Abstract
Background
The aim of this study was to evaluate the color-changing effect and adverse effects after Nd: YAG laser application on the iris surface of rabbit eyes.
Material/Methods
The study was performed on right eyes of 12 pigmented rabbits. A laser device that produces frequency doubled 532 nm wavelength Nd: YAG laser with 900 μm spot diameter was used. The laser was applied in 3 sessions at 2-week intervals, at energy levels of 0.8 mJ in Group A and 1.5 mJ in Group B. Slit-lamp examinations and measurements of intraocular pressure (IOP) using a Tono-Pen were performed before and 1 day after each laser session. Iris thickness (IT) was measured at the beginning and the end using an ultrasonic biomicroscope. The eyes were enucleated for histopathologic examination on day 60.
Results
On the first day after each laser session, maximum grade 1 anterior chamber flare and cells were observed in both groups. In all eyes, flare and cells disappeared at the end of the first week. There was no significant difference in the IOP and IT values between measurements performed prior to and after laser sessions during the study (p>0.05). None of the eyes showed complications such as corneal edema, hypopyon, posterior synechia, transillumination defect, or pupillary defect. In histopathological examinations, reduction in pigment density was more profound in Group B compared to Group A, which was statistically significant (p<0.019).
Conclusions
There were no serious complications apart from mild transient inflammatory signs. Change in iris color was more evident at the end of the second month.
MeSH Keywords: Eye Color; Intraoperative Complications; Iris; Lasers, Solid-State
Background
The iris is the anterior part of the uvea; it is made up of connective and muscle tissue, is approximately 11–12 mm in diameter, and has an opening at the center called the pupil. Iris tissue consists of 2 layers: on the inside there is iris pigment epithelia, which is made of cuboidal pigment cells, and on the outside there is a looser stromal layer consisting of fibroblasts and melanocytes. Two kinds of pigment have been defined: eumelanin pigment is brown-black in color and pheomelanin pigment is yellow-red. These pigments are stored inside the melanosome organelles of melanocyte pigment cells [1]. The main factors that determine various eye colors are the density of the melanocytes located at the anterior superficial layer of the stroma and the nature of the pigments inside the melanocytes [1–4]. Studies have shown that there is approximately 15% less melanin in blue irises than in brown irises [5].
Ophthalmological lasers are commonly used in diagnosis and treatment of various eye diseases. The Nd: YAG (neodymium: yttrium-aluminium-garnet) laser is successfully used in the treatment of glaucoma, where it is applied directly on the iris for iridotomy or selectively to pigmented cells in the trabecular meshwork. The laser that is used in selective laser trabeculoplasty (SLT) is a 532 nm wavelength Q-switched Nd: YAG laser, and it shows affinity to melanin pigment. The typical property of this laser is that it lyses intracytoplasmic melanosomes via selective photothermolysis. This effect has been demonstrated in the treatment of glaucoma and in treatment of cutaneous pigmentations in dermatological studies [6]. In addition, it has been reported that Nd: YAG laser applied together with surgical treatment yields satisfactory cosmetic results and is safe in treatment of oculodermal melanocytosis [7]. There are several studies in the literature related with applications on trabecular meshwork; these studies have shown that SLT is effective and safe [8,9]. However, little is known about the morphological and functional adverse effects that could occur after laser applications on the iris surface.
The current study aimed to evaluate the adverse effects following application of wide spot diameter 532 nm wavelength Q-switched Nd: YAG laser on the anterior iris surface of rabbit eyes at different energy levels.
Material and Methods
Animals
The right eyes of 12 pigmented rabbits, weighting 2.5 to 3.0 kg each, were included in the study. All animals had brown irides. All the experimental procedures followed the ARVO (Association for Research in Vision and Ophthalmology) statement for the use of animals in ophthalmic and vision research. Approval for animal studies was obtained from the Gulhane Military Medical Academy Haydarpasa Training Hospital, Animal Ethics Committee for Medical Research. The rabbits were anesthetized by an intramuscular injection of a mixture containing ketamine hydrochloride (50 mg/kg) and xylazine solution (2 mg/kg). Topical anesthesia (Proparakain HCl 0.5%) was administered to reduce the animals’ discomfort.
