Abstract
Background and aim
Centrilobular zonal necrosis (CZN) is a known histological variant of autoimmune hepatitis (AIH). However, the significance of CZN is yet to be fully elucidated. This study aimed to determine whether CZN is a hallmark of a distinctive subtype of AIH.
Methods
Histological changes in the centrilobular zones of liver biopsies from 113 AIH patients were assessed by a single pathologist and classified into three categories: typical zonal necrosis defined as CZN (15 patients); other necroinflammatory change (NIC; 24 patients); and absence of necrosis (non-NIC; 74 patients). The clinicopathological features and immunogenetic background of CZN patients were then assessed.
Results
The clinicopathological features of AIH with CZN were distinct from other types of AIH, including a higher frequency of acute onset, lower frequency of antinuclear antibodies, lower antinuclear antibody titers, lower serum immunoglobulin G levels, lower grade interface hepatitis, less prominent lymphoplasmacytic infiltration, and lower AIH score. Increased and decreased frequencies of HLA-DR9 and HLA-DR4, respectively, were identified as immunogenetic features of AIH with CZN. Conversely, the clinicopathological characteristics of AIH with NIC were similar to those of non-NIC AIH, including the majority of the AIH patients. The therapeutic outcomes of AIH with CZN were excellent when precise diagnoses were made without delay.
Conclusion
The clinicopathological features and immunogenetic background of AIH with CZN differed from AIH without CZN. CZN may be a hallmark of a distinct subtype of AIH.
Keywords: antinuclear antibody, autoimmune hepatitis, centrilobular zonal necrosis, HLA-DR antigen
Introduction
Autoimmune hepatitis (AIH) is an organ-specific autoimmune disease characterized by immune-mediated cellular damage of hepatocytes. AIH is clinically diagnosed on the basis of the presence of hypergammaglobulinemia and autoantibodies, particularly antinuclear antibodies (ANAs) in type 1 AIH 1,2. In 1999, the International AIH group revised the diagnostic criteria of AIH and presented a scoring system that has been used widely to define AIH 3. The pathological characteristics of typical AIH are interface hepatitis, rosetting of hepatocytes, and predominant lymphocytic/plasmacytic infiltration 1–3. However, necrosis of the centrilobular zone (Rappaport zone 3; zone 3), characterized by extending confluent hepatic necrosis preferentially affecting zone 3, may be a hallmark of histologically atypical AIH 2–5. The necrosis in zone 3 has been termed centrilobular zonal necrosis (CZN) 6, centrilobular (central) necrosis (CN) 7, or zone 3 necrosis 8. Zen et al. 6 applied the term CZN to typical confluent necrosis in zone 3 with relative sparing of the portal area. However, there is a lack of consensus on the definition of CN or necrosis in zone 3. Furthermore, typical CZN is yet to be differentiated clearly from other types of significant necrosis in zone 3.
In the present study, marked confluent necrosis affecting a considerable proportion of zone 3 was defined as CZN. To clarify the clinicopathological features of AIH with CZN, other types of necrosis such as spotty or focal necrosis in zone 3 or bridging necrosis extending from the portal area to zone 3 were definitely differentiated from CZN. Subsequently, we performed an extensive evaluation of whether CZN was a hallmark of a distinctive subtype of AIH.
Methods
Patients
A total of 113 consecutive AIH patients who underwent liver biopsy and provided adequate tissue samples were enrolled in the present study. All patients were treated at the Jikei University Katsushika Medical Center or Tokyo Metropolitan Bokutoh Hospital between 2000 and 2014. The diagnosis of AIH was made on the basis of the classification of definitive or probable according to the diagnostic criteria of the International Autoimmune Hepatitis Group 3 or on empirical judgment by experienced hepatologists. Other etiologies of liver disease such as primary biliary cirrhosis, drug-induced liver injury, hemochromatosis, primary sclerosing cholangitis, Wilson’s disease, α1-antitrypsin deficiency, cytomegalovirus infection, Epstein–Barr virus infection, and nonalcoholic steatohepatitis were strictly ruled out. Hepatitis C virus-RNA positive, hepatitis B-virus positive, and HIV-positive patients were excluded from the present study.
Definition of the pattern of clinical presentation
On the basis of the study by Miyake et al. 9, clinical presentations were classified into acute or chronic according to the following criteria. Acute clinical presentations (acute onset) were defined as follows: acute elevation of serum alanine aminotransferase (ALT) or aspartate aminotransferase levels of greater than 10 times the upper normal limit or acute development of liver-related symptoms (fatigue, jaundice, and appetite loss) without evidence of previous liver dysfunction (more than 6 months before the time of diagnosis). Other clinical presentations were defined as chronic.
