Abstract
Reductions in function within the serotonin (5HT) neuronal system have long been proposed as etiological factors in depression. Serotonin selective reuptake inhibitors (SSRIs) are the most common treatment for depression and their therapeutic effect is generally attributed to their ability to increase the synaptic levels of 5HT. Tryptophan hydroxylase 2 (TPH2) is the initial and rate-limiting enzyme in the biosynthetic pathway of 5HT in the CNS and losses in its catalytic activity lead to reductions in 5HT production and release. The time differential between the onset of 5HT reuptake inhibition by SSRIs (minutes) and onset of their anti-depressant efficacy (weeks to months), when considered with their overall poor therapeutic effectiveness, has cast some doubt on the role of 5HT in depression. Mice lacking the gene for TPH2 are genetically depleted of brain 5HT and were tested for a depression-like behavioral phenotype using a battery of valid tests for affective-like disorders in animals. The behavior of TPH2−/− mice on the sucrose preference test, tail suspension test and forced swim test and their responses in the unpredictable chronic mild stress and learned helplessness paradigms was the same as wild-type controls. While TPH2−/− mice as a group were not responsive to SSRIs, a subset responded to treatment with SSRIs in the same manner as wild-type controls with significant reductions in immobility time on the tail suspension test, indicative of antidepressant drug effects. The behavioral phenotype of the TPH2−/− mouse questions the role of 5HT in depression. Furthermore, the TPH2−/− mouse may serve as a useful model in the search for new medications that have therapeutic targets for depression that are outside of the 5HT neuronal system.
Keywords: serotonin, TPH2, TPH2 knock out, depression-like behavior, SSRIs, SERT
The serotonin (5HT) neuronal system innervates nearly the entire neuraxis from cell bodies located in the mesencenphalon.1 In its role as a neurotransmitter, 5HT regulates a diverse array of physiological functions to include feeding, aggression and sleep.2 Defects in 5HT neurochemical function have been implicated in a large number of neuropsychiatric and developmental conditions to include schizophrenia, attention deficit hyperactivity disorder, sudden infant death syndrome and autism. Perhaps the strongest association between impaired 5HT function and clinically significant psychopathology is for depression. Since the monoamine theory of depression was posited about 50 years ago,3–5 a great deal of work has sharpened focus on the role played by reduced 5HT levels in the synapse in this condition.6 The most widely used pharmacotherapy for depression is the class of drugs referred to as serotonin-selective reuptake inhibitors (SSRIs7,8). Fluoxetine and other SSRIs block the 5HT transporter and are thought to exert their anti-depressant effects by increasing the synaptic levels of 5HT.9
Depression is a very serious medical condition and accounts for a disproportionate amount of disability and loss of productivity among all of the major psychiatric diseases. The life-time prevalence of depression/mood disorders is approximately 20%,10 and depression is highly co-morbid with anxiety and other medical conditions.11 Unfortunately, therapeutic options for depression are somewhat limited and the best treatments leave ~60–70% of patients without symptomatic relief.12,13 Some even question if SSRIs have clinically significant therapeutic value beyond a placebo effect in treating any form of depression.14,15 Despite the long-held hypothesis that 5HT neuronal dysfunction is an underlying cause of depression,16–18 the relatively poor efficacy of the SSRIs in treating this condition and the high rates of remission13 have renewed interest in achieving a better understanding of the neurobiological bases of this disorder and in developing more effectively targeted drug therapies for it.
As part of a larger project to explore the involvement of 5HT in neurodevelopment and psychiatric conditions, we developed a mouse lacking the gene for the brain-specific form of tryptophan hydroxylase (TPH), TPH2.19 TPH2 is the initial and rate-limiting enzyme in the biosynthesis of 5HT. Mice lacking the gene for TPH2 are viable and fertile but show some degree of developmental lag by comparison to wild-type mice.20–22 TPH2−/− mice have no TPH2 protein and brain tissue from these mice cannot hydroxylate tryptophan to 5-hydroxytryptophan.19 Consequently, their central nervous systems are devoid of 5HT. Other major elements of the 5HT neuronal system (i.e., receptors, neurons, axons, dendrites, raphe unit firing and the 5HT transporter) remain essentially intact in these mice and there appear to be few if any compensatory alterations in other neurotransmitter systems.19,20,23–28 In the course of characterizing the behavioral phenotype of the TPH2−/− mouse, we and others have noted numerous interesting and novel aspects of 5HT-behavioral relations. TPH2−/− mice display intense impulsive and compulsive behaviors and extreme aggressiveness,23,29 as well as autistic-like behavioral traits.30 Surprisingly, these mice show decreased anxiety-like behaviors by comparison to wild-type control mice.23,29,30
In light of the suspected roles played by deficits in 5HT neurochemistry in depression, we carried out a battery of extensively validated behavioral tests of depression-like behaviors in TPH2−/− mice. We hypothesized that these mice would show a profound behavioral phenotype indicative of depression. However, TPH2−/− mice were not different from wild-type controls on any of these tests and in some cases were more resistant to the development of depression-like behaviors than wild-type controls. What is more, a subset of TPH2−/− mice responded to fluoxetine, paroxetine and citalopram like wild-type mice with reductions in immobility time on the tail suspension test, indicative of anti-depressant responses. Together, these results suggest that the absence of brain 5HT does not confer in mice a depression-like behavioral phenotype. The TPH2−/− mouse questions the role of 5HT as an etiological factor in depression and may serve as a valuable and interesting starting point to search for new medications that have therapeutic targets for depression that are outside of the 5HT neuronal system.
RESULTS AND DISCUSSION
TPH2−/− mice were compared to wild-type controls for sucrose preference to test for anhedonic-like behaviors. The results in Fig. 1A show that the main effect of days was significant (F3,66 = 4.73, p = 0.005, 2-way repeated measures ANOVA). Sucrose preference was ~80–85% for both groups. Total fluid intake was identical for both groups of mice on each day of this test (i.e., sucrose plus water; data not shown). Fig. 1B presents results of the quinine preference test and indicates that both genotypes expressed low preference for quinine (~20–25% of total fluid intake). The main effect of days (F3,66 = 4.13, p = 0.009) and genotype (F1,22 = 7.11, p = 0.014) were significant by two-way repeated measures ANOVA whereas their interaction was not. TPH2−/− mice actually drank significantly less quinine solution than wild-type controls on day 2 (Bonferroni’s multiple comparison test, p < 0.05). Food consumption was also measured in addition to fluid intake and the results in Fig. 1C indicate that the main effects of days (F3,66 = 8.16, p = 0.0001) and the days x genotype interaction (F3,66 = 3.94, p = 0.011) were significant whereas the main effect of genotype was not. Post hoc comparisons revealed that food intake in the TPH2−/− mice was significantly lower on days 2–4 (p < 0.05 – 0.0001, Bonferonni’s multiple comparison test) by comparison to their day 1 food intake. Food intake of TPH2+/+ mice did not vary over the 4 day test period. The results presented in Fig. 1 indicate that the behavior of TPH2−/− mice is normal and not indicative of anhedonia.
Figure 1.
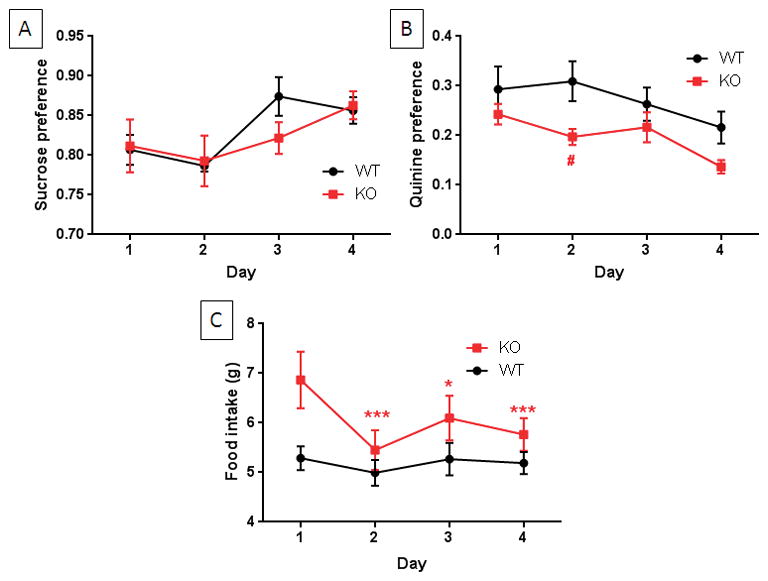
Sucrose and quinine preference and food intake in TPH2−/− and wild-type mice. Wild-type (WT) and TPH2−/− mice (KO) were tested daily for drinking preference over the 4 day test for sucrose (A) or quinine (B) in a two-bottle choice paradigm. KO and WT mice show the same preference for sucrose but KO mice drink significantly less quinine than WT controls while drinking significantly more water. (C) KO mice have slightly but significantly greater food intake than WT controls. Data are presented as preference for liquid intake and in g for food consumption and are means ± SEM for 12 mice per independent group per test. The symbols are *, p < 0.05, ***, p < 0.0001 compared to day 1; #, p < 0.05 compared to WT controls at day 2.