Slit-lamp examinations and photography of anterior segment were performed on all eyes prior to the laser application, on days 1 and 7 after each laser session and on day 60. Measurement of intraocular pressure (IOP) was performed prior to the laser application and 1 day after each laser session using a Tono-Pen tonometer (Reichert, Inc., Depew, NY, USA). IOP of each eye was measured three times under topical anesthesia and the mean data recorded. The anterior chamber flare and cells were graded according to the SUN Working Group Grading Scheme [10].
Ultrasound biomicroscopy (UBM) examination was performed to measure iris thickness (IT) prior to the laser application and 60 days after first laser session with VuMax II (Sonomed, USA), which incorporates a 50-MHz transducer. The pupils were miosed with topical pilocarpine 2% prior to UBM examination. The IT was measured manually using a special caliber included in the instrument software, in two different points at 2 mm (IT1) and 3 mm (IT2) from the edge of pupil. All measurements were made in the temporal meridian. All examinations were obtained by the same examiner (ED) at least three times to confirm reproducibility.
Laser procedure
In this study, a device that produces frequency doubled, 532 nm wavelength Nd: YAG laser with 900 μm spot diameter (A.R.C. Laser, Germany) was used, which was mounted on to the slit-lamp biomicroscope. In order to maximize iris surface area and to minimize topographic differences, the pupils were constricted 30 minutes prior to laser applications by dropping the miotic agent (pilocarpine 2%). Speculums were used during application to keep the eye open. Laser application time was 3 nanoseconds, and energy range was between 0.1 mJ-2.0 mJ in this device. Rabbits were divided into two groups based on laser energy: Group A (n=6), 360 degrees at right eyes with 0,8 mJ; Group B (n=6), 360 degrees at right eyes with 1.5 mJ. In both groups, anterior surface of iris around the peripupillary ring were administered with 120 pulses at first session. At the second session which was two weeks after the first application, the same dose and pulses were repeated again. At the first two sessions, during laser application in single pulses, attention was given not to overlap the applied laser spots and not to direct them on peripupillary ring. At the 3rd laser application session which was performed at the fourth week of the study; both groups were applied with 200 laser pulses to fill in the gaps between spots. Thus, in total, laser was applied minimally as 352 mJ in group A (0.8 mJ ×440 pulses/3 sessions) and maximally as 660 mJ in group B (1.5 mJ ×440 pulses/3 sessions). The characteristics of the groups are summarized in Table 1.
Table 1.
Characteristics of the groups.
| Groups | Group A (n=6) | Group B (n=6) |
|---|---|---|
| Laser frequency | 532 nm | 532 nm |
| Density of energy (mj) | 0.8 | 1.5 |
| Spot size (μm) | 900 | 900 |
| Number of pulses in first session (n) | 120 | 120 |
| Number of pulses in second session (n) | 120 | 120 |
| Number of pulses in third session (n) | 200 | 200 |
| Application field | 360° | 360° |
Histological examination
On day 60, all rabbits were euthanized with an intravenous injection of sodium pentobarbital. The eyes were enucleated and fixed in formaldehyde 10% for histopathologic study. After being dehydrated and embedded in paraffin, the sections were cut and stained with hematoxylin-eosin for routine light microscopy. Sections prepared from the left eyes were included as control group. Evaluations were made for changes in pigment density, vascular structure, inflammatory and atrophic changes. The changes in these factors were graded by one pathologist (blinded as to the sample identity) on a scale from 0–3: none (0), mild (1), moderate (2), and marked (3).
Statistical analysis
Results were presented as mean ± standard deviation. Wilcoxon signed rank test was used to analyze the related variables. Mann-Whitney U test was used for comparison of two groups. A p value of <0.05 was considered statistically significant.