Collection of demographic and laboratory data
Demographic and laboratory data at the time of diagnosis were collected and the clinical courses of patients were retrospectively investigated. Laboratory data included aspartate aminotransferase, ALT, gamma-glutamyltransferase, alkaline phosphatase, total bilirubin, albumin, immunoglobulin G (IgG), platelet counts, prothrombin time, and ANA. ANA was titrated by standard indirect fluorescence using HepG2 cells. A titer of at least 40 was considered positive, whereas that of at least 160 was considered a high positive titer. HLA-DR antigens were identified using a reverse sequence-specific oligonucleotide method 10.
Histological evaluation
Liver tissue was obtained by transcutaneous liver biopsy before diagnosis using a 16 G or an 18 G needle. Biopsy specimens were stained using hematoxylin/eosin and Masson’s trichrome or Heidenhain’s Azan trichrome. Patients with liver biopsy samples shorter than 2 cm were excluded from the present study because of an increased possibility of incorrect assessment of the pattern of necrosis in zone 3.
The grade of interface hepatitis, presence of predominant lymphoplasmacytic infiltrate, and rosetting of liver cells were evaluated and scored according to the histologic score of AIH proposed by the International Autoimmune Hepatitis Group 1. Fibrosis stage was assessed on the basis of the Metavir scoring system as follows: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with few septa; F3, numerous septa without cirrhosis; and F4, cirrhosis.
Necrosis in zone 3 was carefully examined and initially classified into two categories according to the presence (CZN) or absence (non-CZN) of typical CZN. Subsequently, necroinflammatory changes other than typical CZN in zone 3 were evaluated and further subclassified into the presence (necrosis in the centrilobular zone; NIC) or absence (non-NIC) of necrosis other than CZN in zone 3. CZN was strictly defined as marked confluent necrosis that preferentially and extensively affected zone 3 hepatocytes. NIC was defined as significant necroinflammatory change other than CZN in zone 3, including spotty/focal necrosis and central perivenular hepatitis in zone 3, and bridging necrosis predominantly affecting the periportal zone with extension into zone 3.
Typical CZN, shown in Fig. 1a and b, is comparatively easily differentiated from the other type of necrosis in zone 3 shown in Fig. 2a and b. Histologic evaluations were performed by a single pathologist (A.S.) who was blinded to patient clinical information.
Fig. 1.
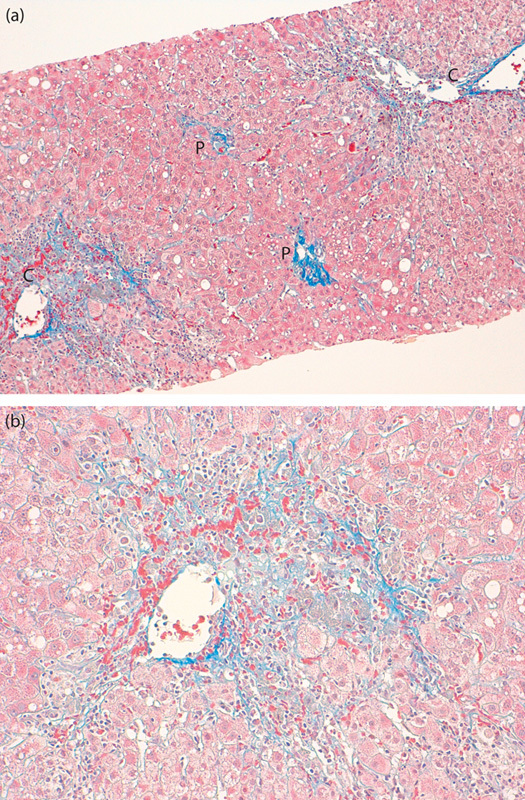
(a) Typical centrizonal necrosis. Marked confluent necrosis in the centrilobular zone with sparing portal tract involvement (Masson trichrome stain, ×100). (b) Higher magnification of the centrilobular zone. Mild to moderate lymphoplasmacytic infiltration in the necrotic region (Masson trichrome stain, ×200). C, central vein; P, portal area.
Fig. 2.
