Abstract
Caffeine is the most widely used neurostimulant in the world. There is considerable debate on its effect on immune cells as it has been shown to antagonize adenosine receptors (ARs), which mediate an anti‐inflammatory switch in activated immune cells. A second target is phosphodiesterase, where it acts as an inhibitor. If the primary effect of caffeine on mononuclear phagocytes were to antagonize ARs we would expect cells exposed to caffeine to have a prolonged proinflammatory response. The aim of this study was to investigate the effects and mechanism of action of caffeine in mononuclear phagocytes. Human mononuclear phagocytes were separated from whole blood and pretreated with protein kinase A inhibitor (PKA) and then exposed to micromolar physiological concentrations of caffeine. Phagocytosis and phagocytosis exhaustion were quantified using flow cytometry. Treatments were analyzed and compared to controls, using a beta regression controlling for factors of age, gender, caffeine intake, and exercise. We found that caffeine suppresses phagocytosis at micromolar physiological concentrations. This suppression was prevented when mononuclear phagocytes were pretreated with PKA inhibitor, suggesting that caffeine's phagocytic suppression may be due to its function as a phosphodiesterase inhibitor, pushing cells towards an anti‐inflammatory response. Additionally, these effects are altered by regular caffeine intake and fitness level, emphasizing that tolerance and immune robustness are important factors in mononuclear phagocyte activation. These results demonstrate that caffeine may be acting as a phosphodiesterase inhibitor and suppressing phagocytosis in mononuclear phagocytes by promoting an anti‐inflammatory response.
Keywords: Adenosine receptors, caffeine, inflammation, phagocytosis
Abbreviations
- cDCs
conventional dendritic cells
- CNS
central nervous system
- DCs
dendritic cells
- MPS
mononuclear phagocyte system
- pDCs
plasmacytoid dendritic cells
- PKA
phosphokinase A
Introduction
Mononuclear phagocytes are a group of cells composed of monocytes, macrophages, and dendritic cells (DCs). They are often referred to mononuclear phagocyte system (MPS). The MPS has important roles in inflammation, cancer, autoimmunity, and overall homeostasis in an organism (Chow et al. 2011). MPS cells share many features, such as the ability to phagocytose foreign molecules or cell debris, but are also known for their plasticity in gene expression and their heterogeneity in populations leading to their inability to be identified based on surface markers (Hume 2008). It was once thought that MPS cells differentiated from a common progenitor in the marrow, but recent studies have challenged the relationship between DCs and macrophages, finding that monocytes differentiate into macrophages in tissue and DCs may have a different marrow precursor (Jenkins and Hume 2014).
Dendritic cells are mainly distinguished from macrophages by their ability to activate T cells by antigen presentation. Hence, DCs are thought to be mediators of immunological tolerance. DCs are categorized into two subtypes: classical or conventional DCs (cDCs), which are able to present antigen to T cells and promote T cell stimulation in the absence of intentional activation, and plasmacytoid DCs (pDCs), which secrete of type I interferon (interferon α/β, IFN‐I) and other cytokines but present antigens inefficiently until after pathogen‐induced activation (Lewis and Reizis 2012). This paper will focus on mononuclear phagocytes derived from whole blood, which are therefore more phenotypically monocytes and premacrophages.
Macrophages are well known for their ability to engulf debris and foreign particles and to repair and remodel damaged tissues (Chawla 2010). Macrophages express a variety of cellular markers including: CD14, CD40, CD64, F4/80 (mice)/EMR1 (human), lysozyme M, MAC‐1/MAC‐3, and CD68 (Khazen et al. 2005). The most commonly used markers in biological assays are CD16 and CD14. CD16 is a low‐affinity Fc receptor that is expressed on natural killer cells, neutrophils, and monocytes. It binds the Fc portion of antibodies that have attached to their target antigen and activates antibody‐dependent cell‐mediated cytotoxicity (Janeway et al. 2001). CD14 is a co‐receptor for toll‐like receptor 4 (TLR‐4) and binds lipopolysaccharide (LPS) with the help of LPS‐ binding protein (LBP). CD14 is expressed almost solely on macrophages, but is also found at a much lower concentration on neutrophils. When LPS binds the CD14/TLR4 complex, it leads to the activation of transcription of NF‐κB, which activates proinflammatory pathways resulting in an inflammatory response (Dauphinee and Karsan 2006).
Macrophages can be activated by the nature of the pathogens they ingest as well as by their environment. The extent of macrophage activation has been shown to play a major role in many diseases such as cancer, arthritis, sepsis, and autoimmune diseases (Ayala and Chaudry 1996; Movahedi et al. 2010). Extensive research on the signals involved in macrophage activation has revealed that at least two main subtypes of macrophages exist. Classically activated macrophages are stimulated by proinflammatory signals, while alternatively activated macrophages are usually stimulated by anti‐inflammatory signals. These two phenotypes have also been termed M1 and M2, mirroring the Th1 and Th2 phenotypes of T cells (Mantovani et al. 2002).