Results
In biomicroscopic examination which was made on the first day after laser application, it was observed that air bubbles which had formed during laser application disappeared in all eyes. No cells were detected in either group by the slit lamp examination at baseline. On the first day after all laser sessions, maximum grade 1 anterior chamber flare and cells were observed in both groups. Mild ciliary injection was observed in three eyes of Group B at first day after third laser session. In all cases, flare and cells at the anterior chamber and the ciliary injection disappeared at the end of the first week. None of the eyes showed complications such as corneal edema, hypopyon, posterior synechia, transillumination defect, lens opacity, vitreous haze or pupillary defect. No change was observed in pupillary functions (persistent miosis or mydriasis).
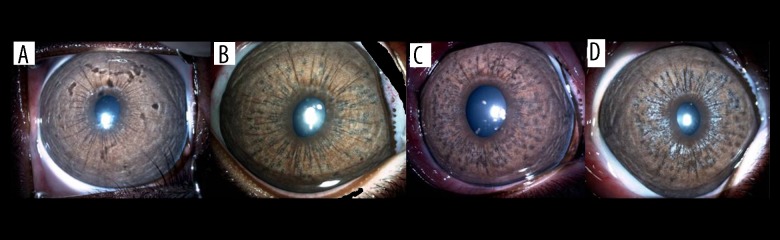
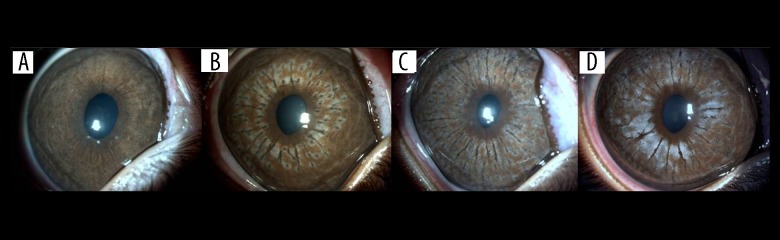
At the end of the first week following laser application, there were lighter ring and hyperpigmented mottling in the centre of spots which was marked in Group B. On day 30, these spots turned diffusely hyperpigmented spots in group A, that cause patchy appearance. But in group B, mottling became more extensive on day 30 with a grayish appearance. Hyperpigmented granular areas diminished on day 60, and had a broken white appearance which was marked in group B (Figures 1, 2).
Figure 1.
Photographs of the iris of a rabbit in Group A, (A) prior to laser application, (B) after first session, (C) after second session, (D) on day 60.
Figure 2.
Photographs of the iris of a rabbit in Group B, (A) prior to laser application, (B) after first session, (C) after second session, (D) on day 60.
There was no statistically significant difference in IOP levels between measurements performed prior to and after laser sessions (Table 2). IT measurements performed with UBM are summarized in Table 2. According to results, there was no statistically significant difference in IT values at the 60th day measurements compared to pre-laser values. We did not find significant difference in the IOP and IT values between Group A and Group B during the study period (Tables 2, 3).
Table 2.
Comparisons of intraocular pressure values during the study period.
| Time | Group A | Group B | p** |
|---|---|---|---|
| First session | |||
| Pre-laser | 10.22±0,50 | 10.05±0.97 | 0.69 |
| 1st day | 10.99±1.11 | 10.38±0.57 | 0.18 |
| p* | 0.24 | 0.33 | |
|
| |||
| Second session | |||
| Pre-laser | 10.10±0.93 | 10.77±0.83 | 0.18 |
| 1st day | 10.72±1.06 | 11.44±0.96 | 0.24 |
| p* | 0.14 | 0.41 | |
|
| |||
| Third session | |||
| Pre-laser | 11.05±0.92 | 10.27±1.45 | 0.31 |
| 1st day | 11.16±0.65 | 10.44±0.65 | 0.93 |
| p* | 0.45 | 0.83 | |
Comparison of related variables (pre-laser-1st day), Wilcoxon signed rank test;
Comparison of IT values of Group A and Group B, Mann-Whitney U test
Table 3.
