Abstract
Different carbohydrate diets have been administrated to diabetic patients to evaluate the glycemic response, while Poor-controlled diabetes is increasing world wide. To investigate the role of an alternative carbohydrate diet on glycemic control, we explored the effect of a low glycemic load (Low GL)-high fat diet on glycemic response and also glycated hemoglobin (HbA1c) of poor-controlled diabetes patients. Hundred poorly-controlled diabetes patients, HbA1c > 8, age 52.8 ± 4.5 y, were administrated a low GL diet, GL = 67 (Energy 1800 kcal; total fat 36%; fat derived from olive oil and nuts 15%; carbohydrate 42%; protein 22%) for 10 weeks. Patients did their routine life style program during intervention. Fasting blood glucose and HbA1c before and after intervention with significant reduction were: 169 ± 17, 141 ± 12; 8.85% (73 mmol/mol) ± 0.22%, and 7.81% (62 mmol/mol) ± 0.27%; respectively (P < 0.001). Mean fasting blood glucose reduced by 28.1 ± 12.5 and HbA1c by 1.1% (11 mmol/mol) ± 0.3% (P=0.001). There was positive moderate correlation between HbA1c concentration before intervention and FBS reduction after intervention (P < 0.001, at 0.01 level, R =0.52), and strong positive correlation between FBS before intervention and FBS reduction (P < 0.001, at 0.01 level, R = 0.70). This study demonstrated that our alternative low glycemic load diet can be effective in glycemic control.
Keywords: Poorly-controlled diabetes, Glycemic load, Glycated hemoglobin
1. Introduction
Poorly-controlled diabetes that is characterized with increased glycated hemoglobin (HbA1c) > 8% (64 mmol/mol) (Mahan and Escott-Stump, 2007) is increasing world wide, especially in North America and Europe which resulted in an increasing prevalence of disease associated with poor glycemic control (Livesey and Tagami, 2009). Different interventions to lower the glycemic response to carbohydrate foods have been introduced. Theses approaches included: Diets containing 50-60% calories from carbohydrates (Arora and McFarlance, 2005), consumption of soluble fiber, non-soluble fiber, low viscosity fiber (resistant maltodextrin) (Livesey and Tagami, 2009), and administration of low glycemic load diet (100 g) (glucose equivalents per day) without elevating fat intake (Livesey and Tagami, 2009). High carbohydrate intake recommended in diabetes, resulting in suboptimal glycemic control and lipoprotein profile, gradually increasing insulin and/or oral hypoglycemic medication requirement and eventually weight gain (Arora and McFarlance, 2005, 2:16). Several studies have demonstrated that viscous soluble fibers suppress the glycemic response to carbohydrate foods, (Garcia et al., 2007; Livesey et al., 2008), and beneficial effect of insoluble dietary fiber for glycemic control has been reported in different studies (De Munter et al., 2007; Schulze et al., 2007); however such polysaccharides have limited palatability and insoluble dietary fiber produce flatus and is not suitable for most subjects suffering from gastrointestinal disease. In addition, in prospective cohort studies, it is mainly insoluble cereal dietary fiber (i.e., cellulose and hemicelluloses) and whole grains, not soluble dietary fiber, that associated with reduced diabetes risk (De Munter et al., 2007; Schulze et al., 2007). In relation to consumption of non-viscous soluble palatable polysaccharides (resistant maltdextrin, RMD) a systematic review of randomized, placebo controlled trials revealed that administration of ≤ 10 g RMD per meal significantly reduces the postprandial glycemic response to a carbohydrate meal in acute studies (Livesey and Tagami, 2009), however its effect in relation to reducing risk of diabetes in long period consumption is not clear. Also RMD is fermented; it increases the production of flatus and has potential to contribute to abdominal discomfort in higher doses and continues use. (Ohkuma and Takahashi, 1990). Also RMD is more potent in drinks consumed with starch foods than when placed directly into such foods (Livesey and Tagami, 2009).
Therefore the aim of the present study was to investigate the role of low glycemic load diet having lower amount of carbohydrate and higher fat content than traditionally introduced diets as an alternative approach to reduce glycemic response to carbohydrate and also reducing HbA1c concentration of poor-controlled diabetes. We hypothesized that carbohydrate-based low glycemic load diet (GL ≈ 67), with 36% fat, and 42% carbohydrate suppress glycemic response and reduces HbA1c concentration in poor-controlled diabetes.
