Abstract
A close association between early-life experience and cognitive and emotional outcomes is found in humans. In experimental models, early-life experience can directly influence a number of brain functions long-term. Specifically, and often in concert with genetic background, experience regulates structural and functional maturation of brain circuits and alters individual neuronal function via large-scale changes in gene expression. Because adverse experience during sensitive developmental periods is often associated with neuropsychiatric disease, there is an impetus to create realistic models of distinct early-life experiences. These can then be used to study causality between early-life experiential factors and cognitive and emotional outcomes, and to probe the underlying mechanisms. Although chronic early-life stress has been linked to the emergence of emotional and cognitive disorders later in life, most commonly used rodent models of involve daily maternal separation and hence intermittent early-life stress. We describe here a naturalistic and robust chronic early-life stress model that potently influences cognitive and emotional outcomes. Mice and rats undergoing this stress develop structural and functional deficits in a number of limbic-cortical circuits. Whereas overt pathological memory impairments appear during adulthood, emotional and cognitive vulnerabilities emerge already during adolescence. This naturalistic paradigm, widely adopted around the world, significantly enriches the repertoire of experimental tools available for the study of normal brain maturation and of cognitive and stress-related disorders including depression, autism, post-traumatic stress disorder, and dementia.
Keywords: early-life experience, depression, dementia, post-traumatic stress disorder, memory, hippocampus, maternal care, stress, animal models, fragmentation
INTRODUCTION: A RATIONALE FOR NATURALISTIC MODELS OF CHRONIC EARLY-LIFE STRESS
Early postnatal life represents a period when the quality of the infant’s experience and specifically of the interaction with the mother is associated with emotional and cognitive outcomes (Ammerman, Van Hasselt, & Hersen, 1991; Fernald & Gunnar, 2009). Epidemiological data suggest that adverse early-life experience, especially chronic early-life stress (CES) can have a life-long impact on cognitive and emotional functions. Adverse early-life conditions, including poverty, loss of a parent, substance abuse by the mother or maternal depression are associated with vulnerability to psychopathologies later in life (Halligan, Herbert, Goodyer, & Murray, 2007; Lupien, McEwen, Gunnar, & Heim, 2009; Repetti, Taylor, & Seeman, 2002; Schore, 2000). Stress-related disorders, including depression, anxiety and post-traumatic stress disorders, appear to be especially sensitive to the effects of CES (Bremner, Southwick, Johnson, Yehuda, & Charney, 1993; Browne & Finkelhor, 1986; Heim, Newport, Mletzko, Miller, & Nemeroff, 2008; Lynch & Cicchetti, 1998; MacMillan et al., 2001; Paolucci, Genuis, & Violato, 2001). In addition, cognitive and executive functions seem to be affected by childhood adversity in epidemiological studies (Kaplan et al., 2001; Nelson et al., 2007; Wilson et al., 2007). Among the most influential studies of these effects are those of institutionally reared children, where chronically impoverished care was associated with cognitive and emotional deficits, and these were partially reversible by early fostering (Gunnar, Frenn, Wewerka, & Van Ryzin, 2009; Gunnar, Morison, Chisholm, & Schuder, 2001; Nelson et al., 2007). Because prevention of chronic childhood stress is unlikely, approaches promising post hoc clinical interventions are needed. These, in turn, require an understanding of the processes by which CES influences the growing brain.
Although the epidemiological studies described above suggest that CES influences later pathology, the correlational nature of these studies precludes direct causal inferences. Indeed, ethical concerns prevent direct manipulation of early environment in children, and uncontrollable factors including genetic predisposition cannot be fully accounted for. Animal models enable testing direct causal relationships, as well as control of genetic background and prenatal environment. In addition, parameters of interest can be manipulated and subsequent experiences can be controlled throughout the entire period of investigation. Finally, direct access to specific brain regions coupled with neuroanatomical, biochemical and genetic approaches can tease out the regions, circuits, mediators and signaling cascades that might contribute to the profound effects of early-life experience on adult outcome (Claessens et al., 2011; Enoch, 2011; Gillespie, Phifer, Bradley, & Ressler, 2009; Korosi & Baram, 2009; Roth & Sweatt, 2011; Schmidt, Wang, & Meijer, 2011).
TIMING ASPECTS RELEVANT TO CREATING OPTIMAL CHRONIC EARLY-LIFE STRESS MODELS
Brain maturation involves multiple dynamic processes that are regulated both by genetic factors and environmental input. Some of these processes are complete at birth, whereas others continue into early postnatal life and into adolescence. The human fetal brain undergoes dramatic growth, characterized by neurogenesis and differentiation; by 28 weeks of gestation, the number of neurons is 40% greater than in the adult (Levitt, 2003). Far from mature, the early postnatal brain continues to undergo significant developmental processes, including axonal and dendritic growth, synaptic stabilization and synaptic pruning (Levitt, 2003). Thus, maturational processes span developmental periods including prenatal, perinatal, infancy, and adolescence. In addition, brain regions mature at different velocities and trajectories for each one, and these trajectories differ across species (Avishai-Eliner, Brunson, Sandman, & Baram, 2002). These dynamic multiple and co-incident developmental trajectories raise several issues in conceptualizing suitable animal models for CES (Baram et al., 2012).
Because of the consensus about the potential importance of adversity to the “developing brain,” this area has been a focus of intense research. However, the term “early-life” has often been employed to describe different developmental windows including prenatal, early postnatal (until weaning at postnatal day (PND) 21) or even the adolescent period (peri-pubertal, PND 25–35 in rodent). As mentioned, brain regions mature at different velocities and trajectories for each one, and these trajectories differ across species (Avishai-Eliner et al., 2002). Therefore, it is difficult to directly compare the age of the developing rodent or primate to a specific age of a human in terms of overall brain development. It might be more informative to focus on a specific brain structure or network, (e.g., amygdala, hippocampus, sensory processing network, visual network, etc), and adjust the age of experimental animals to the specific stage of development targeted in humans. An example of this approach is provided in the table in Avishai-Eliner et al. (2002).
Timing of Stress Can Profoundly Influence Its Consequences
It was initially assumed that the neonatal period consisted of a more or less stable, unidirectional set of processes of brain development, so that the effects of stress across this period were consistent and cumulative. However, this assumption has turned out to be largely inaccurate, because even within the early postnatal period defined here, the direction and magnitude of stress-induced changes can vary dramatically according to the timing of the stress. In addition, the consequences of the timing vary for different outcome measures.
When the Outcome Measure Is the Neuroendocrine Response to Subsequent Stress
The timing effects were found for several early-life stress paradigms. For example, in studies using a single 24-hr maternal separation (MS) a 24-hr MS during PND 3–4 led to a hyper-responsive hypothalamic-pituitary-adrenal (HPA) axis later in life, whereas the same MS procedure just days later (PND 7–8 or 11–12) resulted in a hypo-responsive or attenuated stress system (Avishai-Eliner, Yi, Newth, & Baram, 1995; van Oers, de Kloet, & Levine, 1997, 1998). In the model provoking CES by limited bedding and nesting material and the resulting fragmentation of maternal care, the timing is also important. When imposed during PND 2–9 or on PND 1–7 or 3–8 elevated plasma corticosterone were found (Moriceau, Shionoya, Jakubs, & Sullivan, 2009; Raineki, Moriceau, & Sullivan, 2010). Adrenal hypertrophy in the pups was found acutely in the PND 2–9 paradigm (Avishai-Eliner, Gilles, Eghbal-Ahmadi, Bar-El, & Baram, 2001; Gilles, Schultz, & Baram, 1996). Notably, when pups reach adulthood, the neuroendocrine parameters of their stress system have returned to baseline (Brunson et al., 2005) and the hormonal response to stress did not appear grossly aberrant.