Comparisons of iris thickness values during the study period.
| Time | Group A | Group B | p** |
|---|---|---|---|
| Pre-laser-IT1 | 0.423±0,042 | 0.398±0.075 | 0.25 |
| 60th day-IT1 | 0.418±0.047 | 0.380±0.044 | 0.18 |
| p* | 0.083 | 0.202 | |
|
| |||
| Pre-laser-IT2 | 0.476±0.076 | 0.463±0.080 | 0.80 |
| 60th day-IT2 | 0.460±0.095 | 0.441±0.078 | 0.93 |
| p* | 0.102 | 0.066 | |
Comparison of related variables (pre-laser-60th day), Wilcoxon signed rank test;
Comparison of IT values of Group A and Group B, Mann-Whitney U test.
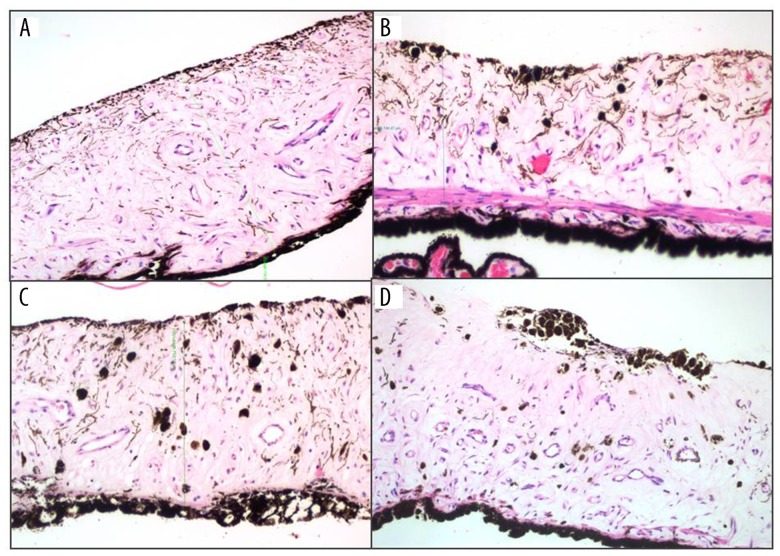
In histopathological examinations, when compared to normal tissue (left eyes), average pigment density reduction score at the anterior layer of iris stroma was found to be 1.16±0.40 in Group A and 2.33±0.81 in Group B. Reduction in pigment density was more profound in Group B compared to Group A, which was statistically significant (p<0.019). It was observed that melanocytes that were regularly lined on anterior layer of iris stroma decreased or disappeared. The mean number of melanophages (pigment laden histiocytes) was 9,33±7.4 in Group A and 8±2,9 in Group B in the normal tissue (lefts eyes), and 23.17±5.64 and 41.83±13.86 in the right eyes of Group A and Group B, respectively. When compared to the normal tissue, changes in both groups were found to be statistically significant (Group A: p=0.027, Group B: p=0.028). When Group A and Group B were compared, number of melanophages were significantly higher in Group B (p=0.02). It was observed that melanophages accumulated and formed clumps in the anterior stroma in some of the sections (Figure 3). There were no inflammation, fibrosis, change in vascular structure in iris stroma, and no change in iris pigment epithelia, pupillary sphincter or dilatory muscle in neither of the eyes in both groups (Figure 3).
Figure 3.
Photomicrograph of hematoxylin and eosin stained iris cross-section of a rabbit eye in group B, (A) section from normal tissue where laser was not applied at left eye; (B, C) increase in number of melanophages at areas where laser is applied; (D) pigment reduction in anterior surface layer of iris and melanophage clumps are seen.