The results in Fig. 2A show that TPH2−/− mice were immobile for the same amount of time on the tail suspension test (TST) as wild-type controls. Performance of TPH2−/− and wild-type mice on the TST was not complicated by tail climbing which can limit the use of the C57BL/6 strain on this test.31 Results in Fig. 2B present data for the forced swim test (FST). The main effects of genotype (F1,22 = 5.05, p = 0.034) and trial (F1,22 = 32.41, p < 0.0001) were significant by two-way repeated measures ANOVA but the genotype x trial interaction was not. TPH2−/− mice remained immobile for significantly less time than wild-type controls (i.e., less “depressed”) on the first trial (p < 0.05, Bonferroni multiple comparison test) but this difference was no longer apparent on the second trial when the behavior of TPH2−/− mice was the same as wild-type controls. Taken together, results presented in Fig. 2 indicate that TPH2−/− mice do not exhibit depression-like behaviors.
Figure 2.
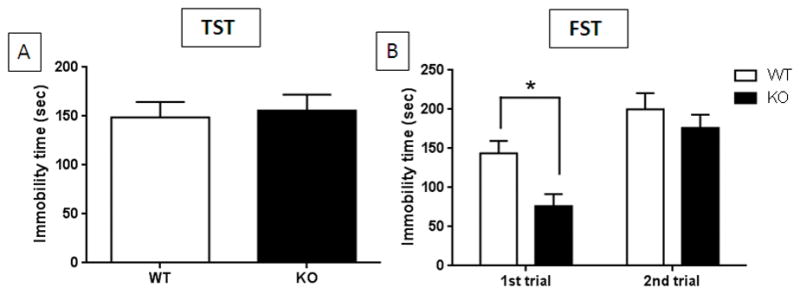
Immobility times for TPH2−/− and wild-type control mice on the TST and FST. (A) TPH2−/− (KO) and wild-type controls (WT) have the same immobility times in the TST. (B) KO mice spend significantly less time immobile than WT controls on the first trial of the FST but have the same immobility times on the second trial. Immobility times are in sec for both tests and are means ± SEM of 13 WT and 14 KO for panel A and 12 WT and 12 KO mice for panel B. The symbol is *, p < 0.05.
Results from the unpredictable chronic mild stress (UCMS) model of depression are presented in Fig. 3. Scores for coat status are included in Fig. 3A. The main effects of weeks (F6,126 = 25.71, p < 0.0001) and genotype (F1,21 = 9.85, p < 0.005) were significant by two-way repeated measures ANOVA but their interaction was not. Post hoc analyses indicated that both TPH2−/− (p < 0.0001, Bonferroni’s multiple comparison test) and TPH2+/+ mice (p < 0.05 – 0.0001, Bonferroni’s multiple comparison test) degraded significantly from their starting coat status at weeks 3–6. Total grooming times are presented in Fig. 3B and show that the main effect of weeks was significant (F6,126 = 4.88, p = 0.0002, two-way repeated measures ANOVA) while the main effect of genotype and the weeks x genotype interaction were not. Post-hoc analyses revealed that TPH2−/− mice grooming time was significantly decreased from starting values at weeks 4–6 (p < 0.05–0.001, Bonferroni’s multiple comparison test) whereas wild-type mice did not vary over the weeks 0–6. Body weights of mice were measured throughout the UCMS protocol and the results are presented in Fig. 3C. The main effect of weeks was significant (F6,120 = 17.9, p < 0.0001) whereas the main effect of genotype and the weeks x genotype interaction were not. Post-hoc analyses revealed that the body weights of TPH2−/− mice increased slightly at weeks 3–6 compared to starting body weights (p < 0.001 – 0.0001, Bonferroni’s multiple comparison test) and wild-type controls differed significantly from their starting weights at weeks 1–6 (p < 0.05 – 0.001, Bonferroni’s multiple comparison test). These results indicate that TPH2−/− mice maintain normal appetitive behavior over the extended UCMS protocol. The post-UCMS sucrose preference test results in Fig. 3D show that the main effects of treatment (F1,113 = 281.7, p < 0.0001) and genotype (F1,113 = 8.65, p = 0.004) and their interaction (F1,113 = 10.78, p = 0.001) were significant by two-way ANOVA. Wild-type mice developed depression-like anhedonia with a significant reduction in sucrose preference from 80% to 30% (p < 0.0001, Bonferroni’s multiple comparison test). TPH2−/− mice also showed a significant reduction in sucrose preference from 80% to 45% after exposure to the UCMS protocol (Bonferroni’s multiple comparison test, p < 0.0001). The extent of the reduction in sucrose preference after the UCMS procedure was significantly greater in wild-type controls by comparison to TPH2−/− mice (Bonferroni’s multiple comparison test, p < 0.001). Together, the data in Fig. 3 show that stress-induced depression-like behaviors are induced in TPH2−/− mice to the same extent displayed by wild-type mice.
Figure 3.
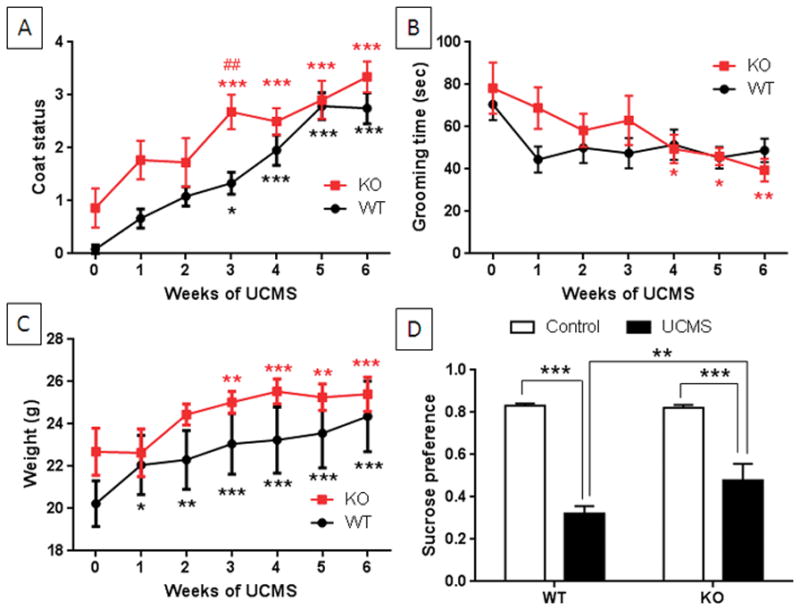
Effects of UCMS on depression-like behavior of TPH2−/− and wild-type control mice. TPH2−/− (KO) and wild-type controls (WT) were exposed to UCMS for 6 weeks and the emergence of depression-like behaviors was monitored weekly. (A) Coat status for both KO and WT mice degraded significantly at weeks 3–6 and KO mice differed from WT only at the 3 week time point in the stress protocol. (B) Grooming time (in sec) in the splash test diminished significantly in KO mice but not in WT controls and no difference was seen between genotypes. (C) Body weights (in g) of both WT and KO mice increased significantly over time. (D) Sucrose preference (ml sucrose divided by total water plus sucrose intake) after completion of the UCMS. Both WT and KO mice showed significant decreases in sucrose preference by comparison to their respective controls and WT mice showed a significantly greater reduction in sucrose preference compared to KO mice. Data in each panel are means ± SEM for 12 mice per group. The symbols are *, p < 0.05, **, p < 0.001, *** 0.0001 by comparison to the respective 0 time point for KO and WT mice. ##, p < 0.001 by comparison to the WT control at week 3.
TPH2−/− mice and wild-type controls were exposed to the learned helplessness (LH) paradigm and the results are presented in Fig. 4. The main effect of treatment was significant (F3,20 = 5.92, p = 0.005, one-way ANOVA) and both genotypes showed a significant increase in latency to escape on the test day (p < 0.05 for both, Tukey’s multiple comparison test). The magnitude of the effect was the same for both genotypes, indicating that TPH2−/− mice develop depression-like behavior in the learned helplessness model like wild-type controls.
Figure 4.
