Abstract
The long-term weight management of obesity remains a very difficult task, associated with a high risk of failure and weight regain. However, many people report that they have successfully managed weight loss maintenance in the long term. Several factors have been associated with better weight loss maintenance in long-term observational and randomized studies. A few pertain to the behavioral area (eg, high levels of physical activity, eating a low-calorie, low-fat diet; frequent self-monitoring of weight), a few to the cognitive component (eg, reduced disinhibition, satisfaction with results achieved, confidence in being able to lose weight without professional help), and a few to personality traits (eg, low novelty seeking) and patient–therapist interaction. Trials based on the most recent protocols of lifestyle modification, with a prolonged extended treatment after the weight loss phase, have also shown promising long-term weight loss results. These data should stimulate the adoption of a lifestyle modification-based approach for the management of obesity, featuring a nonphysician lifestyle counselor (also called “lifestyle trainer” or “healthy lifestyle practitioner”) as a pivotal component of the multidisciplinary team. The obesity physicians maintain a primary role in engaging patients, in team coordination and supervision, in managing the complications associated with obesity and, in selected cases, in the decision for drug treatment or bariatric surgery, as possible more intensive, add-on interventions to lifestyle treatment.
Keywords: obesity, lifestyle modification, cognitive behavior therapy, multidisciplinary treatment
Introduction
The main challenge of obesity treatment is not weight loss, but long-term weight loss maintenance. This widely accepted view is supported by several studies indicating that a healthy weight loss of 5%–10% can be achieved through both behavioral1 and pharmacological treatments,2 but weight is gradually regained in a large percentage of individuals.
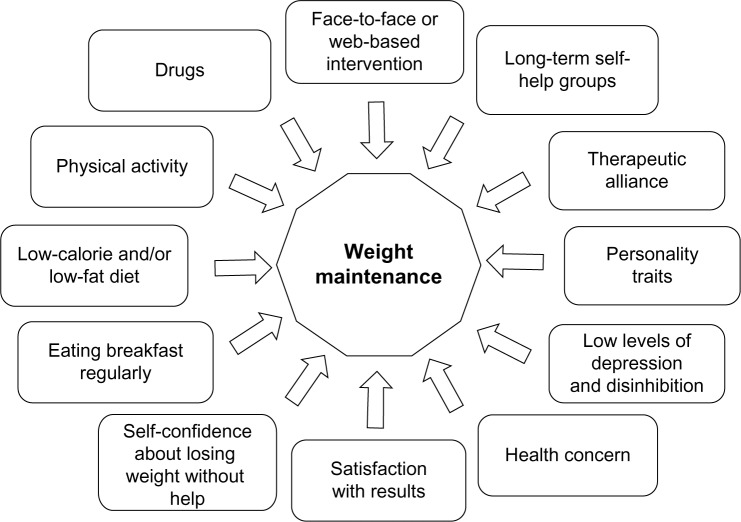
Weight loss maintenance is hindered by a complex interaction of environmental, biological, behavioral, and cognitive factors, which are only partly known.3 Their constellation is presented in Figure 1; they variably interact in individual patients to an extent that is difficult to forecast. This explains why a high number of individuals, after a successful weight loss period, regain most of the weight lost. However, a proportion of individuals successfully maintain a long-term weight loss,4 and the study of this cohort, who achieve the goal despite the strong pressures to regain weight, may help identify the factors associated with this desired outcome.
Figure 1.
Constellation of factors associated with long-term weight loss maintenance in the general population of obese subjects attending nonsurgical weight loss programs.
The most recent developments of comprehensive lifestyle modification programs combine dietary and physical activity recommendations with specific cognitive and behavior strategies to improve patients’ adherence to a long-term weight management. They confirm that a large subgroup of treated patients is able to maintain a healthy weight loss in the long term.5 These promising data have stimulated the development of multidisciplinary lifestyle modification teams aimed at providing patients with a comprehensive long-term management of obesity.
The aims of this narrative review are: 1) to provide a definition of weight maintenance; 2) to review the data on long-term weight loss maintenance; 3) to describe the characteristics of individuals who successfully achieve long-term weight loss; 4) to review the evidence-based strategies to promote weight loss maintenance; 5) to describe a multidisciplinary approach, based on lifestyle modification aimed at providing patients with a comprehensive long-term management of obesity and its complications.
Definition of weight loss maintenance
In the past 20 years, several definitions have been proposed to define “successful weight loss maintenance”. Wing and Hill6 suggested that successful weight loss maintainers should be defined as “individuals who have intentionally lost at least 10% of their body weight and kept it off at least 1 year”. Rossner7 proposed that a sustained weight loss of about 5%–10% of baseline body weight represents a definite degree of success. This goal has also been recommended by the 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults.8
The aforementioned definitions share the notion that successful weight maintenance does not necessarily imply a large weight loss, but that a modest 5%–10% amount is sufficient. From a clinical point of view, this amount of weight loss significantly reduces the risk of developing type 2 diabetes in susceptible people,9 and eliminates most of the other risks associated with obesity.8 Moreover, this modest weight loss also improves psychological functioning, in particular, mood, body image, and binge eating.10,11
The Wing and Hill definition6 introduces two additional indicators of weight maintenance. First, weight loss should be intentional. This criterion is important because several studies reported that unintentional weight loss is common and may have causes and consequences totally different from intentional weight loss.12 Second, weight loss should be maintained at least 1 year. This criterion was set as a reasonable target for research on the factors that enable individuals to maintain weight loss. However, it is obvious that the term “successful” would require a much longer period of weight maintenance, hopefully life-long.
