Abstract
Background
Despite growing evidence that N-terminal pro-brain natriuretic peptide (NT-proBNP) has an important prognostic value for patients with cardiovascular disease, chronic kidney disease, etc, the prognostic significance of NT-proBNP levels in the general population has not been established. The aim of this study was to evaluate the clinical significance of NT-proBNP in a community population.
Methods
This is a community-based prospective survey of residents from two communities in Beijing conducted for a routine health status checkup. Out of 1,860 individuals who were eligible for inclusion from 2007 to 2009, 1,499 completed a follow-up and were assessed for the prognostic value of NT-proBNP in 2013. A questionnaire was used for end point events. Anthropometry and blood pressure were measured. Plasma NT-proBNP, creatinine, lipids, and glucose were determined.
Results
A total of 1,499 subjects with complete data were included in the analysis. Participants were divided into four groups according to baseline NT-proBNP levels (quartile 1, <19.8 pg/mL; quartile 2, 19.8–41.6 pg/mL; quartile 3, 41.7–81.8 pg/mL; quartile 4, ≥81.9 pg/mL). During a median 4.8-year follow-up period, the all-cause mortality rate rose from 0.8% in the lowest concentration NT-proBNP group (<19.8 pg/mL) to 7.8% in the highest NT-proBNP group (≥81.9 pg/mL; P<0.001). The incidence of major adverse cardiovascular events (MACEs) increased from 3.1% in the lowest NT-proBNP group to 18.9% in the highest group (P<0.001). Individuals in the highest NT-proBNP group (≥81.9 pg/mL) were associated with higher risk of all-cause death and MACEs compared with the lowest NT-proBNP group using Kaplan–Meier survival curves and the Cox proportional hazard model after adjusting for age, sex, and traditional risk factors.
Conclusion
The plasma NT-proBNP level is a strong and independent prognosis factor for all-cause death and MACEs in the community population. The NT-proBNP cut-point for the prognostic value remains to be further studied. NT-proBNP is a strong and independent prognostic factor for all-cause death and MACEs in individuals older than 65 years and MACEs in individuals younger than 65 years.
Keywords: NT-proBNP, community population, prognosis
Introduction
Brain natriuretic peptide (BNP) is synthesized as preproBNP, enzymatically cleaved to proBNP in response to myocyte stretch, and subsequently released in the circulation as biologically active BNP and inactive N-terminal fragment (N-terminal pro-brain natriuretic peptide, NT-proBNP). Many studies have shown that BNP and NT-proBNP are important prognostic factors.1 Compared with BNP, NT-proBNP has a longer half-life, and is more stable in vitro, and thus, it is more advantageous to detect. Clinical laboratories should consider the appropriate implementation of the BNP as a diagnostic test to assist in ruling out heart failure (HF); however, in the application of prognosis and guiding therapy, a number of questions remain to be answered.2 A recent study showed that both NT-proBNP and BNP are associated with future cardiovascular events in the general population; however, NT-proBNP seems to be superior due to its higher prognostic value.3 NT-proBNP is established as a biomarker for the diagnosis, prognosis, and management of established cardiovascular disease and HF.4,5 Although some studies have reported that NT-proBNP has a positive prognostic value in the general population, these studies were conducted on European and American people, and some participants had documented cardiovascular disease6–8 with a high risk of cardiovascular events. We were interested in examining the relationship between NT-proBNP and the incidence of all-cause death and major adverse cardiovascular events (MACEs) in the Chinese general population, and excluded those who had documented cardiovascular disease. We prospectively studied a large, community-based sample of persons in whom plasma natriuretic peptide levels were routinely measured and who were followed for the occurrence of the MACEs and death.
Methods
Study population
This is a prospective observational study on inhabitants (age ≥18 years) of the Pingguoyuan area of the Shijingshan district in Beijing, People’s Republic of China. Subjects with severe systemic diseases such as collagenosis, endocrine and metabolic disease (except diabetes mellitus [DM]), inflammation, neoplastic, severe liver or renal diseases, and a history of ischemic heart disease or HF were excluded. Ultimately, a total of 1,680 participants were initially eligible for cross-sectional analysis between September 2007 and January 2009. The follow-up visits were conducted from February 1 to September 30, 2013. During these visits, all participants received a questionnaire survey in community medical centers. The median follow-up interval for the original 1,680 participants was 4.8 years (range, 4.5–5.2 years). During the period between the initiation of the study and the follow-up, 181 participants were lost to follow-up and were excluded from the analysis. Complete follow-up data were obtained from 1,499 participants (follow-up rate, 89.2%). All participants in the study gave written informed consent, and the Medical Ethics Commission of the Chinese People’s Liberation Army (PLA) General Hospital (Beijing, People’s Republic of China) approved the study.
Questionnaire and anthropometric measurements
Information about the age, smoking status, and history of hypertension and DM was obtained using standardized self-reporting questionnaires. These questionnaires were administered using a face-to-face counseling method. The investigation was completed by physicians from the Department of Geriatric Cardiology, the PLA General Hospital, who were trained by the research team.
