DOI] [PubMed] [Google Scholar]
- Chen IC, Hill JK, Ohlemuller R, Roy DB, Thomas CD. Rapid range shifts of species associated with high levels of climate warming. Science. 2011;333:1024–1026. doi: 10.1126/science.1206432. [DOI] [PubMed] [Google Scholar]
- Chena R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Global Change Biology. 2013;20:2356–2367. doi: 10.1111/gcb.12475. [DOI] [PubMed] [Google Scholar]
- Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlid J, Finlay RD, Wardle DA, Lindahl BD. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science. 2013;339:1615–1618. doi: 10.1126/science.1231923. [DOI] [PubMed] [Google Scholar]
- Cleveland CC, Reed SC, Townsend AR. Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology. 2006;87:492–503. doi: 10.1890/05-0525. [DOI] [PubMed] [Google Scholar]
- Colwell RK, Brehm G, Cardelus CL, Gilman AC, Longino JT. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science. 2008;322:258–261. doi: 10.1126/science.1162547. [DOI] [PubMed] [Google Scholar]
- Conant RT, Steinweg JM, Haddix ML, Paul EA, Plante AF, Six J. Experimental warming shows that decomposition temperature sensitivity increases with soil organic matter recalcitrance. Ecology. 2008;89:2384–2391. doi: 10.1890/08-0137.1. [DOI] [PubMed] [Google Scholar]
- Conant RT, et al. Temperature and soil organic matter decomposition rates: Synthesis of current knowledge and a way forward. Global Change Biology. 2011;17:3392–3404. [Google Scholar]
- Corre MD, Veldkamp E, Arnold J, Wright SJ. Impact of elevated N input on soil N cycling and losses in old-growth lowland and montane forests in Panama. Ecology. 2010;91:1715–1729. doi: 10.1890/09-0274.1. [DOI] [PubMed] [Google Scholar]
- Couteaux MM, Sarmiento L, Bottner P, Acevedo D, Thiery JM. Decomposition of standard plant material along an altitudinal transect (65–3968 m) in the tropical Andes. Soil Biology and Biochemistry. 2002;34:69–78. [Google Scholar]
- Cox PM, Pearson D, Booth BB, Friedlingstein P, Huntingford C, Jones CD, Luke CM. Sensitivity of tropical carbon to climate change constrained by carbon dioxide variability. Nature. 2013;494:341–344. doi: 10.1038/nature11882. [DOI] [PubMed] [Google Scholar]
- Craine JM, Fierer N, McLauchlan KK. Widespread coupling between the rate and temperature sensitivity of organic matter decay. Nature Geoscience. 2010;3:854–857. [Google Scholar]
- Cusack DF, Silver WL, Torn MS, McDowell WH. Effects of nitrogen additions on above- and belowground carbon dynamics in two tropical forests. Biogeochemistry. 2011;104:203–225. [Google Scholar]
- Davidson EA, Janssens IA. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature. 2006;440:165–173. doi: 10.1038/nature04514. [DOI] [PubMed] [Google Scholar]
- Davidson EA, Janssens IA, Luo Y. On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Global Change Biology. 2006;12:154–164. [Google Scholar]
- Feeley KJ, Silman MR, Bush MB, Farfan W, Cabrera KG, Malhi Y, Meir P, Revilla NS, Quisiyupanqui MNR, Saatchi S. Upslope migration of Andean trees. Journal of Biogeography. 2011;38:783–791. [Google Scholar]
- Fierer N, McCain CM, Meir P, Zimmermann M, Rapp JM, Silman MR, Knight R. Microbes do not follow the elevational diversity patterns of plants and animals. Ecology. 2011;92:797–804. doi: 10.1890/10-1170.1. [DOI] [PubMed] [Google Scholar]
- Fisher JB, Malhi Y, Torres IC, Metcalfe DB, van de Weg MJ, Meir P, Silva-Espejo JE, Huasco WH. Nutrient limitation in rainforests and cloud forests along a 3000-m elevation gradient in the Peruvian Andes. Oecologia. 2013;172:889–902. doi: 10.1007/s00442-012-2522-6. [DOI] [PubMed] [Google Scholar]