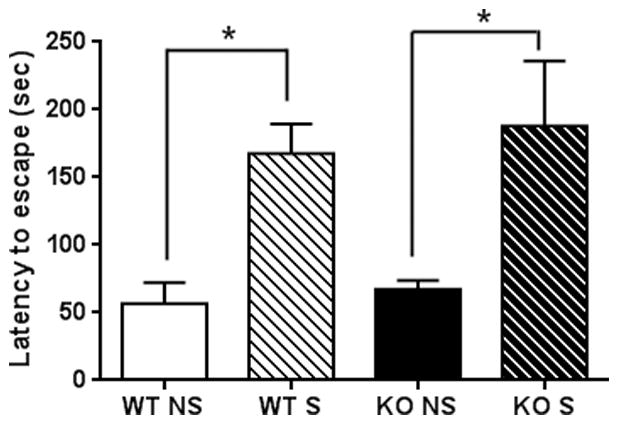
Effects of LH on depression-like behavior of TPH2−/− and wild-type control mice. TPH2−/− (KO) and wild-type controls (WT) not exposed to foot shocks (NS) or exposed to inescapable foot shocks (S) were tested for shock escape. Both WT and KO mice showed significant increases in latency to escape on the test day compared to the NS condition. WT and KO mice did not differ in the NS and S test conditions, respectively. Data are expressed as means ± SEM for 6 mice per group. The symbol is * p < 0.05.
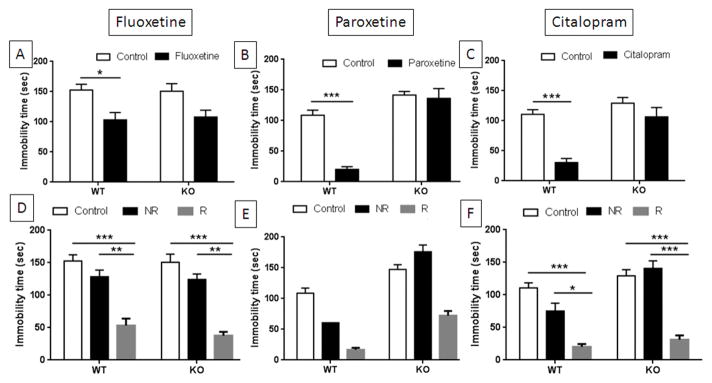
Many strains of wild-type mice show decreases in immobility in the FST or the TSTs when treated acutely with SSRIs.32–36 Therefore, we tested TPH2−/− mice for their response to the SSRIs fluoxetine, paroxetine and citalopram in the TST with the expectation that they would be unresponsive to these drugs (i.e., TPH2−/− mice should not respond to SSRIs because they lack brain 5HT and inhibition of the SERT could not possibly increase synaptic 5HT levels). The results presented in Fig. 5A show that when mice were treated with fluoxetine, the main effect of drug was significant (F1,53 = 16.13, p = 0.0002, two-way ANOVA) but the main effect of genotype and the drug x genotype interaction were not. Wild-type mice showed a significant reduction in immobility time after treatment with fluoxetine (p < 0.05, Bonferroni’s multiple comparison test). When mice were treated with paroxetine (Fig. 5B), the main effects of genotype (F1,43 = 52.4, p < 0.0001) and drug (F1,43 = 21.85, p < 0.0001) as well as their interaction (F1,43 = 13.12, p = 0.0008) were significant when analyzed by two-way ANOVA. Post hoc comparisons showed that paroxetine significantly reduced immobility times on the TST for wild-type controls (p < 0.0001, Bonferroni’s multiple comparison test) but did not change the behavior of TPH2−/− mice. The effect of citalopram on TST performance is presented in Fig. 5C and show that the main effects of genotype (F1,50 = 17.3, p = 0.0001) and drug (F1,50 = 20.6, p < 0.0001) and their interaction (F1,50 = 6.42, p = 0.014) were significant by two-way ANOVA. Wild-type mice showed significant reductions in immobility times after citalopram (p < 0.0001, Bonferroni’s multiple comparison test) whereas the slight reduction seen in TPH2−/− mice did not reach significance.
Figure 5.
Effects of SSRIs on immobility times in the TST for TPH2−/− and wild-type control mice. Independent groups of TPH2−/− (KO) mice or wild-type controls (WT) were injected acutely with (A) fluoxetine (20 mg/kg), (B) paroxetine (10 mg/kg) and (C) citalopram (20 mg/kg) or with vehicle controls and immobility times were tested 30 min after treatment. Only WT mice responded significantly to each SSRI with reductions in immobility times. Data from drug treated WT and KO mice was re-examined to classify mice as responders (R) or non-responders (NR) as defined in SI Tables 1 and 2. Both WT and KO mice responded significantly to (D) fluoxetine and (F) citalopram by comparison to controls and non-responsive mice for each respective drug. Statistical tests for mice treated with (E) paroxetine could not be carried out because the WT non-responder group contained just one mouse. WT and KO controls did not differ from NR mice in the fluoxetine and citalopram groups. Immobility times are in sec and are means ± SEM for 27 WT and 29 KO mice in panels A and D, 23 WT and 24 KO mice in panels B and E, and 27 WT and 27 KO mice in panels C and F. Symbols are *, p < 0.05, **, p < 0.001, ***, 0.0001.
Although TPH2−/− mice did not respond as groups to SSRI treatment with significant reductions in TST immobility times (Fig. 5A–C), we noted that some individual TPH2−/− mice clearly displayed substantial reductions after drug treatment. Depressed patients treated in clinical trials with SSRIs are commonly classified as responders and non-responders,37–39 generally based on a pre-determined reduction in depression scores (e.g., usually 50%). The concept of responsive and non-responsive subjects has also been recognized in animal models of antidepressant-resistance.40 Therefore, we re-examined the data from SSRI treatment of wild-type and TPH2−/− mice presented above and classified mice in either group as drug responders if their immobility times were 2 standard deviations lower than the mean of their respective non-treated controls. Mice that had the same immobility (< 2 standard deviations from the control mean) or higher times as non-treated controls were classified as non-responders. Using this classification, it was observed that 33% of WT-controls and 19% of TPH2−/− mice were responders to fluoxetine. The results of this analytical approach are presented in Figs. 5D–F. Treatment with fluoxetine resulted in a significant main effect of drug (F2,51 = 27.38, p < 0.0001, two-way ANOVA). The main effect of genotype and the drug x genotype interaction were not significant. Post-hoc comparisons revealed that TPH2+/+ mice that responded to fluoxetine had significantly shorter immobility times than controls (p < 0.0001, Bonferroni’s multiple comparison test) and non-responsive drug-treated mice (p < 0.001, Bonferroni’s multiple comparison test). Fluoxetine non-responders were not different from untreated controls. Similarly, TPH2−/− responders showed significantly shorter immobility times by comparison to both untreated controls (p < 0.0001, Bonferroni’s multiple comparison test) and non-responsive drug-treated mice (p < 0.001, Bonferroni’s multiple comparison test) and among TPH2−/− mice, untreated controls were not different from non-responders. The pattern of response to paroxetine was similar to that of fluoxetine and is presented in Fig. 5E. Approximately 92% of TPH2+/+ mice responded to paroxetine whereas only 38% of TPH2−/− mice were responders. Statistical testing of paroxetine TPH2+/+ non-responders to other groups could not be done because this group contained only one mouse. Results from treatment of mice with citalopram are presented in Fig. 5F. Approximately 81% of TPH2+/+ mice were classified as citalopram responders whereas only 31% of treated TPH2−/− mice were responders. The main effects of genotype (F1,48 = 13.82, p = 0.0005) and drug (F2,48 = 55.48, p < 0.0001) and the genotype x drug interaction (F2,48 = 3.22, p = 0.048) were significant when analyzed by two-way ANOVA. Post-hoc comparisons revealed that TPH2+/+ mice that responded to citalopram had significantly shorter immobility times than controls (p < 0.0001, Bonferroni’s multiple comparison test) and non-responsive drug-treated mice (p < 0.05, Bonferroni’s multiple comparison test). Citalopram non-responders were not different from untreated controls. Similarly, TPH2−/− responders showed significantly shorter immobility times by comparison to both untreated controls (p < 0.0001, Bonferroni’s multiple comparison test) and non-responsive drug-treated mice (p < 0.0001, Bonferroni’s multiple comparison test) and among TPH2−/− mice, untreated controls were not different from non-responders. The number of TPH2+/+ and TPH2−/− mice classified as responders and non-responders (and specified by sex) to the SSRIs along with the immobility time cutoff values used to make these classifications are presented in SI Tables 1 and 2, respectively. Taken together, the results in Fig. 5 show that while TPH2−/− mice did not respond to SSRIs as full groups, some individual mice did show substantial and significant reductions in immobility times on the TST.
