Abstract
Much of the social behavior in which rodents engage is related to reproduction, such as maintaining a breeding territory, seeking mates, mating, and caring for young. Rodents belong to the internally fertilizing species that require sexual behavior for reproduction. The dyadic, heterosexual patterns of most mammalian species are sexually dimorphic, but they also share mutual components in both sexes: sexual attraction is reciprocal, sexual initiative is assumed, appetitive behavior is engaged in and mating involves consummatory and postconsummatory phases in females as well as in males. Serotonin, a phylogenetically ancient molecule, is the most widely distributed neurotransmitter in the brain and its signaling pathways are essential for numerous functions including sexual behavior. Since the late 1960’s, brain serotonergic neurotransmission has been considered to exert an inhibitory influence on the neural mechanisms mediating sexual behavior. This contention was based mainly on the observations that a decrease in central serotonergic activity facilitated the elicitation of sexual behavior while an increase in central serotonergic activity attenuated it. However, the discovery of over 14 types of serotonin receptors has added numerous layers of complexity to the study of serotonin and sexual behavior. Evidence shows that upon activation, certain receptor subtypes facilitate while some others suppress sexual behavior as well as sexual arousal and motivation. Furthermore, the role of these receptors has been shown to be differential in males versus females. The use of serotonergic pharmacological interventions, mouse strains with genetic polymorphisms causing alterations in the levels of brain serotonin as well as animal models with genetic manipulations of various serotonin effectors has helped delineate the fundamental role of this neurotransmitter in the regulation of sexual behavior. This review aims to examine the basics of the components of female and male sexual behavior and the participation of the serotonin system in the modulation of these behaviors with emphasis on rodents.
Keywords: serotonin, sexual behavior, copulation, female receptivity, mounting
Introduction
Much of the social behavior in which rodents engage is related to reproduction, such as maintaining a breeding territory, seeking mates, mating, and caring for young (Bonthuis, et al., 2010). Rodents belong to the internally fertilizing species that require sexual behavior for reproduction (Wallen and Zehr, 2004), and an optimal reproductive fitness is essential for the biological success and survival of species (Oboti, et al., 2014). Sexual behavior emerges when animals reach adulthood and engage in behaviors that result in the joining of a male and a female, ending in copulation, with the intent to reproduce (Olivier, et al., 2011). Early studies have identified that the heterosexual patterns of most mammalian species are sexually dimorphic, but they also share mutual components in both sexes: sexual attraction is reciprocal, sexual initiative is assumed, appetitive behavior is engaged in and mating involves consummatory and postconsummatory phases in females as well as in males (Beach, 1976). Similarly, there are reports indicating that sexual behavior in females and males may depend upon different anatomical and neurochemical systems in the brain (Beach, 1976). Serotonin, a phylogenetically ancient molecule, is the most widely distributed neurotransmitter in the brain and its signaling pathways are essential for numerous functions including sexual behavior (Olivier, 2014). Brain areas critical for the orchestration of sexual behaviors include several hypothalamic nuclei (Floody and Czipri, 2014), the medial preoptic area (Hull and Dominguez, 2007), septum (Kondo, et al., 1990), the bed nucleus of the stria terminalis (Lau, et al., 2011), the amygdala (Veening and Coolen, 2014) and olfactory areas (Bonthuis, et al., 2010): all of them with serotonergic innervations. Since the late 1960’s, brain serotonergic neurotransmission has been considered to exert an inhibitory influence on the neural mechanisms mediating sexual behavior (Tagliamonte, et al., 1969). This contention was based mainly on the observations that a reduction in brain serotonergic pathways facilitated the elicitation of sexual behavior (Albinsson, et al., 1996) while an increase in central serotonergic activity attenuated it (Olivier, et al., 2011; Uphouse, 2014). However, the discovery of over 14 types of serotonin receptors has added numerous layers of complexity to the study of serotonin and sexual behavior. Evidence shows that certain receptor subtypes facilitate (Snoeren, et al., 2014) while some others suppress (Olivier, et al., 2011; Snoeren, et al., 2010; Uphouse, 2014) sexual behavior as well as sexual arousal and motivation. Furthermore, the role of these receptors has been shown to be differential in males versus female (Snoeren, et al., 2014). The use of serotonergic pharmacological interventions, mouse strains with genetic polymorphisms causing alterations in the levels of brain serotonin as well as animal models with genetic manipulations of various serotonin effectors has helped delineate the fundamental role of this neurotransmitter in the regulation of sexual behavior. This review aims to examine the basics of the components of female and male sexual behavior, the neuroanatomical regions involved and the participation of the serotonin system in the modulation of these behaviors with emphasis on rodents.