Data on long-term weight loss maintenance
Only a few studies have tracked successful weight losers over long-term follow-up or assessed the effect of lifestyle modification programs on long-term weight maintenance.
The National Weight Control Registry (NWCR) was established in 1994 as a prospective investigation of long-term successful weight loss maintenance. In 2014, Thomas et al4 reported a 10-year observation of self-reported weight loss and behavior change in 2,886 participants, recruited primarily through newspaper and magazine articles, who had lost at least 30 pounds (13.6 kg) and kept it off for at least 1 year. The mean weight loss of participants was 31.3 kg at baseline, 23.8 kg at 5 years and 23.1 kg at 10 years. Of note, 87% of participants were still maintaining at least a 10% weight loss after 5 and 10 years. These impressive data show that long-term weight loss maintenance is possible in self-selected weight losers.
The 1999–2006 National Health and Nutrition Examination Survey examined the prevalence and the correlates of long-term weight loss maintenance, defined as weight loss maintained for at least 1 year, in 14,306 US adults.13 The study found that more than one out of every six US adults who have ever been overweight or obese has accomplished long-term weight loss maintenance of at least 10%. Although the period of weight maintenance was much shorter than in the case of the NWCR, the study confirms that also in nonselected individuals in the community, long-term weight loss is possible.
A recent systematic review on the outcome of weight loss lifestyle modification programs found that at 1 year, about 30% of participants had a weight loss ≥10%, 25% between 5% and 9.9%, and 40% ≤4.9%.1 Weight loss reaches its peak within 6 months of the start of treatment, and in the absence of a weight maintenance program, the trend begins to reverse thereafter, with 50% of patients returning to their original weight after about 5 years.14 These data indicate that traditional lifestyle modification programs require a greater focus on long-term maintenance to be considered successful in real terms.15
Hopefully, trials based on the latest generation of weight loss lifestyle modification programs, including the most innovative and powerful cognitive behavioral procedures, should produce even better long-term results. The most striking example is the Look AHEAD (Action for Health in Diabetes) study, which assessed the effects of intentional weight loss on cardiovascular morbidity and mortality in 5,145 overweight/obese adults with type 2 diabetes, randomly assigned to intensive lifestyle intervention (ILI) or usual care (ie, diabetes support and education [DSE]).16 At year 1, more ILI than DSE participants had lost ≥5% of their initial weight (68.0% vs 13.3%), with the ILI group showing an average weight loss of 8.5%, significantly greater than the 0.6% seen in DSE participants. At year 8, 88% of both groups completed an outcomes assessment, which revealed that ILI and DSE participants lost, on average, 4.7% and 2.1% of their initial weight, respectively. Among the ILI and DSE participants, 50.3% and 35.7%, respectively, lost ≥5%, and 26.9% and 17.2% lost ≥10%.17 These impressive figures show that well-conducted lifestyle modification programs can produce clinically meaningful long-term weight loss, but also that the Look AHEAD was not able to completely solve the problem of weight regain in a large percentage of its participants.
The 10-year follow-up of the data of the randomized clinical trial Diabetes Prevention Program (DPP) showed that the cumulative incidence of diabetes, among adults at high risk, remained lower in the lifestyle group, compared with the metformin and placebo arms. This outcome occurred even if the original lifestyle group partly regained weight.18 The results of DPP underline that the effect of lifestyle modification programs may produce significant health benefits even if the weight lost is partly regained.
A few data are also available on the long-term weight loss maintenance with pharmacotherapy. The XENDOS (XENical in the prevention of Diabetes in Obese Subjects) trial, for example, randomized 3,305 patients to lifestyle changes plus either orlistat 120 mg or placebo, three times daily in a double-blind, prospective study. After 4 years, the mean weight loss was significantly greater with orlistat than placebo (5.8 vs 3.0 kg), and the cumulative incidence of diabetes was only 6.2% with orlistat and 9.0% with placebo, corresponding to a risk reduction of 37.3%.19 Therefore, adding weight loss drugs to the lifestyle modification programs not only improves long-term weight loss, but also reduces the incidence of diabetes.
Clinical characteristics of individuals who successfully achieve long-term weight loss
The study of the characteristics of individuals who successfully achieve long-term weight loss is a potential useful strategy to elucidate the factors implicated in the long-term maintenance of intentional weight loss. The pivotal studies and the most recent published reports are presented in Table 1.4,13,15,16,19–29
Table 1.