Discussion
It is a fact that physical appearance is important for everyone, and a nice and beautiful appearance makes people feel better. Because iris color is an important aspect of physical appearance, people desire to have colored eyes. Moreover, different eye colors can develop in one person as a result of some disorders such as Fuchs’ heterochromic iridocyclitis, Horner syndrome, or due to use of prostaglandin analogs, trauma or surgery. Focal color changes can occur due to iris nevi. As a result, people feel uncomfortable when they have heterochromic eye colors. In recent years, this problem has been resolved to some extent with use of colored contact lenses. However, use of contact lenses has its own disadvantages such as allergic reactions, feeling of foreign body, dryness and infections [11]. Another method that is used to change eye color is the artificial intraocular iris implants that are placed on the anterior surface of iris. This technique has not been commonly adopted due to its invasive nature and the developing complications [12,13]. A novel method to change the eye color which has been developed in recent years is application of laser on to the iris surface. There is no literature data regarding the safety of these applications which have been in clinical use for aesthetic purposes without obtainment of a license yet. The overall structure of the iris stroma is similar in irides of all colors. Differences in color are related to the amount of pigmentation in the anterior border layer and the deep stroma, thickness of stroma and accumulation type of white collagen fibers [14]. The stroma of blue irides is lightly pigmented, and brown irides have a densely pigmented stroma that absorbs light. Therefore, It is proposed that eye color could be changed with this laser by reduction or elimination of melanin pigments on the anterior surface of iris. The laser used for this purpose has the same properties with the laser that is used in the treatment of glaucoma with SLT device; it is Q-switched, frequency doubled, 532 nm wavelength Nd: YAG laser, with the only difference of having 900 μm spot diameter instead of 400 μm.
In SLT, the target for the laser is melanin content of trabecular cells. “Selective photothermolysis” occurs by restriction of the thermal damage to the target, which is the melanin pigment. Thus, only pigmented trabecular tissue is being treated, where intracytoplasmic melanosomes are lysed without damaging the cell membranes or neighboring nonpigmented tissues [15]. Another factor for prevention of damage to neighboring structures is the very short duration of laser pulse provided by the device (3 nanoseconds), and very short heat relaxation time of the target melanin pigment (1 microseconds). Q-switched, frequency doubled, 532 nm wavelength Nd: YAG laser bearing SLT applications have been shown not to cause coagulation damage or scarring [16,17]. In our study, unlike with the SLT device, we had the opportunity to apply laser to iris tissue with fewer pulses, at a larger area with 900 μm spot size. Application duration was 3 nanoseconds, being at two different energy levels as 0.8 mJ and 1.5 mJ. Total numbers of pulses were 440 in both groups. At the first week following laser application, there were lighter spots in iris tissue in patchy appearance, as well as areas showing hyperpigmentation from place to place due to pigment clumping. These dark granular areas were present especially at the early period after laser application in the center of laser spots; but they diminished at the end of eighth week. In the result, lightening of the iris color became more profound and permanent. This change is thought to be resulting from clearing of pigment laden macrophages which had increased in stroma after laser application. Because 900 μm spot size instead of 400 μm is supposed to lessen patchy appearance by decreasing cumulative pigmentation, and also to act on larger areas by applying fewer pulses, inflammation would be milder.
SLT stimulates proliferation of endothelial cells in trabecular cells and Schlemm’s canal, release of cytokines, monocytes and phagocytosis. Increased migration and phagocytosis functions of monocytes help eliminate the debris in trabecular meshwork, and endothelial cells are biologically stimulated by the effect of chemokines released from the cells [18]. Mononuclear phagocytes become pigment laden macrophages after engulfing melanin granules; they have been reported to enter systemic venous circulation after passing to the lumen of Schlemm’s canal [19]. In SLT applications, biological effects of the release of chemotactic and vasoactive cytokines such as interleukin-1 beta and tumor necrosis factor alpha are known to play an important role in the control of IOP [20]. Increased levels of lipid peroxidase in aqueous humor and free oxygen radicals that are released from trabecular meshwork have been suggested to take part in inflammation occurring after SLT application [21]. In an animal experiment by Gulati et al. [22] that investigated response to steroid treatment in anterior uveitis which was developed by applying laser to anterior surface of iris with SLT device, histopathological examinations revealed that there was pigment clumping at anterior surface of iris stroma, and that the changes were limited to anterior layers of iris. Additionally, after measurements conducted with flarimetry, they reported that laser applications resulted in anterior chamber inflammation that was responsive to steroids [22]. In our study, we observed ciliary injection and mild anterior chamber reaction in all groups on the first day, being more prominent in the Group B in which higher dose of laser was applied; and these signs disappeared in the examination at first week. In our study, in which we did not administer anti- inflammatory treatment, there was no hypopyon or prolonged anterior chamber reaction.
