Abstract
Severe ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) deficiency causes thrombotic thrombocytopenic purpura (TTP), which is characterized by microangiopathic hemolytic anemia, thrombocytopenia, and the absence of oliguric or anuric renal failure. However, some patients with this constellation of findings do not have ADAMTS13 deficiency, and some patients with ADAMTS13 deficiency have renal failure or relatively normal blood counts. Consequently, many investigators and clinicians have incorporated severe ADAMTS13 deficiency into the case definition of TTP. This change has facilitated the timely initiation of treatment for patients with atypical clinical features who otherwise would not be recognized as having TTP. Conversely, excluding severe ADAMTS13 deficiency focuses attention on the diagnosis and treatment of other causes of thrombotic microangiopathy that require different treatment. The rapid return of ADAMTS13 data is important to make the best use of this information.
During the past 20 years, thrombotic thrombocytopenic purpura (TTP) has morphed from a clinical diagnosis of exclusion into a pathophysiologic diagnosis based on specific laboratory results. As a consequence of advances in diagnosis, we are learning the limitations of traditional clinical criteria for TTP while also devising better treatments by targeting the mechanism of disease.
Pathophysiology of TTP
Failure to regulate von Willebrand factor (VWF)-dependent platelet adhesion causes the widespread microvascular thrombosis that characterizes TTP (Figure 1). After secretion, long VWF multimers are released into the blood, and some remain adherent to endothelial cells. Platelets bind to VWF on endothelial cells or at sites of vascular injury and can form aggregates large enough to cause microangiopathic hemolysis, consume platelets, and induce tissue ischemia or infarction. These consequences are prevented by the plasma metalloprotease ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), which cleaves a specific Tyr-Met bond in the A2 domain of VWF. This bond is buried in native VWF but is exposed when VWF is stretched by fluid shear force, especially as blood flows through a growing platelet-rich thrombus. Homozygous or compound heterozygous ADAMTS13 mutations that result in loss of function disrupt this feedback regulation and cause congenital TTP or Upshaw–Schulman syndrome. Autoantibodies that inhibit ADAMTS13 or clear it from the blood cause acquired TTP.1
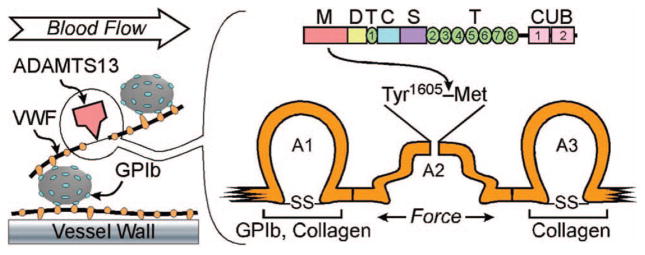
Figure 1.
VWF-dependent platelet adhesion and TTP. VWF multimers adhere to endothelial cell surfaces or to subendothelial connective tissue through A1 or A3 domains (orange) in each VWF subunit. Platelets adhere to the VWF A1 domain through platelet membrane glycoprotein Ib (GPIb, blue). Flowing blood exerts a force on the platelet that stretches the attached VWF multimer. A1 and A3 domains have a disulfide bond (SS) that stabilizes them against this force. Without such a disulfide bond, the A2 domain unfolds and exposes the Tyr1605-Met bond to cleavage by ADAMTS13, which releases adherent platelets and prevents microvascular thrombosis. Without sufficient ADAMTS13 activity, thrombi grow large enough to cause TTP. ADAMTS13 consists of metalloprotease (M), disintegrin-like (D), thrombospondin 1-like (T), cysteine-rich (C), spacer (C), and CUB [complement C1r/C1s, Uegf, Bmp1] domains.