When the Outcome Measures Were Learning and Memory
MS on PND 4 impaired active avoidance and conditioned freezing, whereas MS on PND 9 improved performance in these tests (Lehmann, Pryce, Bettschen, & Feldon, 1999), as did daily 3-hr MS (postnatal Days 1–16) (Schable, Poeggel, Braun, & Gruss, 2007). Middle-aged rats exposed to repeated 6-hr MS (PND 12, 14, 16, and 18) tended to demonstrate shorter escape latencies in the Morris watermaze test (MWM) (Lehmann et al., 2002). Learning and memory were impaired in middle-aged rats experiencing the limited bedding/nesting model (PND 2–9), manifest by increased escape latencies in the MWM (Brunson et al., 2005). When the limited bedding/nesting model was employed on PND 1–7 attachment learning deficits were found (Moriceau et al., 2009).
When the Outcome Measure Was Emotional Function
Three-hour MS during PND 2–14 did not induce depressive-like behavior in the forced swim test (FST) (Marais, van Rensburg, van Zyl, Stein, & Daniels, 2008), whereas the same MS occurring from PND 2 to 21 increased the immobility time in young adult (Aisa, Tordera, Lasheras, Del Rio, & Ramirez, 2007). Adolescent rats exposed to limited bedding/nesting environment (PND 8–12) demonstrated higher immobility time in the FST (Raineki, Cortes, Belnoue, & Sullivan, 2012). Other, employing the CES model during PND 2–9 reported anxiety-like behaviors in young adult rats in the elevated plus maze test (EPM) (Dalle Molle et al., 2012). Anxiety-like behaviors were found upon daily 3-hr MS (PND 2–21) (Huot, Thrivikraman, Meaney, & Plotsky, 2001), but not after MS during PND 1–10 (Benetti et al., 2009).
These data suggest that both the timing of the chronic stress period within development and the nature of the stress (intermittent vs. chronic), drastically influence the outcomes in a domain-specific manner.
Age-Specific Aspects of the Hormonal Stress Response: Is There a Stress Hypo-Responsive Period?
In the neonatal rodent, qualitative, and quantitative aspects of the response to stress may differ from those in the adult (Brunson, Avishai-Eliner, Hatalski, & Baram, 2001; Harbuz, Russell, Sumner, Kawata, & Lightman, 1991; Lightman & Young, 1988). Initial work on the ontogeny of the stress system suggested that the first 2 weeks of life in rodents (PND 4–14 in rats; 1–12 in mice) are characterized by a stress hypo-responsive period (SHRP) in which the responsiveness of the HPA system to stress is attenuated (Levine, Glick, & Nakane, 1967; Rosenfeld, Suchecki, & Levine, 1992; Schapiro, Geller, & Eiduson, 1962; Schoenfeld, Leathem, & Rabii, 1980). This characterization was based on low basal cortico-sterone plasma levels (Schapiro et al., 1962), reduced sensitivity to corticotropin-releasing hormone (CRH) (Walker, Perrin, Vale, & Rivier, 1986), and the apparent absence of a stress response to a variety “typical” adult stressors (Levine et al., 1967; Rosenfeld et al., 1992; Schoenfeld et al., 1980). However, this initial concept has been proven to not fully represent the stress-responsiveness of immature humans and rodents. In humans, stress responses to pain exist throughout the neonatal period (Stang, Gunnar, Snellman, Condon, & Kestenbaum, 1988). In rodents, immature pups can mount a 300–400% increases in plasma corticosterone levels in response to age-appropriate stressors. These include MS or hypothermia (Pihoker, Owens, Kuhn, Schanberg, & Nemeroff, 1993; Plotsky & Meaney, 1993; Schoenfeld et al., 1980; Walker, Scribner, Cascio, & Dallman, 1991; Yi & Baram, 1994). These hormonal responses are mediated by stress-induced activation of CRH release and are associated with stress-induced enhancement of CRH expression in stress-responsive hypothalamic neurons (Dent, Okimoto, Smith, & Levine, 2000; Hatalski & Baram, 1997; Hatalski, Guirguis, & Baram, 1998; Yi & Baram, 1994). Thus, rather than being unresponsive, the developing stress system seems to be tuned specifically to stresses that may be relevant to the early-life period. For example, cold exposure did not induce a strong stress response in the adult in contrast to restraint stress (Harbuz et al., 1991; Lightman & Young, 1988), whereas cold exposure was a strong stressor in neonatal rats (Walker et al., 1991; Yi & Baram, 1994), and restraint stress was not.
EARLY-LIFE STRESS: CHRONIC VERSUS INTERMITTENT MODELS
Commonly, early-life stress is generated by maternal separation (MS), a manipulation believed to be stressful. Simply removing the dam for extended periods of time would lead to hypothermia and starvation, so many models use intermittent maternal deprivation, resulting in intermittent stress. This paradigm modulates the quantity of maternal care. Although MS models have provided a vast amount of data on the effects of reducing maternal input on pup development, this manipulation may differ from salient human conditions. When infants and children grow up in severe poverty, famine, war or in the presence of drug-abusing mothers, the stress is typically chronic rather than intermittent. Importantly, the mother is typically present and her behavior may be stress-provoking to the child (Baram et al., 2012; Kendall-Tackett, 2007; Koenen, Moffitt, Caspi, Taylor, & Purcell, 2003; Whipple & Webster-Stratton, 1991). An alternative approach for generating sustained stress is to provoke chronic and persistent changes in maternal care (Avishai-Eliner et al., 2001; Ivy, Brunson, Sandman, & Baram, 2008; Moriceau et al., 2009; Raineki et al., 2012; Rice, Sandman, Lenjavi, & Baram, 2008; Roth & Sullivan, 2005; Wang et al., 2011, 2012).
The Baram group developed a model of continuous CES (simulated poverty by limiting bedding and nesting material in the cage to recapitulate these important elements of the human condition). The altered cage environment resulted in abnormal maternal care, that is, fragmented and erratic maternal-derived sensory input to the pups, generating chronic (rather than intermittent) unpredictable and uncontrollable early-life “emotional stress” (Avishai-Eliner et al., 2001; Baram et al., 2012; Gilles et al., 1996; Ivy et al., 2008). Analysis of maternal behaviors in this paradigm revealed little or no change in the overall duration of maternal care or of specific aspects of care (licking and grooming, nursing, etc. Ivy et al., 2008). However, in both mice and rats, maternal care was fragmented and unpredictable: each nurturing behavior was shorter in duration and often interrupted, and the sequence of nurturing behaviors was unpredictable (Baram et al., 2012; Rice et al., 2008). Interestingly, a hallmark of maternal behavior in neglect/abuse situation is its unpredictable and fragmented quality (Gaudin, Polansky, Kilpatrick, & Shilton, 1996; Whipple & Webster-Stratton, 1991). Further, when the limited bedding/nesting model was employed by the Sullivan group (PND 1–7, 3–8, or 8–12), rough handling of the pups by the dam was noted as a strong element of maternal behavior (Moriceau et al., 2009; Raineki et al., 2010, 2012).