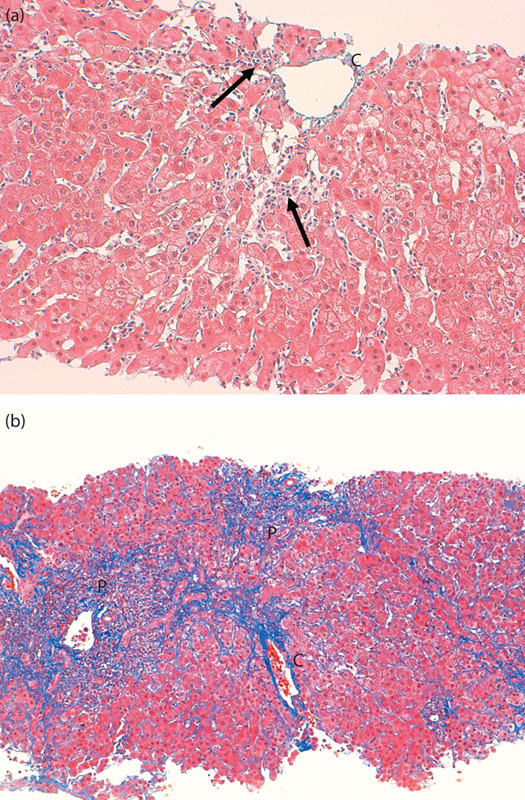
(a) Spotty necrosis with inflammation (arrows) in the centrilobular zone (Masson trichrome stain, ×200). (b) Marked periportal necrosis extending to the centrilobular zone forming bridging necrosis (Masson trichrome stain, ×100). C, central vein; P, portal area.
Study design
AIH patients were initially classified into two groups: the CZN and the non-CZN groups. Subsequently, the non-CZN group was subdivided into the NIC and non-NIC groups. Demographic, clinical, and histologic features, including HLA-DR genotypes, were investigated in each group and the differences between the CZN and non-CZN groups were compared by statistical analyses. Furthermore, differences between the NIC and the non-NIC groups were investigated.
The present study complied with the standards of the Declaration of Helsinki and current ethical guidelines as reflected by the approval of the human ethics review committee of each participating institution. All participants were informed of the protocol of this study. Informed consent was obtained from all patients.
Statistical analysis
Continuous variables are expressed as medians (Q1–Q3) and categorical data are expressed as number (%). Differences between two groups were analyzed using the Mann–Whitney U-test for continuous data and Fisher’s exact test or χ2-test for nominal data. Two-tailed P-values equal to or less than 0.05 were defined as statistically significant. Values 0.05<P<0.1 were considered marginal. Statistical analyses were carried out using Statistica for Windows, version 6 (StatSoft, Tulsa, Oklahoma, USA).
Results
Classification of AIH according to central zone necrosis