The M2 macrophage acts as a support cell by promoting the healing of damaged cells by angiogenesis and tissue remodeling via secretion of anti‐inflammatory cytokines and by decreasing phagocytic activity (Mantovani et al. 2004) The M2 group can be further subdivided by the mechanism of activation: M2a, M2b, and M2c. Il‐4 and IL‐13 drive M2a activation and type II immune responses, whereas TLR/IL‐1R ligands activate the M2b suppression and immunoregulation. M2c macrophages are stimulated by IL‐10, resulting in tissue remodeling and matrix deposition (Mantovani et al. 2005).
While these two polarities exist, it is widely believed that macrophages exist mainly in the continuum between these two phenotypes (Mantovani et al. 2005). Regulation of this continuum involves complex processes that have been extensively studied, yet still remain relatively unclear. Recently it was shown that macrophages will respond to injury or infection by upregulating proinflammatory (M1) responses, but later switch to an anti‐inflammatory (M2) response once the infection is under control (Martinez et al. 2006; Csóka et al. 2007). One proposed mechanism for this switch involves the activation of adenosine receptors (ARs) in macrophages as they mature, to decrease the initial proinflammatory response, and promote vascularization and wound repair (Csóka et al. 2007). Analogs of adenosine are therefore of interest as therapeutic agents in inflammatory diseases.
Caffeine is an analog of adenine and one of the most widely used neuroactive compounds in the world (Gilbert 1976). It has been studied intensely as an analog of adenosine to investigate its role in inflammatory regulation. Caffeine is metabolized by cytochrome P450 oxidase into three metabolites also in the xanthine family: theophylline (4%), theobromine (12%), and paraxanthine (84%). Theophylline and paraxanthine have neurotropic and immunomodulatory activity similar to caffeine (Snyder et al. 1981; Horrigan et al. 2004). van Furth et al. (1997) showed that theophylline, theobromine, paraxanthine, and caffeine all have a suppressive effect on TNF‐α production in human leukocytes. The mechanism of action of these methylxanthines has been a subject of study for many years, yet still remains somewhat unclear.
There is a significant body of evidence to suggest that many of the physiological effects of dietary caffeine are mediated by antagonism of ARs (Mandel 2002). Caffeine binds nonselectively to ARs. The activation of ARs on immune cells generally leads to the suppression of proinflammatory cytokines (Ohta and Sitkovsky 2001). This suppression of cytokine production has been observed following stimulation with agonists of adenosine A1, A2, and A3 receptors, as well as adenosine itself (Link et al. 2000; Kreckler et al. 2006). Therefore, if the primary effect of caffeine on mononuclear phagocytes was to antagonize ARs, we would expect caffeine to prolong proinflammatory responses. However, caffeine suppresses proinflammatory cytokine production in whole‐blood macrophages (Horrigan et al. 2004). This suppression may be due to increased cyclic adenosine monophosphate (cAMP) levels caused by caffeine acting as a phosphodiesterase inhibitor. Increasing cAMP levels in cells activates downstream targets like phosphokinase A (PKA). PKA belongs to a family of kinases that have two regulatory subunits controlled by cAMP binding. When cAMP levels are high, two cAMP molecules bind the regulatory subunits thus removing them from the active site allowing the catalytic subunits to interact with protein kinases to phosphorylate Ser or Thr residues and cause suppression of some proinflammatory pathways (Horrigan et al. 2004).
In order to investigate the effect of caffeine on immune function, we studied the inflammatory profile of mononuclear phagocytes after exposure to caffeine and PKA inhibitor. Using a phagocytic assay, we sought to elucidate caffeine's mechanism of action at a range of time points. We observed suppression of phagocytosis in caffeine‐treated cells and its prevention by pretreatment with PKA inhibitor indicating that caffeine acts as an immunosuppressant by blocking cAMP phosphodiesterase.
Materials and Methods
Tissue culture
Blood was drawn intravenously from volunteers ages 20–30, and information was recorded on age, gender, caffeine intake, and exercise level (BYU IRB # X 14194). Patients who regularly consumed caffeine (500 mg week−1) were considered regular caffeine users. Patients who exercised for 30 min, more than twice a week, were considered regular exercisers.
Mononuclear cells were separated from whole blood using lymphocyte separation medium (Stemcell Tech, Tukwila, WA, USA), then washed and resuspended in RPMI 1640 medium supplemented with 20% human serum from the blood donor. Mononuclear cells were then seeded in 12‐well plates at a concentration of 106 cells mL−1 and incubated (37°C, 5% CO2) for 1 h ‐ 3 day to allow monocytes to adhere to the plate (Wahl and Wahl 2006). Adherent cells collected after 1 h were termed “Day 0” and used as a baseline control for phagocytosis and AR expression. Remaining cells were allowed to culture for 1 day or 3 day after isolation with or without PKA inhibitor (Rp‐8‐Br‐cAMPS; Santa Cruz Biotech, Santa Cruz, CA, USA), final concentration 10−5 mol/L for 0.5 h. Then caffeine‐supplemented media (Sigma, St. Louis, MO, USA, CAS # C8960) was added, final concentration 35 μmol/L‐15 mmol/L, for 2 h followed by LPS stimulation (1 μg mL−1) and further incubation (37°C, 5% CO2) for 22 h.