This model of CES that is based on aberrant yet existing maternal care activities has resulted in robust derangements of emotional and cognitive functions later in life in both mouse and rat studies. Therefore, it is being widely adopted and modified throughout the world (Dalle Molle et al., 2012; Green, Chen, Alvarez, Ferrari, & Levine, 2011; Gunn et al., 2013; Machado et al., 2013; Malter Cohen et al., 2013; Moriceau et al., 2009; Raineki et al., 2010, 2012; Roth, Lubin, Funk, & Sweatt, 2009; Roth & Sullivan, 2005; Wang et al., 2011, 2012). This naturalistic rodent model of CES will be described in the next section.
A NATURALISTIC RODENT MODEL OF CHRONIC EARLY-LIFE STRESS: LIMITED BEDDING/NESTING AND FRAGMENTED MATERNAL CARE
Active parental care, leading to sensory input to the developing brain, is crucial for normal development. Beyond simply providing nutrition and safety to the nest, it is maternal-derived sensory stimulation that influences appropriate development and levels of gene expression patterns and stress-related neuronal circuits: sensory stimulation (licking and grooming by the dam, as well as suckling motion of the pup) appeared to act directly on CRH-Adrenocorticotropic hormone (ACTH) expression and release (Eghbal-Ahmadi, Avishai-Eliner, Hatalski, & Baram, 1999; Suchecki, Nelson, Van Oers, & Levine, 1995) and are important in maintaining basal levels of activity of enzymes and hormones necessary for normal growth (Kuhn, Butler, & Schanberg, 1978; Kuhn & Schanberg, 1998). This input also seems to influence neural stem cell levels and neuronal and glial development (Kippin, Cain, Masum, & Ralph, 2004; Zhang et al., 2002). It has since become clear that both quantity and quality of specific elements of maternal care are important for normal infant development. Indeed, these can be manipulated bi-directionally and lead to diametrically opposing cognitive and emotional outcomes (Brunson, Chen, Avishai-Eliner, & Baram, 2003; McEwen & Gianaros, 2011). For example, several models of “enhanced” early-life experience, designed to increase levels of maternal care, report improved cognitive function and even later resilience to stress and psychopathology (Fenoglio et al., 2005; Fenoglio, Brunson, & Baram, 2006; Hofer, 1994; Korosi & Baram, 2009; Levine, 1967). On the opposite end of the spectrum, reduction of maternal care may lead to adverse emotional and cognitive outcomes, as found in humans (Gunnar et al., 2001, 2009; Nelson et al., 2007).
The limited bedding/nesting approach to CES does not significantly reduce the quantity of maternal care but leads to fragmentation and chaotic nature of this care that provoke profound chronic stress. The latter is manifest by both elevated plasma corticosterone and adrenal hypertrophy in the pups that is apparent already at the end of the stress (PND 9), and disappears later. (Avishai-Eliner et al., 2001; Brunson et al., 2005; Gilles et al., 1996; Ivy et al., 2008) (Fig. 1). Remarkably, augmented excitatory innervation of CRH-expressing, stress-sensitive cells hypothalamic neurons has been reported on PND 18–26 CES mice (Gunn et al., 2013). This fact, together with the adrenal hypertrophy indicates that the CES model promotes rapid plasticity already during the stress period, in addition to long-lasting consequences.
FIGURE 1.
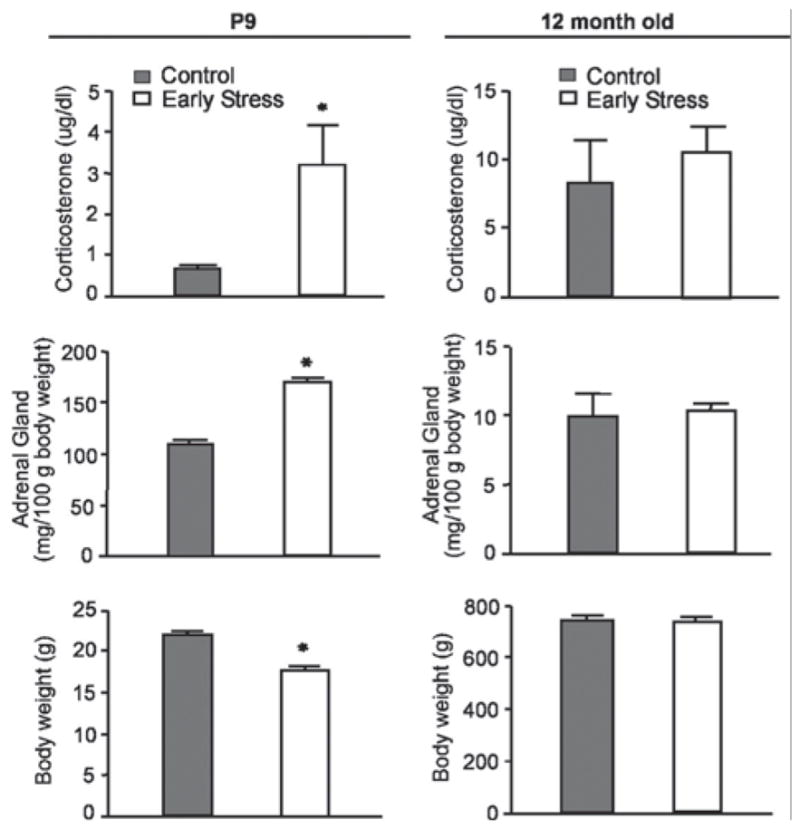
Parameters indicative of chronic stress in 9-day-old pups experiencing a week of CES; (left column) and in adult male rats (12 months of age; right column). Elevated basal corticosterone levels, higher adrenal weights, and modestly lower body weight are found in chronically stressed 9-day old rats. These changes are no longer apparent in adult rats. *p <.05; Student’s t-test. (Modified from Brunson et al. (2005), with permission).
Here we describe the original model, and important modifications in subsequent studies:
As devised originally in the Baram lab, the onset of the stress period is on PND 2. Pups from several litters are mixed among dams and those assigned to the CES groups are transferred to cages with limited bedding and nesting material. Specifically, the cages are fitted with a plastic coated aluminium mesh platform to sit approximately 2.5 cm above the cage floor. Bedding is reduced to only cover cage floor sparsely, and one-half of a single paper towel is provided for nesting maternal. The impoverished cage environment prevents the dam from constructing a satisfactory nest which might be dispersed (Fig. 2). The impoverished environment leads to significant maternal stress, and this stress is associated with alterations in the pattern of care manifest as fragmented and erratic nurturing behaviors (Ivy et al., 2008). Detailed analysis of maternal care in this model demonstrates a pattern characterized by shortened bouts of each nurturing behavior and frequent shifts between different behaviors (Baram et al., 2012; Ivy et al., 2008). As mentioned above, it appears that the disrupted maternal care is a main source of CES in the pups. Indeed, when, on PND 9, dams and pups are returned to normal bedding/nesting cages, maternal behavior returns to normal within hours, and stress hormone levels are reduced (Ivy et al., 2008).