Of the 113 AIH patients, typical CZN was observed in 15 patients (CZN group). Of the remaining 98 patients without typical CZN (non-CZN group), significant necrosis other than typical CZN in zone 3 was observed in 24 patients (NIC group), whereas significant necrosis was not observed in the remaining 74 patients (non-NIC group). The non-NIC group represented the majority of the AIH patients.
Comparison of the clinicopathological features between the CZN and non-CZN groups and the NIC and non-NIC groups
The clinicopathological characteristics of the CZN and non-CZN groups and those of the NIC and non-NIC groups are summarized in Tables 1 and 2, respectively.
Table 1.
Comparisons of demographic and clinical characteristics between AIH patients with and without histologic evidence of CZN
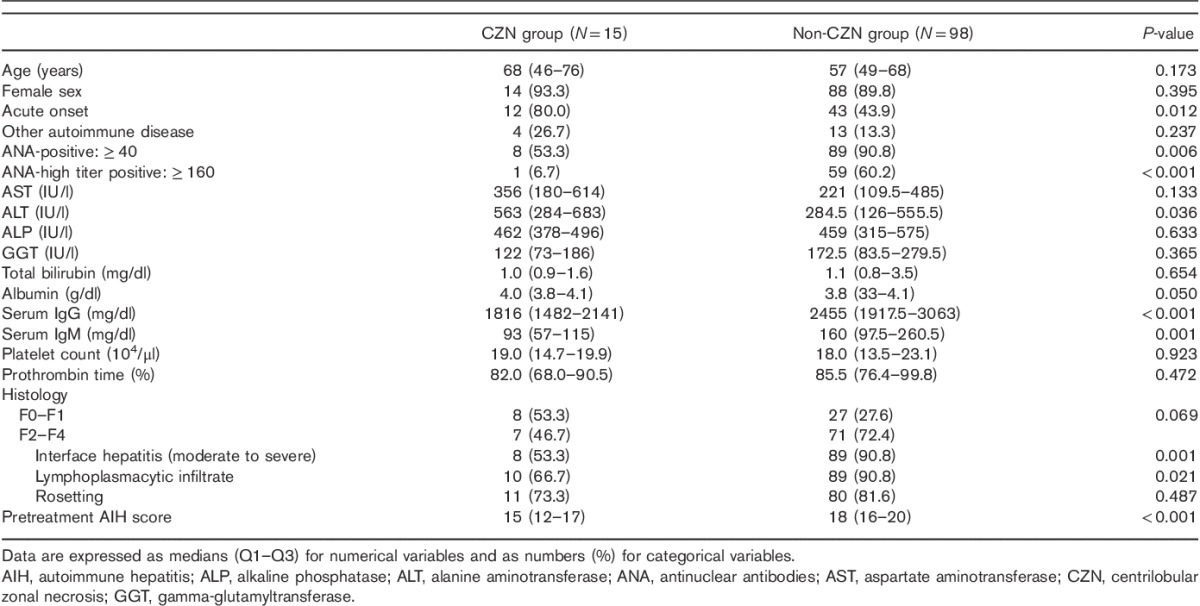
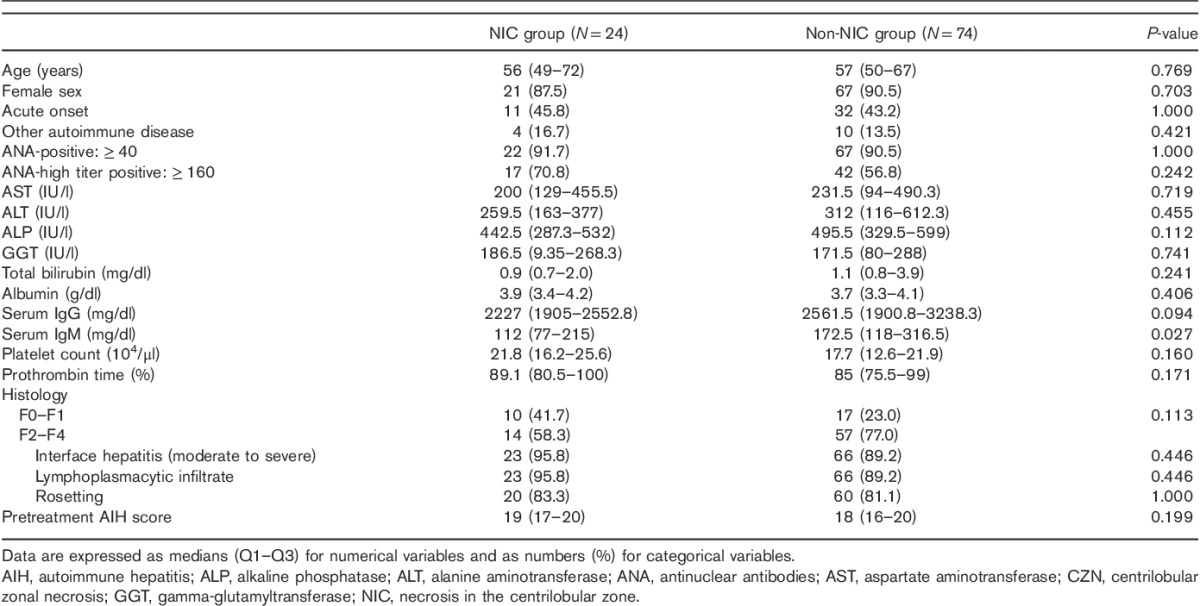
Table 2.
Comparisons of demographic and clinical characteristics between AIH patients with and without significant necrosis other than CZN in the central zone
A number of clinicopathological features differed between the CZN and the non-CZN groups. A higher rate of acute onset, lower prevalence of ANA positivity, lower prevalence of high ANA titers, and lower serum IgG and IgM levels were observed in the CZN group compared with the non-CZN group. Serum ALT and albumin levels were higher in the CZN group compared with the non-CZN group. Histologically, fewer patients had typical features of AIH, except rosetting, fibrosis stages were marginally less advanced, and AIH scores were lower in the CZN group compared with the non-CZN group (Table 1).
In contrast, the clinicopathological characteristics of the NIC and non-NIC groups were comparable, except for lower serum IgM levels and marginally lower serum IgG levels in the NIC group compared with the non-NIC group. No significant differences were observed in the histological findings or AIH scores between the NIC and the non-NIC groups (Table 2).