In histopathological examinations in our study, we observed that the regularly placed melanocytes at the anterior layers of the iris decreased or disappeared, and that also there was increase in the number of melanophages located at anterior or middle stroma in this area. In some of the sections, it was observed that these melanophages formed patchy clumps at the anterior stroma (Figure 3). These sections belong to the regions where patchy pigment clumps are detected at anterior layer of the iris with biomicroscope. It is assumed that laser applications with large spot diameters would cause less patchiness compared to smaller spot diameters.
Several studies have reported that transient increases in IOP may occur after SLT applications [23–25]. There is no consensus on use of post-procedure anti-inflammatory treatment for suppression of inflammation; it has been proposed that inflammatory process is a requirement for the effectiveness of the procedure [26]. In glaucoma patients, because SLT application is directly on the trabecular meshwork that provides outward flow of aqueous, low-grade inflammatory process taking place here could play a role in the increment of IOP. For this reason, we did not administer anti-inflammatory treatment to rabbits after the procedure. IOP measured on all eyes at all times did not differ from the values measured prior to the procedure. In the study by Gulati et al., although they detected flare in lower rates in the group treated with dexamethasone compared to the control group, they found that IOP was statistically higher [22]. This situation might have been due to dexamethasone administration rather than the laser. In our study, as we did not administer steroid or non-steroid medication, there was no increase in IOP in both groups.
Rabbit eyes also have peripupillary ring, the same as in humans, that is darker than the color of the eye [27]. As this ring is very slender, it is important considering that it provides minor vascular circulation of the iris and includes the sphincter muscle. Additionally, because this region is in close proximity to the crystalline lens, there is risk of damaging the crystalline lens during laser applications. In our study, since there was no laser application to the peripupillary area, which includes the sphincter muscle, miotic function is not supposed to be affected. However, possible damage by laser to neural and vascular network that are located along the iris should be kept in mind. Miotic response by contraction of the iris sphincter muscle following pilocarpine drops at each examination can be regarded as proof of healthiness of this tissue. Besides the sphincter muscle, another functional component of the iris tissue is pupillary dilator muscle. Composed of thin fibers, these smooth muscle fibers are located intrastromally close to posterior surface, where iris pigment epithelia is located. In histological examinations on sections of rabbit eyes, there was no change in either of sphincter and dilator muscles in comparisons with normal areas. There was no change in vascular structure and no development of new vessels (Figure 3). In addition, there was no problem in miotic and mydriatic functions in examination of pupillary light reflexes carried out under slit lamp before anesthesia and also with drops that have miotic or mydriatic effects. There was no change in the iris pigment epithelial layer in any of the rabbits, and there was no transillumination defect in biomicroscopic examination.
IT was measured with UBM to determine the changes in iris stromal thickness. Although measurements with UBM were performed by calculating the mean of measurements at 3 different axes, measurement errors could arise from the presence of circular or ventricular contraction rings and crypts in the structure of the iris.
Conclusions
Following application of 532 nm wavelength Q-switched Nd: YAG laser on iris tissue in rabbit eyes, there were no serious adverse effects or complications apart from mild inflammatory signs. Change in iris color occurred as a result of reduction in pigmentation in the anterior surface of the iris, being especially evident at the end of the second month.