The physical examination included anthropometric and blood pressure measurements. Height, weight, and waist and hip circumferences were measured. The body mass index (BMI) and waist-to-hip ratio were calculated. BMI was calculated as the weight in kilograms divided by the height in meters squared (kg/m2). Waist-to-hip ratio was calculated as waist circumference divided by hip circumference. The blood pressure of the participants was measured in the sitting position. The blood pressure measurement was performed using a calibrated desktop sphygmomanometer (Yuyue; Armamentarium Limited Company, Jiangsu, People’s Republic of China) after the participants had been in the sitting position for ≥5 minutes, which is consistent with current recommendations.9 Blood pressure was measured three times consecutively, with ≥1 minute between measurements. The mean value of blood pressure was used for the statistical analysis.
Biomarker variable determination
All subjects underwent full laboratory evaluation (lipid profile and liver and kidney function tests). Blood samples were collected from subjects between 8 am and 10 am after overnight fasting for at least 12 hours. Plasma aliquots were frozen at −80°C until the assays were performed. Peripheral blood samples were obtained to measure the following parameters: fasting blood glucose, total cholesterol, triglyceride (TG), low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, uric acid, hypersensitive C-reactive protein (Hs-CRP), homocysteine (HCY), creatinine (Cr), and NT-proBNP. Concentrations of plasma Cr were measured by an enzymatic assay (Roche Diagnostics GmbH, Basel, Switzerland) on a Hitachi 7600 autoanalyzer (Hitachi, Tokyo, Japan). NT-proBNP was determined with an electrochemiluminescence immunoassay (Roche Diagnostics GmbH) using a Roche analyzer (Roche Diagnostics).
Definition of variables
All participants without a history of DM were given a standard 75 g oral glucose tolerance test. DM was indicated by (i) a fasting glucose level ≥7.1 mmol/L, (ii) a 2-hour venous blood glucose level ≥11.1 mmol/L, or (iii) the use of a hypoglycemic drug or insulin.10 Estimated glomerular filtration rate (eGFR) was calculated using the Chinese modified Modification of Diet in Renal Disease equation as follows:11 eGFR (mL/min/1.73 m2) =175× standard Cr (mg/dL)−1.234 × age (year)−0.179 ×0.79 (if female), and chronic kidney disease was defined according to published guidelines.12 Cigarette smoking was defined as smoking one or more cigarettes per day for at least 1 year. Hypertension was indicated by the following: (i) systolic blood pressure (SBP) ≥140 mmHg, (ii) diastolic blood pressure (DBP) ≥90 mmHg, and/or (iii) the use of an antihypertensive drug.13 Alcohol users were defined as drinking once a week (white spirit, beer, or red wine). Waist circumference was measured at the level of the umbilicus. Exercise status was defined as exercising for at least 30 minutes every day regardless of exercise type.
End points
The major end points assessed were all-cause death and MACEs. Death was ascertained from the death record, a legal document including time, site, and other information. MACEs include cardiovascular death, nonfatal myocardial infarction, coronary revascularization therapy, and coronary artery disease diagnosed by coronary artery imaging or stroke.14
Statistical analysis
Continuous variables with a normal distribution are expressed as the mean and standard deviation. NT-proBNP is given as a median with an interquartile range (IQR). Categorical variables are expressed as numbers and percentages. NT-proBNP levels are presented as a continuous variable (after natural logarithmic transformation) and as categorical variables when appropriate. All analyses were performed at a median follow-up interval of 4.8 years. Plasma NT-proBNP levels at baseline were categorized as quartile 1 (≤19.8 pg/mL, n=389), quartile 2 (19.8–41.6 pg/mL, n=370), quartile 3 (41.7–81.8 pg/mL, n=370), and quartile 4 (≥81.9 pg/mL, n=370). The comparison of the continuous variables between demographic and clinical characteristics among the groups was done using analysis of variance or Cuzik’s nonparametric trend test. Differences in proportions were tested using chi-square test and Fisher’s exact test.
The relationship between NT-proBNP levels and major end points was evaluated using Cox proportional hazard regression model, and group 1 was the control group. Model 1 adjusted for age and sex. Model 2 adjusted for the variables in model 1 plus hypertension, diabetes, current smoking, BMI, SBP, fasting blood glucose, and high-density and low-density lipoprotein cholesterol. Model 3 adjusted for the variables in model 2 plus eGFR. Model 4 adjusted for the variables in model 3 plus Hs-CRP and HCY. Cumulative mortality and MACE curves were estimated using the Kaplan–Meier method. A receiver operating characteristic (ROC) curve was generated to evaluate the accuracy of NT-proBNP in the prediction of the all-cause death and MACEs.
All analyses were conducted using SPSS software for Windows, version 13.0 (SPSS, Chicago, IL, USA) and State software (version 11.0; Stata Corporation, College Station, TX, USA). P-values <0.5 were considered statistically significant.
Results
Baseline clinical characteristics of participants
A total of 1,499 subjects were included in the analysis. There were 629 males (42%) and 870 females (58%). The age range was 45–98 years (mean, 61.4±11.4 years). There were 247 (16.5%) current smokers, 292 (19.5%) participants with DM, and 690 (46%) participants with hypertension. At baseline, the median NT-proBNP level was 41.6 pg/mL (IQR, 19.8–81.9). Demographic characteristics, cardiovascular risk factors, and related laboratory test results in each group are shown in Table 1. Individuals in the highest quartile of the NT-proBNP were generally older, more often female, and had a history of hypertension and/or DM. Furthermore, individuals in the highest quartile had higher SBP, Cr, and HCY levels, while they had lower DBP, TG, and eGFR levels at baseline in comparison to subjects in the lower three quartiles (P-value for all, <0.01).