In view of the results showing that a subset of TPH2−/− mice respond to SSRIs with anti-depressant-like reductions in immobility time and considering that SSRIs are thought to exert their antidepressant effects via inhibition of SERT, it was important to confirm that SSRIs would bind to the SERT in TPH2−/− mice. We tested this possibility by determining if SSRIs would block the synaptosomal uptake of [3H]-5HT in TPH2−/− mice. It can be seen in all panels of Fig. 6 that [3H]-5HT uptake was slightly but not significantly higher in TPH2−/− mice as compared to wild-type controls. This result is consistent with our previous finding of slightly increased synaptosomal [3H]-5HT uptake in TPH2−/− mice.23 The main effect of drug on uptake was significant for fluoxetine (F3,17 = 9.75, p = 0.0006), paroxetine (F3,18 = 7.59, p = 0.001) and citalopram (F3,23 = 5.76, p = 0.0043) when analyzed by a one-way ANOVA. The main effect of genotype was not significant for any drug. Fig. 6A shows that fluoxetine leads to a significant reduction in [3H]-5HT uptake in synaptosomes from both wild-type (p < 0.05, Tukey’s multiple comparison test) and TPH2−/− mice (p < 0.001, Tukey’s multiple comparison test). Paroxetine also significantly reduced [3H]-5HT uptake in wild-type (p < 0.05, Tukey’s multiple comparison test) and TPH2−/− mice (p < 0.05, Tukey’s multiple comparison test) as shown in Fig. 6B. Finally, it can be seen in Fig. 6C that citalopram significantly reduced the uptake of [3H]-5HT in wild-type (p < 0.05, Tukey’s multiple comparison test) and TPH2−/− mice (p < 0.05, Tukey’s multiple comparison test). The results in Fig. 6 show that SERT function in TPH2−/− mice is the same as wild-type controls and SSRIs block [3H-5HT] synaptosomal uptake to the same extent in each group.
Figure 6.
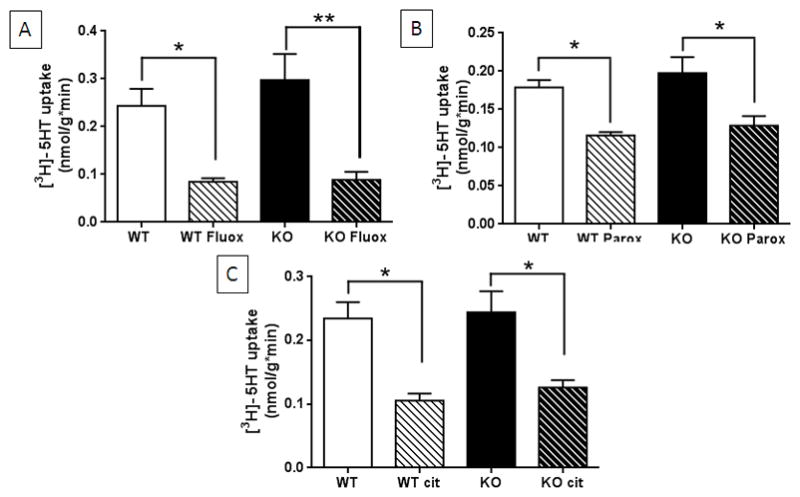
Effects of SSRIs on SERT-mediated uptake of [3H]-5HT by hippocampal synaptosomes. Uptake of [3H]-5HT into synaptosomes from TPH2−/− (KO) and wild-type control mice (WT) was measured in the absence or presence of (A) fluoxetine (10 μM), (B) paroxetine (50 μM) and (C) citalopram (50 μM). All SSRIs significantly inhibited uptake to the same extent in KO and WT tissue. Uptake of [3H]-5HT is expressed as nmol/g*min and is the mean ± SEM of 4 independent experiments. The symbols are *, p < 0.05, **, p < 0.001 comparing drug to control for each genotype.
The data presented in this manuscript document two rather surprising behavioral characteristics of a mouse lacking brain 5HT. First, TPH2−/− mice do not display a depression-like behavioral phenotype. Second, a subset of TPH2−/− mice shows an anti-depressant response to the SSRIs fluoxetine, citalopram and paroxetine on the TST. Defects in 5HT neurotransmission have long been implicated as causal factors in depression.16–18 Three different tests widely used to assess mood disorders in animals, and which have high predictive validity for anti-depressant medications—the sucrose preference test, the TST and the FST—yielded results that were in excellent agreement and demonstrated conclusively that the lack of 5HT in brain does not result in depression-like behaviors. The UCMS and LH protocols, which elicit depression-like behaviors in wild-type mice (e.g., diminished self-grooming and coat status, reduced sucrose preference, increased shock escape times), have the same effect on TPH2−/− mice. Thus, the genetic depletion of 5HT from brain does not induce a depression-like phenotype and it does not prevent mice from developing a depression-like phenotype upon exposure to chronic mild stress or inescapable footshock. Because results using 1 or 2 different tests of depressive-like behavior can yield conflicting outcomes (see below), we felt it was important to use a larger number of behavioral tests for comparative purposes in the event that some tests indicated that TPH2−/− mice displayed a depression-like phenotype while others did not. Fortunately, the results of the sucrose preference test, TST, FST, UCMS and LH were in excellent agreement, adding strength to the conclusion that TPH2−/− mice do not express a depression-like phenotype. In addition, we have previously shown that TPH2−/− mice display significantly less novelty suppressed feeding than wild-type controls.23 This test has been used widely to probe the anxiety-related component of depression in rodents41,42 and performance of TPH2−/− mice on this test was consistent with the other tests used presently. We did not test TPH2−/− mice on the repeated social defeat stress test of depression because their extreme aggressiveness23,29 prevents social defeat by other mice.
It may appear that the present results stand in contrast to a substantial body of research that has linked decreased 5HT function with depression for the past five decades. However, prior studies that have manipulated brain 5HT in animals via pharmacological or genetic approaches actually reveal very mild changes in behavior. For example, partial reductions in brain 5HT content with p-chlorophenylalanine, an inhibitor of TPH2, while having minor effects itself on behavior on the TST, does block reductions in immobility by SSRIs.43,44 Mice lacking the gene for PET-1 have ~80% reductions in brain 5HT neurons but do not differ from wild-type controls in the TST and FST45,46. Savelieva and colleagues26 reported that only male TPH2−/− mice spent significantly less time immobile on the FST than wild-type controls, indicating that these mice were non-depressive. These same investigators also showed that male TPH2−/− mice were not different from wild-type controls on the TST and female TPH2−/− mice had no phenotype on either the TST or FST.26 Mosienko and colleagues reported that TPH2−/− mice displayed increased immobility in the FST but not on the TST.29
The possibility exists that moderate reductions in brain 5HT, versus the more drastic depletions seen in PET-1 and TPH2−/− mice, would reveal a depression-like phenotype. Testing of this possibility has been done using mice with partial reductions in TPH2 expression. For instance, Beaulieu and colleagues47 generated a knockin mouse line that expressed a R439H mutant of TPH2. This mutant is equivalent to a very rare human TPH2 variant (R441H) seen in unipolar major depression.48 This loss of function mutant of TPH2 has 60–80% reductions in brain 5HT as a result of lowered TPH2 enzymatic activity.47,49 These TPH2 knockin mice indeed show increased immobility time on the TST, indicative of depression-like behavior.47,50 However, this result is confusing because mice heterozygous for the altered R439H TPH2 gene have normal brain 5HT levels but, like homozygotes, show significant increases in immobility time on the TST.47
Zhang and colleagues51 reported a functional single nucleotide polymorphism in the mouse TPH2 gene. This C1473G mutation replaces proline-447 with an arginine and results in significant reductions in TPH2 catalytic activity and 5HT synthesis.51,52 These investigators also made the very interesting observation that BALB/c and DBA/2 mice are homozygous for the 1473G allele and show much lower levels of TPH2 activity and 5HT than C57BL/6 and 129X1/SvJ strains which are homozygous for the 1473C allele.51 Studies comparing mouse strains for depression-like behavior and responsiveness to SSRIs are inconsistent in supporting a role for TPH2 and reduced brain 5HT in depression. For instance, a very large number of studies have shown that C57BL/6 mice (1473C TPH2 allele) show more, less or the same immobility times on the TST or FST as BALB/c or DBA/2 mice which bear the 1473G TPH2 allele.36 Mice with the same TPH2 1473G allele can even display the highest (BALB/c) or lowest (DBA/2) immobility times among various mouse strains when compared in the same study.53
With regard to responsiveness to SSRIs on the TST or FST, it also appears that the TPH2 genotype is not a determinant. For example, it has been shown that mice with the 1473C allele (C57BL/6 and 129Sv) are responsive to SSRIs in the FST whereas strains bearing the 1473G allele (BALB/c and DBA/2) are not responsive.54 Mice with the same 1473C allele (C57BL/6 and NMRI) are responsive or non-responsive respectively on the TST.55 Mice with different alleles (C57BL/6- C allele and DBA/2- G allele) can be the most responsive34 or the least responsive (C57BL/6- C allele and A/J- G allele) to SSRIs on the TST among compared strains.33 These complex findings show that while some of the variation in responsiveness to SSRIs can be attributed to the specific drug used or the behavioral test employed (TST versus FST), the TPH2 allele is not a determining factor. Perhaps the most conclusive demonstration that the TPH2 C1473G polymorphism is not related to depression-like behaviors has been presented by Tenner and colleagues.56 These investigators bred the 1473G allele from DBA/2 mice onto a C57BL/6 background to generate congenic strains bearing the 1473C or the 1473G alleles. These strains did not differ in either brain 5HT levels or immobility time on the FST.56 Similarly, Berger et al.57 showed that mice bearing the TPH2 C1473G polymorphism were not different from wild-type controls in brain 5HT content and they did not show a depression-like phenotype on the TST, FST, sucrose preference test or the LH paradigm. Taken together, our results agree with the majority of mouse genetic/strain studies and do not support a role for TPH2 or brain 5HT in the expression of a depression-like behavioral phenotype in mice.