2. Materials and Methods
One hundred and twenty Poor-controlled (HbA1c > 8%) (Mahan and Escott-Stump, 2007) diabetes patients who were referred to endocrine clinic during 6 months (January 2009 to Jun 2010), and were receiving either insulin or oral medication during study were volunteers for this study. Patients were receiving conventional high carbohydrate low fat diabetes diet. Subjects were excluded if they were unwilling to consume the administrated diet and their medications have not been changed during the study. The procedures were followed in accordance with the ethical standards of the Qazvin University of Medical Science and the study was approved by the Human Research Ethics Committee of the institution. Subjects underwent on low glycemic load diet, GL = 67 (Energy = 1800 kcal, total fat = 36%, fat derived from olive oil and nuts 15%, carbohydrate = 42%, protein = 22% (Table 1) for 10 weeks. Patients were recommended to do their routine daily life style program during intervention. Fasting blood glucose (FBS), HbA1c, weight and BMI were measured before and after intervention. Data were inspected for normality of distribution before use of parametric statistics with SPSS version16 (SPSS Inc, Cary, NC). Data are reported as means ± SDs. Data were analyzed by using paired t-test and Pearson correlation to compare weight, BMI, FBS, and HbA1c of patients before and after intervention.
Table 1.
Low glycemic load diet administrated to poor-controlled diabetes patients*
| Food | Weight (g) | Protein (g) | Fat (g) | Carbohydrate (g) | GI | GL | Energy (kcal) |
|---|---|---|---|---|---|---|---|
| 4 exchange from starch list, (whole-wheat bread, rice, backed beans, sliced fried potato), all low GIs | different | 12 | --- | 60 | 47 | 28 | 320 |
| 4 exchange from milk list (low fat milk, yogurt) | 1000 | 32 | 20 | 48 | 30 | 14 | 480 |
| 8 exchange from meat and meat substitutes list (lean meat, low fat cheese, egg whites) | different | 49 | 21 | ----- | --- | ---- | 440 |
| 2 exchange from vegetable list (letus, cucumber, tomato) | 2 cups raw vegetable | 4 | 10 | 1 | 1 | 50 | |
| 4 exchange from fruit list (fresh low GI fruits, apple, orange) | 480 | ----- | 60 | 40 | 24 | 240 | |
| 6 exchange from fat list (olive oil, olives, nuts, walnut) | different | 30 | ---- | 270 (15%) | |||
| Total | 97 (22%) | 71 (36%) | 178 (42%) | 67 | 1800 |
Source of analysis of ingredients foods: GI, & GL of foods (Taleban and Esmaeili, 1999)
3. Results
Hundred subjects (55 M, 45 F), aged 52.8 ± 4.5 y, weight 74.0 ± 5 kg, BMI = 27.2 ± 1.9 kg/m2 who had recruitment criteria took part in this study. Fifteen persons had BMI ≤ 25, while 85 persons had BMI > 25. The mean values for data are shown in Table 2. FBS concentration, HbA1c percentage, weight and BMI was significantly different between two values of before and after intervention (P <0.001). Mean fasting blood glucose reduced by 28.1 ± 12.5 mg/dl (16.6%), HbA1c by 1.1% (11 mmol/mol) ± 0.3%, weight by 3.3 ± 1 kg and BMI by 1.2 ± 0.4 kg/m2 after diet intervention (P <0.001). There were positive weak correlation between BMI kg/m2 before intervention and HbA1c level reductions (P = 0.01, at 0.05 level, R = 0.27), between BMI kg/m2 reduction and HbA1c reduction (P = 0.01, at 0.05 level, R = 0.25), and between HbA1c concentration before intervention and HbA1c reduction (P < 0.001, at 0.01 level, R = 0.36). Also there was positive moderate correlation between HbA1c concentration before intervention and FBS reduction (P < 0.001, at 0.01 level, R = 0.52), and strong positive correlation between FBS before intervention and FBS reduction (P < 0.001, at 0.01 level, R = 0.70), (Table 3, Figure 1). Observed variable changes were significant in both normal and overweight groups.
Table 2.
Blood glucose profile of diabetic patients before and after diet intervention
| patients | no | age | weight | BMI | FBS | HbA1c |
|---|---|---|---|---|---|---|
| at baseline | 100 | 52.8±4.5 | 74.0±5 CV=6.7% |
27.2±1.9, CV=7% |
169±17 CV=10% |
8.85% (73 mmol/mol) ±0.22% CV=2% |
| after 10 weeks | 70.7±4.6 CV=6.5% |
26.0±1.8, CV=7% |
141±12 CV=8% |
7.81% (62 mmol/mol) ±0.27% CV=3% |
||
| P | P<0.001 | P<0.001 | P<0.001 | P<0.001 |
Table 3.