Caffeine concentration
Caffeine concentration was measured before and after cell culture to ensure uniform concentration across the 24 h incubation period. Caffeine's absorbance was read at 300 nm using a Synergy HT Multi‐Mode Microplate reader (Biotek Instruments, Winooski, VT, USA) at time zero, 2 and 24 h postincubation.
Phagocytosis assay
After 24 h incubation with caffeine, 2 μm phycoerythrin (PE)‐conjugated polychromatic red latex microspheres, or beads, (Polysciences, Inc., Warrington, PA, USA) were added to wells (~109 particles mL−1) and allowed to incubate for 1 h. This concentration was chosen to ensure the beads were not a limiting factor in phagocytosis rates. The beads were suspended in fetal bovine serum (FBS) to allow proper phagocytosis by macrophages and to prevent beads from sticking to the cell membranes (Fig. 1D).
Figure 1.
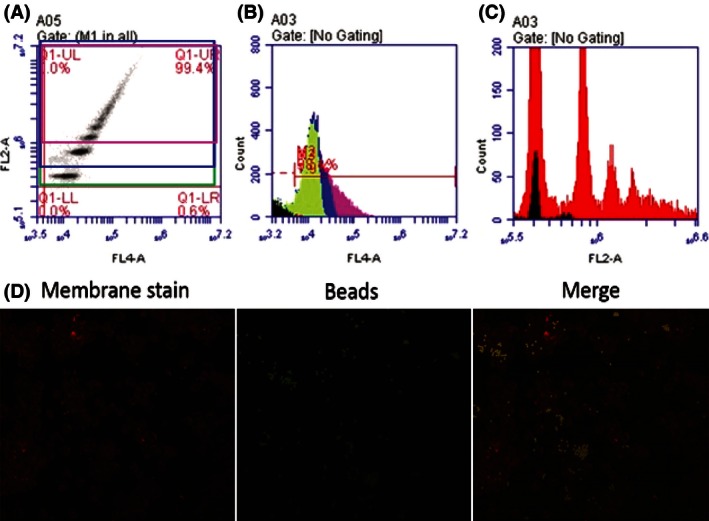
(A) FL4‐A represents CD14+ events, FL2‐A represents PE + events. Distinct populations of cells engulfing 1, 2, and 3+ beads can be seen ascending in PEfluorescence. (B) CD14+ events are separated to show cells that are engulfing 1 bead (green), 2 beads (blue), and 3+ beads (pink). (C) PE + events overlayed with CD14+ in red. The 1 bead population shows slight contamination with nonCD14+ events (black). This may be residual B cells which might have beads attached on the membrane. The 1 bead population was not used in analysis because of this contamination. (D) Mononuclear phagocytes postaddition of beads. We can observe there are very few beads on the membrane or free beads after 1 h incubation and washes. Most beads are fully internalized.
Flow cytometry analysis
After 1 h, cells were placed on ice to stop phagocytosis and mononuclear phagocytes were isolated by their adherence to the plate. Mononuclear phagocytes were collected using a cell scraper, washed twice and stained with human CD14‐APC/Cy7 antibody (BioLegend, San Diego, CA, USA) for monocyte/macrophage identification (DCs are CD14−). After washing, samples were analyzed using a BD Accuri C6 flow cytometer and software.
Statistical analysis
Treatments were analyzed and compared to controls, using a beta regression controlling for factors of age, gender, caffeine intake, and exercise. Matched pair t‐tests were used to further analyze the effect of each factor on macrophage aggressiveness.
Results
Flow cytometry analysis
Cell phagocytosis was stratified by gating on PE+ peaks representing 1, 2, and 3+ beads (Fig. 1A). FL4‐A represents CD14+ events and FL2‐A represents PE+ events (Fig. 1B and C). The “2 bead” and “3+ bead” populations are used in analysis of phagocytic aggressiveness and are used to calculate the percentage of cells that are “highly aggressive.” This percentage was compared to all CD14+ events to measure mononuclear phagocyte activation.
Caffeine concentration
To ensure that caffeine concentrations remained uniform in culture throughout the experiment, caffeine's absorbance was read at 0, 2, and 24 h postincubation with caffeine. No significant difference was seen in caffeine concentration after incubation (Fig. 2).
Figure 2.
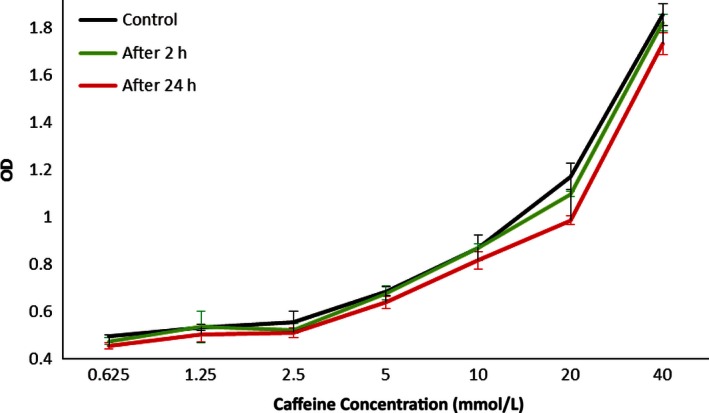
Media from cell culture was sampled and measured using spectrophotometry to identify concentration of caffeine. Wavelength = 300 nm. All other variables besides caffeine concentration were kept constant. No significant differences were seen between time points, suggesting that caffeine concentrations remained relatively stable throughout the 24 h incubation.