Diagnosing TTP
TTP has been defined clinically as microangiopathic hemolytic anemia and thrombocytopenia, without disseminated intravascular coagulation or another apparent cause and without acute renal failure at presentation. This collection of findings can occur in many illnesses, but across several studies, ~80% of patients that fit this case definition (with a broad range of 33% to 100%) turn out to have severe ADAMTS13 deficiency (<10% of normal), usually associated with acquired TTP in adults. Conversely, a small fraction of patients with autoimmune ADAMTS13 deficiency do not meet this definition of acquired TTP because they do have renal insufficiency, as discussed below. Congenital TTP is much less common than acquired TTP.1
Other clinical and laboratory features that correlate with severe ADAMTS13 deficiency include blood group O, preserved renal function, severe thrombocytopenia, African ancestry, female gender, obesity, and the absence of systemic infection, cancer, or drugs associated with renal injury.2–4 Some combinations of routine laboratory tests are fairly good predictors of severe ADAMTS13 deficiency. For example, in one study of 214 patients with thrombotic microangiopathy, a platelet count <30 000/μL and serum creatinine ≤200 μM (2.26 mg/dL) occurred in 157 of 160 patients with severe ADAMTS13 deficiency and did not occur in 48 of 54 patients without severe ADAMTS13 deficiency.2 However, algorithms based on clinical or laboratory criteria always miscategorize some patients, and the case definition of TTP has evolved to include ADAMTS13 activity <10% along with microangiopathic hemolytic anemia and thrombocytopenia.5 This change is appropriate because thrombotic microangiopathy without severe ADAMTS13 deficiency often requires treatment other than plasma exchange. Therefore, ADAMTS13 testing should be performed if possible for all patients who could have TTP.
The clinical spectrum of TTP
Because ADAMTS13 testing has been applied more widely in suspected thrombotic microangiopathy, the association of ADAMTS13 deficiency with severe thrombocytopenia and preserved renal function has survived, but exceptions have proved to be more common than expected. These cases demonstrate the value of laboratory testing for identifying atypical presentations of TTP.
Renal failure
The urinalysis is usually abnormal with microhematuria, hemoglobinuria, proteinuria, and casts, but acute renal failure is not a classic feature of TTP. On the contrary, thrombotic microangiopathy with acute oliguric or anuric renal failure is referred to as hemolytic uremic syndrome (HUS), which occurs in two major subtypes. Ingestion of Shiga toxin-producing Escherichia coli (STEC) causes STEC–HUS with a typical prodrome of abdominal pain and bloody diarrhea. Atypical HUS (aHUS) is “atypical” because it is not preceded by painful blood diarrhea, and aHUS often is caused by inherited mutations in proteins of the alternative complement pathway or by autoantibodies against complement factor H.
Although the presence or absence of renal failure correlates with the underlying pathophysiology of HUS or TTP, the correspondence is imperfect, and recent studies that rely on ADAMTS13 deficiency as a diagnostic criterion describe a surprisingly high incidence of acute renal failure in TTP. In a series of 92 patients with TTP, 27% had stage 3 acute kidney injury (serum creatinine ≥4 mg/dL or ≥3-fold increased over baseline, with oliguria or anuria), and 15% required renal replacement therapy. Six months after successful treatment, one patient had an estimated glomerular filtration rate of 15 to 29 mL/min, and 3 patients required long-term dialysis. Two of the patients on dialysis had systemic lupus erythematosis, and one had Sjogren syndrome, suggesting an alternative potential cause for renal failure.6 In another series of 60 patients with TTP, 6 had moderately severe acute renal failure (an increase in serum creatinine ≥1 mg/dL above baseline or ≥4 mg/dL plus dialysis within 7 days of diagnosis); only 2 of these patients survived, and they eventually regained normal renal function.3 Significant renal disease also occurs sometimes in congenital TTP, especially if patients have a catastrophic first event or a chronic relapsing course before the diagnosis is made and plasma treatment is initiated.7 Thus, renal failure is unusual in TTP but does not exclude the diagnosis.
Conversely, although oliguric or anuric renal failure is characteristic of aHUS, in one series, ~20% of patients with aHUS had a normal serum creatinine at presentation.8 The incidence of renal failure depends on the underlying complement abnormality, and some patients, particularly those with MCP mutations, never develop renal failure.9,10 Consequently, ADAMTS13 testing is crucial because patients with severe ADAMTS13 deficiency are likely to respond to plasma exchange, whereas those without severe ADAMTS13 deficiency may have complement-driven disease that will respond to eculizumab.
Neurological involvement
Similar concerns apply to neurological symptoms. Recent case series report neurological findings at presentation in ~50% of patients with acquired TTP2,3 and ~35% of patients with congenital TTP.7 However, symptoms ranging from confusion to stroke, coma, and seizures have been reported in 10% to 30% of patients with aHUS.9,10 Severe neurological symptoms also occur in at least 10% to 30% of patients with STEC–HUS and are more common in older patients.11,12 Admittedly, comparisons among these reports are difficult because they may use different criteria for neurological involvement, and attributing some symptoms to neurological injury is subjective. Nevertheless, neurological symptoms do not require severe ADAMTS13 deficiency.