FIGURE 2.

Photograph of a cage at the moment of onset of the limited bedding/nesting chronic early-life stress paradigm in rats. Note the fine, plastic-coated aluminium-mesh floor and the shredded paper towel. The cage is placed on a metal cart.
The model of limited bedding/nesting and fragmented maternal care developed in rats, that truly recapitulates CES, has been adapted to mice (Rice et al., 2008), and adopted and modified by several other groups. It has proven powerful, predictable and reproducible in both rat and mouse models (Dalle Molle et al., 2012; Malter Cohen et al., 2013; Moriceau et al., 2009; Wang et al., 2011, 2012). To facilitate future studies, a step-by step description of the protocol is provided in Table 1.
Table 1.
Setting Up the Limited Bedding/Nesting Chronic Early-Life Stress (CES) Paradigm in Rats and Mice
| Day | Procedure | Note |
|---|---|---|
| Time-pregnancy | Order time-pregnant females from your animal supplier or arrange to breed females in-house. | |
| To limit the effects of previous experience on the dam’s maternal behavior and response to stress, always use virgin naïve females. | ||
| Minimize disturbances and other stress sources throughout pregnancy. | ||
| Check for births at least twice daily on the days surrounding expected parturition; at least two dams will give birth within the same 10–12 hr period to mix the pups across litters on the day of manipulation. | ||
|
| ||
| Postnatal Day 2 | Prepare limited bedding and nesting cages: | |
| Start with clean, empty standard housing cages. | Plastic-coated aluminium mesh dimensions: 0.4 cm × 0.9 cm. McNichols Co., Tampa, FL catalog no. 4700313244. | |
| Position a fine-gauge, plastic coated aluminium mesh platform to sit approximately 2.5 cm above the cage floor. Folding edges of mesh along the length approximately 3 cm so that platform sits above the bottom of the cage, permitting droppings to fall below the platform without trapping the pups. | ||
| Cover cage floor with a small amount of standard bedding. This should not reach the top of the mesh. | ||
| Provide a limited amount of nesting material. For rats, add one-half of a single paper towel to cage. For mice, add one-half of a single NESTLET square (Ancare, Bellmore, NY). | ||
| Limited bedding/nesting manipulation: | ||
| To minimize genetic factors, pups from several dams are mixed and matched and assigned to CES or control dams at random. | Be careful the separation time is limited to under 15–20 minutes. | |
| For each litter, quickly and gently remove all pups from the home cage; identify the sex of each pup (using anogenital distance) and place males and females into separate, euthermic holding cages. | ||
| Repeat for each litter, keeping separate holding cages for male and female pups. | ||
| Once all litters are removed and sorted, randomly assign dams to the control or limited bedding/nesting conditions. | ||
| Place dam into fresh, clean standard cage (with normal amounts of bedding and nesting material). Randomly transfer pups from the male and female holding cages to control cage with the dam. | Litter size: because it influences both pup weight and hence maturation of the brain, as well as maternal behaviors per pup, we typically have 4 – 6 pups per mouse litter and 10–12 per rat litter. Final sex ratio should be approximately 1:1. | |
| Limited bedding/nesting cages: Place dam into cage with mesh platform and limited bedding and nesting material. Randomly transfer pups from the male and female holding cages to the experimental cage with the dam. | Counter-balance the order in which you replace pups with the dams between control and limited bedding/nesting conditions to limit differences in the total duration pups are separated. | |
|
| ||
| Postnatal Day 2–9 | Leave control and limited bedding/nesting cages undisturbed (and unchanged) until postnatal Day 9. | Observe maternal behavior during that period at least twice daily, as described in Ivy et al., 2008. Alternatively, video behavior. Consider looking at continuous behaviors and patterns of care over 60–90 minutes. |
|
| ||
| Postnatal Day 10 | In the morning, change all cages (control and limited bedding/nesting) to fresh, standard cages with normal bedding and nesting material. | Return cages to standard husbandry and changing schedules. |
|
| ||
| Postnatal Day 21 | Wean animals from dam. House same-sex litter-mates in the same cage. | All animals should be housed in temperature-controlled, quiet, un-crowded conditions on a 12-hr light, 12-hr dark schedule, with free access to food and water. |
Prof. Sullivan and her group have adopted and modified the paradigm in several ways, most importantly modifying the timing of CES during development (PND 1–7, 3–8, or 8–12). They aim to simulate rough and abusive maternal care patterns (Moriceau et al., 2009; Raineki et al., 2010, 2012). Several additional groups have now published on the use of the model, typically commencing on PND 2 (Dalle Molle et al., 2012; Gunn et al., 2013; Machado et al., 2013; Malter Cohen et al., 2013; Wang et al., 2011, 2012). The availability of a potent mouse and rat model of CES has spurred studies in numerous labs on CES consequences and the underlying mechanisms (Tables 2 and 3). In the paragraphs below we discuss the established consequences of the CES model on cognitive and emotional functions, focusing on those subserved by the hippocampus and the amygdala.
Table 2.
Major Outcomes Provoked by the Limited Bedding/Nesting Chronic Early-Life Stress (CES) Paradigm in Rats
| CES Period | Sex | Strain | Acute/Long Term | Outcomes | References |
|---|---|---|---|---|---|
| Stress system perturbations | |||||
| PND 2–9 | Both | Sprague-Dawley | PND 9 | Elevated basal corticosterone levels, higher adrenal weights. |
Gilles et al. (1996) Avishai-Eliner et al. (2001) Brunson et al. (2005) |
| PND 3–8 | Both | Long-Evans | PND 8 | Elevated basal corticosterone levels. | Raineki et al. (2010) |
| Cognitive and emotional functions | |||||
| PND 1–7 | Both | Long-Evans | PND 7 | Attachment learning deficits. | Moriceau et al. (2009) |
| PND 3–8 | Both | Long-Evans | PND 8 | Disrupted social attachment behaviors. | Raineki et al. (2010) |
| PND 8–12 | Both | Long-Evans | PND 20 and 45 PND 45 |
Impaired social behaviors. Depressive-like behaviors in FST test. |
Raineki et al. (2012) |
| PND 2–9 | Male | Wistar | PND 60 | Anxiety-like behaviors in EPM test. | Dalle Molle et al. (2012) |
| PND 2–9 | Male | Sprague-Dawley | 10–12 months | Memory deficits in MWM and NOR tests. | Brunson et al. (2005) |
| No anxiety-like behavior in EPM test. | Ivy et al. (2010) | ||||
| Brain changes | |||||
| PND 1–7 | Both | Long-Evans | PND 7 | Amygdala-locus ceruleus-olfactory bulb network perturbations. | Moriceau et al. (2009) |
| PND 3–8 | Both | Long-Evans | PND 8 | Enhanced amygdala neural activity. | Raineki et al. (2010) |
| PND 2–9 | Both | Sprague-Dawley | PND 9 | Reduced CRH mRNA expression in the PVN and CRF1 mRNA expression in CA1 and dentate gyrus. Reduced CRH receptor binding capacities in pituitaries. Reduced GR gene expression in the PVN and frontal cortex. |
Avishai-Eliner et al. (2001) |
| PND 8–12 | Both | Long-Evans | PND 45 | Enhanced amygdala neural activity. | Raineki et al. (2012) |
| PND 2–9 | Both | Wistar | PND 60 | Elevated plasma BDNF levels. | Dalle Molle et al. (2012) |
| PND 2–9 | Male | Sprague-Dawley | 10–12 months | Dendritic atrophy of CA1 pyramidal cells and mossy fiber expansion in CA3. | Brunson et al. (2005) |
| Synaptic plasticity defects in CA3 and CA1 associated with physiological abnormalities in CA3. Augmented CRH expression in the hippocampus. |
Ivy et al. (2010) | ||||
PND, postnatal day; CRH, corticotropin releasing hormone; CRF1, CRH receptor 1; PVN, paraventricular nucleus of hypothalamus; GR, glucocorticoid receptor; MWM, Morris water maze; NOR, novel object recognition; EPM, elevated plus maze; BDNF, brain-derived neurotrophic factor; FST, forced swim test.