Table 1.
Prevalence or mean of relevant covariables in each quartile of NT-proBNP at baseline
| Variables | NT-proBNP (pg/mL)
|
P-value | |||
|---|---|---|---|---|---|
| <19.8 (n=389) | 19.8–41.6 (n=370) | 41.7–81.8 (n=370) | ≥81.9 (n=370) | ||
| Age (years) | 57.6±8.2 | 60.5±9.2 | 63.4±9.3 | 67.8±9.5 | <0.001 |
| Men, n (%) | 216 (55.5) | 154 (41.6) | 136 (36.7) | 123 (33.2) | <0.001 |
| HNT, n (%) | 140 (36.0) | 166 (44.9) | 176 (47.6) | 208 (56.2) | <0.001 |
| DM, n (%) | 65 (16.7) | 73 (19.7) | 75 (20.3) | 82 (22.2) | 0.013 |
| Current smoking, n (%) | 84 (21.6) | 70 (18.9) | 45 (12.2) | 48 (13.0) | <0.001 |
| BMI (kg/m2) | 25.8±3.6 | 26.1±3.4 | 25.3±3.4 | 25.4±3.6 | 0.008 |
| SBP (mmHg) | 130.6±15.8 | 133.1±16.8 | 132.9±17.4 | 134.5±18.2 | 0.02 |
| DBP (mmHg) | 78.9±9.8 | 78.0±9.8 | 75.9±10.1 | 73.8±10.7 | <0.001 |
| Heart rate (bpm) | 76.6±10.1 | 75.7±9.7 | 74.4±9.6 | 74.3±10.1 | 0.004 |
| FBG (mmol/L) | 5.5±1.5 | 5.4±1.7 | 5.3±1.7 | 5.4±1.6 | 0.555 |
| PBG (mmol/L) | 7.9±3.9 | 7.8±4.1 | 8.0±4.4 | 8.2±4.1 | 0.598 |
| TG (mmol/L) | 2.0±1.4 | 1.9±1.4 | 1.6±0.9 | 1.6±1.0 | <0.001 |
| LDL-C (mmol/L) | 3.0±0.7 | 3.0±0.7 | 3.0±0.7 | 3.0±0.7 | 0.415 |
| Cr (μmol/L) | 65.9±15.2 | 62.9±15.5 | 64.2±16.5 | 68.7±19.1 | 0.001 |
| UA (μmol/L) | 300.9±70.8 | 285.1±76.6 | 283.2±69.4 | 296.5±74.9 | 0.001 |
| HCY (μmol/L) | 17.0 (13.9, 21.5) | 16.6 (13.7, 22.2) | 17.1 (14.0, 21.0) | 18.6 (15.7, 23.0) | <0.001 |
| Hs-CRP (mg/L) | 2.2 (1.4, 3.4) | 2.2 (1.3, 3.5) | 2.2 (1.3, 3.4) | 2.4 (1.5, 3.6) | 0.207 |
| eGFR (mL/min/1.73 m2) | 91.6±13.7 | 91.5±15.5 | 88.5±15.7 | 83.8±16.6 | <0.001 |
Notes: Continuous variables (age, BMI, TG, TC, HDL-C, LDL-C, HCY, SBP, DBP, PBG, eGFR, UA, Cr) were expressed as mean (±SD) or median (interquartile range), and categorical variables (men, current smoking, diabetes, and hypertension) were expressed as counts and percentages.
Abbreviations: NT-proBNP, N-terminal pro-brain natriuretic peptide; HNT, hypertension; DM, diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; PBG, postprandial blood glucose; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; Cr, creatinine; UA, uric acid; HCY, homocysteine; Hs-CRP, high-sensitive C-reactive protein; eGFR, estimated glomerular filtration rate; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; SD, standard deviation.
Association of NT-proBNP with clinical variables at baseline
At baseline, older age, female sex, hypertension, SBP, HCY, and Hs-CRP were positively associated with NT-proBNP (logarithmically transformed). Current smoking, DBP, heart rate, TG, and eGFR were inversely associated with NT-proBNP by univariate analyses. In multivariable linear regression analysis, only female sex, older age, and SBP were positively associated with NT-proBNP, while eGFR and DBP were inversely associated with NT-proBNP (Table 2).
Table 2.