Three additional factors support the contention that mice genetically depleted of 5HT do not show a depression-like behavioral phenotype. First, it is well-known that anxiety symptoms and syndromes are highly prevalent among patients with depressive disorders10,58,59 which suggests that if TPH2−/− mice were indeed depressed, they would also show anxiety-like behaviors. It has been shown clearly that TPH2−/− mice are the same as wild-type controls23,30 or show decreases in behaviors on tests that model anxiety.29 Likewise, PET-1 knockout mice do not express anxiety-like behaviors.46 Second, mouse strains characterized by poor maternal care are highly vulnerable to developing depression-like behaviors.60 TPH220 and PET-1 knockout dams61 show very poor maternal behavior in the presence of their newborn pups, yet their offspring do not develop depression-like behaviors despite drastic reductions in brain 5HT. Third, it has been proposed that increased hippocampal neurogenesis is a correlate of the antidepressant effects of SSRIs, which increase synaptic 5HT, but mice genetically modified to have low or no brain 5HT levels (i.e., PET-1, VMATf/f:SERT and TPH2−/− mice) exhibit normal levels of hippocampal neurogenesis.62,63
Experiments designed to test the responsiveness of TPH2+/+ and TPH2−/− mice to SSRIs on the TST yielded interesting results. The immobility times of TPH2+/+ mice were significantly decreased by fluoxetine, paroxetine and citalopram whereas those of TPH2−/− mice were not. These results confirm numerous previous studies showing that wild-type mice respond to SSRIs with anti-depressant-like reductions in immobility times. The failure of TPH2−/− mice to respond to these drugs when group-analyzed is consistent with the expectation that the anti-depressant effects of SSRIs are mediated by 5HT.33,34,36 However, we noted that a subset TPH2−/− mice responded to SSRIs with substantial reductions in immobility times and some TPH2+/+ mice were non-responders. Because of this unexpected finding, we classified both TPH2−/− and wild-type controls as drug responders and non-responders much as depressed individuals are classified with respect to their responsiveness to SSRIs. Mice of both genotypes that showed reductions in immobility times after drug treatment that was > 2 standard deviations lower than the means of the respective untreated controls were designated as responders. Using this alternative analytical approach that takes into account the individual responses of mice to drug treatment, a unique finding emerged and confirmed that some TPH2−/− mice indeed responded to SSRI treatment with significant reductions in immobility times on the TST. Assuming that the SSRIs exert this effect in wild-type controls through blockade of the SERT to cause an increase in synaptic 5HT, it is very interesting that they could have anti-depressant behavioral effects in an animal totally lacking brain 5HT. TPH2−/− mice have normal levels of a functional SERT (i.e., the SERT transports 5HT into synaptosomes and uptake can be blocked by SSRI drugs as shown in Fig. 6) but SSRI binding to it cannot lead to increases in synaptic 5HT because there is no 5HT available for transport into the presynaptic process. Clearly, far fewer TPH2−/− mice responded significantly to SSRI treatment than TPH2+/+ mice (20–36% TPH2−/− responders compared to 33–92% TPH2+/+ responders). Nevertheless, the reductions in TST immobility times for responsive TPH2−/− mice were quite large and highly statistically significant. In many ways, this result is similar to the situation in depressed humans that are non-responsive to SSRI treatment.13 It has also been recognized that large proportions of animals are non-responsive to SSRIs in behavioral models of depression-like behaviors.40 Therefore, while the results on the partial responsiveness of TPH2−/− mice to SSRIs must be interpreted with caution and should be expanded to include additional behavioral tests, it is still quite interesting that these mice show “anti-depressant-like” changes in behavior that are not mediated by increases in synaptic 5HT caused by drug-induced inhibition of the SERT.
The SERT is not the only target in brain with which the SSRIs interact.64,65 In particular, fluoxetine, sertraline and paroxetine fail to reduce TST immobility times in mice lacking brain norepinephrine66 suggesting that drug-induced increases in synaptic norepinephrine are playing a role in the anti-depressant actions of selected SSRIs. In light of this, it is certainly possible that the effects of the SSRIs on selected TPH2−/− mice are mediated by norepinephrine in the absence of 5HT. In addition, these drugs can have broad influence on brain function via interactions with ion channels,67 protein kinases,68–70 phosphatases71 and phosphodiasterases.72 At least fluoxetine reduces acid sphingomyelinase activity and lowers brain ceramide levels while improving depressive-like behavior in the UCMS model.73 Long-term treatment with SSRIs can also alter gene expression via interactions with transcription factors74,75 and their ability to stimulate neurogenesis in the adult brain76 may play a role in therapeutic efficacy in the treatment of depression.77–79 Together, the present results suggest the possibility that SSRIs can exert anti-depressant effects by acting at targets other than the SERT and do so in a manner that is independent of 5HT. In light of the modest therapeutic efficacy of current treatments for depression and considering the high rates of treatment resistance and remission,13 the TPH2−/− mouse model is ideally suited to allow new studies that search for new therapeutic sites of action for the SSRIs. In the process, these studies are also likely to yield new information on the neurobiological bases of depression.
METHODS
Subjects
TPH2−/− mice were generated by deleting exon 1 of the TPH2 gene as described.19 These mice have no brain TPH2 protein and they express no other compensatory enzymatic or chemical mechanism to hydroxylate tryptophan, so their brains are devoid of 5HT and 5-hydroxyindoleacetic acid (5HIAA). 5HT neurons and processes remain intact in TPH2−/− mice and show normal expression of the SERT and 5HT receptors. The SERT is also capable of transporting 5HT into synaptosomes to the same extent as wild-type mice.30 Wild-type and TPH2−/− mice used in this study were derived from matings of heterozygous (TPH2+/−) males and heterozygous (TPH2+/−) females on a mixed C57BL/6-Sv129 background. Genome scanning analysis by The Jackson Laboratory revealed that our strain background was 95.4% C57BL/6. Heterozygous TPH2+/− mice have the same brain levels of 5HT, SERT, and TPH2 as wild-type mice, and were not tested presently. Offspring were housed with their mothers until weaning at PND21 and thereafter litters were housed together for an additional week. Litters were then separated by sex and males and females from the same litter were housed as groups (4–6 mice per cage) in 27 × 48 × 20 cm cages for at least 4 weeks prior to testing. For tests that required individual housing of mice during the test (e.g., sucrose preference test), subjects were housed singly overnight before experimentation. Results of all tests reflect groups of mice from at least 3–4 independent litters. Separate cohorts of adult mice (10–12 weeks of age) were acclimated to the behavioral testing rooms for 1 hr prior to all testing (10am–4pm daily). Independent groups were used for each behavioral test and experimenters scoring behaviors were blind to genotype. Equal numbers of male and female mice were used in all tests. However, because we did not observe a main effect of sex on any test so data from male and female mice was pooled for each genotype. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Care and Use Committee of Wayne State University (Permit Number A3310-01).
Sucrose and quinine preference and food intake
The sucrose preference test was used as a measure of hedonia/anhedonia and was carried out as previously described.80,81 Singly housed mice were habituated to the presence of two graduated drinking tubes (100 ml glass tubes with 1 ml graduations; Braintree Scientific, Braintree, MA) containing tap water for 2 days. Sipper tubes contained ball bearings to minimize loss of fluid to drippage. For the following 4 days, mice were given a two-bottle choice of 3% sucrose in tap water versus tap water. To eliminate potential side preferences, the position of the bottles was switched daily. Consumption of water, sucrose, and total liquid intake was measured once daily. Fluid intake was determined by weighing each bottle at the start of the test period and the weight of the bottles after 24 hr on each cage was subtracted from the starting weight to yield fluid intake (i.e., 1 g = 1 ml of fluid). To assess fluid loss to drippage during the test periods, 10 bottles were placed on empty cages (1 per cage) throughout the cage rack and loss was determined as described above. Loss of fluid from sipper bottles was negligible (0.8% of total volume over 24 hr). Preference for sucrose is expressed as % of consumed sucrose divided by the total volume of liquid consumed. Consumption of a solution of 0.0024% quinine was measured in the same manner described above for sucrose. Diminished preference for sucrose versus water is indicative of depression-like behavior. For measures of food intake, mice were individually housed and water was available ad libitum. Animals had access to standard chow and their food consumption was quantified by weighing food pellets daily for 5 consecutive days.