Table 3.
Major Outcomes Provoked by the Limited Bedding/Nesting Chronic Early-Life Stress (CES) Paradigm in Mice
| CES Period | Age | Strain | Acute/Long Term | Outcomes | References |
|---|---|---|---|---|---|
| Stress system perturbations | |||||
|
| |||||
| PND 2–9 | Both | C57BL/6J | PND 9 and 4–8 months | Elevated basal corticosterone levels. | Rice et al. (2008) |
|
| |||||
| Cognitive and emotional functions | |||||
|
| |||||
| PND 2–9 | Male Female |
C57BL/6J | PND 21, 29 and 63 PND 63 |
Enhanced anxiety-like behaviors in the novelty-induced hypophagia paradigm. | Malter Cohen et al. (2013) |
| PND 2–9 | Male | 129S2/Sv × C57BL/6J | 3 months | Enhanced anxiety-like behaviors in OF and light-dark box tests. | Wang et al. (2012) |
| PND 2–9 | Male | C57BL/6J | 4–8 months | Memory impairments in MWM and NOR tests No anxiety-like behavior in OF test. |
Rice et al. (2008) |
| PND 2–9 | Male | 129S2/Sv × C57BL/6J | 6 months | Memory impairments in MWM and Y-maze tests. | Wang et al. (2011) |
|
| |||||
| Brain changes | |||||
|
| |||||
| PND 2–9 | Both | C57BL/6J | PND 9 | Reduced CRH mRNA expression in the PVN. | Rice et al. (2008) |
| PND 2–9 | Both | C57BL/6J × 129Sv-SvJ | PND 18–26 | Upregulation of CRH expression in the PVN Astrocytic glutamate reuptake impairments and enhanced glutamatergic drive onto dorsal-medial neurons of the hypothalamus. |
Gunn et al. (2013) |
| PND 2–9 | Both | C57BL/6J × 129Sv-SvJ | 8 weeks | Upregulation of CRH expression in the PVN. | Gunn et al. (2013) |
| PND 2–9 | Male | 129S2/Sv × C57BL/6J | 3 months | No change in the gene expression of CRH and arginine vasopressin in the PVN, MR and GR in the hippocampus and CRH in the central amygdala. | Wang et al. (2012) |
| PND 2–9 | Male | C57BL/6J | 4–8 months | Reduced CRH mRNA expression in the PVN. | Rice et al. (2008) |
|
| |||||
| PND 2–9 | Male | 129S2/Sv × C57BL/6J | 6 months | LTP deficits in CA3 Reduced number of dendritic spines in CA3 Reduced inhibitory synaptic density in CA1 and excitatory synaptic density in CA1 and CA3 Reduced Neurexin-1 mRNA levels in CA3 and Neuroligin-3 mRNA levels in CA1. |
Wang et al. (2011) |
PND, postnatal day; CRH, corticotropin releasing hormone; PVN, paraventricular nucleus of hypothalamus; MWM, Morris water maze; NOR, novel object recognition; OF, open field; MR, mineralocorticoid; GR, glucocorticoid receptor.
COGNITIVE CONSEQUENCES OF CHRONIC EARLY-LIFE STRESS
Initial studies using the CES model focused on cognitive functions and specifically on learning and memory. During young adulthood, rats that were exposed to the limited bedding/nesting environment perform reasonably well in the MWM test of spatial learning and memory (Brunson et al., 2005). Long-term potentiation (LTP) in response to high-frequency stimulation is also normal in both areas CA1 and CA3, although subtle changes in the properties of CA3 pyramidal cells are evident at this age. However, when these early-life stressed rats reach middle-age, impairments of synaptic and behavioral measures of hippocampus are found (Brunson et al., 2005). Cognitive deficits in MWM test and LTP disruptions in hippocampal area have also been reported in adult mice (Wang et al., 2011). More recent studies have suggested that this CES graduates harbor cognitive vulnerabilities that are unmasked already in adolescence by an additional challenge or stress (Molet et al., unpublished). In addition, memory test more stringent than the MWM may unmask these incipient cognitive vulnerabilities (ibid).
Because deficits in hippocampus-dependent learning and memory were observed, attention focused on maturation of hippocampus structure and connectivity. It has been well-established that the hippocampus is particularly vulnerable to the effects of stress, and when that stress occurs early in life, the deleterious effects are often persistent (Maras & Baram, 2012). Although in mice (Rice et al., 2008) or rats exposed to the limited bedding/nesting model described above neuroendocrine parameters of their stress system have returned to baseline in adulthood (Brunson et al., 2005) (Fig. 1), profound and overt changes in hippocampal structure and function were found (Brunson et al., 2005; Ivy et al., 2010) (Fig. 3). Pharmacological approaches in rats, and the use of mouse genetics have suggested a major role for the neuropeptide CRH in these hippocampal structural and cognitive impairments (Ivy et al., 2010; Wang et al., 2011).
FIGURE 3.
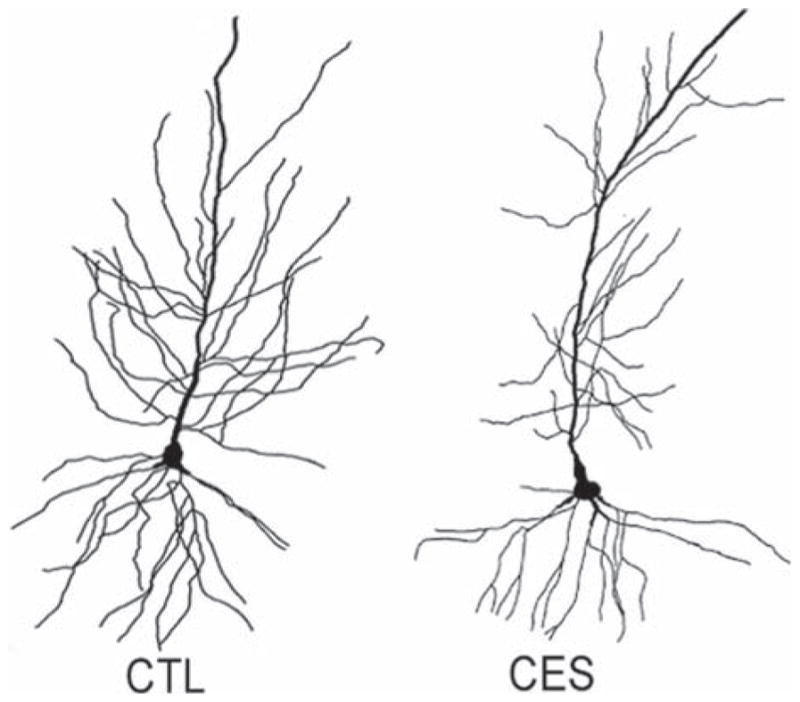
Dendritic atrophy of CA1 pyramidal cells from early-life stressed middle-aged rats. Golgi-impregnated CA1 pyramidal cells were reconstructed using camera Lucida. Representative neurons from control (left) and CES (right) illustrate the reduction in dendritic branching in a neuron from the early-life stressed group. (Modified from Ivy et al. (2010), with permission).