Correlation analyses on the association of clinical variables with NT-proBNP levels at baseline
| Variates | Univariate
|
Multivariates
|
||
|---|---|---|---|---|
| r-value | P-value | Standard β-value | P-value | |
| Age (years) | 0.400 | <0.001 | 0.334 | <0.001 |
| Sex (female) | 0.175 | <0.001 | 0.203 | <0.001 |
| Current smoking | −0.114 | <0.001 | – | – |
| HNT | 0.145 | <0.001 | – | – |
| DM | 0.011 | 0.682 | – | – |
| BMI (kg/m2) | −0.043 | 0.101 | – | – |
| SBP (mmHg) | 0.092 | <0.001 | 0.107 | 0.001 |
| DBP (mmHg) | −0.176 | <0.001 | −0.082 | 0.017 |
| Heart rate (bpm) | −0.078 | 0.003 | – | – |
| TG (mmol/L) | −0.135 | <0.001 | – | – |
| LDL-C (mmol/L) | −0.036 | 0.166 | – | – |
| FBG (mmol/L) | −0.022 | 0.403 | – | – |
| PBG (mmol/L) | 0.032 | 0.242 | – | – |
| UA (μmol/L) | −0.013 | 0.629 | – | – |
| HCY (μmol/L) | 0.107 | <0.001 | – | – |
| Hs-CRP (mg/L) | 0.064 | 0.014 | – | – |
| eGFR (mL/min/1.73 m2) | −0.206 | <0.001 | −0.124 | <0.001 |
Notes: – indicates that there was no significant correlation between this factor and NT-proBNP in multivariate analysis, so the standard beta value and P-value were not indicated.
Abbreviations: NT-proBNP, N-terminal pro-brain natriuretic peptide; r, correlation coefficient; β, regression coefficient; HNT, hypertension; DM, diabetes mellitus; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; FBG, fasting blood glucose; PBG, postprandial blood glucose; UA, uric acid; HCY, homocysteine; Hs-CRP, high-sensitive C-reactive protein; eGFR, estimated glomerular filtration rate.
Association of baseline NT-proBNP levels with all-cause mortality
During a median follow-up of 4.8 years, the all-cause mortality rate significantly rose from 0.8% in the lowest concentration NT-proBNP group (<19.8 pg/mL) to 7.8% of the highest concentration NT-proBNP group (≥81.9 pg/mL) by an unadjusted model (Figure 1).
Figure 1.
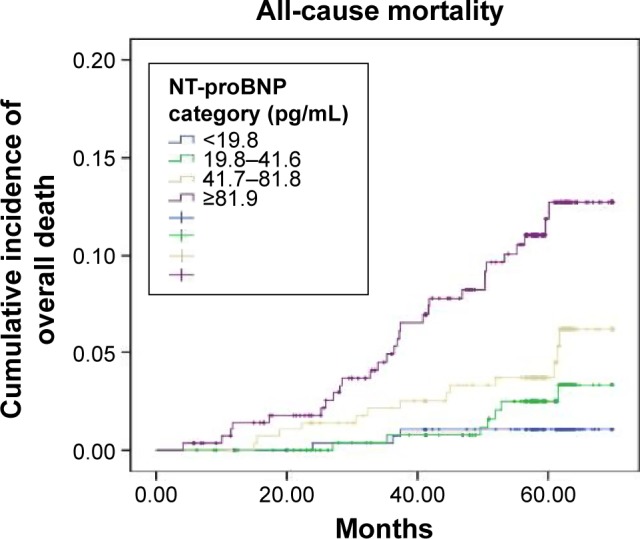
The Kaplan–Meier curves demonstrating incidence of all-cause mortality in community residents with different NT-proBNP levels (quartile 1: <19.8 pg/mL, quartile 2: 19.8–41.6 pg/mL, quartile 3: 41.7–81.8 pg/mL, quartile 4: ≥81.9 pg/mL).
Note: The all-cause mortality risk in the quartile 4 (7.8%) was significantly higher than that in the quartile 1 (0.8%) (HR, 3.59; P<0.001; 95% CI, 1.22–8.81).
Abbreviations: NT-proBNP, N-terminal pro-brain natriuretic peptide; HR, hazard ratio; CI, confidence interval.
A higher NT-proBNP level was associated with a higher risk of all-cause death with Cox proportional hazard model after adjusting for age, sex, blood pressure, plasma lipids, renal function, and other traditional risk factors. Even though the risk ratio was reduced after adjustment for Hs-CRP and HCY (model 4), participants in the highest NT-proBNP (≥81.9 pg/mL) group still had a significantly higher risk of death (hazard ratio [HR], 3.59; 95% confidence interval [CI], 1.22–8.81) compared with ones in the three remaining groups with NT-proBNP <81.9 pg/mL (Table 3).
Table 3.
Association of baseline NT-proBNP level with death
| Model Cox | HR (95% CI)
|
|||
|---|---|---|---|---|
| <19.8 pg/mL (n=389) |
19.8–41.6 pg/mL (n=370) |
41.7–81.8 pg/mL (n=370) |
≥81.9 pg/mL (n=370) |
|
| All-cause mortality | 3 (0.8%) | 7 (1.9%) | 13 (3.5%) | 29 (7.8%) |
| HR unadjusted | 1 (control) | 2.47 (0.63–6.55) | 4.53 (1.29–10.90) | 11.34 (3.46–27.26) |
| Model 1 | 1 (control) | 1.97 (0.51–4.66) | 3.10 (0.87–8.01) | 5.81 (1.71–12.77) |
| Model 2 | 1 (control) | 2.21 (0.57–6.63) | 3.35 (0.92–10.18) | 5.77 (1.63–15.44) |
| Model 3 | 1 (control) | 2.20 (0.56–5.61) | 3.34 (0.92–11.16) | 5.79 (1.63–17.54) |
| Model 4 | 1 (control) | 1.29 (0.35–4.29) | 2.19 (0.70–6.45) | 3.59 (1.22–8.81) |
Notes: Model 1: adjusted for age and sex; model 2: adjusted for age, sex, current smoking, BMI, SBP, DBP, FBG, TC, HDL-C, and LDL-C; model 3: adjusted for age, sex, current smoking, BMI, SBP, DBP, FBG, TC, HDL-C, LDL-C, and eGFR; and model 4: adjusted for age, sex, current smoking, BMI, SBP, DBP, FBG, TC, HDL-C, LDL-C, eGFR, Hs-CRP, and HCY.