Tissue injury without hemolysis or thrombocytopenia
The advent of effective plasma exchange therapy led to a sharp decline in the progression of signs and symptoms beyond microangiopathic hemolysis and thrombocytopenia, and the prevention of renal and neurological injury became an important goal of therapy. Now, experience with ADAMTS13 testing suggests that life-threatening microvascular thrombosis can occur even without hemolysis or thrombocytopenia.
For example, a 40-year-old woman with no signs of thrombotic microangiopathy had a myocardial infarction caused by a right coronary artery thrombus. She returned 10 days later with acute right posterior and middle cerebral infarcts, many schistocytes, and severe thrombocytopenia, at which time ADAMTS13 was undetectable.13 At least 13 other women have been reported to present with stroke symptoms but without microangiopathic hemolytic anemia, usually with a normal platelet count and normal lactate dehydrogenase, and were later found to have severe ADAMTS13 deficiency. For 6 of these patients, a remote history of TTP prompted immediate ADAMTS13 testing and treatment with plasma exchange. All responded with resolution of symptoms. Two others were treated with a delay of 1 to 3 weeks when their platelet count decreased and schistocytes appeared. For the 5 women with no previous history of TTP, treatment was delayed until they finally developed microangiopathic hemolysis and thrombocytopenia 8 days to 8 months after presenting with stroke symptoms.13–17
These cases exhibit thrombotic features that seem atypical because they occurred independent of microangiopathic hemolysis or thrombocytopenia. However, they are compatible with the pathophysiology of TTP, which involves the inadequate regulation of VWF-dependent platelet adhesion. Excessive platelet adhesion can cause thrombocytopenia. Platelet-rich thrombi or strands of VWF can disrupt blood flow enough to cause hemolysis and produce schistocytes. However, life-threatening injury of the brain or heart requires only one well-placed thrombus, which need not be accompanied by other signs of thrombotic microangiopathy. Thus, the archetypal clinical features of TTP can occur singly or in any combination, and sometimes ADAMTS13 testing is the only way to identify a patient who should receive plasma exchange.
Arterial and venous thrombosis
TTP is characterized by microvascular thrombosis, but occasionally patients also have macrovascular thrombosis. For example, cardiac injury usually reflects small vessel disease, but proximal coronary artery thrombosis has been described in TTP.13 As might be expected for microvascular thrombosis, neurological findings in TTP frequently cannot be explained by a simple ischemic event. However, patients may also have neurological deficits and imaging studies consistent with the occlusion of large cerebral arteries.18 Angiography often fails to show a responsible thrombus, but thrombi have been visualized in proximal middle cerebral artery segments of several patients with acquired or familial TTP and stroke.15,17,19
Venous thromboembolism also has been reported in TTP. In a series of 36 patients with acquired TTP and undetectable ADAMTS13 activity, 10 had central catheter-related thrombosis, 4 had deep venous thrombosis, and 4 had pulmonary embolism.18 In another series of 68 adults with TTP, 7 patients had thromboembolic events, including 5 deep venous thromboses (all associated with central venous catheters) and 3 pulmonary emboli.20 A male with congenital TTP had a splenectomy at age 18 years, and during the following 18 months had several pulmonary emboli, resulting in chronic oral anticoagulant therapy.21 Whether these episodes of venous thromboembolism can be attributed directly to the pathophysiology of TTP is uncertain, but the incidence is high enough to consider routine thromboprophylaxis when the platelet count exceeds a minimum threshold such as 50 000 platelets/μL.
ADAMTS13 assay methods
Plasma ADAMTS13 activity is usually assayed by diluting citrated plasma into a reaction mixture that contains a fluorogenic peptide substrate, FRETS-VWF73. Cleavage of the substrate increases its fluorescence, and the rate of increase is proportional to the concentration of active ADAMTS13.22 Another common assay is not based on fluorescence but instead uses an enzyme-linked monoclonal antibody to detect the new C terminus produced when ADAMTS13 cleaves the substrate.23 These and similar assays have very good reproducibility, although the lower limit of detection is rather close to the threshold of 5% to 10% ADAMTS13 activity for diagnosing TTP. As a consequence, substantial skill is required to perform these assays reliably enough for diagnostic use.