Phagocytic exhaustion
In order to observe the phagocytic activity of mononuclear phagocytes as they mature, exhaustion was measured over 3 days using phagocytosis as an indicator of aggressiveness. Figure 3 shows that in noncaffeine‐treated (control) mononuclear phagocytes, cell maturity reduced phagocytosis after 3 days.
Figure 3.
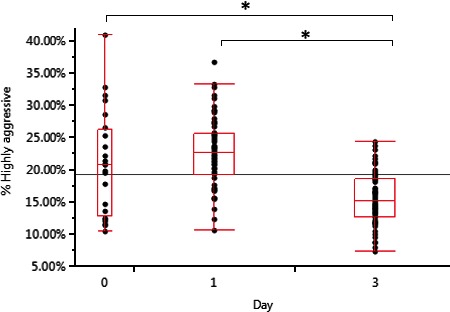
Mononuclear phagocyte aggressiveness as measured by percent highly aggressively engulfing. Over 3 days, macrophage aggressiveness decreases significantly by 34.4% (P = 0.0003), compared to control day 0. Data was generated using a paired t‐test.
On day three, total phagocytosis was reduced by 34.4% (P = 0.0003, 95% CI [13.8%, 37.6%]). This reduction confirms previous work showing that under homeostatic conditions cells that are potentially macrophages shift to an M2 phenotype (Martinez et al. 2006).
Caffeine and phagocytosis
Caffeine‐treated mononuclear phagocytes were analyzed for aggressiveness based on the patient's caffeine intake, gender, age, and exercise level. PKA inhibitor was also added to determine caffeine's mechanism of action. A beta regression accounting for the overall effect of patient factors was performed (Fig. 4).
Figure 4.
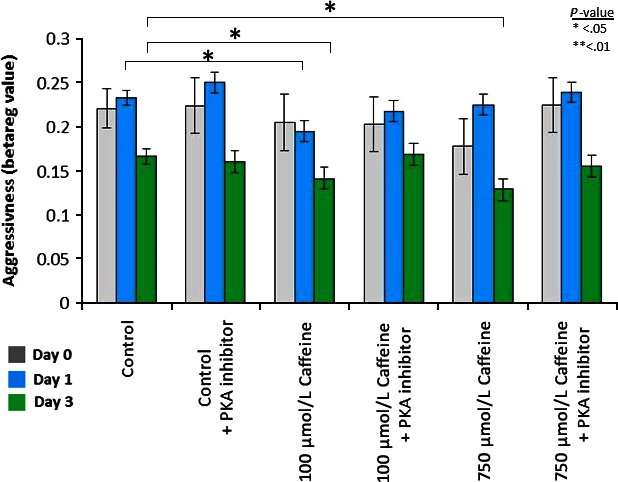
Beta regression accounting for gender, age, caffeine intake, and regular exercise was performed for each day of analysis. Bars represent the average beta regression value of aggressiveness. Day 0 (gray) showed no significant difference in any treatments with no factor having a significant effect. Day 1 (blue) showed a significant decrease in aggressiveness after 100 μmol/L caffeine treatment (P = 0.0428), but addition of PKA inhibitor reversed this effect (P = 0.496). Day 3 (green) showed significant decreases in aggressiveness when treated with both 100 μmol/L caffeine (P = 0.012) and 750 μmol/L caffeine (P = 0.019). Addition of PKA inhibitor reversed this suppression in both concentrations (P = 0.92 and 0.509, respectively). n = 11.
Caffeine's effect becomes more significant with cell maturity, with day three having the most profound effect. Pretreatment with PKA inhibitor negates the effect of caffeine. This suggests that the effect on aggressiveness seen in caffeine‐treated mononuclear phagocytes is due to the phosphodiesterase inhibitory activity of caffeine. Beta regression revealed that patient caffeine intake and regular exercise have a significant effect on mononuclear phagocyte response to caffeine treatment, while other factors had no significant effect or interaction.
Following the beta regression results, further analysis using a matched pair t‐test was done to reveal the effect of patient caffeine intake and exercise for each day. Day 0 results showed no significant difference regardless of caffeine treatment, caffeine intake, or exercise level (data not shown). Figure 5A shows the effect of caffeine treatment on non‐caffeine drinkers’ mononuclear phagocytes on day 1.
Figure 5.