Abbreviations: NT-proBNP, N-terminal pro-brain natriuretic peptide; HR, hazard ratio; CI, confidence interval; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; Hs-CRP, high-sensitive C-reactive protein; HCY, homocysteine.
Association of baseline NT-proBNP level with MACEs
There were 154 participants with MACEs during the follow-up period. The incidence of MACEs significantly increased from 3.1% in the lowest NT-proBNP group to 18.9% in the highest group as shown in Figure 2. Furthermore, the risk of MACEs increased with the NT-proBNP level (Table 4) when adjusted for multiple factors. Compared with group 1, the HRs for MACEs in groups 2, 3, and 4 were 2.15 (95% CI, 1.09–4.24), 2.40 (95% CI, 1.24–4.66), and 3.16 (95% CI, 1.64–6.09), respectively, in model 4 adjustment.
Figure 2.
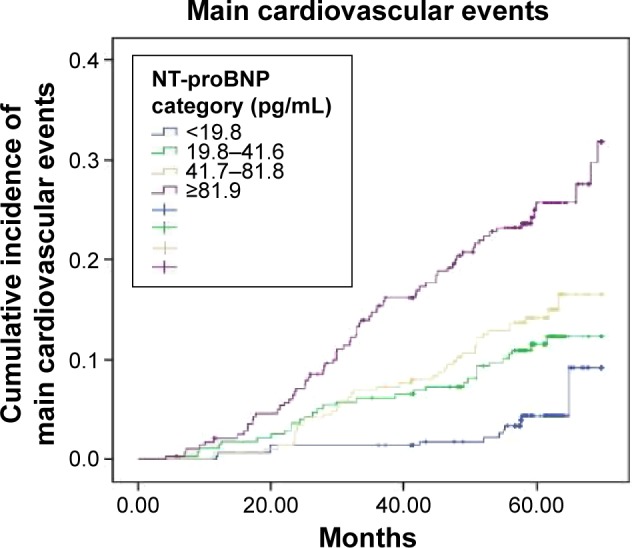
Kaplan–Meier cumulative incidence of MACEs in the four groups of individuals was divided according to the level of the NT-proBNP.
Note: The incidence of MACEs in the quartile 4 (18.9%) was significantly higher than in the quartile 1 (3.1%) (HR, 6.52; P<0.001; 95% CI, 3.53–12.03).
Abbreviations: MACEs, major adverse cardiovascular events; NT-proBNP, N-terminal pro-brain natriuretic peptide; HR, hazard ratio; CI, confidence interval.
Table 4.
Association of baseline NT-proBNP levels with MACEs
| Model Cox | HR (95% CI)
|
|||
|---|---|---|---|---|
| <19.8 pg/mL (n=389) |
19.8–41.6 pg/mL (n=370) |
41.7–81.8 pg/mL (n=370) |
≥81.9 pg/mL (n=370) |
|
| MACEs | 12 (3.1%) | 31 (8.4%) | 41 (11.1%) | 70 (18.9%) |
| HR unadjusted | 1 (control) | 2.72 (1.40–5.30) | 3.44 (1.81–6.55) | 6.52 (3.53–12.03) |
| Model 1 | 1 (control) | 2.37 (1.22–4.63) | 2.68 (1.40–5.15) | 4.23 (2.24–7.99) |
| Model 2 | 1 (control) | 2.44 (1.24–4.78) | 2.92 (1.51–5.66) | 4.46 (2.32–8.59) |
| Model 3 | 1 (control) | 2.34 (1.19–4.61) | 2.90 (1.50–5.63) | 4.46 (2.32–8.59) |
| Model 4 | 1 (control) | 2.15 (1.09–4.24) | 2.40 (1.24–4.66) | 3.16 (1.64–6.09) |
Notes: Model 1: adjusted for age and sex; model 2: adjusted for age, sex, current smoking, BMI, SBP, DBP, FBG, TC, HDL-C, and LDL-C; model 3: adjusted for age, sex, current smoking, BMI, SBP, DBP, FBG, TC, HDL-C, LDL-C, and eGFR; model 4: adjusted for age, sex, current smoking, BMI, SBP, DBP, FBG, TC, HDL-C, LDL-C, eGFR, Hs-CRP, and HCY.
Abbreviations: NT-proBNP, N-terminal pro-brain natriuretic peptide; HR, hazard ratio; CI, confidence interval; MACEs, major adverse cardiovascular events; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; Hs-CRP, high-sensitive C-reactive protein; HCY, homocysteine.