Differences in HLA-DR antigen phenotypes between the CZN and the non-CZN groups and the NIC and the non-NIC groups
HLA-DR antigen phenotype distributions are presented in Tables 3 and 4. HLA-DR4 was less frequent in the CZN group than in the non-CZN group. The frequency of HLA-DR9 was significantly higher in the CZN group than in the non-CZN group. The distribution of HLA-DR antigens other than HLA-DR4 and HLA-DR9 was similar between the CZN and the non-CZN groups (Table 3).
Table 3.
Difference in HLA-DR antigen distributions between AIH patients with and without histological evidence of CZN
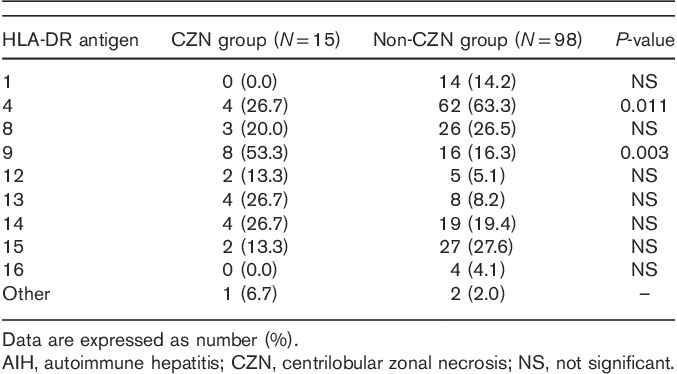
Table 4.
HLA-DR antigen distribution between AIH patients with and without significant necrosis other than CZN in the central zone
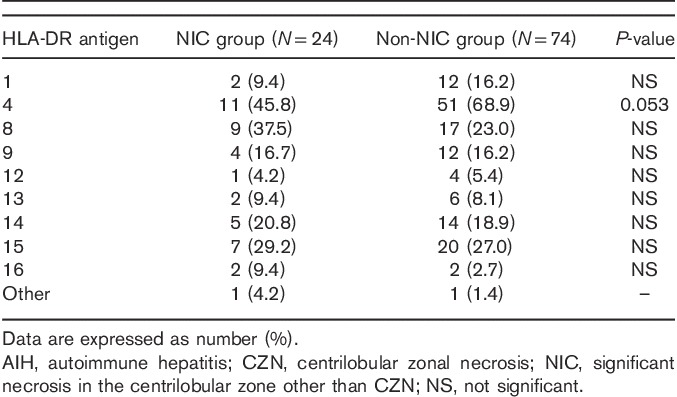
When comparing the distribution of HLA-DR phenotypes between the NIC and the non-NIC groups, no significant differences were observed, except for a marginal decrease in the frequency of DR4 in the NIC group (Table 4).
Clinicopathological features of non-CZN patients compared with CZN patients with lower severity of fibrosis
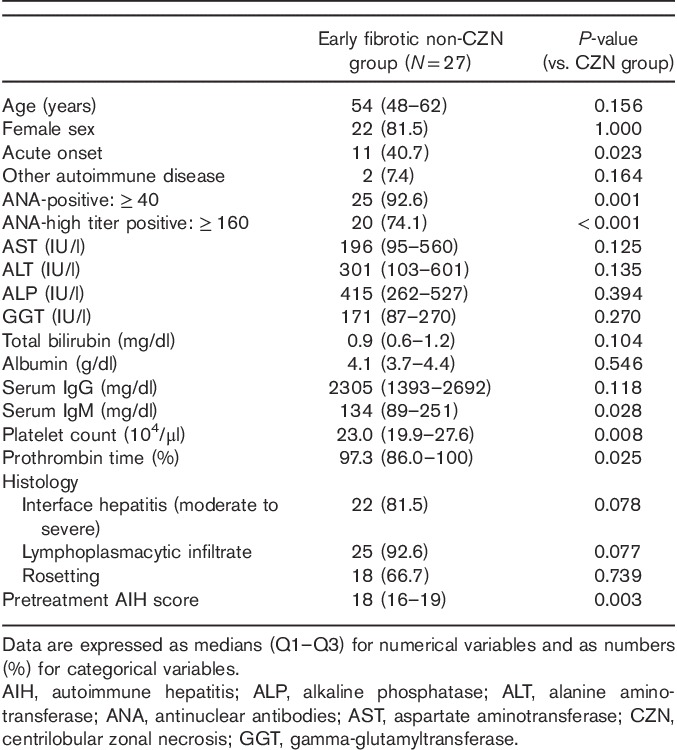
In a further study including 27 non-CZN patients with mild fibrosis (F0–F1), a number of clinicopathological features differed between CZN and non-CZN patients with mild fibrosis. The frequencies of acute onset, ANA positivity, and high ANA titers were higher in non-CZN patients with mild fibrosis. Moreover, higher serum IgM levels, platelet counts, and %prothrombin time values were observed in non-CZN patients with mild fibrosis compared with CZN patients. Histologically, a marginal increase in the frequency of interface hepatitis and lymphoplasmacytic infiltration was observed in non-CZN patients with mild fibrosis. AIH scores were significantly higher in non-CZN AIH patients with mild fibrosis (Table 5).