Correlation between different variables of before and after intervention
| Variables | FBS reduction 28.1 ± 12.5 mg/dl |
HbA1c reduction 1.1 ± 0.3 |
|---|---|---|
| Weight 74.0±5 kg Before intervention |
____________ | _________ |
| BMI 27.2±1.9 kg/m2 Before intervention |
____________ | P = 0.01, at 0.05 level, R= 0.27 |
| FBS 169 ±17 mg/dl Before intervention |
P < 0.001, at 0.01 level, R = 0.70 | __________ |
| HbA1c 8.85 ±0.22 Before intervention |
P < 0.001, at 0.01 level, R = 0.52 | P < 0.001, at 0.01 level, R = 0.36 |
| Weight reduction 3.3 ± 1 kg |
_____________ | __________ |
| BMI reduction 1.2 ± 0.4 kg/m2 |
_____________ | P = 0.01, at 0.05 level, R = 0.25 |
Figure 1.
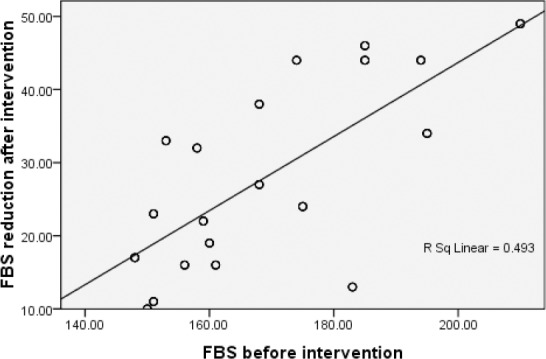
Correlation between fasting blood glucose before intervention and fasting blood glucose reduction after intervention in diabetes patients
4. Discussion
This study showed a significant effect of low glycemic load diet on FBS and HbA1c. In our study as we hypothesized, the administrated low glycemic load diet suppressed the HbA1c of poor-controlled diabetes patients to 7.8% (62 mmol/mol) ± 0.3% level which does not considered as poorly-controlled level (Mahan and Escott-Stump, 2007) and was our target in current study.
This study revealed that the more sever the dysglycemia, the greater effect of low GI diet on glycemic control was observed. This finding was parallel with point view of conducted workshop by Howlett and colleagues (Howlett and Ashwell, 2008). Similarly, researchers (Kiens and Richter, 1996) in their study found that both two isoenergetic diets which were composed of 46%, 41%, and 13% as carbohydrate, fat, and protein respectively and the carbohydrate contents were either a high GI (90) or a low GI (66), both didn’t have significant effect on normal blood glucose of healthy subjects at the end of 30 days of intervention. In addition it is reported that unavailable carbohydrate reduces fasting blood glucose or HbA1c in persons with diabetes but not in individuals having normal fasting blood glucose (Livesey et al., 2008). These studies support our finding in which lower blood glucose levels and also normal blood glucose were less affected by low glycemic load diets.
Diets having composition of 50-60% of total energy as carbohydrates is recommended for diabetics and subjects with metabolic syndrome. Even recommendation of some health organizations is 55-70% carbohydrate, 15-20% proteins and 20-30% fats (Krauss et al., 2000; Liu et al., 2000; Franz et al., 2002). However, epidemiological studies such as the Nurses Health Study and Health Professional Follow-Up Study (Hu and Willett, 2001), and also Framingham Offspring Study (Mckeown et al., 2004) have demonstrated the association between glycemic load with type 2 diabetes, CVD and metabolic syndrome. High carbohydrate intake results in suboptimal glycemic control and lipoprotein profile, and subsequently increasing insulin and/or oral hypoglycemic medication requirement and weight gain (Surender and Samy, 2005), while the effect of low carbohydrate diets with 20% of total energy as carbohydrate on glycemic control was greater and independent of weight loss. However in long term compelling with restricted carbohydrate diet is difficult and adherence to such a diet having around 100 g carbohydrates a day which is far away from patients’ food habits is weak. In addition physicians are reluctant to advice such a diet to their patients. Considering accumulating evidence for benefits of restricted carbohydrate diets, the American Diabetes Association (ADA) agrees with role of carbohydrate restriction “in weight management of type 2 diabetes, replacing carbohydrate with monounsaturated fats reduces post prandial glycemia and triglyceridemia” and recommends that carbohydrates and monounsaturated fat together should provide 60-70% of the energy intake in which their ratio should be individualized. However, alternatively, there is statement from ADA which limits carbohydrate intake to 45-65% of the calories intake (Blades et al., 1997). In our study the moderate carbohydrate diet with GL = 67g/day, including 42% carbohydrate as energy intake, and 15% of fat intake from monounsaturated fatty acids sources was almost similar to ADA’s recommendation which is more appropriate and compelling for glycemic control in long period. The GL < 80 g/day is considered low GL diet (Brand-Miller, 2005). The higher the GL, the greater the glycemic effect (Afaghi et al., 2007) and insulinogenic effect (Foster-Powell et al., 2002). The GL of diet in our study was 67 g/d which was even lower than maximum g/day recommendation for low GL diet.