ROC analysis of the NT-proBNP value on the all-cause death and MACEs
The ROC analysis indicated that NT-proBNP had reasonable accuracy for the prediction of all-cause death and MACEs. The area under the ROC curve was 0.74 (95% CI, 0.66–0.83) for all-cause death (Figure 3). The cut-off of NT-proBNP levels for predicting all-cause death was 81.7 pg/mL and had a max Yonden index of 0.447. The area under the ROC curve was 0.64 (95% CI, 0.57–0.70) for MACEs (Figure 4). The cut-off of NT-proBNP levels for predicting MACEs was 81.7 pg/mL and had a max Yonden index of 0.272.
Figure 3.
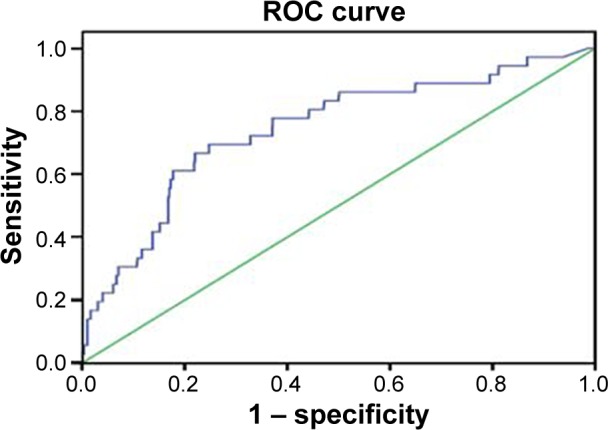
An ROC curve of NT-proBNP to predict the all cause death.
Note: The AUC was 0.74 (95% CI, 0.66–0.83).
Abbreviations: ROC, receiver operating characteristic; NT-proBNP, N-terminal pro-brain natriuretic peptide; AUC, area under curves; CI, confidence interval.
Figure 4.
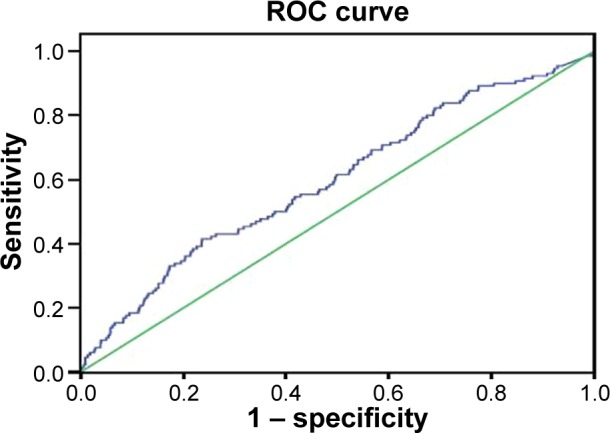
An ROC curve of NT-proBNP to predict MACEs.
Note: The AUC was 0.64 (95% CI, 0.57–0.70).
Abbreviations: ROC, receiver operating characteristic; NT-proBNP, N-terminal pro-brain natriuretic peptide; MACEs, major adverse cardiovascular event; AUC, area under curves; CI, confidence interval.
The predictive value of NT-proBNP in different age groups on death and MACEs
The cumulative mortality rate was 1.87% in individuals <65 years old, 5.32% in those >65 years old, and these differences were statistically significant. NT-proBNP was independently associated with all-cause death after adjustment by multivariates (model 4 in Table 5) in individuals older than 65 years, and the HR was 1.68 (95% CI, 1.22–2.31; P=0.001), but not in those <65 years (HR, 1.17; 95% CI, 0.83–1.42; P>0.05).
Table 5.
The predictive value of NT-proBNP in different age groups
| Endpoint events | <65 years (n=804) |
≥65 years (n=695) |
P-value |
|---|---|---|---|
| MACEs | 46 (5.72%) | 108 (15.54%) | <0.001 |
| HR (95% CI)* | 1.45 (1.09–1.94); P=0.011 | 1.40 (1.17–1.68); P<0.001 | |
| Death | 15 (1.87%) | 39 (5.32%) | <0.001 |
| HR (95% CI)* | 1.17 (0.83–1.42); P>0.05 | 1.68 (1.22–2.31); P=0.001 |
Note:
Multivariable adjusted model 4.
Abbreviations: NT-proBNP, N-terminal pro-brain natriuretic peptide; MACEs, major adverse cardiovascular events; HR, hazard ratio; CI, confidence interval.
The cumulative incidence of MACEs was 5.72% in individuals <65 years old and 15.54% in ≥65 years old. NT-proBNP was independently associated with MACEs in both individuals <65 years old (HR, 1.45; 95% CI, 1.09–1.94; P=0.011) and those ≥65 years old (HR, 1.40; 95% CI, 1.17–1.68; P<0.001) after a multivariate (model 4 in Table 5).
Discussion
The main findings of this study include the following: first, even though NT-proBNP levels are in the normal range, the risk of all-cause death and MACEs gradually increases with NT-proBNP level increment by adjusted models using the Chinese community-based population; second, the baseline NT-proBNP level is an independent predictive factor for all-cause death in the population with age ≥65 years but not age <65 years; thirdly, the risk of death and MACEs was particularly increased in individuals with NT-proBNP levels ≥81.9 pg/mL in our study. This may be suggested as the cut-off NT-proBNP level for prediction of all-cause death and MACEs in apparently healthy people.