Selection of the more relevant/recent trials analyzing factors associated with long-term weight loss maintenance
| Reference | Number of cases, and characteristics | Study design – follow-up | Results | Factors associated with WL maintenance |
|---|---|---|---|---|
| Ryan et al16; 2003 – Look AHEAD (Action for Health in Diabetes) study | 5,145 following a WL program delivered over 4 years | RCT of intensive lifestyle intervention (ILI) vs diabetes support and education (DSE) – 8 years | At 8 years, ILI participants maintained 4.7% WL, vs 2.1% of DSE group | Having attended an ILI group, exercising, reducing calorie and fat intake and using meal replacements are correlated with greater WL maintenance |
| Torgerson et al19; 2004 – XENDOS study | 3,305 obese participants included in a continuing education program | RCT of lifestyle + orlistat 120 mg tid or lifestyle + placebo – 4 years | Dietary counseling every 2 weeks for 6 months and monthly thereafter. Mean weight loss significantly greater with orlistat (5.8 vs 3.0 kg with placebo) | Adding orlistat to lifestyle changes increased WL and reduced weight regain |
| Dalle Grave et al15,20; 2005 and 2014 – QUOVADIS study | 1,944 obese participants enrolled by 24 obesity centers | Observational: 1,000 subjects contacted by phone – 3 years | 5% WL (63.7% of cases) associated with higher dietary restraint and lower disinhibition. The number of cases who achieved 5% WL higher in continuers (40.8%) vs dropouts (22.7%) | The dropouts satisfied with the results (7.0% of participants), and those confident to lose additional weight without professional help (11.7%) reported a larger WL than continuers |
| Wing et al21; 2006 | 314 individuals who had lost a mean of 19.3 kg in 2 years | RCT: newsletter group vs face-to-face intervention vs web-based group – 1.5 years | Weight regain significantly lower in the face-to-face group (2.5 kg) vs 4.7 kg in the Internet group and 4.9 kg in controls | Daily self-weighing associated with a decreased risk of weight regain |
| Svetkey et al22; 2008 | 1,032 individuals who had lost >4 kg during a WL program | RCT: self-directed control vs monthly personal contact vs access to an interactive technology- based intervention – 2.5 years | Personal-contact group regained less weight (4.0 kg) than self-directed group (5.5 kg). Weight regain lower in the technology-based vs the self-directed group at 18–24 months, but no longer different at 30 months (5.2 vs 5.5 kg) |
Monthly brief personal contact associated with lower weight regain. Interactive technology- based intervention provides early but transient benefit |
| Perri et al23; 2008 – TOURS study | 234 individuals who lost a mean of 10 kg in the initial 6-month WL program | RCT of extended care (phone or face-to-face) vs control group – 1 year | Participants in the telephone and face-to-face groups regained less weight (1.3 and 1.2 kg, respectively) than those in the control group (3.7 kg) | Frequent telephone or face-to- face contact with a counselor associated with less weight regain |
| Kraschnewski et al13; 2010 – National Health and Nutrition Examination Survey | 14,306 randomly selected participants in the National survey | Retrospective observational – 1 year | 36.6, 17.3, 8.5, and 4.4% of participants reported long-term WL maintenance of at least 5, 10, 15, and 20%, respectively | Women, older adults (age, 75–84 years), non-Hispanic whites, and those with low education more frequently maintained WL; married individuals less frequently |
| Thomas et al4; 2014 – National Weight Control Registry | 2,886 subjects in the registry | Observational – 10 years | 88.4% and 86.6% of people maintained a WL of at least 10% at 5 and 10 years, respectively | Higher initial WL and duration of WL associated with maintenance. Decrease in leisure-time caloric expenditure, increase in % intake of calories from fat, disinhibition, restraint and self-weighing all associated with weight regain |
| Spark et al28; 2015 | 40 participants in an RCT of a phone WL program vs usual care. Extended care via messages | Patients received text message for the first 6 months, followed by a no-contact 12-month period – 1 year | There was a small but nonsignificant increase in weight during the extended contact intervention (1.3 kg), with weight remaining relatively stable vs the no-contact follow-up period (−0.1 kg) | Text message-delivered extended contact attenuates weight regain and promotes the maintenance of physical activity |
| Fuller et al24; 2014 | 171 of 739 individuals completing a 1-year WL program | RCT: commercial program (Weight Watchers) vs standard care – 1 year | During follow-up, both groups gained weight, but weight gain was significantly greater for the commercial vs the standard group (difference +2.0 kg) | Individuals achieving ≥10% WL at 24 months practiced more physical activity, but the difference was not statistically significant |
| Santos et al26; 2015 – Promotion of Exercise and Health in Obesity (PESO) | 154 women following a 1-year WL program | RCT: 2 years of motivational intervention vs general education – 2 years | Average WL higher in the intervention group (−3 kg) than in the general education control group (−1 kg) | Body image, intrinsic motivation, exercise autonomous motivation significantly associated with WL maintenance |
| Anastasiou et al25; 2015 – MedWeight study | Analysis of LOC (internals vs externals) in 239 WL maintainers (≥10%) vs regainers | Observational, cross-sectional – 2 years | WL more commonly associated with expert aid or drugs in externals. Maximum WL significantly higher for internals. Among maintainers, internals maintained higher % WL through physical activity vs externals |
WL maintenance is associated with an internal LOC |
| Simpson et al27; 2015 | RCT: 170 individuals who lost ≥5% in the previous 12 months (feasibility trial) | RCT: high-intensity motivational intervention vs less intensive intervention vs usual care – 1 year | The mean weights in the highly intensive and the less intensive arms remained 2.8 kg and 0.7 kg lower than in controls. The participants in the intensive arm had a 43% higher chance of maintaining WL than controls | Intensive motivational interviewing may facilitate long- term weight maintenance |
| Young et al29; 2015 – SHED-IT Weight Loss Maintenance Trial | 92 men who had experienced a 5% WL in 6 months | RCT: text messages or emails vs self-help control – 1 year | No differences in WL maintenance between the intervention and control groups at 6 or 12 months (1.5 kg mean between-group difference in weight regain at 12 months) | At 12 months, both the intervention and control groups had maintained medium-to-large increases in physical activity; dietary changes were less efficiently maintained |
Abbreviations: LOC, locus of control; RCT, randomized controlled trial; WL, weight loss.