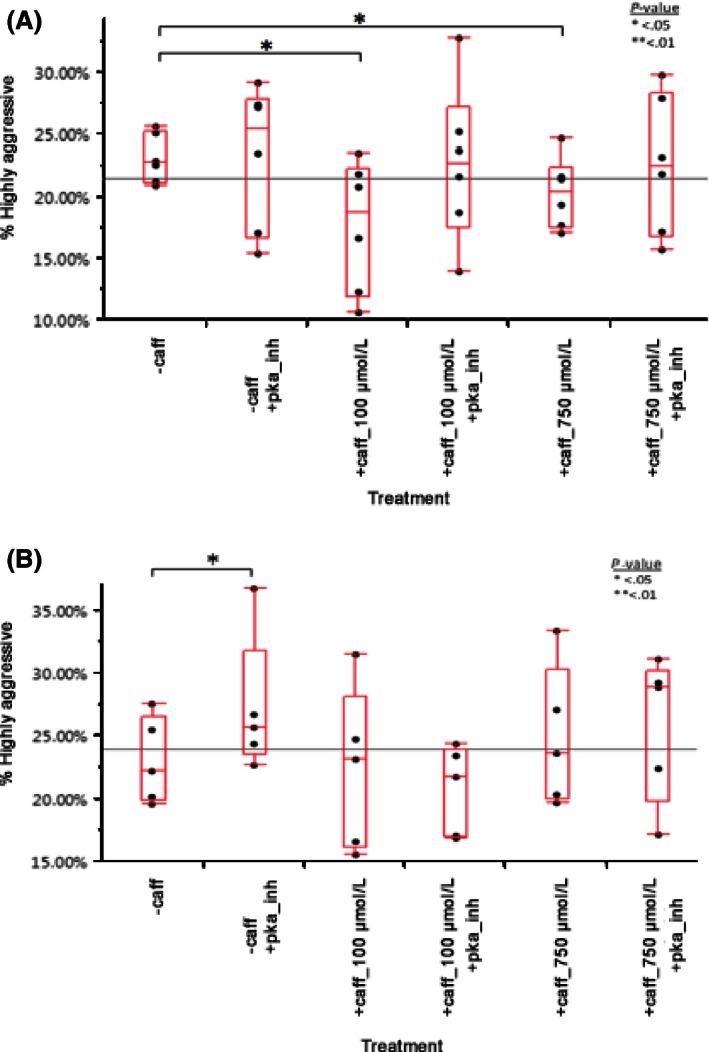
(A) Caffeine reduces mononuclear phagocyte aggressiveness in noncaffeine drinkers at 100 μmol/L (P = 0.0480) and 750 μmol/L (P = 0.0116). Differences report was generated using a matched pair t‐test. n = 6. (B) With caffeine drinkers’ mononuclear phagocytes, there was no significant difference in aggressiveness in any concentration or with PKA inhibitor except a small difference between the control and control + PKA inhibitor (P = 0.0439). This could be due to the inhibition of caffeine already in the serum of these patients. Data generated using a matched pair t‐test. n = 5.
Mononuclear phagocytes treated with 100 μmol/L caffeine (+caff_100 μmol/L) or 750 μmol/L caffeine (+caff_750 μmol/L) showed a 23.5% and 11.7% reduction in aggressiveness, respectively (P = 0.0480 and 0.0116, respectively). However, mononuclear phagocytes treated with caffeine and PKA inhibitor (+pka_inh) showed no significant decrease in aggressiveness (P = 0.8773 and 0.8177, respectively). Figure 5B shows the effect of caffeine treatment on caffeine drinkers’ mononuclear phagocytes on day 1. A significant difference in aggressiveness is seen in the noncaffeine‐treated cells between the untreated mononuclear phagocytes control and untreated + PKA inhibitor control. This may be due to residual caffeine in the serum of these patients.
Figure 6A shows the effect of caffeine treatment on noncaffeine drinkers’ mononuclear phagocyte aggressiveness after day 3 of incubation.
Figure 6.
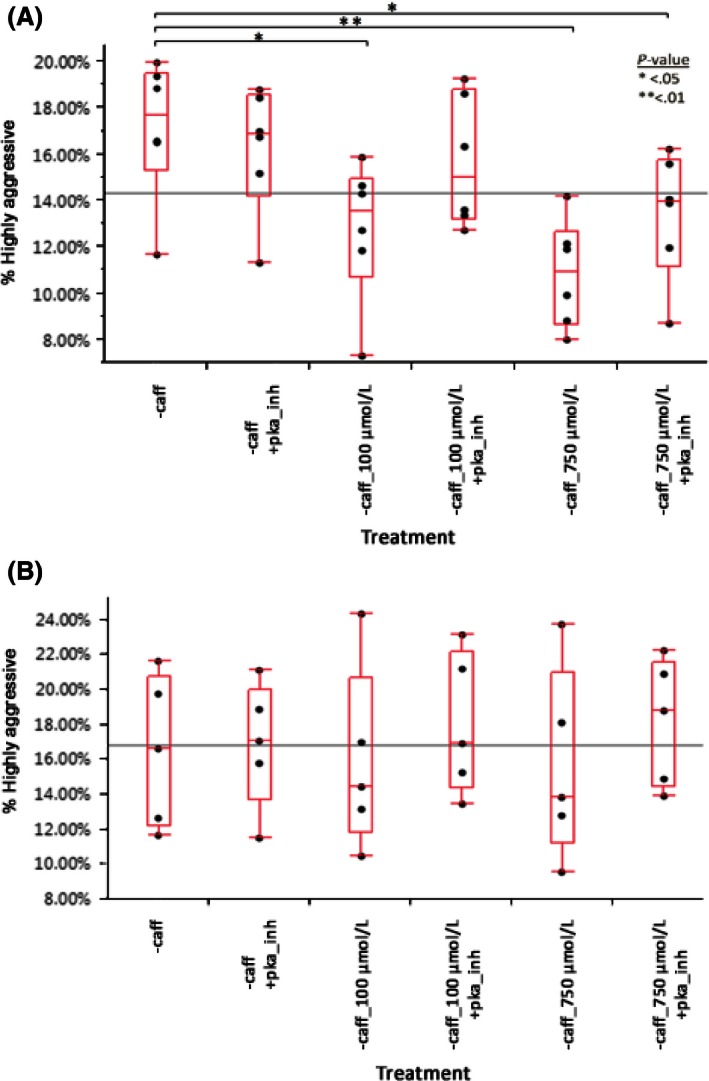