In recent years, NT-proBNP has been used as an important biomarker for diagnosis, prognosis evaluation, and therapeutic monitoring of the effect in patients with cardiac insufficiency.1,15,16 There also have been some researchers who have evaluated the prognostic value of NT-proBNP in the general population.3,6–8 In the present study, conducted in the Chinese community, the median NT-proBNP level (41.6 pg/mL) is lower than in previous studies;3,6,7 however, it has independent predictive value for all-cause death and MACEs. This suggests that even if the NT-proBNP level is in lower normal range, there is still a risk of cardiovascular events and mortality. The exact mechanism of this is not clear, and subclinical myocardial ischemia, myocardial hypertrophy, or fibrosis might be the main reason. Meanwhile, other factors including endothelin, angiotensin II, and tumor necrosis factor-alpha have been found to stimulate secretion of in vitro BNP,17,18 and all of these factors have been associated with adverse events. A lot of studies4–8 have confirmed that plasma NT-proBNP is a good prognostic factor in different populations; however, its prognostic value was impaired because many factors affect plasma NT-proBNP, especially in the elderly population. A study has found that serial NT-proBNP measurements may be an effective method for improving its prognostic value.19
Keeping with previous studies, we found an independent prognostic value for NT-proBNP in terms of death and MACEs in older adults (age ≥65 years).20–22 The exact mechanism is not yet clear; however, NT-proBNP can be seen as a general measure of decreased vitality.23 The prevalence of cardiovascular disease, diabetes, and chronic kidney disease increased, and the function of each organ gradually declined with aging, which are the causes of higher mortality, as well as higher level of NT-proBNP. In our study, the value of eGFR in older adults is significantly lower than that in the younger (mean, 97.4±11.1 vs 116.8±7.8 mL/min/1.73 m2; P<0.01), the prevalence of diabetes in the older is significantly higher than that in the younger (23.8% vs 16.7%; P<0.01), and both low eGFR24 and diabetes25 have been demonstrated to be the independent risks for cardiovascular events and death. In addition, we failed to find that NT-proBNP independently predicts death in younger (<65 years), which is not consistent with previous studies.6,8,26 It may be related to the number of deaths which is only 15 (1.87%) in younger adults but 39 (5.32%) in older adults.
Current guidelines recommend that NT-proBNP levels >125 pg/mL should be considered abnormal, and the cut-off value for evaluating the prognosis is usually higher than in patients with HF.27 The National Institute for Health and Care Excellence clinical guideline on the management of chronic HF recommends an NT-proBNP of 400 pg/mL as the threshold for referring to echocardiography, whereas the European Society of Cardiology suggests a threshold level of 125 pg/mL to exclude HF. Taylor et al28 suggest that the current National Institute for Health and Care Excellence cut-off is too high and that 150 pg/mL is a more reliable threshold for further investigations. In our study, although the median NT-proBNP was only 41.6 pg/mL (IQR, 19.8–81.9 pg/mL), it showed an independent prognostic value for death and MACEs. The ROC curve showed that the risks of all-cause death and MACEs were significantly increased in individuals with NT-proBNP levels ≥81.7 pg/mL, indicating that the 81.7 pg/mL may be a reasonable cut-off point for MACEs or death in this population. Consistent with our study, Linssen et al8 previously found the NT-proBNP level of 87.5 pg/mL as a significant cut-off for predicting cardiovascular outcomes. So far, there is not a prevailing cut-point value for NT-proBNP in predicting adverse events in the general population. Therefore, further studies are required to establish optimal cut-points for the prediction of an adverse prognosis in the general population.
Limitations of this study should also be taken into account. First, the present study was performed with Chinese residents from two communities in Beijing, and thus, the results may not represent Chinese residents from other areas of People’s Republic of China, or not be applicable to other ethnic groups. Second, this study did not include echocardiographic examinations, so it could not evaluate heart structure. Third, although the results were adjusted for multiple covariates that may be associated with circulating NT-proBNP levels, the possibility of the existence of residual confounding factors, such as silent myocardial ischemia, remains undetermined in atrial fibrillation and subclinical infection.
Conclusion
The NT-proBNP level is a strong and independent prognostic factor for all-cause death and MACEs in the community population. Furthermore, participants with NT-proBNP ≥81.7 pg/mL have a significantly higher risk of death and MACEs. This may be suggested as the NT-proBNP cut-point for the prediction of adverse events in the appearing healthy population. NT-proBNP is a strong and independent prognosis factor for all-cause death and MACEs in individuals older than 65 years and MACEs in individuals younger than 65 years.