Tail suspension test
The tail suspension test (TST) was carried out as originally described by Steru et al.82 This model of “behavioral despair” was originally devised as a test for screening antidepressant drugs but is now used widely in phenotyping depression-like behaviors in rodents.83,84 Mice are secured by the tail to a plastic band (~4 cm wide) with medical adhesive tape (1–1.5 cm of the distal tail) and suspended head-down 30 cm above the lab bench. Mice are scored for immobility over a 6 min test period. Immobility is defined as a lack of movement/struggling and motionless hanging. The time spent immobile by mice was recorded by 2–3 investigators blinded to the genotype of the subject undergoing testing. Increased immobility is indicative of depression-like or resignation behavior.
Forced swim test
The forced swim test (FST) was carried out according to the method of Porsolt.83,85 This model, like the TST, was originally designed as a test for screening antidepressant drugs but is now used widely to assess depression-like behaviors.83,86 Mice are tested on two separate occasions. On the first test, mice are placed individually into a 2 liter glass beaker (OD = 13.1 cm, H = 19.3 cm) containing 1.5 liter of tap water (12 cm deep) at 25°C for 15 min and the time of immobility is scored only in the first 5 min. The second test is administered 24 hr later and mice are placed into the testing chamber for 5 min and the time of immobility was scored throughout. Immobility is defined as minimal movement required for a mouse to keep its head and nostrils above the water level. Increased immobility is indicative of depression-like or resignation behavior.
Unpredictable chronic mild stress (UCMS)
The UCMS procedure was based on those designed for rats65,87,88 and mice.89 In this test, mice were exposed to a series of mild stressors including altered cage bedding, social stress, cage tilt, light/dark cycle disruption, cage exchange, predator sounds, presented in random order over a period of 6 weeks basically as described previously.36,90,91 The order of presentation of all stressors in the UCMS is included in SI Table 3. Body weight and coat status was assessed before initiation of the UCMS protocol and weekly during the procedure. Coat status from 8 body parts (head, neck, dorsal coat, ventral coat, tail, forepaws and hind paws) was scored as 0 for well groomed and 1 for unkempt by 2–3 observers blind to mouse genotype. The total score per test was derived by summing the individual scores for each body part.78,87,92 Immediately after scoring coat status, mice were exposed to the splash test93,94 which involves spraying a 10% sucrose solution onto the dorsal coat of mouse in its home cage. This mildly sticky solution induces self-grooming the duration of which was recorded for 5 min by 2–3 observers blinded to mouse genotype. Finally, after completion of the 6 week UCMS procedure, mice were assessed for sucrose preference in a single overnight session as described above. Worsening coat status scores over time and decreased self-grooming are indicative of depression-like behavior.
Learned Helplessness (LH)
The LH paradigm was carried out essentially as described by Fukui et al.95 The apparatus consisted of a shuttle cage (36 × 18 × 30 cm) where the compartments were separated by a sliding door (Coulbourn Instruments, Whitehall, PA). Briefly, group-housed animals were divided evenly and designated as foot-shock (FS) or no-foot-shock (NFS). Training was given in two sessions separated by 24 h. Learned helplessness was induced in FS mice by administering 100 inescapable 2-sec foot shocks (0.2 mA) with an intertrial interval of 10 s. NFS mice were placed in the cage but did not receive any shocks and were allowed to explore for an equivalent time period. Approximately 24 h after the second training session, escape testing was performed and both groups received 30 trials of escapable 0.2 mA foot shocks. Animals had a 5 min acclimatization period with the door between the compartments closed prior to initiation of the escape testing. The door opened with shock onset and the trial terminated when the mouse crossed through the gate into the adjacent safe chamber or if they failed to escape within 20 sec.
Treatment of mice with SSRIs
In order to test the response of TPH2−/− mice and their wild-type controls to SSRIs, mice of both genotypes were injected intraperitoneally with fluoxetine (20 mg/kg), paroxetine (10 mg/kg) or citalopram (20 mg/kg). Drugs were dissolved in 0.9% phosphate-buffered saline and injected in volumes of 0.2 ml per 20g of body weight. Mice were subsequently tested in the TST (see above) 30 min after injection. Doses of SSRIs were selected from previous studies showing their effectiveness in the TST after acute treatment and without causing disruption in locomotor activity.33,66,86 We noted that subsets of mice from both genotypes were responsive to SSRIs in the TST and some were not responsive. Human subjects given SSRIs in clinical studies are frequently classified as responders and non-responders37–39,96 as are mice treated with antidepressants in behavioral tests,40,86 so we followed this convention presently. Data from SSRI treatment of wild-type and TPH2−/− groups of mice was re-examined and classified mice in either group as drug responders if their immobility times were 2 standard deviations lower than the mean of their respective non-treated controls. Mice that had the same immobility (< 2 standard deviations from the control mean) or higher times as non-treated controls were classified as non-responders.
Functional Characterization of the SERT in TPH2−/− mice
The functional status of the SERT in wild-type and TPH2−/− mice was assessed by measuring [3H]-5HT uptake into hippocampal synaptosomes as previously described.23 The amount of [3H]-5HT accumulated by synaptosomes in a 10 min reaction was determined by liquid scintillation counting and is expressed as nmol 5HT/g/min. The ability of the SSRIs fluoxetine (10 μM), paroxetine (50 μM) and citalopram (50 μM) to block uptake of 5HT by the SERT was determined by adding drugs to synaptosomes 15 min prior to initiation of the uptake reaction with the addition of substrate. The SSRI concentrations used were selected from published reports showing inhibition of synaptosomal [3H]-5HT uptake.
Data analysis
Data from the sucrose preference test (Fig. 1A), quinine preference test (Fig. 1B), food intake (Fig. 1C), FST (Fig. 2B), UCMS coat status (Fig. 3A), UCMS grooming time (Fig. 3B), and UCMS body weights (Fig. 3C) were analyzed using a two-way repeated measures ANOVA and post hoc comparisons were made using Bonferonni’s multiple comparison test. Results from the TST (Fig. 2A) were analyzed using a Student’s T-test. Results from the post-UCMS sucrose preference test (Fig. 3D), LH test (Fig. 4), and all tests of the effects of SSRIs on SERT uptake of 5HT (Fig. 6) were analyzed using a 1-way ANOVA and post hoc comparisons were made using Tukey’s multiple comparison test. Results on the effects of SSRIs on the TST (Fig. 5) were analyzed using a 2-way ANOVA and post hoc comparisons were made using Bonferroni’s multiple comparison test. p values < 0.05 were deemed statistically significant. All statistical analyses were carried out using GraphPad Prism version 6.01 for Windows, GraphPad Software, San Diego, CA, www.graphpad.com.
Supplementary Material
Acknowledgments
Funding
A Department of Veterans Affairs grant to DMK (RX000458) supported this research.
We thank Dr. Cynthia Arfken for her advice on the statistical analyses of our data.
ABBREVIATIONS
- FST
forced swim test
- LH
learned helplessness
- SERT
serotonin transporter
- 5HT
serotonin
- SSRI
serotonin-selective reuptake inhibitor
- TST
tail suspension test
- TPH2
tryptophan hydroxylase 2
- TPH
tryptophan hydroxylase
- UCMS
unpredictable chronic mild stress
Footnotes
Author Contributions
MAP, MJK, DIB, NHM and CES conducted the in vivo behavioral and pharmacological experiments. DMF and MAP conducted the in vitro synaptosomal 5HT uptake experiments. MAP, MJK and DMK interpreted the data. MAP, MJK and DMK conceived of project and wrote the paper. All authors edited and approved the final version of the manuscript.
Notes
The authors declare no competing financial interest.