In practice, laboratories often begin by measuring only ADAMTS13 activity. Values are expressed as a fraction or percentage of the ADAMTS13 activity in pooled normal plasma, defined as 1 U/mL or 100%. Ideally, the laboratory will calibrate its assay against the new World Health Organization international standard ADAMTS13 plasma.24 ADAMTS13 levels are strikingly bimodal in patients thought to have thrombotic microangiopathy. Almost all patients diagnosed with TTP have ADAMTS13 <5%, and almost all of the remaining patients have ADAMTS13 levels >25%.2,3
If the ADAMTS13 activity is less than a predefined cutoff, perhaps 30% of normal plasma, then the sample is assayed for ADAMTS13 inhibitors by a method analogous to that used for factor VIII inhibitors in hemophilia, in which an inhibitor of 1 U reduces the activity in an equal volume of normal plasma by 50%. Polyclonal antibodies that inhibit ADAMTS13, usually immunoglobulin (Ig) G but sometimes IgA or IgM, are detected in at least 65% of patients with acquired TTP. So far, only a single patient with congenital TTP has been reported to develop an apparent alloantibody to ADAMTS13.25 Therefore, severe ADAMTS13 deficiency with an inhibitor supports a diagnosis of acquired TTP.
If no ADAMTS13 inhibitor is detected, some laboratories then assay for antibodies that bind ADAMTS13 with an enzyme-linked immunosorbent assay or Western blotting method. Anti-ADAMTS13 antibodies are found in almost all patients with acquired TTP. However, up to 4% of healthy controls and 13% of patients with systemic lupus erythematosis have levels of anti-ADAMTS13 antibodies that overlap with the range observed in TTP despite also having normal ADAMTS13 activity.26 Conversely, severe ADAMTS13 deficiency without anti-ADAMTS13 antibodies is consistent with a diagnosis of congenital TTP, which may be confirmed by genetic testing or a rapid complete response to simple plasma infusion.
ADAMTS13 antigen can also be measured and may be useful to compare with ADAMTS13 activity. ADAMTS13 antigen can be much greater than activity if an inhibitor is present that does not also promote ADAMTS13 clearance.
Limitations of ADAMTS13 assays
ADAMTS13 deficiency may be a hallmark of TTP, but the assays available to demonstrate it are not perfect. Most assays are done at pH 6 and low ionic strength, conditions under which ADAMTS13 has maximal activity, to achieve the sensitivity required to diagnose TTP.22,23 Patient plasma also is diluted 15-fold to 30-fold to minimize interference from colored compounds, such as bilirubin and free hemoglobin.22
In principle, the nonphysiologic pH, low ionic strength, and high dilution might promote the dissociation of inhibitory antibodies from ADAMTS13, thereby increasing ADAMTS13 activity artifactually. Thus, a patient could have severe ADAMTS13 deficiency in vivo but only moderately low or normal ADAMTS13 activity in the laboratory. One instance has been reported of a patient with relapsing TTP who initially had normal plasma ADAMTS13 activity but developed severe ADAMTS13 deficiency with episodes 4, 5, and 6 of disease. Examination of the progress curves for plasma samples from episodes 1 and 3 shows that the rate of substrate cleavage increased substantially during the assay, indicating that dilution allowed dissociation of a clinically important inhibitor.27 With one such case reported during 20 years of ADAMTS13 testing, the frequency of this problem is unknown and likely to be very low, but the possibility should be remembered.
Extensive dilution of plasma in ADAMTS13 assays also necessarily impairs the detection of autoantibody inhibitors. Both decreased sensitivity for inhibitors, and potential overestimation of ADAMTS13 activity could be avoided by performing assays with minimally diluted plasma. Several assays use such conditions or could be adapted to them23,28 but are not widely available.
Conditions other than TTP that may affect ADAMTS13
A few conditions other than TTP are associated with variably decreased ADAMTS13 activity but rarely cause severe ADAMTS13 deficiency and generally do not pose a diagnostic dilemma.
Hemolytic anemias
In addition to interfering with product detection for some ADAMTS13 assays,28 hemoglobin directly inhibits ADAMTS13 with an IC50 of ~5 to ~15 mg/mL. Moderately decreased AD-AMTS13 activity has been reported in some patients with malaria29,30 and sickle cell disease,31 but ADAMTS13 <10% does not seem to occur. Plasma hemoglobin rarely exceeds 1 mg/mL in these conditions, which appears insufficient to inhibit ADAMTS13. Although not yet reported, ADAMTS13 might be inhibited in vivo by extremely high free hemoglobin concentrations in other settings, such as hemolytic transfusion reactions.