- Ghannoum O, Way DA. On the role of ecological adaptation and geographic distribution in the response of trees to climate change. Tree Physiology. 2011;31:1273–1276. doi: 10.1093/treephys/tpr115. [DOI] [PubMed] [Google Scholar]
- Giardina CP, Litton CM, Crow SE, Asner GP. Warming-related increases in soil CO2 effux are explained by increased below-ground carbon flux. Nature Climate Change. 2014;4:822–827. [Google Scholar]
- Girardin CAJ, et al. Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes. Global Change Biology. 2010;16:3176–3192. [Google Scholar]
- González G, Willig MR, Waide RB. Ecological Gradient Analyses in a Tropical Landscape. Wiley-Blackwell; 2013. [Google Scholar]
- Hartley IP, Ineson P. Substrate quality and the temperature sensitivity of soil organic matter decomposition. Soil Biology and Biochemistry. 2008;40:1567–1574. [Google Scholar]
- [IPCC] Intergovernmental Panel on Climate Change . Climate Change 2013: The Physical Science Basis. Cambridge University Press; 2013. [Google Scholar]
- Jobbagy EG, Jackson RB. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecological Applications. 2000;10:423–436. [Google Scholar]
- Karhu K, et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature. 2014;513:81–84. doi: 10.1038/nature13604. [DOI] [PubMed] [Google Scholar]
- Kitayama K, Aiba SI. Ecosystem structure and productivity of tropical rain forests along altitudinal gradients with contrasting soil phosphorus pools on Mount Kinabalu, Borneo. Journal of Ecology. 2002;90:37–51. [Google Scholar]
- Körner C. The use of “altitude” in ecological research. Trends in Ecology and Evolution. 2007;22:569–574. doi: 10.1016/j.tree.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Lloyd J, Farquhar GD. Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philosophical Transactions of the Royal Society B. 2008;363:1811–1817. doi: 10.1098/rstb.2007.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi Y, Silman M, Salinas N, Bush M, Meir P, Saatchi S. Introduction: Elevation gradients in the tropics: Laboratories for ecosystem ecology and global change research. Global Change Biology. 2010;16:3171–3175. [Google Scholar]
- Meier CL, Rapp J, Bowers RM, Silman M, Fierer N. Fungal growth on a common wood substrate across a tropical elevation gradient: Temperature sensitivity, community composition, and potential for above-ground decomposition. Soil Biology and Biochemistry. 2010;42:1083–1090. [Google Scholar]
- Melillo JM, Steudler PA, Aber JD, Newkirk K, Lux H, Bowles FP, Catricala C, Magill A, Ahrens T, Morrisseau S. Soil warming and carbon-cycle feedbacks to the climate system. Science. 2002;298:2173–2176. doi: 10.1126/science.1074153. [DOI] [PubMed] [Google Scholar]
- Melillo JM, et al. Soil warming, carbon–nitrogen interactions, and forest carbon budgets. Proceedings of the National Academy of Sciences. 2011;108:9508–9512. doi: 10.1073/pnas.1018189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser G, Leuschner C, Hertel D, Graefe S, Soethe N, Iost S. Elevation effects on the carbon budget of tropical mountain forests (S Ecuador): The role of the belowground compartment. Global Change Biology. 2011;17:2211–2226. [Google Scholar]
- Nottingham AT, Turner BL, Stott AW, Tanner EVJ. Nitrogen and phosphorus constrain labile and stable carbon turnover in lowland tropical forest soils. Soil Biology and Biochemistry. 2015a;80:26–33. [Google Scholar]
- Nottingham AT, Turner BL, Whitaker J, Ostle N, McNamara NP, Bardgett RD, Salinas N, Meir P. Soil microbial nutrient constraints along a tropical forest elevation gradient: A belowground test of a biogeochemical paradigm. Biogeosciences Discuss. 2015b;12:6489–6523. [Google Scholar]