The NWCR, described in the “Data on long-term weight loss maintenance” section, is the most important and longest study assessing the characteristics of individuals who successfully lost and maintained their weight loss, as well as the strategies they use to maintain their weight loss.30 In a large NWCR analysis, the members reported an average weight loss of 33 kg, which was maintained for more than 5 years. The main strategies adopted by members to keep a stable weight in the long term were:31 1) high levels of physical activity (about 1 hour per day); 2) eating a low-calorie, low-fat diet; 3) eating breakfast regularly; 4) self-monitoring weight; and 5) maintaining a consistent eating pattern across weekdays and weekends. An encouraging result is that the chance of longer-term success increases in members who had managed to keep their weight off for 2 years or more. Finally, low levels of depression and disinhibition, and medical triggers (ie, general practitioners or specialists promoting weight loss for medical reasons and/or having a family member with a heart attack) were also associated with weight maintenance.
A random digit-dial telephone survey that used a representative sample of the US adult population compared the behavioral strategies adopted by successful individuals who maintained weight loss (ie, an average weight loss of 37 pounds maintained for over 7 years) with those of individuals who regained weight and weight-stable controls. This study confirmed that maintainers reported higher levels of strenuous physical activity, greater frequency of self-weighing, and continued use of more behavioral strategies to control their dietary fat intake.32
These reports, unfortunately, failed to answer the key question, namely, why some individuals persist in practicing weight-control behaviors, and therefore maintain long-term weight loss, while others abandon it. The fact that several individuals are able to maintain weight loss in the long term clearly demonstrates that the biological pressure on individuals to overeat in order to restore their original weight (the set-point theory)33 is not the only mechanism involved in weight regain. “Complex behaviors” involved in maintenance of long-term weight loss are in part influenced by conscious cognitive processes. Nevertheless, cognitive factors have largely been neglected in traditional weight-loss lifestyle modification interventions, which are based mainly on the principles of behaviorism. The lack of an intensive cognitive intervention has been suggested as one of the reasons for their limited effectiveness in promoting long-term weight loss.34
The QUOVADIS (QUality of life in Obesity: eVAluation and DIsease Surveillance) study, an observational study on 1,944 treatment-seeking obese patients in 25 medical centers authorized to treat obesity by the Italian National Health Service,35 investigated several cognitive factors involved in the long-term weight loss. The study confirmed that some cognitive factors are associated with the amount of weight lost (ie, increased dietary restraint and reduced disinhibition), while others are associated with long-term weight loss maintenance (ie, satisfaction with results achieved, confidence in being able to lose weight without professional help).15 According to these data, there is an urgent need to evaluate the effectiveness of specific procedures and strategies designed to address the cognitive variables associated with negative treatment outcomes. These strategies might help improve the long-term efficacy of weight-loss lifestyle modification programs.
The individual beliefs in the control over personal life (expressed by the concept of “locus of control”) have long been implicated in weight loss and weight loss maintenance, with conflicting results. Very recently, the large MedWeight study confirmed an association between an “internal” locus of control and weight loss maintenance; individuals with an internal locus of control (ie, subjects perceiving they have control over the environment and feeling able to control stimuli) tend to have longer and more sustained weight loss maintenance compared with subjects with “external” locus of control (ie, subjects perceiving that their life is regulated by something outside their control).25 The internal orientation of locus of control is intimately connected with self-efficacy, another psychological domain of interest for weight maintenance.36 Notably, individuals with internal locus of control are more likely to lose weight and maintain weight loss without any external support.25
Personality traits are habitual patterns of behavior, thought, and emotion that are relatively stable over the years, differ across individuals, and may play a role in long-term weight loss by influencing behavior.37 Personality traits, psychological well-being, body image, and eating behaviors have been measured in the QUOVADIS II, an observational study of patients with obesity seeking treatment at eight Italian medical centers. Among personality traits and after adjusting for general confounders and eating disorder scores, only novelty seeking was significantly associated with the ≥10% weight loss goal at 12-month follow-up.38 Similar data were reported in a weight loss lifestyle modification program, in which low novelty seeking was the only personality trait discriminating between patients who were successful in losing ≥10% and those who were unsuccessful.39 These data support the observation that healthy dietary control and dietary restraint (ie, the cognitive control of food intake) are associated with low novelty seeking scores.40
The poor compliance with long-term weight loss programs may also be attributable to the attachment style of individuals. Support for this hypothesis comes from a study showing that 12-month weight loss was significantly greater in patients with secure attachment than in those with insecure attachment, and that the patient–therapist relationship was rated more positively by those with secure attachment.41 These data require replication in a different setting; they indirectly confirm that securely attached adults are more cooperative in a patient–therapist context,42 and that therapeutic alliance is pivotal in the treatment outcome of obesity.