(A) Noncaffeine drinkers’ mononuclear phagocytes show significant reductions in aggressiveness when treated with either 100 μmol/L (P = 0.0314) or 750 μmol/L caffeine (P = 0.0037). Addition of PKA inhibitor reversed this reduction for 100 μmol/L caffeine, but not totally for 750 μmol/L caffeine (P = 0.0117). Data generated using a matched paired t‐test. n = 6. (B) Caffeine drinkers’ mononuclear phagocytes show no sensitivity to caffeine at 100 or 750 μmol/L concentrations. PKA inhibitor also had no effect on these cells’ aggressiveness. Data generated using a matched paired t‐test. n = 5.
Addition of 100 μmol/L caffeine resulted in a 25.4% reduction in aggressiveness (P = 0.0314). Addition of 750 μmol/L caffeine reduced aggressiveness by 36.9% (P = 0.0037). PKA inhibitor prevented the reduction caused by 100 μmol/L caffeine (P = 0.2147), but was unable to completely reverse the effect of 750 μmol/L caffeine, which still had a 21.9% decrease in aggressiveness compared to controls (P = 0.0117). Figure 6B shows caffeine drinkers’ mononuclear phagocyte aggressiveness 3 days poststimulation and after caffeine treatment. No significant difference was observed.
Exercise and caffeine suppression
Figures 7 and 8 show the effect of caffeine on mononuclear phagocytes in exercising versus nonexercising patients.
Figure 7.
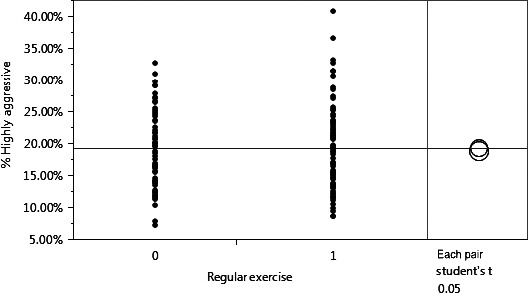
A student's t‐test revealed no effect on overall aggressiveness was observed in exercising versus nonexercising patients (P = 0.5997). n = 5 (nonexerciser) and n = 6 (exerciser). 0 = no regular exercise, 1 = regular exercise.
Figure 8.
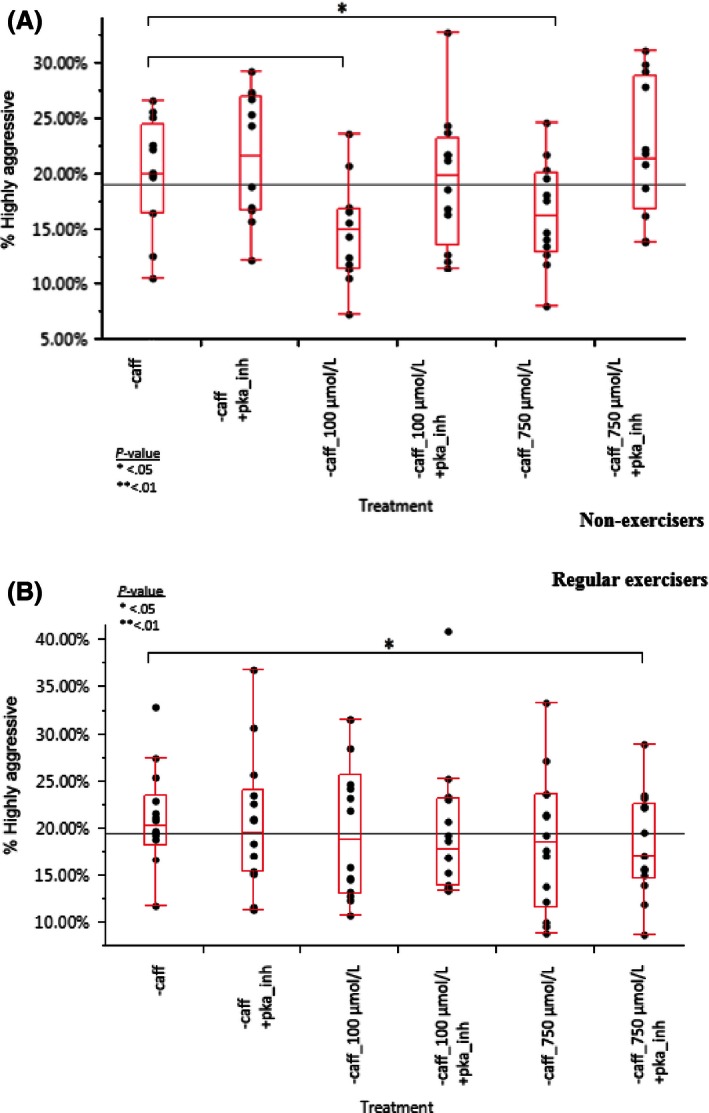
(A) Caffeine has a significant effect on nonexercisers (P = 0.0207 at 100 μmol/L, P = 0.0394 at 750 μmol/L) and PKA inhibitor reversed the effect. However, in regular exercisers (B), caffeine had no significant effect. Data were combined across all days poststimulation to see an overall effect. Differences report generated using a matched pair t‐test.