SUPPORTING INFORMATION AVAILABLE
The numbers of TPH2+/+ and TPH2−/− mice classified as responders or non-responders (specified by sex) to the SSRIs fluoxetine, citalopram and paroxetine is included in SI Table 1. The immobility time cutoff values for defining whether a subject was classified as a responder or non-responder to SSRIs is presented in SI Table 2. Information on the specific stressors used in the UCMS is included in SI Table 3. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 2.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 3.Prange AJ. The pharmacology and biochemistry of depression. Dis Nerv Syst. 1964;25:217–221. [PubMed] [Google Scholar]
- 4.Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 5.Bunney WE, Davis JM. Norepinephrine in depressive reactions. A review. Arch Gen Psychiatry. 1965;13:483–494. doi: 10.1001/archpsyc.1965.01730060001001. [DOI] [PubMed] [Google Scholar]
- 6.Serretti A, Artioli P. The pharmacogenomics of selective serotonin reuptake inhibitors. Pharmacogenomics J. 2004;4:233–244. doi: 10.1038/sj.tpj.6500250. [DOI] [PubMed] [Google Scholar]
- 7.Wenthur CJ, Bennett MR, Lindsley CW. Classics in chemical neuroscience: fluoxetine (Prozac) ACS Chem Neurosci. 2014;5:14–23. [Google Scholar]
- 8.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 9.Gartside SE, Umbers V, Hajos M, Sharp T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 11.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 13.Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011;34:1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan A, Faucett J, Lichtenberg P, Kirsch I, Brown WA. A systematic review of comparative efficacy of treatments and controls for depression. PLoS One. 2012;7:e41778. doi: 10.1371/journal.pone.0041778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araragi N, Lesch KP. Serotonin (5-HT) in the regulation of depression-related emotionality: insight from 5-HT transporter and tryptophan hydroxylase-2 knockout mouse models. Curr Drug Targets. 2013;14:549–570. doi: 10.2174/1389450111314050005. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen JP, Medvedev IO, Caron MG. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. 2012;367:2444–2459. doi: 10.1098/rstb.2012.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lesch KP, Araragi N, Waider J, van den Hove D, Gutknecht L. Targeting brain serotonin synthesis: insights into neurodevelopmental disorders with long-term outcomes related to negative emotionality, aggression and antisocial behaviour. Philos Trans R Soc Lond B Biol Sci. 2012;367:2426–2443. doi: 10.1098/rstb.2012.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas DM, Angoa Perez M, Francescutti-Verbeem DM, Shah MM, Kuhn DM. The role of endogenous serotonin in methamphetamine-induced neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2010;115:595–605. doi: 10.1111/j.1471-4159.2010.06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci USA. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narboux-Neme N, Angenard G, Mosienko V, Klempin F, Pitychoutis PM, Deneris E, Bader M, Giros B, Alenina N, Gaspar P. Postnatal growth defects in mice with constitutive depletion of central serotonin. ACS Chem Neurosci. 2013;4:171–181. doi: 10.1021/cn300165x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutknecht L, Jacob C, Strobel A, Kriegebaum C, Muller J, Zeng Y, Markert C, Escher A, Wendland J, Reif A, Mossner R, Gross C, Brocke B, Lesch KP. Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. Int J Neuropsychopharmacol. 2006:1–12. doi: 10.1017/S1461145706007437. [DOI] [PubMed] [Google Scholar]
- 23.Angoa-Perez M, Kane MJ, Briggs DI, Sykes CE, Shah MM, Francescutti DM, Rosenberg DR, Thomas DM, Kuhn DM. Genetic depletion of brain 5HT reveals a common molecular pathway mediating compulsivity and impulsivity. J Neurochem. 2012;121:974–984. doi: 10.1111/j.1471-4159.2012.07739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araragi N, Mlinar B, Baccini G, Gutknecht L, Lesch KP, Corradetti R. Conservation of 5-HT1A receptor-mediated autoinhibition of serotonin (5-HT) neurons in mice with altered 5-HT homeostasis. Front Pharmacol. 2013;4:97. doi: 10.3389/fphar.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waider J, Proft F, Langlhofer G, Asan E, Lesch KP, Gutknecht L. GABA concentration and GABAergic neuron populations in limbic areas are differentially altered by brain serotonin deficiency in Tph2 knockout mice. Histochem Cell Biol. 2013;139:267–281. doi: 10.1007/s00418-012-1029-x. [DOI] [PubMed] [Google Scholar]
- 26.Savelieva KV, Zhao S, Pogorelov VM, Rajan I, Yang Q, Cullinan E, Lanthorn TH. Genetic disruption of both tryptophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutknecht L, Araragi N, Merker S, Waider J, Sommerlandt FM, Mlinar B, Baccini G, Mayer U, Proft F, Hamon M, Schmitt AG, Corradetti R, Lanfumey L, Lesch KP. Impacts of brain serotonin deficiency following Tph2 inactivation on development and raphe neuron serotonergic specification. PLoS One. 2012;7:e43157. doi: 10.1371/journal.pone.0043157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kriegebaum C, Song NN, Gutknecht L, Huang Y, Schmitt A, Reif A, Ding YQ, Lesch KP. Brain-specific conditional and time-specific inducible Tph2 knockout mice possess normal serotonergic gene expression in the absence of serotonin during adult life. Neurochem Int. 2010;57:512–517. doi: 10.1016/j.neuint.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Mosienko V, Bert B, Beis D, Matthes S, Fink H, Bader M, Alenina N. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl Psychiatry. 2012;2:e122. doi: 10.1038/tp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kane MJ, Angoa-Perez M, Briggs DI, Sykes CE, Francescutti DM, Rosenberg DR, Kuhn DM. Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PLoS One. 2012;7:e48975. doi: 10.1371/journal.pone.0048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayorga AJ, Lucki I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology (Berl) 2001;155:110–112. doi: 10.1007/s002130100687. [DOI] [PubMed] [Google Scholar]
- 32.David DJ, Renard CE, Jolliet P, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the forced swimming test. Psychopharmacology (Berl) 2003;166:373–382. doi: 10.1007/s00213-002-1335-4. [DOI] [PubMed] [Google Scholar]
- 33.Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology (Berl) 2005;183:257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- 34.Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- 35.Ripoll N, David DJ, Dailly E, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the tail suspension test. Behav Brain Res. 2003;143:193–200. doi: 10.1016/s0166-4328(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 36.Jacobson LH, Cryan JF. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav Genet. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- 37.Illi A, Setala-Soikkeli E, Viikki M, Poutanen O, Huhtala H, Mononen N, Lehtimaki T, Leinonen E, Kampman O. 5-HTR1A, 5-HTR2A, 5-HTR6, TPH1 and TPH2 polymorphisms and major depression. Neuroreport. 2009;20:1125–1128. doi: 10.1097/WNR.0b013e32832eb708. [DOI] [PubMed] [Google Scholar]
- 38.Tzvetkov MV, Brockmoller J, Roots I, Kirchheiner J. Common genetic variations in human brain-specific tryptophan hydroxylase-2 and response to antidepressant treatment. Pharmacogenet Genomics. 2008;18:495–506. doi: 10.1097/FPC.0b013e3282fb02cb. [DOI] [PubMed] [Google Scholar]
- 39.Peters EJ, Slager SL, McGrath PJ, Knowles JA, Hamilton SP. Investigation of serotonin-related genes in antidepressant response. Mol Psychiatry. 2004;9:879–889. doi: 10.1038/sj.mp.4001502. [DOI] [PubMed] [Google Scholar]
- 40.Samuels BA, Leonardo ED, Gadient R, Williams A, Zhou J, David DJ, Gardier AM, Wong EH, Hen R. Modeling treatment-resistant depression. Neuropharmacology. 2011;61:408–413. doi: 10.1016/j.neuropharm.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollak DD, Rey CE, Monje FJ. Rodent models in depression research: classical strategies and new directions. Ann Med. 2010;42:252–264. doi: 10.3109/07853891003769957. [DOI] [PubMed] [Google Scholar]
- 42.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 43.O’Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, Page ME, Lucki I. Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology (Berl) 2007;192:357–371. doi: 10.1007/s00213-007-0728-9. [DOI] [PubMed] [Google Scholar]
- 44.Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl) 1999;147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- 45.Wellman CL, Camp M, Jones VM, Macpherson KP, Ihne J, Fitzgerald P, Maroun M, Drabant E, Bogdan R, Hariri AR, Holmes A. Convergent effects of mouse Pet-1 deletion and human PET-1 variation on amygdala fear and threat processing. Exp Neurol. 2013;250C:260–269. doi: 10.1016/j.expneurol.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaefer TL, Vorhees CV, Williams MT. Mouse plasmacytoma-expressed transcript 1 knock out induced 5-HT disruption results in a lack of cognitive deficits and an anxiety phenotype complicated by hypoactivity and defensiveness. Neuroscience. 2009;164:1431–1443. doi: 10.1016/j.neuroscience.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, Schwartz DA, Krishnan KR, Caron MG. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 49.Jacobsen JP, Siesser WB, Sachs BD, Peterson S, Cools MJ, Setola V, Folgering JH, Flik G, Caron MG. Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of-function mice. Mol Psychiatry. 2012;17:694–704. doi: 10.1038/mp.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sachs BD, Jacobsen JP, Thomas TL, Siesser WB, Roberts WL, Caron MG. The effects of congenital brain serotonin deficiency on responses to chronic fluoxetine. Transl Psychiatry. 2013;3:e291. doi: 10.1038/tp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- 52.Sakowski SA, Geddes TJ, Kuhn DM. Mouse tryptophan hydroxylase isoform 2 and the role of proline 447 in enzyme function. J Neurochem. 2006;96:758–765. doi: 10.1111/j.1471-4159.2005.03604.x. [DOI] [PubMed] [Google Scholar]