Table 5.
Characteristics of AIH patients with mild fibrosis and no histological evidence of CZN
Response to immunosuppressive therapy in CZN patients
In the 13 CZN patients, responses to prednisolone therapy were generally satisfactory without disease relapse, except for those in two patients. One patient was refractory to prednisolone following a considerable delay (>6 months) in the initiation of prednisolone therapy. The other patient relapsed following the interruption of a short duration (3 months) of prednisolone therapy. In both these patients, a definitive diagnosis of AIH was not confirmed despite the availability of histological findings.
Discussion
In the present study, we assessed clinicopathological features and immunogenetic backgrounds in AIH patients with histological features of marked and extensive necrosis in zone 3, termed CZN.
The histological characteristics of CZN were first described by Pratt et al. 4 and Te et al. 5 as a prominent central zone (zone 3) necrosis with relative sparing of portal regions in liver biopsy specimens from patients with glucocorticoid-responsive chronic hepatitis. Subsequently, the clinicopathological features of CZN without classical histologic features of AIH were assessed and summarized 6,11. However, consensus on the histological features of CZN is still lacking. In addition, the natural clinical course of AIH with CZN is yet to be fully elucidated. However, Singh et al. 11 reported a case with a marked predominance of centrizonal injury in an initial liver biopsy that later transformed into a typical histological appearance over the course of several months. This finding may indicate that CZN represents a very early histologic phenotype of typical AIH.
Fulminant AIH, a severe form of CZN that progresses to pan-lobular necrosis, is considered distinct from other types of fulminant AIH with massive hepatic necrosis involving zone 3 12. However, the significance of typical CZN in nonfulminant AIH remains unclear. The frequency of significant necrosis in zone 3 has been reported to be 17.5–29% of all AIH cases 7,8 and 31.7% of all acute-onset AIH cases 13. However, the reported frequency of necrosis in zone 3 without obvious portal inflammation is relatively low, accounting for only 3.5% of all AIH cases 7.
A limitation of previous studies is the ambiguous histologic definition and clarification of necrosis in zone 3. In an earlier report, confluent necrosis and spotty necrosis in zone 3 were concomitantly abbreviated as CN 14. Although spotty or focal necrosis in zone 3 is often observed in AIH, marked confluent necrosis (typical CZN) is a relatively rare histological finding. Therefore, to clarify the significance of CZN, the distinction of typical CZN from other types of necrosis is necessary 15.
In the present study, we examined the significance of CZN after distinctive differentiation of typical CZN from other types of necrosis. As a result, we found that AIH patients with CZN had distinctive clinicopathological features such as a higher prevalence of acute-onset liver disease, lower frequency of ANA positivity, lower ANA titers, higher serum ALT levels, lower serum IgG levels, and a lower frequency of histological features of AIH compared with non-CZN patients. Accordingly, AIH scores were significantly lower in the CZN group.
Compared with previous reports, the prevalence of AIH with CZN was relatively low in the present study, accounting for only 13.3% of all AIH cases and 21.8% of acute-onset AIH cases. In addition, the observed clinicopathological features of CZN cases in the present study did not corroborate previous reports 7,8. Although the high frequency of acute-onset, higher serum ALT levels and less advanced fibrosis reported in previous studies were in agreement with the findings of the present study, other features differed markedly. In a previous European study 7, more severe portal and lobular inflammation was observed in cases of AIH with CN. Moreover, the proportion of definite AIH defined according to AIH was similar between AIH patients with CN and without CN. In a study of Japanese AIH patients 8, the reported clinical features associated with necrosis in zone 3 were lower serum albumin and higher serum total bilirubin levels; however, the observed frequency of cirrhosis was similar between AIH patients with and without necrosis in zone 3. In a further Japanese study of acute-onset AIH 13, AIH with CN was found to be characterized by lower serum IgG levels, lower prevalence of ANA, and less advanced fibrosis. The findings of the present study corroborate these reports. However, the frequencies of characteristic histological findings of AIH and ANA titers were reportedly similar between AIH patients with and without CN. The discordant clinicopathological features among previous reports and the findings of the present study are likely to be predominantly because of the different definitions of necrosis in zone 3 used.