There was no difference in overall aggressiveness between exercising and nonexercising patients.
However, in nonexercising patients, mononuclear phagocyte aggressiveness significantly decreased after treatment with 100 μmol/L or 750 μmol/L caffeine, by 23.8% (P = 0.0265) and 24.7% (P = 0.0215), respectively. Pretreatment with PKA inhibitor prevented this effect (P = 0.8687, and 0.2796, respectively). Caffeine intake was not suspected to be a confounding variable since both regular exercisers and nonexercisers both had an equal ratio of caffeine/noncaffeine drinkers and beta regression revealed no significant interaction. This finding adds confidence to recent suspicions that regular exercise may boost immune robustness and protect against immunosuppression by modulators such as caffeine (Starnbach et al. 2010).
Discussion
The regulation of mononuclear phagocyte activation is a complex and dynamic process. It involves many moving pieces that interact at varying time points and concentrations. Signaling events can affect many downstream pathways that may contradict each other, resulting in inconsistent results. Pharmacologic manipulation of these signaling events is heavily dependent on dosage and timing, as well as the state of the cell at the time of exposure. Caffeine, a neurostimulant and analog of adenine, is thought to have an effect on immune function. This study investigated the effect of caffeine on the inflammatory profile of mononuclear phagocytes.
Although antagonism of ARs is expected to prolong proinflammatory responses, the literature suggests that caffeine effectively suppresses the expression of proinflammatory cytokines (Link et al. 2000; Horrigan et al. 2004). This suppression may be due to increased caffeine's function as phosphodiesterase inhibitor that causes a rise in cAMP levels. Higher cAMP levels activate downstream targets like PKA, which will cause suppression of some proinflammatory pathways. (Horrigan et al. 2004). Here, we suggest that caffeine also suppresses phagocytosis by inhibiting cAMP phosphodiesterase. This effect is further complicated by regular dietary caffeine intake and fitness level of the patient. These results suggest that at these concentrations, caffeine's effect on ARs is not phenotypically manifest.
We observe that caffeine suppresses phagocytosis at 100 μmol/L in mononuclear phagocytes at day 1 and at day 3 after isolation (Fig. 4). Interestingly, we observe a slight increase between phagocytic activities at 750 μmol/L caffeine compared to 100 μmol/L caffeine treatments at day 1. We think that this increase in aggressiveness is due to caffeine saturating ARs. At 100 μmol/L, there isn't enough AR saturation by caffeine, therefore the effects of caffeine as a phosphodiesterase inhibitor increase cAMP levels, leading to a reduction in inflammation (less aggressive phagocytes) while the saturation of ARs at 750 μmol/L increases inflammation regardless of the phosphodiesterase inhibitor action of caffeine, thus restoring phagocyte aggressiveness.
In Figure 5B we observe that in the caffeine drinkers’ mononuclear phagocytes (day 1), there was a significant difference between the control and the control + PKA inhibitor, whereas in Figure 6B (day 3) the same treatment shows no significant difference. The results from day 1 suggest that phagocytic inhibition may be due to the caffeine already present in the serum of patients while the results from day 3 may confirm a form of caffeine tolerance that has been shown to exist in chronic caffeine drinkers (Corti 2002).
It is difficult to assess the practical significance of phagocytic suppression, but our previous work (unpublished) has shown that cancer cells can suppress phagocytosis in a similar manner. If this is the case, caffeine may be aiding in the suppression of immune response in the tumor microenvironment. It has long been thought that regular exercise could boost immune robustness, and our results confirm that higher fitness levels prevent immune suppression by caffeine. However, this study only shows preliminary data on the effects of caffeine on phagocytic suppression of mononuclear phagocytes and further studies will need to include a greater sample size and number of experimental runs, which will provide much more representative data and better inform the average consumer on the role of caffeine as a modulator of the immune system and the health risks and benefits associated to caffeine consumption.
Author Contributions
R P Steck designed and performed experiments and all aspects of data accrual and interpretation and wrote the manuscript.
S L Hill contributed to experimental design and data interpretation, performed experiments and helped revise the manuscript.
E G Weagel contributed to experimental design and data interpretation, helped draft and revise the manuscript.