Hyperbilirubinemia
Bilirubin often is increased in thrombotic microangiopathy, and concentrations >10 mg/dL can interfere with some fluorogenic ADAMTS13 assays.32 Whether bilirubin also inhibits ADAMTS13 is not settled. In two reports, bilirubin had no effect at 20 mg/mL28 or 40 mg/mL,32 but in one report, unconjugated bilirubin partially inhibited ADAMTS13 ~60%, with an IC50 of 10 mg/mL.33 These inconsistencies probably depend on specific features of the assay methods.
Liver failure
Hepatic stellate cells synthesize ADAMTS13, and plasma ADAMTS13 activity correlates inversely with the severity of liver disease. For example, ~10% of patients with severe cirrhosis (Child class C) may have ADAMTS13 activity <10%.34 However, preexisting cirrhosis does not exclude the possibility of typical acquired TTP with severe ADAMTS13 deficiency that will respond to plasma exchange.
Profound ADAMTS13 deficiency can occur during liver transplantation but resolves within the first postoperative day if the transplanted liver functions properly.35
Sepsis and multiorgan failure
ADAMTS13 activity tends to decrease modestly in patients with sepsis, disseminated intravascular coagulation, severe inflammatory response syndrome, or multiorgan failure. However, the incidence of severe ADAMTS13 deficiency in these conditions is uncertain. Some studies, covering hundreds of patients in total, report severe ADAMTS13 deficiency in ~9% of them,36–38 whereas others report no instances of severe ADAMTS13 deficiency.39,40 Differences in the study populations, possibly the prevalence of liver failure, may account for the different results.
Using ADAMTS13 data to diagnose TTP
The better we define the molecular pathophysiology of thrombotic microangiopathy, the less surprised we are about variations in the clinical features of these disorders. As a consequence, laboratory testing to identify the underlying mechanism is supplementing or superseding our customary dependence on signs and symptoms that typify TTP, STEC–HUS, and aHUS. Compared with other causes of thrombotic microangiopathy, TTP is relatively easy to diagnose because, despite a few caveats, severe ADAMTS13 deficiency is both sensitive and specific for congenital or acquired TTP.
In many hospitals, ADAMTS13 assays are sent out to a reference laboratory and the results return many days later. Consequently, the initial diagnosis of TTP must be based mainly on the presence of microangiopathic hemolysis, severe thrombocytopenia, and preserved renal function (Figure 2A). This approach is effective but misclassifies a significant number of patients, especially those with unusual clinical features. Patients with severe ADAMTS13 deficiency who happen to have renal failure can be misdiagnosed with HUS, and those without schistocytes or thrombocytopenia will be overlooked entirely. Conversely, patients with aHUS but little renal injury may be diagnosed with TTP. As a result, some patients will not receive appropriate treatment in a timely manner.
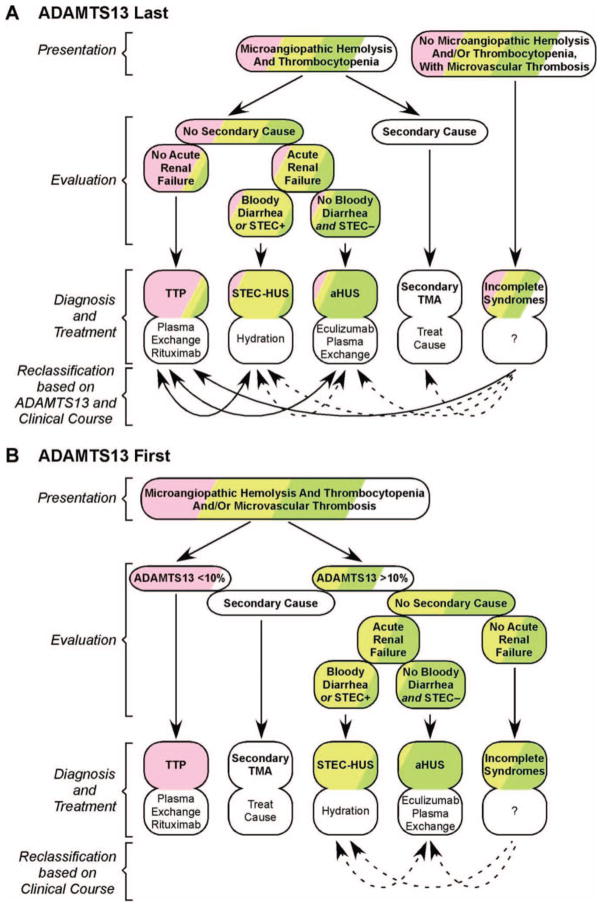
Figure 2.