Footnotes
Source of support: Departmental sources
Conflict of Interest
None of the authors has conflict of interest with the submission. All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All the experimental procedures followed the ARVO (Association for Research in Vision and Ophthalmology) statement for the use of animals in ophthalmic and vision research. Approval for animal studies was obtained from the Gulhane Military Medical Academy Haydarpasa Training Hospital, Animal Ethics Committee for Medical Research.
References
- 1.Prota G, Hu DN, Vincensi MR, et al. Characterization of melanins in human irides and cultured uveal melanocytes from eyes of different colors. Exp Eye Res. 1998;67:293–99. doi: 10.1006/exer.1998.0518. [DOI] [PubMed] [Google Scholar]
- 2.Eagle RC. Iris pigmentation and pigmented lesions: an ultrastructural study. Trans Am Ophthalmol Soc. 1988;86:581–87. [PMC free article] [PubMed] [Google Scholar]
- 3.Imesch PD, Bindley CD, Khademian Z, et al. Melanocytes and iris color. Electron microscopic findings. Arch Ophthalmol. 1996;114:443–47. doi: 10.1001/archopht.1996.01100130439015. [DOI] [PubMed] [Google Scholar]
- 4.Wilkerson CL, Syed NA, Fisher MR, et al. Melanocytes and iris color. Light microscopic findings. Arch Ophthalmol. 1996;114:437–42. doi: 10.1001/archopht.1996.01100130433014. [DOI] [PubMed] [Google Scholar]
- 5.Menon IA, Wakeham DC, Persad SD, et al. Quantitative determination of the melanin contents in ocular tissues from human blue and brown eyes. J Ocular Pharmacol. 1992;1:35–42. doi: 10.1089/jop.1992.8.35. [DOI] [PubMed] [Google Scholar]
- 6.Anderson RR, Margolis RJ, Watenabe S, et al. Selective photothermolysis of cutaneous pigmentation by Q-switched Nd: YAG laser pulses at 1064, 532, and 355 nm. J Invest Dermatol. 1989;93:28–32. doi: 10.1111/1523-1747.ep12277339. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, Kim JY, Kim MJ, Tchah H. Efficacy and safety of combination treatment for oculodermalmelanocytosis: surgical reduction and use of 532-nm Q-switched Nd: YAG laser. Cornea. 2014;33:832–37. doi: 10.1097/ICO.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 8.Klamann MK, Maier AK, Gonnermann J, Ruokonen PC. Adverse effects and short-term results after selective laser trabeculoplasty. J Glaucoma. 2014;23:105–8. doi: 10.1097/IJG.0b013e3182684fd1. [DOI] [PubMed] [Google Scholar]
- 9.Ayala M, Chen E. Comparison ofselective laser trabeculoplasty (SLT) in primary open angle glaucoma and pseudoexfoliation glaucoma. Clin Ophthalmol. 2011;5:1469–73. doi: 10.2147/OPTH.S25636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabs DA, Nussenblatt RB, Rosenbaum JT Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data.Results of the first international workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beljan J, Beljan K, Beljan Z. Complications caused by contact lens wearing. Coll Antropol. 2013;37:179–87. [PubMed] [Google Scholar]
- 12.Hoguet A, Ritterband D, Koplin R, et al. Serious ocular complications of cosmetic iris implants in 14 eyes. J Cataract Refract Surg. 2012;38:387–93. doi: 10.1016/j.jcrs.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 13.Hull S, Jayaram H, Mearza AA. Complications and management of cosmetic anterior chamber iris implants. Cont Lens Anterior Eye. 2010;33:235–38. doi: 10.1016/j.clae.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Sturm RA, Larsson M. Genetics of human iris colour and patterns. Pigment Cell Melanoma Res. 2009;22:544–62. doi: 10.1111/j.1755-148X.2009.00606.x. [DOI] [PubMed] [Google Scholar]