In addition to hippocampus-mediated spatial learning, CES has profound consequences on other types of learning: major disturbances of attachment learning, a process involving amygdala-locus ceruleus-olfactory bulb network, have been described and elegantly delineated following CES perturbation (Moriceau et al., 2009).
EMOTIONAL CONSEQUENCES OF CHRONIC EARLY-LIFE STRESS
The consequences of CES on emotional function are profoundly important. Recent research employed the limited bedding/nesting model to examine for depressive and anxiety-like behaviors and their underlying mechanisms.
Even during preweaning period, deficits in social behaviors were observed (Raineki et al., 2012). Importantly, Raineki et al., found depressive-like behaviors manifest by enhanced immobility time in the FST in adolescent rats experiencing rough and abusive maternal behaviors in the CES model. These behaviors were associated with increased amygdala neural activity in adolescent rats (Raineki et al., 2012). These results support the fundamental role of the amygdala in emotional disorders (Bremner, 2003; Drevets, 1999; Ressler & Mayberg, 2007; Roozendaal, McEwen, & Chattarji, 2009; Sibille et al., 2009).
Several additional groups reported on anxiety-like behaviors in young adult mice exposed to the limited bedding/nesting paradigm. Some behaviors, including the novelty-induced hypophagia, were associated with alteration in amygdala circuitry (Malter Cohen et al., 2013). In the open field test (OF), anxiety-like behaviors have been reported in adult mice experiencing CES, and the generation of these behaviors required intact forebrain CRH receptor type 1 (Wang et al., 2012). In young adult rats experiencing CES, anxiety-like behaviors were found in the EPM test, associated with increased peripheral BDNF levels (Dalle Molle et al., 2012).
These emotional aberrations may be age-dependent, because unlike in adolescent and young adult rodents, anxiety levels in older mice or rats did not distinguish CES graduates from controls (Brunson et al., 2005; Rice et al., 2008).
LIMITATIONS AND SUMMARY
The majority of CES models, including the one discussed here employ rodents. Rodents are obviously incapable of reproducing the rich repertoire of human development, as well as cognitive and emotional behaviors. Non-human primates, whose brains and sociality most closely represent those of humans, have provided powerful insights into the development of complex psychiatric disorders. The seminal work of Harlow and colleagues using maternally isolated rhesus monkeys as a model was the first to demonstrate that maternal–infant interactions are in fact required for normal cognitive and emotional brain development (Mason & Harlow, 1958; Seay, Hansen, & Harlow, 1962; Seay & Harlow, 1965). More recently, using a model of maternal maltreatment in rhesus monkeys, Sanchez and colleagues suggested that this adverse early experience affects the development of brain systems involved in stress responses as well as emotional reactivity and adult abusive parenting (Maestripieri et al., 2006; Sanchez et al., 2010). Although primate models of CES continue to provide important insights, the many practical and ethical concerns associated with the use of primates preclude their widespread use. Rodents provide tractable models of CES and have therefore become widely adopted. Maternal separation models have provided a vast amount of data on the effects of reducing maternal input on pup development, yet these focus on reduced quantity of maternal care and on intermittent stress. Because in human scenarios stress is often chronic and the mother is present but provides sub-optimal care, the current model may more closely recapitulate many forms of CES in humans. These include inconsistency and lack of sensitivity of the mother and even abuse (Baram et al., 2012; Kendall-Tackett, 2007; Koenen et al., 2003; Whipple & Webster-Stratton, 1991).
As illustrated above, it appears that though relatively new, the continued adoption of robust CES models in rodents may provide a valuable tool for identifying the long-term consequences of CES on brain development and vulnerability to disease, and the underlying mechanisms. Whereas much has already been discovered, numerous research avenues have not been explored, including the role of CES on the bed nucleus of the stria terminalis and prefrontal cortex function and structure, gender differences, and importantly, gene-environment interactions. CES models should be very useful to examine the interaction of single gene and multiple gene variations and mutations with early-life stress as determinants of psychopathology (Caspi et al., 2002; Heim & Binder, 2012; Martin, Ressler, Binder, & Nemeroff, 2009).
In summary, animal models of CES provide important tools to study normal adaptation as well as the role of early-life experience in resilience and vulnerability to cognitive and emotional stress-related disorders. Given the importance of mother–infant interactions, it is reasonable for most animal models to focus on manipulating maternal care. Because in infants chronic early-life psychological stress typically results from abnormal behaviors of a present mother, CES models described here, based on simulated poverty and fragmented, erratic maternal care patterns, offer opportunities to recapitulate important aspects of the human condition.
Acknowledgments
NOTE
The authors thank Barbara Cartwright for editorial help.