Diagnosing TTP. (A) Without immediate access to ADAMTS13 data, other criteria must be used to diagnose patients with microangiopathic hemolysis and thrombocytopenia who may have TTP (pink), STEC–HUS (yellow), aHUS (green), or secondary thrombotic microangiopathy (TMA; white). This approach misclassifies some patients. In addition, a few patients without microangiopathic hemolysis or thrombocytopenia will not be recognized promptly. Some of these errors can be corrected when ADAMTS13 results become available (solid arrows), and others become evident from the clinical course (dotted arrows). (B) Immediate access to ADAMTS13 data prevents the misclassification of patients with TTP, after excluding rare secondary causes of ADAMTS13 deficiency, such as liver failure. In addition, immediate testing facilitates the identification of patients with atypical presentations of ADAMTS13 deficiency, including the absence of microangiopathic hemolysis or thrombocytopenia.
Many of these misdiagnoses can be avoided if ADAMTS13 data are available on the day of presentation (Figure 2B). For patients with microangiopathic hemolysis and thrombocytopenia, a finding of severe ADAMTS13 deficiency supports a diagnosis of congenital or acquired TTP. Other potential causes of low ADAMTS13, such as liver failure or sepsis, seldom cause diagnostic problems. Ideally, ADAMTS13 assays should be performed on samples obtained before the initiation of plasma therapy, which can increase ADAMTS13 levels. However, the majority of patients with acquired TTP still have ADAMTS13 <10% even after 3 daily plasma exchange treatments. Consequently, assays of samples obtained suboptimally during treatment can be diagnostically useful.41
If a patient has a normal complete blood count but also has neurological deficits or cardiac injury, TTP may still be suspected if the patient has other risk factors for TTP or has had TTP in the past, even if they happen to have other risk factors for cardiovascular disease. Rapid ADAMTS13 testing can settle the question and expedite treatment. Conversely, excluding severe ADAMTS13 deficiency focuses attention on distinguishing patients who need mainly supportive care (STEC–HUS), eculizumab (aHUS), or treatment of an underlying disorder (secondary thrombotic microangiopathy).
Learning Objectives.
To understand the role of ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) and von Willebrand factor in the pathogenesis of thrombotic thrombocytopenic purpura (TTP)
To appreciate the strengths and limitations of ADAMTS13 assays
To understand how ADAMTS13 data can be used to diagnose TTP and identify patients with other causes of thrombotic microangiopathy
Footnotes
Conflict-of-interest disclosure: The author is on the board of directors or an advisory committee for Baxter HealthCare and XO1 Limited and has consulted for Band Therapeutics and BioMarin.
References
- 1.Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112(1):11–18. doi: 10.1182/blood-2008-02-078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coppo P, Schwarzinger M, Buffet M, et al. Predictive features of severe acquired ADAMTS13 deficiency in idiopathic thrombotic microangiopathies: the French TMA reference center experience. PLoS One. 2010;5(4):e10208. doi: 10.1371/journal.pone.0010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kremer Hovinga JA, Vesely SK, Terrell DR, Lammle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115(8):1500–1511. doi: 10.1182/blood-2009-09-243790. [DOI] [PubMed] [Google Scholar]
- 4.Terrell DR, Motto DG, Kremer Hovinga JA, Lammle B, George JN, Vesely SK. Blood group O and black race are independent risk factors for thrombotic thrombocytopenic purpura associated with severe ADAMTS13 deficiency. Transfusion. 2011;51(10):2237–2243. doi: 10.1111/j.1537-2995.2011.03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654–666. doi: 10.1056/NEJMra1312353. [DOI] [PubMed] [Google Scholar]
- 6.Zafrani L, Mariotte E, Darmon M, et al. Acute renal failure is prevalent in patients with thrombotic thrombocytopenic purpura associated with low plasma ADAMTS13 activity. J Thromb Haemost. 2015;13(3):380–389. doi: 10.1111/jth.12826. [DOI] [PubMed] [Google Scholar]