- 53.Trullas R, Jackson B, Skolnick P. Genetic differences in a tail suspension test for evaluating antidepressant activity. Psychopharmacology (Berl) 1989;99:287–288. doi: 10.1007/BF00442824. [DOI] [PubMed] [Google Scholar]
- 54.Cervo L, Canetta A, Calcagno E, Burbassi S, Sacchetti G, Caccia S, Fracasso C, Albani D, Forloni G, Invernizzi RW. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J Neurosci. 2005;25:8165–8172. doi: 10.1523/JNEUROSCI.1816-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobsen JP, Nielsen EO, Hummel R, Redrobe JP, Mirza N, Weikop P. Insensitivity of NMRI mice to selective serotonin reuptake inhibitors in the tail suspension test can be reversed by co-treatment with 5-hydroxytryptophan. Psychopharmacology (Berl) 2008;199:137–150. doi: 10.1007/s00213-008-1142-7. [DOI] [PubMed] [Google Scholar]
- 56.Tenner K, Qadri F, Bert B, Voigt JP, Bader M. The mTPH2 C1473G single nucleotide polymorphism is not responsible for behavioural differences between mouse strains. Neurosci Lett. 2008;431:21–25. doi: 10.1016/j.neulet.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 57.Berger SM, Weber T, Perreau-Lenz S, Vogt MA, Gartside SE, Maser-Gluth C, Lanfumey L, Gass P, Spanagel R, Bartsch D. A functional Tph2 C1473G polymorphism causes an anxiety phenotype via compensatory changes in the serotonergic system. Neuropsychopharmacology. 2012;37:1986–1998. doi: 10.1038/npp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vazquez GH, Baldessarini RJ, Tondo L. Co-occurrence of anxiety and bipolar disorders: clinical and therapeutic overview. Depress Anxiety. 2014;31:196–206. doi: 10.1002/da.22248. [DOI] [PubMed] [Google Scholar]
- 59.D’Avanzato C, Martinez J, Attiullah N, Friedman M, Toba C, Boerescu DA, Zimmerman M. Anxiety symptoms among remitted depressed outpatients: Prevalence and association with quality of life and psychosocial functioning. J Affect Disord. 2013;151:401–404. doi: 10.1016/j.jad.2013.06.040. [DOI] [PubMed] [Google Scholar]
- 60.Belzung C. Innovative drugs to treat depression: did animal models fail to be predictive or did clinical trials fail to detect effects? Neuropsychopharmacology. 2014;39:1041–1051. doi: 10.1038/npp.2013.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lerch-Haner JK, Frierson D, Crawford LK, Beck SG, Deneris ES. Serotonergic transcriptional programming determines maternal behavior and offspring survival. Nat Neurosci. 2008;11:1001–1003. doi: 10.1038/nn.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diaz SL, Narboux-Neme N, Trowbridge S, Scotto-Lomassese S, Kleine Borgmann FB, Jessberger S, Giros B, Maroteaux L, Deneris E, Gaspar P. Paradoxical increase in survival of newborn neurons in the dentate gyrus of mice with constitutive depletion of serotonin. Eur J Neurosci. 2013;38:2650–2658. doi: 10.1111/ejn.12297. [DOI] [PubMed] [Google Scholar]
- 63.Klempin F, Beis D, Mosienko V, Kempermann G, Bader M, Alenina N. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J Neurosci. 2013;33:8270–8275. doi: 10.1523/JNEUROSCI.5855-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:54–63. doi: 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 65.Willner P, Scheel-Kruger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev. 2013;37:2331–2371. doi: 10.1016/j.neubiorev.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 66.Cryan JF, O’Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, Page ME, Dalvi A, Thomas SA, Lucki I. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci USA. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tytgat J, Maertens C, Daenens P. Effect of fluoxetine on a neuronal, voltage-dependent potassium channel (Kv1.1) Br J Pharmacol. 1997;122:1417–1424. doi: 10.1038/sj.bjp.0701545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prasad HC, Zhu CB, McCauley JL, Samuvel DJ, Ramamoorthy S, Shelton RC, Hewlett WA, Sutcliffe JS, Blakely RD. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci USA. 2005;102:11545–11550. doi: 10.1073/pnas.0501432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carneiro AM, Blakely RD. Serotonin-, protein kinase C-, and Hic-5-associated redistribution of the platelet serotonin transporter. J Biol Chem. 2006;281:24769–24780. doi: 10.1074/jbc.M603877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steiner JA, Carneiro AM, Wright J, Matthies HJ, Prasad HC, Nicki CK, Dostmann WR, Buchanan CC, Corbin JD, Francis SH, Blakely RD. cGMP-dependent protein kinase Ialpha associates with the antidepressant-sensitive serotonin transporter and dictates rapid modulation of serotonin uptake. Mol Brain. 2009;2:26. doi: 10.1186/1756-6606-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- 72.Zhu CB, Hewlett WA, Francis SH, Corbin JD, Blakely RD. Stimulation of serotonin transport by the cyclic GMP phosphodiesterase-5 inhibitor sildenafil. Eur J Pharmacol. 2004;504:1–6. doi: 10.1016/j.ejphar.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 73.Gulbins E, Palmada M, Reichel M, Luth A, Bohmer C, Amato D, Muller CP, Tischbirek CH, Groemer TW, Tabatabai G, Becker KA, Tripal P, Staedtler S, Ackermann TF, van Brederode J, Alzheimer C, Weller M, Lang UE, Kleuser B, Grassme H, Kornhuber J. Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs. Nat Med. 2013;19:934–938. doi: 10.1038/nm.3214. [DOI] [PubMed] [Google Scholar]
- 74.Di Benedetto M, D’Addario C, Candeletti S, Romualdi P. Alterations of CREB and DARPP-32 phosphorylation following cocaine and monoaminergic uptake inhibitors. Brain Res. 2007;1128:33–39. doi: 10.1016/j.brainres.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 75.Frechilla D, Otano A, Del Rio J. Effect of chronic antidepressant treatment on transcription factor binding activity in rat hippocampus and frontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:787–802. doi: 10.1016/s0278-5846(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 76.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, Lange C, Higley JD, Rosoklija G, Hen R, Sackeim HA, Coplan JD. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One. 2011;6:e17600. doi: 10.1371/journal.pone.0017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 79.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- 81.Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- 82.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 83.Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci Chapter. 2001;8(Unit 8 10A) doi: 10.1002/0471142301.ns0810as14. [DOI] [PubMed] [Google Scholar]
- 84.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 86.Thompson BJ, Jessen T, Henry LK, Field JR, Gamble KL, Gresch PJ, Carneiro AM, Horton RE, Chisnell PJ, Belova Y, McMahon DG, Daws LC, Blakely RD. Transgenic elimination of high-affinity antidepressant and cocaine sensitivity in the presynaptic serotonin transporter. Proc Natl Acad Sci USA. 2011;108:3785–3790. doi: 10.1073/pnas.1011920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 88.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 89.Monleon S, D’Aquila P, Parra A, Simon VM, Brain PF, Willner P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology (Berl) 1995;117:453–457. doi: 10.1007/BF02246218. [DOI] [PubMed] [Google Scholar]
- 90.Ibarguen-Vargas Y, Surget A, Touma C, Palme R, Belzung C. Multifaceted strain-specific effects in a mouse model of depression and of antidepressant reversal. Psychoneuroendocrinology. 2008;33:1357–1368. doi: 10.1016/j.psyneuen.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 91.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 92.Farooq RK, Isingrini E, Tanti A, Le Guisquet AM, Arlicot N, Minier F, Leman S, Chalon S, Belzung C, Camus V. Is unpredictable chronic mild stress (UCMS) a reliable model to study depression-induced neuroinflammation? Behav Brain Res. 2012;231:130–137. doi: 10.1016/j.bbr.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 93.Ducottet C, Belzung C. Correlations between behaviours in the elevated plus-maze and sensitivity to unpredictable subchronic mild stress: evidence from inbred strains of mice. Behav Brain Res. 2005;156:153–162. doi: 10.1016/j.bbr.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 94.Yalcin I, Aksu F, Belzung C. Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur J Pharmacol. 2005;514:165–174. doi: 10.1016/j.ejphar.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 95.Fukui M, Rodriguiz RM, Zhou J, Jiang SX, Phillips LE, Caron MG, Wetsel WC. Vmat2 heterozygous mutant mice display a depressive-like phenotype. J Neurosci. 2007;27:10520–10529. doi: 10.1523/JNEUROSCI.4388-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.