Contract grant sponsor: NIH
Contract grant numbers: MH73136, NS29012, P50MH096889
References
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32(3):256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Ammerman RT, Van Hasselt VB, Hersen M. Parent–child problem-solving interactions in families of visually impaired youth. Journal of Pediatric Psychology. 1991;16(1):87–101. doi: 10.1093/jpepsy/16.1.87. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends in Neurosciences. 2002;25(10):518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. Journal of Neuroendocrinology. 2001;13(9):799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, Newth CJ, Baram TZ. Effects of maternal and sibling deprivation on basal and stress induced hypothalamic-pituitary-adrenal components in the infant rat. Neuroscience Letters. 1995;192(1):49–52. doi: 10.1016/0304-3940(95)11606-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A, Stern H. Fragmentation and unpredictability of early-life experience in mental disorders. The American Journal of Psychiatry. 2012;169(9):907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetti F, Mello PB, Bonini JS, Monteiro S, Cammarota M, Izquierdo I. Early postnatal maternal deprivation in rats induces memory deficits in adult life that can be reversed by donepezil and galantamine. International Journal of Developmental Neuroscience. 2009;27(1):59–64. doi: 10.1016/j.ijdevneu.2008.09.200. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child and Adolescent Psychiatric Clinics of North America. 2003;12(2):271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. The American Journal of Psychiatry. 1993;150(2):235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Browne A, Finkelhor D. Impact of child sexual abuse: A review of the research. Psychological Bulletin. 1986;99(1):66–77. [PubMed] [Google Scholar]
- Brunson KL, Avishai-Eliner S, Hatalski CG, Baram TZ. Neurobiology of the stress response early in life: Evolution of a concept and the role of corticotropin releasing hormone. Molecular Psychiatry. 2001;6(6):647–656. doi: 10.1038/sj.mp.4000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Chen Y, Avishai-Eliner S, Baram TZ. Stress and the developing hippocampus: A double-edged sword? Molecular Neurobiology. 2003;27(2):121–136. doi: 10.1385/MN:27:2:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, … Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. The Journal of Neuroscience. 2005;25(41):9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, … Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Claessens SE, Daskalakis NP, van der Veen R, Oitzl MS, de Kloet ER, Champagne DL. Development of individual differences in stress responsiveness: An overview of factors mediating the outcome of early life experiences. Psychopharmacology. 2011;214(1):141–154. doi: 10.1007/s00213-010-2118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Molle R, Portella AK, Goldani MZ, Kapczinski FP, Leistner-Segal S, Salum GA, … Silveira PP. Associations between parenting behavior and anxiety in a rodent model and a clinical sample: Relationship to peripheral BDNF levels. Translational Psychiatry. 2012;2:e195. doi: 10.1038/tp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent GW, Okimoto DK, Smith MA, Levine S. Stress-induced alterations in corticotropin-releasing hormone and vasopressin gene expression in the paraventricular nucleus during ontogeny. Neuroendocrinology. 2000;71(6):333–342. doi: 10.1159/000054554. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Annals of the New York Academy of Sciences. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski CG, Baram TZ. Differential regulation of the expression of corticotropin-releasing factor receptor type 2 (CRF2) in hypothalamus and amygdala of the immature rat by sensory input and food intake. The Journal of Neuroscience. 1999;19(10):3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. 2011;214(1):17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Stone BA, Kapadia BJ, Baram TZ. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology. 2005;146(9):4090–4096. doi: 10.1210/en.2004-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: Functional and molecular aspects. Frontiers in Neuroendocrinology. 2006;27(2):180–192. doi: 10.1016/j.yfrne.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald LC, Gunnar MR. Poverty-alleviation program participation and salivary cortisol in very low-income children. Social Science & Medicine. 2009;68(12):2180–2189. doi: 10.1016/j.socscimed.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin JM, Jr, Polansky NA, Kilpatrick AC, Shilton P. Family functioning in neglectful families. Child Abuse & Neglect. 1996;20(4):363–377. doi: 10.1016/0145-2134(96)00005-1. [DOI] [PubMed] [Google Scholar]
- Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatric Neurology. 1996;15(2):114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: Genetic and environmental influences on development of the stress response. Depression and Anxiety. 2009;26(11):984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PG, Chen X, Alvarez P, Ferrari LF, Levine JD. Early-life stress produces muscle hyperalgesia and nociceptor sensitization in the adult rat. Pain. 2011;152(11):2549–2556. doi: 10.1016/j.pain.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn BG, Cunningham L, Cooper MA, Corteen NL, Seifi M, Swinny JD, … Belelli D. Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: Relevance to neurosteroids and programming of the stress response. The Journal of Neuroscience. 2013;33(50):19534–19554. doi: 10.1523/JNEUROSCI.1337-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009;34(1):62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from romanian orphanages. Development and Psychopathology. 2001;13(3):611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry. 2007;62(1):40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Harbuz M, Russell JA, Sumner BE, Kawata M, Lightman SL. Rapid changes in the content of proenkephalin A and corticotrophin releasing hormone mRNAs in the paraventricular nucleus during morphine withdrawal in urethane-anaesthetized rats. Molecular Brain Research. 1991;9(4):285–291. doi: 10.1016/0169-328x(91)90074-8. [DOI] [PubMed] [Google Scholar]
- Hatalski CG, Baram TZ. Stress-induced transcriptional regulation in the developing rat brain involves increased cyclic adenosine 3′,5′-monophosphate-regulatory element binding activity. Molecular Endocrinology. 1997;11(13):2016–2024. doi: 10.1210/mend.11.13.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatalski CG, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. Journal of Neuroendocrinology. 1998;10(9):663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental Neurology. 2012;233(1):102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatrica Suppl. 1994;397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology. 2001;158(4):366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: A clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dube C, Maras PM, Grigoriadis DE, … Baram TZ. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. The Journal of Neuroscience. 2010;30(39):13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. International Journal of Epidemiology. 2001;30(2):256–263. doi: 10.1093/ije/30.2.256. [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett KA. Violence against women and the perinatal period: The impact of lifetime violence and abuse on pregnancy, postpartum, and breastfeeding. Trauma Violence & Abuse. 2007;8(3):344–353. doi: 10.1177/1524838007304406. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Cain SW, Masum Z, Ralph MR. Neural stem cells show bidirectional experience-dependent plasticity in the perinatal mammalian brain. The Journal of Neuroscience. 2004;24(11):2832–2836. doi: 10.1523/JNEUROSCI.0110-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Caspi A, Taylor A, Purcell S. Domestic violence is associated with environmental suppression of IQ in young children. Development and Psychopathology. 2003;15(2):297–311. doi: 10.1017/s0954579403000166. [DOI] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. The pathways from mother’s love to baby’s future. Frontiers in Behavioral Neuroscience. 2009;3:27. doi: 10.3389/neuro.08.027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn CM, Butler SR, Schanberg SM. Selective depression of serum growth hormone during maternal deprivation in rat pups. Science. 1978;201(4360):1034–1036. doi: 10.1126/science.684424. [DOI] [PubMed] [Google Scholar]
- Kuhn CM, Schanberg SM. Responses to maternal separation: Mechanisms and mediators. International Journal of Developmental Neuroscience. 1998;16(3–4):261–270. doi: 10.1016/s0736-5748(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Bettschen D, Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacology Biochemistry and Behavior. 1999;64(4):705–715. doi: 10.1016/s0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Jongen-Relo AL, Stohr T, Pothuizen HH, Feldon J. Comparison of maternal separation and early handling in terms of their neurobehavioral effects in aged rats. Neurobiololgy of Aging. 2002;23(3):457–466. doi: 10.1016/s0197-4580(01)00320-7. [DOI] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156(3772):258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- Levine S, Glick D, Nakane PK. Adrenal and plasma corticosterone and vitamin A in rat adrenal glands during postnatal development. Endocrinology. 1967;80(5):910–914. doi: 10.1210/endo-80-5-910. [DOI] [PubMed] [Google Scholar]