The finding of less prominent portal inflammation in the CZN group was also reported by the original studies of CZN 4,5. We observed less prominent histological features of AIH with lower serum IgG levels, lower frequency of ANA, and lower ANA titers in the CZN group. Therefore, more importantly, AIH scores were significantly lower in the CZN group compared with other types of AIH. Thus, a definitive diagnosis of AIH was often challenging in these patients even after histological examination. In contrast to the CZN group, the clinicopathological features of AIH in the NIC group were similar to those of the non-NIC group (majority of AIH cases), except for lower serum IgM levels in the NIC group. Therefore, AIH with NIC does not appear to represent a separate subtype of AIH.
As discussed earlier, AIH with CZN may represent a very early histologic phenotype of typical AIH 11. However, the observed clinicopathological features of the CZN group in the present study differed markedly from those of the non-CZN group, with the observation of mild fibrosis in the CZN group strongly indicating that CZN was not a very early phenotype of typical AIH, but a specific phenotype of AIH that could be differentiated from the majority of the AIH cases. Although the consequence of AIH with CZN is currently unclear, we observed the presence of moderate to severe fibrosis in our series of CZN patients. Therefore, AIH with CZN may progress to advanced fibrosis and cirrhosis. Furthermore, even in cases of CZN with liver fibrosis progression, patients remained ANA negative with low titers 16.
Our proposal of CZN as a hallmark of a definite AIH subtype was further strengthened by the analysis of HLA-DR antigens. The prevalence of DR9 was significantly higher and the prevalence of DR4 was lower in the CZN group compared with the non-CZN group. The relative risk of HLA-DR9 in AIH with CZN was calculated to be 3.72 (95% confidence interval; 1.35–10.37) in a large-scale population study of healthy Japanese individuals by the HLA Laboratory (Kyoto, Japan) (http://www.hla.or.jp/). These findings indicate that HLA-DR9 is an immunogenetic factor associated specifically with AIH with CZN. However, there is an ethnic variation in the frequency of HLA-DR9 17. HLA-DR9 is very rare in the White population (1.5–2.4%), whereas it is ∼10 times more prevalent in the Asian population. Therefore, the association between HLA-DR9 and a histological finding pertaining to CZN may be limited to the Asian population. HLA-DR9 may play a role in the development of Japanese autoimmune fatigue syndrome 18. DR9 has been shown to contribute toward the acute onset and complete destruction of β-cells in Japanese patients with type 1 diabetes 19. Thus, HLA-DR9 may be involved in the acute onset and severity of immune-mediated diseases such as AIH, as indicated by the findings of the present study.
In contrast to HLA-DR9, HLA-DR4 was less frequent in the CZN group compared with the non-CZN group and marginally less frequent in the NIC group compared with the non-NIC group (majority of the AIH cases). HLA-DR4 is an established immunogenetic factor known to increase the susceptibility to type 1 AIH, particularly in Japanese patients 20. The findings of the present study indicate that HLA-DR4 may contribute toward the development of typical clinicopathological features of AIH.
To date, there has been a lack of consensus on the clinicopathological features and immunogenetic backgrounds of AIH with CZN. AIH is a relatively rare disease and the frequency of typical CZN is low. Thus, the size of our study may have been inadequate in conclusively elucidating the significance of CZN. Larger studies are required to validate our assertion that AIH with CZN is a distinctive subtype of AIH.
In conclusion, the clinicopathological features and immunogenetic background of AIH with typical CZN represent a distinct type of AIH. CZN is an unusual histological finding of AIH. Although the existence of this particular type of AIH should be recognized, its precise diagnosis and prompt treatment tend to be delayed because of the lack of typical features of AIH and a low AIH score in this specific subtype of AIH.
Acknowledgements
The authors thank all the staff of the Department of Pathology at the Jikei University Katsushika Medical Center and Tokyo Metropolitan Bokutoh Hospital for the preparation and staining of the liver biopsy samples.