- 7.Loirat C, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura in children. Curr Opin Pediatr. 2013;25(2):216–224. doi: 10.1097/MOP.0b013e32835e7888. [DOI] [PubMed] [Google Scholar]
- 8.Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA, et al. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007;18(8):2392–2400. doi: 10.1681/ASN.2006080811. [DOI] [PubMed] [Google Scholar]
- 9.Fremeaux-Bacchi V, Fakhouri F, Garnier A, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8(4):554–562. doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5(10):1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braune SA, Wichmann D, von Heinz MC, et al. Clinical features of critically ill patients with Shiga toxin-induced hemolytic uremic syndrome. Crit Care Med. 2013;41(7):1702–1710. doi: 10.1097/CCM.0b013e31828a24a8. [DOI] [PubMed] [Google Scholar]
- 12.Takanashi J, Taneichi H, Misaki T, et al. Clinical and radiologic features of encephalopathy during 2011 E coli O111 outbreak in Japan. Neurology. 2014;82(7):564–572. doi: 10.1212/WNL.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 13.Imanirad I, Rajasekhar A, Zumberg M. A case series of atypical presentations of thrombotic thrombocytopenic purpura. J Clin Apher. 2012;27(4):221–226. doi: 10.1002/jca.21216. [DOI] [PubMed] [Google Scholar]
- 14.Htun KT, Davis AK. Neurological symptoms as the sole presentation of relapsed thrombotic thrombocytopenic purpura without microangiopathic haemolytic anaemia. Thromb Haemost. 2014;112(4):838–840. doi: 10.1160/TH14-04-0359. [DOI] [PubMed] [Google Scholar]
- 15.Idowu M, Reddy P. Atypical thrombotic thrombocytopenic purpura in a middle-aged woman who presented with a recurrent stroke. Am J Hematol. 2013;88(3):237–239. doi: 10.1002/ajh.23249. [DOI] [PubMed] [Google Scholar]
- 16.Rojas JC, Banerjee C, Siddiqui F, Nourbakhsh B, Powell CM. Pearls and oysters: acute ischemic stroke caused by atypical thrombotic thrombocytopenic purpura. Neurology. 2013;80(22):e235–e238. doi: 10.1212/WNL.0b013e318294b423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sevy A, Doche E, Squarcioni C, et al. Stroke in a young patient treated by alteplase heralding an acquired thrombotic thrombocytopenic purpura. J Clin Apher. 2011;26(3):152–155. doi: 10.1002/jca.20276. [DOI] [PubMed] [Google Scholar]
- 18.Camous L, Veyradier A, Darmon M, et al. Macrovascular thrombosis in critically ill patients with thrombotic microangiopathies. Intern Emerg Med. 2014;9(3):267–272. doi: 10.1007/s11739-012-0851-4. [DOI] [PubMed] [Google Scholar]
- 19.Kelly PJ, McDonald CT, Neill GO, Thomas C, Niles J, Rordorf G. Middle cerebral artery main stem thrombosis in two siblings with familial thrombotic thrombocytopenic purpura. Neurology. 1998;50(4):1157–1160. doi: 10.1212/wnl.50.4.1157. [DOI] [PubMed] [Google Scholar]
- 20.Yarranton H, Cohen H, Pavord SR, Benjamin S, Hagger D, Machin SJ. Venous thromboembolism associated with the management of acute thrombotic thrombocytopenic purpura. Br J Haematol. 2003;121(5):778–785. doi: 10.1046/j.1365-2141.2003.04360.x. [DOI] [PubMed] [Google Scholar]
- 21.Bennett M, Chubar Y, Gavish I, Aviv A, Stemer G, Chap-Marshak D. Experiences in a family with the Upshaw-Schulman syndrome over a 44-year period. Clin Appl Thromb Hemost. 2014;20(3):296–303. doi: 10.1177/1076029613495309. [DOI] [PubMed] [Google Scholar]
- 22.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129(1):93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 23.Kato S, Matsumoto M, Matsuyama T, Isonishi A, Hiura H, Fujimura Y. Novel monoclonal antibody-based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion. 2006;46(8):1444–1452. doi: 10.1111/j.1537-2995.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 24.Hubbard AR, Heath AB, Kremer Hovinga JA Subcommittee on von Willebrand F. Establishment of the WHO 1st International Standard ADAMTS13, plasma (12/252): communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(6):1151–1153. doi: 10.1111/jth.12881. [DOI] [PubMed] [Google Scholar]
- 25.Raval JS, Padmanabhan A, Kremer Hovinga JA, Kiss JE. Development of a clinically significant ADAMTS13 inhibitor in a patient with hereditary thrombotic thrombocytopenic purpura. Am J Hematol. 2015;90(1):E22. doi: 10.1002/ajh.23851. [DOI] [PubMed] [Google Scholar]