Teixeira et al43 have recently published a meta-analysis on the determinants of healthy behavioral changes influencing weight loss and weight loss maintenance of subjects entering treatment programs. Thirty-five studies were included in the analysis; 42 possible mediators were selected, and their effects on weight loss/weight loss maintenance were assessed by stringent criteria. Overall, the analysis of medium-/long-term weight control (limited to 21 studies) identified higher levels of autonomous motivation, self-efficacy and barriers, self-regulation skills (including self-monitoring), flexible eating restraint, and positive body image as positive mediators for weight maintenance. A few of them were also determinants of physical activity, whereas no consistent mediators were identified for healthy dietary intake. Overall, this analysis points to large differences in the psychological characteristics of the treatment-seeking obese population, which suggests it is necessary to move from a standardized to a tailored approach.
Strategies to promote weight loss maintenance
Several strategies have been evaluated to promote weight loss maintenance. Among them is the provision of an extended care model. Some data showed that group sessions delivered twice a month for 1 year after the weight loss phase, keeping patients in active treatment, help patients maintain the weight loss.44,45 There is also evidence that some people maintain large long-term weight losses with a long-term self-help group pressure and support.46 It has been speculated that an extended care model of treatment provides patients with the support and motivation needed to continue to practice weight control behaviors.47
Recent studies assessed the efficacy of different interventions designed to improve weight loss maintenance, randomizing patients, after a period of weight loss, to one of two active interventions or to a control group with 12–30 months of follow-up. The STOP Regain trial found that a face-to-face intervention and an Internet-based intervention, both emphasizing daily self-weighing and self-regulation, improved weight loss maintenance over a period of 18 months, in comparison with a control group, which received quarterly newsletters.21 This was mainly the case with the face-to-face program. The Weight Loss Maintenance trial found that, after 30 months, the participants allocated to the personal-contact group regained 1.5 kg less weight than those in the self-directed group, whereas those in the interactive technology-based group regained only 0.3 kg less than those in the self-directed arm.22 The TOURS (Treatment of Obesity in Underserved Rural Settings) trial found that obese women from rural communities allocated to 26 biweekly sessions via telephone or face-to-face regained less weight (mean 1.2 and 1.2 kg, respectively) than those in the education control group (mean 3.7 kg).23 Given the importance of motivation and support, a very recent feasibility trial of motivational interviewing showed that face-to-face and phone interviews improved retention in the study, and increased weight maintenance (−2.8 kg vs the control group).27 All these studies confirm that the extended care approach, with monthly or more frequent contacts, in person or via telephone or Internet, can improve successful weight loss. They also reduce the risk of weight regain during the maintenance phase, with the exception of eHealth interventions, where evidence for effectiveness is limited28 and was not confirmed in a meta-analysis.48
However, extended care is not easily accepted by all patients. In a study outside the research setting, only about 15% of cases were still in continuous care after 3 years.20 Future studies should be designed to identify the patients for whom extended care is more suitable, and those more likely to benefit from a shorter duration of treatment. Data from the QUOVADIS study indicate that older patients whose primary motivation for weight loss is improving health are more compliant in continuous care, while patients satisfied with the results they achieved with treatment, and those confident of self-managing additional weight loss may avoid weight gain without continuous professional assistance.20 Finally, all the studies of extended (rather than continuous or indefinite) treatment did not report sufficient follow-up to exclude the occurrence of weight regain after the end of treatment.
To maintain weight loss, individuals must adhere to behaviors that counteract physiological adaptations favoring weight regain.49 Physical activity has modest impact during the weight loss period, but becomes essential to weight maintenance.26 Unfortunately, the level of daily energy expenditure necessary to prevent weight regain is high compared with the modern-day lifestyle,50 and subjects with metabolic disorders do not perceive physical activity as a relevant component of healthy behavior.51 Although total daily energy expenditure is a strong predictor of weight maintenance in obese individuals,52 adherence to a prescribed exercise intensity and/or dose is quite low, also considering the perceived fatigue in accomplishing the desired intensity goals.53
In order to increase motivation and adherence, individuals experiencing problems during weight loss maintenance may be addressed to pleasant programs of leisure-time physical activity.54 Among leisure-time activities, dancing has a remarkable place;55 dance stimulates positive emotions, social interaction, and relationships in the community, while the acoustic stimulation and the music might strengthen the beneficial effects of aerobic exercise on cognitive functions.56 In a pilot nonrandomized trial, a 6-month dance course was associated with similar weight changes but with lower dropout rates compared with self-selected physical activity programs.57 This underlines the importance of social support and pleasant activities to increasing adherence to lifestyle intervention programs and to maintaining long-term weight loss in motivated patients. Self-body perception, enhanced self-confidence, and social support may thus increase self-motivation, facilitating weight loss maintenance in the long term, and overall quality of life.58
Another approach is to add long-term drug treatment to lifestyle change in patients who have been unsuccessful with diet and exercise alone.59 The potential effectiveness of this approach has been demonstrated by the XENDOS trial.19 However, this strategy also does not completely fulfill the needs of obese individuals requiring treatment, for at least three reasons:60 1) most patients do not want to be treated with drugs; 2) the long-term use may be associated with adverse physical complications; and 3) there are situations (eg, pregnancy) in which the treatment would be inappropriate.