This study was financially aided by Bristol-Myers KK, Japan.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM. American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology 2010; 51:2193–2213. [DOI] [PubMed] [Google Scholar]
- 2.Zachou K, Muratori P, Koukoulis GK, Granito A, Gatselis N, Fabbri A, et al. Review article: autoimmune hepatitis – current management and challenges. Aliment Pharmacol Ther 2013; 38:887–913. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 1999; 31:929–938. [DOI] [PubMed] [Google Scholar]
- 4.Pratt DS, Fawaz KA, Rabson A, Dellelis R, Kaplan MM. A novel histological lesion in glucocorticoid-responsive chronic hepatitis. Gastroenterology 1997; 113:664–668. [DOI] [PubMed] [Google Scholar]
- 5.Te HS, Koukoulis G, Ganger DR. Autoimmune hepatitis: a histological variant associated with prominent centrilobular necrosis. Gut 1997; 41:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zen Y, Notsumata K, Tanaka N, Nakanuma Y. Hepatic centrilobular zonal necrosis with positive antinuclear antibody: a unique subtype or early disease of autoimmune hepatitis? Hum Pathol 2007; 38:1669–1675. [DOI] [PubMed] [Google Scholar]
- 7.Hofer H, Oesterreicher C, Wrba F, Ferenci P, Penner E. Centrilobular necrosis in autoimmune hepatitis: a histological feature associated with acute clinical presentation. J Clin Pathol 2006; 59:246–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyake Y, Iwasaki Y, Terada R, Onishi T, Okamoto R, Takaguchi K, et al. Clinical features of Japanese type 1 autoimmune hepatitis patients with zone III necrosis. Hepatol Res 2007; 37:801–805. [DOI] [PubMed] [Google Scholar]
- 9.Miyake Y, Iwasaki Y, Kobashi H, Yasunaka T, Ikeda F, Takaki A, Yamamoto K. Autoimmune hepatitis with acute presentation in Japan. Dig Liver Dis 2010; 42:51–54. [DOI] [PubMed] [Google Scholar]
- 10.Herbut B, Woodman R, Kowalick L, Malkki M, Petersdorf EW, McKenna R. Identification of a novel HLA-B*07 allele – HLA-B*0726. Tissue Antigens 2002; 59:60–62. [DOI] [PubMed] [Google Scholar]
- 11.Singh R, Nair S, Farr G, Mason A, Perrillo R. Acute autoimmune hepatitis presenting with centrizonal liver disease: case report and review of the literature. Am J Gastroenterol 2002; 97:2670–2673. [DOI] [PubMed] [Google Scholar]
- 12.Stravitz RT, Lefkowitch JH, Fontana RJ, Gershwin ME, Leung PS, Sterling RK, et al. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology 2011; 53:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abe K, Kanno Y, Okai K, Katsushima F, Monoe K, Saito H, et al. Centrilobular necrosis in acute presentation of Japanese patients with type 1 autoimmune hepatitis. World J Hepatol 2012; 4:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misdraji J, Thiim M, Graeme-Cook FM. Autoimmune hepatitis with centrilobular necrosis. Am J Surg Pathol 2004; 28:471–478. [DOI] [PubMed] [Google Scholar]
- 15.Yasui S, Fujiwara K, Yonemitsu Y, Oda S, Nakano M, Yokosuka O. Clinicopathological features of severe and fulminant forms of autoimmune hepatitis. J Gastroenterol 2011; 46:378–390. [DOI] [PubMed] [Google Scholar]
- 16.Miyake Y, Iwasaki Y, Kobashi H, Yasunaka T, Ikeda F, Takaki A, et al. Clinical features of antinuclear antibodies-negative type 1 autoimmune hepatitis. Hepatol Res 2009; 39:241–246. [DOI] [PubMed] [Google Scholar]
- 17.Brown J, Poles A, Brown CJ, Contreras M, Navarrete CV. HLA-A, -B and -DR antigen frequencies of the London Cord Blood Bank units differ from those found in established bone marrow donor registries. Bone Marrow Transplant 2000; 25:475–481. [DOI] [PubMed] [Google Scholar]
- 18.Itoh Y, Igarashi T, Tatsuma N, Imai T, Yoshida J, Tsuchiya M, et al. Immunogenetic background of patients with autoimmune fatigue syndrome. Autoimmunity 2000; 32:193–197. [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi K, Inoko H. Combination of HLA-A24, -DQA1*03, and -DR9 contributes to acute-onset and early complete beta-cell destruction in type 1 diabetes: longitudinal study of residual beta-cell function. Diabetes 2006; 55:1862–1868. [DOI] [PubMed] [Google Scholar]
- 20.Zeniya M, Watanabe F, Aizawa Y, Toda G. Immunogenetic background of hepatitis B virus infection and autoimmune hepatitis in Japan. Gastroenterol Jpn 1993; 28 (Suppl 4):69–75. [DOI] [PubMed] [Google Scholar]