- Levitt P. Structural and functional maturation of the developing primate brain. The Journal of Pediatrics. 2003;143(4 Suppl):S35–S45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Young WS., III Corticotrophin-releasing factor, vasopressin and pro-opiomelanocortin mRNA responses to stress and opiates in the rat. The Journal of Physiology. 1988;403:511–523. doi: 10.1113/jphysiol.1988.sp017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lynch M, Cicchetti D. An ecological-transactional analysis of children and contexts: The longitudinal interplay among child maltreatment, community violence, and children’s symptomatology. Development and Psychopathology. 1998;10(2):235–257. doi: 10.1017/s095457949800159x. [DOI] [PubMed] [Google Scholar]
- Machado TD, Dalle Molle R, Laureano DP, Portella AK, Werlang IC, Benetti Cda S, … Silveira PP. Early life stress is associated with anxiety, increased stress responsivity and preference for “comfort foods” in adult female rats. Stress. 2013;16(5):549–556. doi: 10.3109/10253890.2013.816841. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, … Beardslee WR. Childhood abuse and lifetime psychopathology in a community sample. The American Journal of Psychiatry. 2001;158(11):1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Higley JD, Lindell SG, Newman TK, McCormack KM, Sanchez MM. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques (Macaca mulatta) Behavioral Neuroscience. 2006;120(5):1017–1024. doi: 10.1037/0735-7044.120.5.1017. [DOI] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(45):18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais L, van Rensburg SJ, van Zyl JM, Stein DJ, Daniels WM. Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neuroscience Research. 2008;61(1):106–112. doi: 10.1016/j.neures.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Maras PM, Baram TZ. Sculpting the hippocampus from within: Stress, spines, and CRH. Trends in Neurosciences. 2012;35(5):315–324. doi: 10.1016/j.tins.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: Brain imaging, genetics, and psychoneuroendocrinology. Psychiatric Clinics of North America. 2009;32(3):549–575. doi: 10.1016/j.psc.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Harlow HF. Performance of infant rhesus monkeys on a spatial discrimination problem. Journal of Comparative and Physiological Psychology. 1958;51(1):71–74. doi: 10.1037/h0040609. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annual Review of Medicine. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early-life stress disrupts attachment learning: The role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. The Journal of Neuroscience. 2009;29(50):15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, III, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318(5858):1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Paolucci EO, Genuis ML, Violato C. A meta-analysis of the published research on the effects of child sexual abuse. The Journal of Psychology. 2001;135(1):17–36. doi: 10.1080/00223980109603677. [DOI] [PubMed] [Google Scholar]
- Pihoker C, Owens MJ, Kuhn CM, Schanberg SM, Nemeroff CB. Maternal separation in neonatal rats elicits activation of the hypothalamic-pituitary-adreno-cortical axis: A putative role for corticotropin-releasing factor. Psychoneuroendocrinology. 1993;18(7):485–493. doi: 10.1016/0306-4530(93)90042-j. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research. 1993;18(3):195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Raineki C, Cortes MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. The Journal of Neuroscience. 2012;32(22):7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biological Psychiatry. 2010;67(12):1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128(2):330–366. [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nature Neuroscience. 2007;10(9):1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149(10):4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10(6):423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Suchecki D, Levine S. Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neuroscience & Biobehavioral Reviews. 1992;16(4):553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological Psychiatry. 2009;65(9):760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry. 2005;57(8):823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sweatt JD. Epigenetic marking of the BDNF gene by early-life adverse experiences. Hormones and Behavior. 2011;59(3):315–320. doi: 10.1016/j.yhbeh.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, McCormack K, Grand AP, Fulks R, Graff A, Maestripieri D. Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Development and Psychopathology. 2010;22(1):45–53. doi: 10.1017/S0954579409990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schable S, Poeggel G, Braun K, Gruss M. Long-term consequences of early experience on adult avoidance learning in female rats: Role of the dopaminergic system. Neurobiology of Learning and Memory. 2007;87(1):109–122. doi: 10.1016/j.nlm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Schapiro S, Geller E, Eiduson S. Neonatal adrenal cortical response to stress and vasopressin. Proceedings of the Society for Experimental Biology and Medicine. 1962;109:937–941. doi: 10.3181/00379727-109-27384. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Wang XD, Meijer OC. Early life stress paradigms in rodents: Potential animal models of depression? Psychopharmacology. 2011;214(1):131–140. doi: 10.1007/s00213-010-2096-0. [DOI] [PubMed] [Google Scholar]
- Schoenfeld NM, Leathem JH, Rabii J. Maturation of adrenal stress responsiveness in the rat. Neuroendocrinology. 1980;31(2):101–105. doi: 10.1159/000123058. [DOI] [PubMed] [Google Scholar]
- Schore AN. Attachment and the regulation of the right brain. Attachment & Human Development. 2000;2(1):23–47. doi: 10.1080/146167300361309. [DOI] [PubMed] [Google Scholar]
- Seay B, Hansen E, Harlow HF. Mother-infant separation in monkeys. The Journal of Child Psychology and Psychiatry. 1962;3:123–132. doi: 10.1111/j.1469-7610.1962.tb02047.x. [DOI] [PubMed] [Google Scholar]
- Seay B, Harlow HF. Maternal separation in the rhesus monkey. The Journal of Nervous and Mental Disease. 1965;140(6):434–441. doi: 10.1097/00005053-196506000-00006. [DOI] [PubMed] [Google Scholar]
- Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, Belzung C, … Lewis DA. A molecular signature of depression in the amygdala. The American Journal of Psychiatry. 2009;166(9):1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang HJ, Gunnar MR, Snellman L, Condon LM, Kestenbaum R. Local anesthesia for neonatal circumcision. Effects on distress and cortisol response. The Journal of the American Medical Association. 1988;259(10):1507–1511. [PubMed] [Google Scholar]
- Suchecki D, Nelson DY, Van Oers H, Levine S. Activation and inhibition of the hypothalamic-pituitary-adrenal axis of the neonatal rat: Effects of maternal deprivation. Psychoneuroendocrinology. 1995;20(2):169–182. doi: 10.1016/0306-4530(94)00051-b. [DOI] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Levine S. Persistent, but paradoxical, effects on HPA regulation of infants maternally deprived at different ages. Stress. 1997;1(4):249–262. doi: 10.3109/10253899709013745. [DOI] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Levine S. Early vs. late maternal deprivation differentially alters the endocrine and hypothalamic responses to stress. Developmental Brain Research. 1998;111(2):245–252. doi: 10.1016/s0165-3806(98)00143-6. [DOI] [PubMed] [Google Scholar]
- Walker CD, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: Role of the pituitary and the hypothalamus. Endocrinology. 1986;118(4):1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128(3):1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- Wang XD, Labermaier C, Holsboer F, Wurst W, Deussing JM, Muller MB, Schmidt MV. Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1. European Journal of Neuroscience. 2012;36(3):2360–2367. doi: 10.1111/j.1460-9568.2012.08148.x. [DOI] [PubMed] [Google Scholar]
- Wang XD, Rammes G, Kraev I, Wolf M, Liebl C, Scharf SH, … Schmidt MV. Forebrain CRF(1) modulates early-life stress-programmed cognitive deficits. The Journal of Neuroscience. 2011;31(38):13625–13634. doi: 10.1523/JNEUROSCI.2259-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple EE, Webster-Stratton C. The role of parental stress in physically abusive families. Child Abuse & Neglect. 1991;15(3):279–291. doi: 10.1016/0145-2134(91)90072-l. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- Yi SJ, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135(6):2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LX, Levine S, Dent G, Zhan Y, Xing G, Okimoto D, … Smith MA. Maternal deprivation increases cell death in the infant rat brain. Developmental Brain Research. 2002;133(1):1–11. doi: 10.1016/s0926-6410(01)00118-5. [DOI] [PubMed] [Google Scholar]