- Ostle N, Ward S. Climate change and soil biotic carbon cycling. In: Wall DH, editor. Soil Ecology and Ecosystem Services. Oxford University Press; 2012. pp. 241–255. [Google Scholar]
- Palin OF, Eggleton P, Malhi Y, Girardin CAJ, Rozas-Davila A, Parr CL. Termite diversity along an Amazon–Andes elevation gradient, Peru. Biotropica. 2011;43:100–107. [Google Scholar]
- Pan Y, et al. A large and persistent carbon sink in the world's forests. Science. 2011;333:988–993. doi: 10.1126/science.1201609. [DOI] [PubMed] [Google Scholar]
- Porder S, Hilley GE. Linking chronosequences with the rest of the world: Predicting soil phosphorus content in denuding landscapes. Biogeochemistry. 2010;102:153–166. [Google Scholar]
- Quesada CA, et al. Variations in chemical and physical properties of Amazon forest soils in relation to their genesis. Biogeosciences. 2010;7:1515–1541. [Google Scholar]
- Rapp JM, Silman MR. Diurnal, seasonal, and altitudinal trends in microclimate across a tropical montane cloud forest. Climate Research. 2012;55:17–32. [Google Scholar]
- Rapp JM, Silman MR, Clark JS, Girardin CAJ, Galiano D, Tito R. Intra- and interspecific tree growth across a long altitudinal gradient in the Peruvian Andes. Ecology. 2012;93:2061–2072. doi: 10.1890/11-1725.1. [DOI] [PubMed] [Google Scholar]
- Rasse DP, Rumpel C, Dignac MF. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant and Soil. 2005;269:341–356. [Google Scholar]
- Read DJ, Perez-Moreno J. Mycorrhizas and nutrient cycling in ecosystems: A journey towards relevance? New Phytologist. 2003;157:475–492. doi: 10.1046/j.1469-8137.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- Salinas N, et al. The sensitivity of tropical leaf litter decomposition to temperature: Results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytologist. 2011;189:967–977. doi: 10.1111/j.1469-8137.2010.03521.x. [DOI] [PubMed] [Google Scholar]
- Sundqvist MK, Sanders NJ, Wardle DA. Community and ecosystem responses to elevational gradients: Processes, mechanisms, and insights for global change. Annual Review of Ecology, Evolution, and Systematics. 2013;44:261–280. [Google Scholar]
- Todd-Brown KEO, Randerson JT, Post WM, Hoffman FM, Tarnocai C, Schuur EAG, Allison SD. Causes of variation in soil carbon predictions from CMIP5 Earth system models and comparison with observations. Biogeosciences. 2012;9:14437–14473. [Google Scholar]
- Turner BL, Wright SJ. The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry. 2014;117:115–130. [Google Scholar]
- Van de Weg MJ, Meir P, Grace J, Atkin OK. Altitudinal variation in leaf mass per unit area, leaf tissue density, and foliar nitrogen and phosphorus content along an Amazon–Andes gradient in Peru. Plant Ecology and Diversity. 2009;2:243–254. [Google Scholar]
- Van de Weg MJ, Meir P, Williams M, Girardin C, Malhi Y, Silva-Espejo J, Grace J. Gross primary productivity of a high elevation tropical montane cloud forest. Ecosystems. 2014;17:751–764. [Google Scholar]
- Vitousek PM. Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology. 1984;65:285–298. [Google Scholar]
- Vitousek PM, Aplet G, Turner D, Lockwood JJ. The Mauna-Loa environmental matrix: Foliar and soil nutrients. Oecologia. 1992;89:372–382. doi: 10.1007/BF00317415. [DOI] [PubMed] [Google Scholar]
- Von Humboldt A, Bonpland A. Essai sur la Géographie des Plantes, Accompagné d'un Tableau Physique des Régions Équinoxiales. Chez Levrault, Schoell, et Campagnie Librarie. 1805 [Google Scholar]