A final, scarcely tested strategy is the provision of portion-controlled meals or meal supplements in the long term. An old study with positive results, reporting an average weight loss of 8% at 4 years,61 received partial confirmation in a study in which continuing education and support (monthly meetings) were continued after an intensive weight loss phase. During this 12-month weight maintenance phase, all subjects were encouraged to consume a minimum of 14 portion-controlled meals they had received, free of charge, during the weight loss phase.62 It is, however, unknown whether and how long this strategy may be maintained.
The role of the multidisciplinary team in long-term weight management
Lifestyle modification programs include a weight loss phase, consisting of 16–24 weekly sessions in 6 months, followed by a weight maintenance phase, which should last at least 1 year with monthly or more frequent contacts in person or by telephone.8 The strategies for weight maintenance differ from those used to achieve weight loss, and should include frequent self-weighing (at least weekly), the consumption of a reduced calorie diet, and high levels of physical activity (>200 minutes/week).8
In research settings, lifestyle modification programs have been usually delivered by trained nonphysician health professionals, such as dietitians, or subjects having master’s degree training in exercise physiology, behavioral psychology, or health education. Participants are educated in individual sessions (as in the DPP),9 in groups of about 10–20 participants44 or in a combination of group and individual sessions (as in the Look AHEAD study).16
The American Medical Association House of Delegates has recently declared obesity a “disease” requiring treatment,63 because of the multiple medical, psychological, and functional complications reducing life expectancy and impairing quality of life.64 In this context, these heterogeneous complications require a comprehensive assessment aimed at developing a multidimensional and individualized treatment,64 which is obviously better managed by a multidisciplinary team.
As mentioned earlier, the promising results from long-term weight maintenance obtained by the new-generation, lifestyle modification programs should stimulate the physicians within the multidisciplinary teams to receive adequate training in cognitive behavioral therapy to engage patients in lifestyle modification.65 Engaged patients should then be referred to trained lifestyle counselors (also called “lifestyle trainers” or “healthy lifestyle practitioners”)66 working closely with any other component of an ideal lifestyle modification unit (eg, dietitians, psychologists, physical activity supervisors), to implement both the weight loss phase and the long-term maintenance phase of the lifestyle modification. The physician supervising the team should also manage the medical and psychosocial complications associated with obesity, referring the patients to other physicians and health professionals according to specific comorbidities. Finally, the supervising physicians should periodically monitor the effects of treatment, both on lifestyle and on weight outcomes, and consider the opportunity to intensify the lifestyle approach with obesity drugs, residential rehabilitative treatment, and, in selected patients with severe obesity, bariatric surgery. This multidisciplinary approach based on lifestyle modification has the potential to address several obstacles to reach the optimum long-term management of obesity.
Conclusion
The difficulty in helping obese patients maintain a long-term weight loss has been challenged by recent studies showing that several individuals are able to maintain acceptable weight loss targets in the long term and by the promising results achieved by the new-generation lifestyle modification programs. These promising results should stimulate the adoption of multidisciplinary approaches based on lifestyle modification for the management of obesity. Only comprehensive programs administered by noneclectic teams addressing any mediator of lifestyle modification, managing the several medical and psychological complications associated with obesity and, if indicated, coupling the lifestyle treatment with other interventions (eg, drugs, residential inpatient treatment, bariatric surgery) might be successful. The effectiveness and the cost-efficacy of a stepped-care approach should be evaluated by future longitudinal observational studies.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Christian JG, Tsai AG, Bessesen DH. Interpreting weight losses from lifestyle modification trials: using categorical data. Int J Obes. 2010;34(1):207–209. doi: 10.1038/ijo.2009.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel D. Pharmacotherapy for the management of obesity. Metabolism. 2015;64(11):1376–1385. doi: 10.1016/j.metabol.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 3.MacLean PS, Wing RR, Davidson T, et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity. 2015;23(1):7–15. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med. 2014;46(1):17–23. doi: 10.1016/j.amepre.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Wadden TA, Neiberg RH, Wing RR, et al. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity. 2011;19(10):1987–1998. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 7.Rossner S. Defining success in obesity management. Int J Obes Relat Metab Disord. 1997;21(Suppl 1):S2–S4. [PubMed] [Google Scholar]
- 8.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalle Grave R, Cuzzolaro M, Calugi S, et al. The effect of obesity management on body image in patients seeking treatment at medical centers. Obesity. 2007;15(9):2320–2327. doi: 10.1038/oby.2007.275. [DOI] [PubMed] [Google Scholar]