In current study we increased the energy derived from fat up to 36%. Adherence to standard dietary advice to reduce fat intake while increasing carbohydrate intake generally increase the glycemic effect of diet. Both the quantity and quality of a carbohydrate influence postprandial glycemia, and the interaction between the two may be synergistic (Brand-Miller et al., 2002). Therefore our meal plan was based on high fat foods that produce a low glycemic response (low- GI foods) and may promote weight control because they increase satiety, minimize postprandial insulin secretion, and maintain insulin sensitivity (Brand-Miller et al., 2002).
Fiber consumption has significant effect on glycemic control (Howlett and Ashwell, 2008). However large amounts of fibers ingestion (25 grams per meal) is needed to achieve 10% reduction in 2 hr postprandial blood glucose level (Afaghi et al., 2011). In practice due to limited palatability, produced flatus and discomfort by insoluble dietary fiber (DF), consumption of large amount of fiber is not pleasure and diabetic subjects will not compel with such a diet.
Different factors in current study may affected on glycemic control including: moderate energy intake (24 kca/per kg bod weight), low glycemic load diet, and consumption of monounsaturated fatty acids. Moderate energy intake lowers body weight (Freedman et al., 2001) and consequently increases insulin sensitivity. Weight loss of 5-10% of initial body weight may significantly improve glycemic and other metabolic abnormalities, and prevents the development of diabetes in high risk populations (Tuomilehto et al., 2001; Knowler et al., 2002; McFarlane et al., 2003). We observed 3.3%, 4.6% and 4.4% weight loss in persons having BMI ≤ 25, BMI > 25, and in total subjects respectively. Due to observed poor correlation between BMI kg/m2 and HbA1c and lack of any correlation between BMI reduction and FBS reduction, the weight loss in our study, less likely affected on glycemic profile improvement. We believe that the effect of administrated low glycemic load diet was dominant for weight reduction, appetite and also suppress postprandial blood glucose through slow absorption and resulting in reducing HbA1c. We did not have control group which was the limitation of our study.
5. Conclusion
Our provided meal plan for glycemic control of poor-controlled diabetes subjects is appropriate and further investigation for long term effect of low GI diet for glycemic control of poor-controlled diabetes patients is suggested.
Footnotes
Sources of support: This study was funded by Qazvin Metabolic Disease Research Center, Qazvin University of Medical Science
References
- Afaghi A, O’Connor H, Chow C. M. High-glycemic-index carbohydrate meals shorten sleep onset. American Journal of Clinical Nutrition. 2007;85:426–30. doi: 10.1093/ajcn/85.2.426. [DOI] [PubMed] [Google Scholar]
- Afaghi A, Omidi R. B, Sarreshtehdari M. Effect of wheat bran on postprandial glucose response in subjects with impaired fasting glucose. Current Topics in Nutrceutical Research. 2011;9(1-2):35–40. [Google Scholar]
- Arora S. K, McFarlance S. I. The case for low carbohydrate diets in diabetes management. Nutrition and Metabolism. 2005. [Online] Available: http://www.nutritionandmetabolism.com/content/2/1/16 . [DOI] [PMC free article] [PubMed]
- Blades M, Morgan J. B, Dickerson J. W. Dietary advice in the management of the diabetes mellitus-history and current practice. J R Soc Health. 1997;117:143–150. doi: 10.1177/146642409711700303. http://dx.doi.org/10.1177/146642409711700303 . [DOI] [PubMed] [Google Scholar]
- Brand-Miller J. C. Home of the glycemic index, glycemic load. 2005. [accessed 20 December, 2005]. [Online] Available: http://www.glycemicindex.com .