Acknowledgments
The authors thank colleagues in the Department of Laboratory Medicine, the PLA General Hospital, for help with NT-proBNP measurements. The authors are also grateful to all study participants for their participation in the study. This work was supported by grants from the Key National Basic Research Program of China (2013CB530804) and Nature Science Foundation of China (81270941) to P Ye, and the Beijing Nova Program (Z121107002513124) to Y Bai.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Santaguida PL, Don-Wauchope AC, Oremus M, et al. BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: a systematic review. Heart Fail Rev. 2014;19(4):453–470. doi: 10.1007/s10741-014-9442-y. [DOI] [PubMed] [Google Scholar]
- 2.Don-Wauchope AC, McKelvie RS. Evidence based application of BNP/NT-proBNP testing in heart failure. Clin Biochem. 2015;48(4–5):236–246. doi: 10.1016/j.clinbiochem.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Kara K, Lehmann N, Neumann T, et al. NT-proBNP is superior to BNP for predicting first cardiovascular events in the general population: the Heinz Nixdorf Recall Study. Int J Cardiol. 2015;183:155–161. doi: 10.1016/j.ijcard.2015.01.082. [DOI] [PubMed] [Google Scholar]
- 4.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 5.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352(7):666–675. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- 6.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 7.Laukkanen JA, Kurl S, la-Kopsala M, et al. Plasma N-terminal fragments of natriuretic propeptides predict the risk of cardiovascular events and mortality in middle-aged men. Eur Heart J. 2006;27(10):1230–1237. doi: 10.1093/eurheartj/ehi878. [DOI] [PubMed] [Google Scholar]
- 8.Linssen GC, Bakker SJ, Voors AA, et al. N-terminal pro-B-type natriuretic peptide is an independent predictor of cardiovascular morbidity and mortality in the general population. Eur Heart J. 2010;31(1):120–127. doi: 10.1093/eurheartj/ehp420. [DOI] [PubMed] [Google Scholar]
- 9.Shankar A, Leng C, Chia KS, et al. Association between body mass index and chronic kidney disease in men and women: population based study of Malay adults in Singapore. Nephrol Dial Transplant. 2008;23(6):1910–1918. doi: 10.1093/ndt/gfm878. [DOI] [PubMed] [Google Scholar]
- 10.Hu J, Wallace DC, Jones E, Liu H. Cardiometabolic health of Chinese older adults with diabetes living in Beijing, China. Public Health Nurs. 2009;26(6):500–511. doi: 10.1111/j.1525-1446.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- 11.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KIDGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 14.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355(25):2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 15.Sarimehmetoglu AC, Gultekin N, Kucukates E, Yildiz A, Kocas C, Ersanli M. The importance of apoptotic activity and plasma NT-proBNP levels in patients with acute exacerbation of decompensated heart failure and their relation to different drugs and comorbidities. J Pak Med Assoc. 2014;64(8):884–891. [PubMed] [Google Scholar]
- 16.Oremus M, Don-Wauchope A, McKelvie R, et al. BNP and NT-proBNP as prognostic markers in persons with chronic stable heart failure. Heart Fail Rev. 2014;19(4):471–505. doi: 10.1007/s10741-014-9439-6. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins WE, Chen Z, Fukagawa NK, Hall C, Knot HJ, LeWinter MM. Increased atrial and brain natriuretic peptides in adults with cyanotic congenital heart disease: enhanced understanding of the relationship between hypoxia and natriuretic peptide secretion. Circulation. 2004;109(23):2872–2877. doi: 10.1161/01.CIR.0000129305.25115.80. [DOI] [PubMed] [Google Scholar]
- 18.Harada M, Saito Y, Kuwahara K, et al. Interaction of myocytes and nonmyocytes is necessary for mechanical stretch to induce ANP/BNP production in cardiocyte culture. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S357–S359. doi: 10.1097/00005344-199800001-00100. [DOI] [PubMed] [Google Scholar]
- 19.Sargento L, Longo S, Lousada N, Palma Dos Reis R. Serial measurements of the Nt-ProBNP during the dry state in patients with systolic heart failure are predictors of the long-term prognosis. Biomarkers. 2014;19(4):302–313. doi: 10.3109/1354750X.2014.910549. [DOI] [PubMed] [Google Scholar]
- 20.Beleigoli AM, Ribeiro AL, Diniz Mde F, Lima-Costa MF, Boersma E. Comparing the value of BNP in predicting mortality among community-dwelling elderly with and without overweight/obesity: the Bambuí (Brazil) Cohort Study of Aging. Int J Cardiol. 2013;168(4):4364–4366. doi: 10.1016/j.ijcard.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 21.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293(13):1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 22.Daniels LB, Laughlin GA, Clopton P, Maisel AS, Barrett-Connor E. Minimally elevated cardiac troponinT and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008;52(6):450–459. doi: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Peet PG, de Craen AJ, Gussekloo J, de Ruijter W. Plasma NT-proBNP as predictor of change in functional status, cardiovascular morbidity and mortality in the oldest old: the Leiden 85-plus study. Age. 2014;36(3):1541–1554. doi: 10.1007/s11357-014-9660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20(8):1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu M, Li J, Li Z, et al. Mortality rates and the causes of death related to diabetes mellitus in Shanghai Songjiang District: an 11-year retrospective analysis of death certificates. BMC Endocr Disord. 2015;15(1):45. doi: 10.1186/s12902-015-0042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Angelantonio E, Chowdhury R, Sarwar N, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120(22):2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 27.Fuat A, Murphy JJ, Hungin AP, et al. The diagnostic accuracy and utility of a B-type natriuretic peptide test in a community population of patients with suspected heart failure. Br J Gen Pract. 2006;56(526):327–333. [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor CJ, Roalfe AK, Iles R, Hobbs FD. The potential role of NT-proBNP in screening for and predicting prognosis in heart failure: a survival analysis. BMJ Open. 2014;4(4):e004675. doi: 10.1136/bmjopen-2013-004675. [DOI] [PMC free article] [PubMed] [Google Scholar]