- 11.Dalle Grave R, Calugi S, Petroni ML, Di Domizio S, Marchesini G, QUOVADIS Study Group Weight management, psychological distress and binge eating in obesity. A reappraisal of the problem. Appetite. 2010;54(2):269–273. doi: 10.1016/j.appet.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 12.French SA, Jeffery RW, Folsom AR, Williamson DF, Byers T. History of intentional and unintentional weight loss in a population-based sample of women aged 55 to 69 years. Obes Res. 1995;3(2):163–170. doi: 10.1002/j.1550-8528.1995.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 13.Kraschnewski JL, Boan J, Esposito J, et al. Long-term weight loss maintenance in the United States. Int J Obes. 2010;34(11):1644–1654. doi: 10.1038/ijo.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wing RR. Behavioral weight control. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York, NY: The Guildford Press; 2002. pp. 301–316. [Google Scholar]
- 15.Dalle Grave R, Calugi S, Marchesini G. The influence of cognitive factors in the treatment of obesity: lessons from the QUOVADIS study. Behav Res Ther. 2014;63C:157–161. doi: 10.1016/j.brat.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24(5):610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 17.Look Ahead Research Group Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity. 2014;22(1):5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diabetes Prevention Program Research Group. Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 20.Dalle Grave R, Melchionda N, Calugi S, et al. Continuous care in the treatment of obesity: an observational multicentre study. J Intern Med. 2005;258(3):265–273. doi: 10.1111/j.1365-2796.2005.01524.x. [DOI] [PubMed] [Google Scholar]
- 21.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 22.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 23.Perri MG, Limacher MC, Durning PE, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med. 2008;168(21):2347–2354. doi: 10.1001/archinte.168.21.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuller NR, Williams K, Shrestha R, et al. Changes in physical activity during a weight loss intervention and follow-up: a randomized controlled trial. Clin Obes. 2014;4(3):127–135. doi: 10.1111/cob.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anastasiou CA, Fappa E, Karfopoulou E, Gkza A, Yannakoulia M. Weight loss maintenance in relation to locus of control: the MedWeight study. Behav Res Ther. 2015;71:40–44. doi: 10.1016/j.brat.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Santos I, Mata J, Silva MN, Sardinha LB, Teixeira PJ. Predicting long-term weight loss maintenance in previously overweight women: a signal detection approach. Obesity. 2015;23(5):957–964. doi: 10.1002/oby.21082. [DOI] [PubMed] [Google Scholar]
- 27.Simpson SA, McNamara R, Shaw C, et al. A feasibility randomised controlled trial of a motivational interviewing-based intervention for weight loss maintenance in adults. Health Technol Assess. 2015;19(50):v–vi. xix–xxv, 1–378. doi: 10.3310/hta19500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spark LC, Fjeldsoe BS, Eakin EG, Reeves MM. Efficacy of a text message-delivered extended contact intervention on maintenance of weight loss, physical activity, and dietary behavior change. JMIR Mhealth Uhealth. 2015;3(3):e88. doi: 10.2196/mhealth.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young MD, Plotnikoff RC, Collins CE, Callister R, Morgan PJ. Impact of a male-only weight loss maintenance programme on social-cognitive determinants of physical activity and healthy eating: a randomized controlled trial. Br J Health Psychol. 2015;20(4):724–744. doi: 10.1111/bjhp.12137. [DOI] [PubMed] [Google Scholar]
- 30.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66(2):239–246. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 31.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(1 Suppl):222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 32.McGuire MT, Wing RR, Klem ML, Hill JO. Behavioral strategies of individuals who have maintained long-term weight losses. Obes Res. 1999;7(4):334–341. doi: 10.1002/j.1550-8528.1999.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 33.Keesey RE, Hirvonen MD. Body weight set-points: determination and adjustment. J Nutr. 1997;127(9):1875S–1883S. doi: 10.1093/jn/127.9.1875S. [DOI] [PubMed] [Google Scholar]
- 34.Cooper Z, Fairburn CG. A new cognitive behavioural approach to the treatment of obesity. Behav Res Ther. 2001;39(5):499–511. doi: 10.1016/s0005-7967(00)00065-6. [DOI] [PubMed] [Google Scholar]
- 35.Melchionda N, Marchesini G, Apolone G, et al. The QUOVADIS Study: features of obese Italian patients seeking treatment at specialist centers. Diabetes Nutr Metab. 2003;16(2):115–124. [PubMed] [Google Scholar]
- 36.Latner JD, McLeod G, O’Brien KS, Johnston L. The role of self-efficacy, coping, and lapses in weight maintenance. Eat Weight Disord. 2013;18(4):359–366. doi: 10.1007/s40519-013-0068-1. [DOI] [PubMed] [Google Scholar]
- 37.Kassin S. Psychology. 4th ed. Upper Saddle River, NJ: Prentice-Hall, Inc; 2003. [Google Scholar]
- 38.Dalle Grave R, Calugi S, Compare A, et al. Personality, attrition and weight loss in treatment seeking women with obesity. Clin Obes. 2015;5(5):266–272. doi: 10.1111/cob.12112. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan S, Cloninger CR, Przybeck TR, Klein S. Personality characteristics in obesity and relationship with successful weight loss. Int J Obes. 2007;31(4):669–674. doi: 10.1038/sj.ijo.0803464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fassino S, Leombruni P, Piero A, et al. Temperament and character in obese women with and without binge eating disorder. Compr Psychiatry. 2002;43(6):431–437. doi: 10.1053/comp.2002.35906. [DOI] [PubMed] [Google Scholar]