- Brand-Miller J. C, Holt S. H, Pawlak D. B, et al. Glycemic index and obesity. Am J Clin Nutr. 2002;76(suppl):281S–5S. doi: 10.1093/ajcn/76/1.281S. [DOI] [PubMed] [Google Scholar]
- De Munter J. S, Hu F. B, Spiegelman D, et al. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. 2007;4:e261. doi: 10.1371/journal.pmed.0040261. http://dx.doi.org/10.1371/journal.pmed.0040261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster-Powell K, Holt S. H, Brand-Miller J. C. International table of glycemic index and glycemic load. American Journal of Clinical Nutrition. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- Franz M. J, Bantle J. P, Beebe C. A, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2002;25:148–198. doi: 10.2337/diacare.25.1.148. http://dx.doi.org/10.2337/diacare.25.1.148 . [DOI] [PubMed] [Google Scholar]
- Freedman M. R, King J, Kennedy E. Popular diets: a scientific review. Obes Res. 2001;9(Suppl 1):1S–40S. doi: 10.1038/oby.2001.113. http://dx.doi.org/10.1038/oby.2001.113 . [DOI] [PubMed] [Google Scholar]
- Garcia A. L, Otto B, Reich S. C. Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur J Clin Nutr. 2007;61:334–41. doi: 10.1038/sj.ejcn.1602525. http://dx.doi.org/10.1038/sj.ejcn.1602525 . [DOI] [PubMed] [Google Scholar]
- Howlett J, Ashwell M. Glycemic response and health: summary of a workshop. Am J Clin Nutr. 2008;87:212S–6S. doi: 10.1093/ajcn/87.1.212S. [DOI] [PubMed] [Google Scholar]
- Hu F. B, Willett W. C. Diet and coronary heart disease: findings from the Nurse’ Heatlth Study and Health Professionals’ Follow-up Study. J Nutr Health Aging. 2001;5:132–138. [PubMed] [Google Scholar]
- Kiens B, Richter E. A. Types of carbohydrate in an ordinary diet affect insulin action and muscle substrate in humans. Am J Clin Nutr. 1996;63:47–53. doi: 10.1093/ajcn/63.1.47. [DOI] [PubMed] [Google Scholar]
- Knowler W. C, Barrett-Connor E, Fowler S. E, et al. Reduction in the incidence of type 2 diabetes with life style intervention or metformin. N Eng J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. http://dx.doi.org/10.1056/NEJMoa012512 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss R. M, Eckel R. H, Howard B, et al. AHA dietary guidlines: revision 2000: A statment for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. doi: 10.1161/01.cir.102.18.2284. [DOI] [PubMed] [Google Scholar]
- Liu S, Willett W. C, Stampfer M. J, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- Livesey G, Tagami H. Interventions to lower the glycemic response to carbohydrate foods with a low-viscosity fiber (resistant maltodextrin): meta-analysis of randomized controlled trials. Am J Clin Nutr. 2009;89:114–25. doi: 10.3945/ajcn.26842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey G, Tylor R, Hulshof T, et al. Glycemic response and health a systemic review and meta-analysis: relation between dietary glycemic properties and health and health outcom. Am J Clin Nutr. 2008;87:258S–68S. doi: 10.1093/ajcn/87.1.258S. [DOI] [PubMed] [Google Scholar]
- Mahan L. K, Escott-Stump S. Krause’s food, nutrition and diet therapy. Philadelphia: W. B. Saunders; 2007. [Google Scholar]
- McFarlane S. I, Shin J. J, Rundek T, et al. Prevention of type 2 diabetes. Curr Diab Rep. 2003;3:235–241. doi: 10.1007/s11892-003-0070-5. http://dx.doi.org/10.1007/s11892-003-0070-5 . [DOI] [PubMed] [Google Scholar]
- Mckeown N. M, Meigs J. B, Liu S, et al. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care. 2004;27:538–546. doi: 10.2337/diacare.27.2.538. http://dx.doi.org/10.2337/diacare.27.2.538 . [DOI] [PubMed] [Google Scholar]
- Ohkuma K, Takahashi R. Development and application of low caloric modified starch. Tech J Food Chem. 1990;6:62–7. [Google Scholar]
- Schulze M, Schulze M, Heidmann C, et al. Fiber and Magnesium intake and incidence of Type 2 diabetes: A prospective study and meta analysis. Arch Intern Med. 2007;167:956–65. doi: 10.1001/archinte.167.9.956. http://dx.doi.org/10.1001/archinte.167.9.956 . [DOI] [PubMed] [Google Scholar]
- Surender K. A, Samy I. M. The case for low carbohydrate diets in diabetes management. Nutr and Meta. 2005. [Retrieved 16, 2]. [Online] Available: http://www.nutritionandmetabolism.com/content . [DOI] [PMC free article] [PubMed]
- Taleban F. A, Esmaeili M. Glycemic index of Iranian foods: guideline for diabetic and hyperlipidemic patients (in persian languge) Tehran: Institute Nutrition and Food Technology of Iran, Shahid Beheshti University of Medical Science; 1999. [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson J. G, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Eng J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]