- Wagai R, Kitayama K, Satomura T, Fujinuma R, Balser T. Interactive influences of climate and parent material on soil microbial community structure in Bornean tropical forest ecosystems. Ecological Research. 2011;26:627–636. [Google Scholar]
- Wallenstein M, Allison S, Ernakovich J, Steinweg JM, Sinsabaugh R. Controls on the temperature sensitivity of soil enzymes: A key driver of in situ enzyme activity rates. In: Shukla G, Varma A, editors. Soil Enzymology. Berlin Heidelberg: Springer; 2011. pp. 245–258. [Google Scholar]
- Wardle DA, Bardgett RD, Klironomos JN, Setala H, van der Putten WH, Wall DH. Ecological linkages between aboveground and belowground biota. Science. 2004;304:1629–1633. doi: 10.1126/science.1094875. [DOI] [PubMed] [Google Scholar]
- Whitaker J, Ostle N, McNamara NP, Nottingham AT, Stott AW, Bardgett RD, Salinas N, Ccahuana AJQ, Meir P. Microbial carbon mineralization in tropical lowland and montane forest soils of Peru. Frontiers in Microbiology. 2014a;5 doi: 10.3389/fmicb.2014.00720. (art. 720), doi:10.3389/fmicb.2014.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J, Ostle N, Nottingham AT, Ccahuana A, Salinas N, Bardgett RD, Meir P, McNamara NP. Microbial community composition explains soil respiration responses to changing carbon inputs along an Andes-to-Amazon elevation gradient. Journal of Ecology. 2014b;102:1058–1071. doi: 10.1111/1365-2745.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker RH, Niering WA. Vegetation of the Santa Catalina Mountains, Arizona: A gradient analysis of the south slope. Ecology. 1965;46:429–452. [Google Scholar]
- Wieder WR, Bonan GB, Allison SD. Global soil carbon projections are improved by modelling microbial processes. Nature Climate Change. 2013;3:909–912. [Google Scholar]
- Wolf K, Veldkamp E, Homeier J, Martinson GO. Nitrogen availability links forest productivity, soil nitrous oxide, and nitric oxide fluxes of a tropical montane forest in southern Ecuador. Global Biogeochemical Cycles. 2011;25 (art. GB4009), doi:10.1029/2010GB003876. [Google Scholar]
- Wood TE, Cavaleri MA, Reed SC. Tropical forest carbon balance in a warmer world: A critical review spanning microbial- to ecosystem-scale processes. Biological Reviews. 2012;87:912–927. doi: 10.1111/j.1469-185X.2012.00232.x. [DOI] [PubMed] [Google Scholar]
- Wright SJ, Muller-Landau HC, Schipper J. The future of tropical species on a warmer planet. Conservation Biology. 2009;23:1418–1426. doi: 10.1111/j.1523-1739.2009.01337.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhou Y, Ruan HH, Luo YQ, Wang JS. Temperature sensitivity increases with soil organic carbon recalcitrance along an elevational gradient in the Wuyi Mountains, China. Soil Biology and Biochemistry. 2010;42:1811–1815. [Google Scholar]
- Zimmermann M, Bird MI. Temperature sensitivity of tropical forest soil respiration increase along an altitudinal gradient with ongoing decomposition. Geoderma. 2012;187–188:8–15. [Google Scholar]
- Zimmermann M, Meir P, Bird MI, Malhi Y, Ccahuana AJQ. Climate dependence of heterotrophic soil respiration from a soil-translocation experiment along a 3000-m tropical forest altitudinal gradient. European Journal of Soil Science. 2009;60:895–906. [Google Scholar]
- Zimmermann M, Meir P, Bird MI, Malhi Y, Ccahuana AJQ. Temporal variation and climate dependence of soil respiration and its components along a 3000-m altitudinal tropical forest gradient. Global Biogeochemical Cycles. 2010;24 (art. GB4012), doi:10.1029/2010GB003787. [Google Scholar]
- Zimmermann M, Leifeld J, Conen F, Bird MI, Meir P. Can composition and physical protection of soil organic matter explain soil respiration temperature sensitivity? Biogeochemistry. 2012;107:423–436. [Google Scholar]