- 15.Latina MA, Park C. Selective targeting of trabecular meshwork cells: In vitro studies of pulsed and CW laser interactions. Exp Eye Res. 1995;60:359–71. doi: 10.1016/s0014-4835(05)80093-4. [DOI] [PubMed] [Google Scholar]
- 16.Cvenkel B, Hvala A, Drnovsek-Olup B, Gale N. Acute ultrastructural changes of the trabecular meshwork after selective laser trabeculoplasty and low power argon laser trabeculoplasty. Lasers Surg Med. 2003;33:204–8. doi: 10.1002/lsm.10203. [DOI] [PubMed] [Google Scholar]
- 17.McHugh D, Marshall J, Ffytche TJ, et al. Ultrastructural changes of human trabecular meshwork after photocoagulation with a diode laser. Invest Ophthalmol Vis Sci. 1992;33:2664–71. [PubMed] [Google Scholar]
- 18.Alvarado JA, Katz LJ, Trivedi S, Shifera AS. Monocyte modulation of aqueous outflow and recruitment to the trabecular meshwork following selective laser trabeculoplasty. Arch Ophthalmol. 2010;128:731–37. doi: 10.1001/archophthalmol.2010.85. [DOI] [PubMed] [Google Scholar]
- 19.Murphy CG, Johnson M, Alvarado JA. Juxtacanalicular tissue in pigmentary and primary open angle glaucoma. Arch Ophthalmol. 1992;110:1779–85. doi: 10.1001/archopht.1992.01080240119043. [DOI] [PubMed] [Google Scholar]
- 20.Bradley JM, Anderssohn AM, Colvis CM, et al. Mediation of laser trabeculoplasty-induced matrix metalloproteinase expression by IL-1beta and TNF alpha. Invest Ophthalmol Vis Sci. 2000;41:422–30. [PubMed] [Google Scholar]
- 21.Guzey M, Vural H, Satici A, et al. Increase of freeoxygen radicals in aqueous humourinduced by selective Nd: YAG laser trabeculoplasty in the rabbit. Eur J Ophthalmol. 2001;11:47–52. doi: 10.1177/112067210101100109. [DOI] [PubMed] [Google Scholar]
- 22.Gulati V, Pahuja S, Fan S, Toris CB. An experimental steroid responsive model of ocular inflammation in rabbits using an SLT frequency doubled Q switched Nd: YAG laser. J Ocul Pharmacol Ther. 2013;29:663–69. doi: 10.1089/jop.2012.0223. [DOI] [PubMed] [Google Scholar]
- 23.Latina MA, Sibayan SA, Shin DH, et al. Q-switched 532-nm Nd: YAG laser trabeculoplasty (selective laser trabeculoplasty): a multicenter, pilot, clinical study. Ophthalmology. 1998;105:2082–88. doi: 10.1016/S0161-6420(98)91129-0. [DOI] [PubMed] [Google Scholar]
- 24.Melamed S, Ben Simon GJ, Levkovitch-Verbin H. Selective laser trabeculoplasty as primary treatment for open-angle glaucoma: a prospective, nonrandomized pilot study. Arch Ophthalmol. 2003;121:957–60. doi: 10.1001/archopht.121.7.957. [DOI] [PubMed] [Google Scholar]
- 25.Harasymowycz PJ, Papamatheakis DG, Latina MA, et al. Selective laser trabeculoplasty complicated by intraocular elevation in eyes with heavily pigmented trabecular meshworks. Am J Ophthalmol. 2005;139:1110–13. doi: 10.1016/j.ajo.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 26.Latina MA, de Leon JM. Selective laser trabeculoplasty. Ophthalmol Clin North Am. 2005;18:409–19. doi: 10.1016/j.ohc.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Sturm RA, Frudakis TN. Eye colour: portals into pigmentation genes and ancestry. Trends Genet. 2004;20:327–32. doi: 10.1016/j.tig.2004.06.010. [DOI] [PubMed] [Google Scholar]