- 26.Rieger M, Mannucci PM, Kremer Hovinga JA, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood. 2005;106(4):1262–1267. doi: 10.1182/blood-2004-11-4490. [DOI] [PubMed] [Google Scholar]
- 27.Froehlich-Zahnd R, George JN, Vesely SK, et al. Evidence for a role of anti-ADAMTS13 autoantibodies despite normal ADAMTS13 activity in recurrent thrombotic thrombocytopenic purpura. Haematologica. 2012;97(2):297–303. doi: 10.3324/haematol.2011.051433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muia J, Gao W, Haberichter SL, et al. An optimized fluorogenic ADAMTS13 assay with increased sensitivity for the investigation of patients with thrombotic thrombocytopenic purpura. J Thromb Haemost. 2013;11(8):1511–1518. doi: 10.1111/jth.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barber BE, William T, Grigg MJ, et al. Parasite biomass-related inflammation, endothelial activation, microvascular dysfunction and disease severity in vivax malaria. PLoS Pathog. 2015;11(1):e1004558. doi: 10.1371/journal.ppat.1004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowenberg EC, Charunwatthana P, Cohen S, et al. Severe malaria is associated with a deficiency of von Willebrand factor cleaving protease, ADAMTS13. Thromb Haemost. 2010;103(1):181–187. doi: 10.1160/TH09-04-0223. [DOI] [PubMed] [Google Scholar]
- 31.Colombatti R, De Bon E, Bertomoro A, et al. Coagulation activation in children with sickle cell disease is associated with cerebral small vessel vasculopathy. PLoS One. 2013;8(10):e78801. doi: 10.1371/journal.pone.0078801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer SC, Sulzer I, Lammle B, Kremer Hovinga JA. Hyperbilirubinemia interferes with ADAMTS-13 activity measurement by FRETS-VWF73 assay: diagnostic relevance in patients suffering from acute thrombotic microangiopathies. J Thromb Haemost. 2007;5(4):866–867. doi: 10.1111/j.1538-7836.2007.02438.x. [DOI] [PubMed] [Google Scholar]
- 33.Lu RN, Yang S, Wu HM, Zheng XL. Unconjugated bilirubin inhibits proteolytic cleavage of von Willebrand factor by ADAMTS13 protease. J Thromb Haemost. 2015;13(6):1064–1072. doi: 10.1111/jth.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uemura M, Fujimura Y, Matsumoto M, et al. Comprehensive analysis of ADAMTS13 in patients with liver cirrhosis. Thromb Haemost. 2008;99(6):1019–1029. doi: 10.1160/TH08-01-0006. [DOI] [PubMed] [Google Scholar]
- 35.Pereboom IT, Adelmeijer J, van Leeuwen Y, Hendriks HG, Porte RJ, Lisman T. Development of a severe von Willebrand factor/ADAMTS13 dysbalance during orthotopic liver transplantation. Am J Transplant. 2009;9(5):1189–1196. doi: 10.1111/j.1600-6143.2009.02621.x. [DOI] [PubMed] [Google Scholar]
- 36.Ono T, Mimuro J, Madoiwa S, et al. Severe secondary deficiency of von Willebrand factor-cleaving protease (ADAMTS13) in patients with sepsis-induced disseminated intravascular coagulation: its correlation with development of renal failure. Blood. 2006;107(2):528–534. doi: 10.1182/blood-2005-03-1087. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen TC, Han YY, Kiss JE, et al. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med. 2008;36(10):2878–2887. doi: 10.1097/ccm.0b013e318186aa49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peigne V, Azoulay E, Coquet I, et al. The prognostic value of ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13) deficiency in septic shock patients involves interleukin-6 and is not dependent on disseminated intravascular coagulation. Crit Care. 2013;17(6):R273. doi: 10.1186/cc13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kremer Hovinga JA, Zeerleder S, Kessler P, et al. ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J Thromb Haemost. 2007;5(11):2284–2290. doi: 10.1111/j.1538-7836.2007.02743.x. [DOI] [PubMed] [Google Scholar]
- 40.Hyseni A, Kemperman H, de Lange DW, Kesecioglu J, de Groot PG, Roest M. Active von Willebrand factor predicts 28-day mortality in patients with systemic inflammatory response syndrome. Blood. 2014;123(14):2153–2156. doi: 10.1182/blood-2013-08-508093. [DOI] [PubMed] [Google Scholar]
- 41.Wu N, Liu J, Yang S, et al. Diagnostic and prognostic values of ADAMTS13 activity measured during daily plasma exchange therapy in patients with acquired thrombotic thrombocytopenic purpura. Transfusion. 2015;55(1):18–24. doi: 10.1111/trf.12762. [DOI] [PubMed] [Google Scholar]