- 41.Kiesewetter S, Kopsel A, Mai K, et al. Attachment style contributes to the outcome of a multimodal lifestyle intervention. Biopsychosoc Med. 2012;6(1):3. doi: 10.1186/1751-0759-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strauss B. Bindungsforschung und therapeutische Beziehung [Attachment research and therapeutic relationship] Psychotherapeut. 2006;51:5–14. German. [Google Scholar]
- 43.Teixeira PJ, Carraca EV, Marques MM, et al. Successful behavior change in obesity interventions in adults: a systematic review of self-regulation mediators. BMC Med. 2015;13:84. doi: 10.1186/s12916-015-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12(Suppl):151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 45.Perri MG, Nezu AM, Patti ET, McCann KL. Effect of length of treatment on weight loss. J Consult Clin Psychol. 1989;57(3):450–452. [PubMed] [Google Scholar]
- 46.Latner JD, Stunkard AJ, Wilson GT, Jackson ML, Zelitch DS, Labouvie E. Effective long-term treatment of obesity: a continuing care model. Int J Obes Relat Metab Disord. 2000;24(7):893–898. doi: 10.1038/sj.ijo.0801249. [DOI] [PubMed] [Google Scholar]
- 47.Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012;125(9):1157–1170. doi: 10.1161/CIRCULATIONAHA.111.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutchesson MJ, Rollo ME, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev. 2015;16(5):376–392. doi: 10.1111/obr.12268. [DOI] [PubMed] [Google Scholar]
- 49.Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes. 2015;39(8):1188–1196. doi: 10.1038/ijo.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schoeller DA, Shay K, Kushner RF. How much physical activity is needed to minimize weight gain in previously obese women? Am J Clin Nutr. 1997;66(3):551–556. doi: 10.1093/ajcn/66.3.551. [DOI] [PubMed] [Google Scholar]
- 51.Centis E, Trento M, Dei Cas A, et al. Stage of change and motivation to healthy diet and habitual physical activity in type 2 diabetes. Acta Diabetol. 2014;51(4):559–566. doi: 10.1007/s00592-013-0551-1. [DOI] [PubMed] [Google Scholar]
- 52.Soleymani T, Daniel S, Garvey WT. Weight maintenance: challenges, tools and strategies for primary care physicians. Obes Rev. doi: 10.1111/obr.12322. Epub October 21, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ekkekakis P, Lind E. Exercise does not feel the same when you are overweight: the impact of self-selected and imposed intensity on affect and exertion. Int J Obes. 2006;30(4):652–660. doi: 10.1038/sj.ijo.0803052. [DOI] [PubMed] [Google Scholar]
- 54.Ford ES, Herman WH. Leisure-time physical activity patterns in the U.S. diabetic population. Findings from the 1990 National Health Interview Survey – Health Promotion and Disease Prevention Supplement. Diabetes Care. 1995;18(1):27–33. doi: 10.2337/diacare.18.1.27. [DOI] [PubMed] [Google Scholar]
- 55.Lee RE, Mama SK, Medina A, Orlando Edwards R, McNeill L. SALSA: SAving Lives Staying Active to promote physical activity and healthy eating. J Obes. 2011;2011:436509. doi: 10.1155/2011/436509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kattenstroth JC, Kolankowska I, Kalisch T, Dinse HR. Superior sensory, motor, and cognitive performance in elderly individuals with multi-year dancing activities. Front Aging Neurosci. 2010;2:31. doi: 10.3389/fnagi.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mangeri F, Montesi L, Forlani G, Dalle Grave R, Marchesini G. A standard ballroom and Latin dance program to improve fitness and adherence to physical activity in individuals with type 2 diabetes and in obesity. Diabetol Metab Syndr. 2014;6:74. doi: 10.1186/1758-5996-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta H. Barriers to and facilitators of long term weight loss maintenance in adult UK people: a thematic analysis. Int J Prev Med. 2014;5(12):1512–1520. [PMC free article] [PubMed] [Google Scholar]
- 59.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 60.Cooper Z, Fairburn CG, Hawker DM. Cognitive-Behavioral Treatment Of Obesity. New York, NY: The Guilford Press; 2003. [Google Scholar]
- 61.Flechtner-Mors M, Ditschuneit HH, Johnson TD, Suchard MA, Adler G. Metabolic and weight loss effects of long-term dietary intervention in obese patients: four-year results. Obes Res. 2000;8(5):399–402. doi: 10.1038/oby.2000.48. [DOI] [PubMed] [Google Scholar]
- 62.Ptomey LT, Willis EA, Goetz JR, et al. Portion-controlled meals provide increases in diet quality during weight loss and maintenance. J Hum Nutr Diet. doi: 10.1111/jhn.12296. Epub February 9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.American Medical Association House of Delegates Recognition of Obesity as a Disease. Resolution. 2013;420(A-13) [Google Scholar]
- 64.Donini LM, Dalle Grave R, Caretto A, et al. From simplicity towards complexity: the Italian multidimensional approach to obesity. Eat Weight Disord. 2014;19(3):387–394. doi: 10.1007/s40519-013-0097-9. [DOI] [PubMed] [Google Scholar]
- 65.Dalle Grave R, Calugi S, Centis E, Marzocchi R, El Ghoch M, Marchesini G. Lifestyle modification in the management of the metabolic syndrome: achievements and challenges. Diabetes Metab Syndr Obes. 2010;3:373–385. doi: 10.2147/DMSOTT.S13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arena R, Lavie CJ, Hivert M-F, Williams MA, Briggs PD, Guazzi M. Who will deliver comprehensive healthy lifestyle interventions to combat non-communicable disease? Introducing the healthy lifestyle practitioner discipline. Expert Rev Cardiovasc Ther. 2016;14(1):15–22. doi: 10.1586/14779072.2016.1107477. [DOI] [PubMed] [Google Scholar]