K S Weber contributed to experimental design, provided critical feedback on the experimental work, revised the work critically and helped revise the manuscript.
R A Robison contributed to experimental design, provided critical feedback, revised the work critically and helped revise the manuscript.
K L O'Neill contributed to the conception and design of the experiment, provided supervision of the project, revised the work critically and helped revise the manuscript.
All authors have given substantial contribution to the design on this project, revising it, and have given their final approval of this publication. Equally, they all assume full accountability for all aspects of this paper and its claims.
Disclosures
None declared.
Acknowledgements
We acknowledge the Simmons Center for Cancer Research for providing the funding for this project.
Steck R. P., Hill S. L., Weagel E. G., Scott Weber K., Robison R. A., O'Neill K. L.. Pharmacologic immunosuppression of mononuclear phagocyte phagocytosis by caffeine, Pharma Res Per, 3(6), 2015, e00180, doi: 10.1002/prp2.180
References
- Ayala A, Chaudry IH (1996). Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock 6: S27–S38. [PubMed] [Google Scholar]
- Chawla A (2010). Control of macrophage activation and function by PPARs. Circ Res 106: 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Brown BD, Merad M (2011). Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol 11: 788–798. [DOI] [PubMed] [Google Scholar]
- Corti R (2002). Coffee acutely increases sympathetic nerve activity and blood pressure independently of caffeine content: role of habitual versus nonhabitual drinking. Circulation 106: 2935–2940. [DOI] [PubMed] [Google Scholar]
- Csóka B, Németh ZH, Selmeczy Z, Koscsó B, Pacher P, Vizi ES, et al. (2007). Role of A(2A) adenosine receptors in regulation of opsonized E. coli‐induced macrophage function. Purinergic Signal 3: 447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinee SM, Karsan A (2006). Lipopolysaccharide signaling in endothelial cells. Lab Invest 86: 9–22. [DOI] [PubMed] [Google Scholar]
- van Furth AM, Verhard‐Seijmonsbergen EM, van Furth R, Langermans JA (1997). Effect of lisofylline and pentoxifylline on the bacterial‐stimulated production of TNF‐alpha, IL‐1 beta IL‐10 by human leucocytes. Immunology 91: 193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RM (1976). Caffeine as a drug of abuse. Res Adv Drug Alchohol Probl 3: 49–77. [Google Scholar]
- Horrigan LA, Kelly JP, Connor TJ (2004). Caffeine suppresses TNF‐alpha production via activation of the cyclic AMP/protein kinase A pathway. Int Immunopharmacol 4: 1409–1417. [DOI] [PubMed] [Google Scholar]
- Hume DA (2008). Differentiation and heterogeneity in the mononuclear phagocyte system. Mucosal Immunol 1: 432–441. [DOI] [PubMed] [Google Scholar]
- Janeway CAJ, Travers P, Walport M, Shlomchik MJ. (2001). Appendix II. CD Antigens. New York, Garland Science, http://www.ncbi.nlm.nih.gov/books/NBK10772/, 03/09/2014 [Google Scholar]
- Jenkins SJ, Hume DA (2014). Homeostasis in the mononuclear phagocyte system. Trends Immunol 35: 358–367. [DOI] [PubMed] [Google Scholar]
- Khazen W, M'bika J‐P, Tomkiewicz C, Benelli C, Chany C, Achour A, et al. (2005). Expression of macrophage‐selective markers in human and rodent adipocytes. FEBS Lett 579: 5631–5634. [DOI] [PubMed] [Google Scholar]
- Kreckler LM, Wan TC, Ge Z‐D, Auchampach JA (2006). Adenosine inhibits tumor necrosis factor‐alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther 317: 172–180. [DOI] [PubMed] [Google Scholar]
- Lewis KL, Reizis B (2012). Dendritic cells: arbiters of immunity and immunological tolerance. Cold Spring Harb Perspect Biol 4: a007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link AA, Kino T, Worth JA, McGuire JL, Crane ML, Chrousos GP, et al. (2000). Ligand‐activation of the adenosine A2a receptors inhibits IL‐12 production by human monocytes. J Immunol 164: 436–442. [DOI] [PubMed] [Google Scholar]
- Mandel HG (2002). Update on caffeine consumption, disposition and action. Food Chem Toxicol 40: 1231–1234. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002). Macrophage polarization: tumor‐associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23: 549–555. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004). The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Locati M (2005). Macrophage polarization comes of age. Immunity 23: 344–346. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A (2006). Transcriptional profiling of the human monocyte‐to‐macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177: 7303–7311. [DOI] [PubMed] [Google Scholar]
- Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, et al. (2010). Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 70: 5728–5739. [DOI] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M (2001). Role of G‐protein‐coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414: 916–920. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW (1981). Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci USA 78: 3260–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnbach M, Allison KC, Bling C, Leighton S. (2010). The truth about your immune system. Harv Med School 30–35. [Google Scholar]
- Wahl L, Wahl S. (2006). Isolation of human monocyte populations. Curr Protoc 70:I:7.6A: 1–10. [DOI] [PubMed] [Google Scholar]