Precopulatory and appetitive behaviors in males and females
Successful reproduction in vertebrates depends critically upon a suite of precopulatory behaviors that occur prior to mating (Martinez and Petrulis, 2011). These behaviors involve sexual communication between sex partners, which can include one sensory modality or a combination, depending upon factors like species, habitat, and context (Hlinak, 1990). Anogenital scent marking and opposite-sex odor preference are components of these behaviors. In females, precopulatory events such as vaginal marking and solicitation behaviors take place in response to male odors (Martinez and Petrulis, 2011), whereas in males, the precopulatory behavior depends on solicitation and other sexual signals produced by a female (Hlinak, 1990). Proceptivity connotes various reactions by the female toward the male which constitute her assumption of initiative in establishing or maintaining sexual interaction (Beach, 1976). Ultrasounds also serve these animals as precopulatory signals that can attract males and help initiate mating (Floody and Czipri, 2014). In males, ultrasonic vocalizations may represent courtship behavior and it is dependent on the presence of testosterone (Burns-Cusato, et al., 2004). Early studies have shown that a universal form of masculine appetitive response is to approach and remain near females when they are in estrous (Beach, 1976). When a sexually experienced male rat runs toward a sexually receptive female, copulation will usually follow within seconds of having established physical contact with her (Hernandez-Gonzalez, et al., 2008). Male rats rarely make sexual approaches towards objects or nonreceptive females, and they may occasionally try to copulate with them, but both approach and copulatory behavior are of lower intensity than those displayed with receptive females (Hernandez-Gonzalez, et al., 2008). Females can influence their attractivity to males through non-behavioral (i.e. visual cues that manifest estrous, odoriferous secretions from the vagina, etc.) and behavioral (i.e. actively soliciting copulation, expression of “invitational” patterns) stimuli or combinations of both types (Beach, 1976). Attractivity refers to the female’s stimulus value in evoking sexual responses by the male. Appetitive behaviors serve to bring sexual partners into contact and include sexually specific investigative behaviors that are not specific to sex, such as altered locomotor activity, decreased emotional responsiveness or aggressive behavior and altered sensory perception (Fabre-Nys, et al., 2003).
The female strongly influences the pattern of mating through the intermittent and periodic display of solicitational behaviors that are classified as precopulatory or proceptive behaviors (Erskine, 1989). Proceptive behaviors may be defined as those species-typical complex set of appetitive activities displayed by the estrous female which encourage the male to mate and which regulate the pattern of copulation (Beach, 1976; Erskine, 1989). Pacing is a component of feminine proceptive behaviors that consists of intermittent approaches and withdrawals from the male, and plays a critical role in determining the types and amounts of cervical-vaginal stimulation received during mating (Erskine, 1989; Paredes and Vazquez, 1999). The ability to control the rate of sexual interactions has important physiological and behavioral consequences, including an increase in density of new cells in the accessory olfactory bulb (Corona, et al., 2011). Solicitation behaviors occur immediately prior to and throughout the course of mating and serve both to signal a readiness to mate and to govern the timing of intromissions received by the female. Female rats wiggle their ears and run away from the male with a hopping and darting type of locomotion which has an excitatory and an orientating function (Beach, 1976). Solicitation behaviors have been used as an index of female sexual motivation (Erskine, 1989). These behaviors occur under semi-natural and under laboratory conditions when females are tested in a small open area (Paredes and Vazquez, 1999). When females are tested in a seminatural environment, the approach-run away sequence to control the coital stimulation is more frequent (Paredes and Vazquez, 1999). Mating can be aversive for females but these consequences can be greatly reduced when the females are able to pace their sexual contacts. Only when the female is able to control the sexual stimulation received, sexual behavior can be reinforced and induce a reward state (Paredes and Vazquez, 1999).
Consummatory behaviors in males and females
Expression of sexual behavior involves a series of complex behavioral interactions and postural adjustments classified as patterns of appetitive (precopulatory) or consummatory behaviors. Consummatory or receptive behaviors comprise postural changes, which allow copulation to take place (Fabre-Nys, et al., 2003). In the actual mating sequence, appetitive and consummatory reactions often alternate: and furthermore the same response, such as assumption of the coital posture, can be appetitive in one instance and part of the consummatory complex in another (Beach, 1976). Each stage of the mating sequence involves reciprocal and bisexual interaction: when females are in estrous, not only are they more attractive to the male but they are most attracted to him (Beach, 1976).
Male rats usually begin a sexual encounter by investigating the female’s face and anogenital region (Hull and Dominguez, 2007). The male displays a series of mounts and intromissions that end in ejaculation. After ejaculating, the male remains quiescent for some time (postejaculatory interval, -PEI-) and a new round of mounts and intromissions begins (Olivier, et al., 2011). Ejaculation is characterized by a long, deep thrust and much slower dismount and it is accompanied by rhythmic contractions of the muscles at the base of the penis, and of anal sphincter and skeletal muscles (Hull and Dominguez, 2007). Previous sexual experience confers greater copulatory “efficiency” and increased resistance to the effects of various lesions, castration, and stress (Hull and Dominguez, 2007). In the male rat, data suggest that copulatory thrusting, the basic motor pattern in mount and intromission, is dependent on cutaneous stimulation in a way similar to the lordosis posture in females. The difference is that the cutaneous receptors involved are located dorsally in females and ventrally in males. In addition, airborne chemicals seem to facilitate mounting in mice, while their effects in other species are unclear (Hernandez-Gonzalez, et al., 2008). The motivational aspects of sexual behavior in males include orientating toward and approaching an estrous female, while the consummatory aspects include the motoric responses of mounting, pelvic thrusting, intromission and ejaculation (Paredes and Vazquez, 1999).
In females, sexual receptivity can be operationally defined as the behavior exhibited by females in response to stimuli normally provided by conspecific males. Receptivity comprises those feminine reactions which are necessary and sufficient for fertile copulation with a potent male. It includes adoption of a posture facilitating the male’s achievement of insertion plus maintenance of appropriately oriented contact long enough so that intravaginal ejaculation can occur (Beach, 1976). To gauge receptivity in female rodents, the most frequently used measure is the stereotypical lordosis reflex (Bonthuis, et al., 2010). The lordosis reflex occurs in response to a mount by a male and could be associated with consummatory aspects of a sexual interaction (Paredes and Vazquez, 1999). This posture is easily recognizable in rat and it is characterized by a concave flexion of the back, extension of the neck, elevation of the hindquarters and rump and deflection of the tail to one side (Chu and Agmo, 2015). However, female sexual behaviors are not limited to the display of lordosis and other stereotyped motor patterns directly associated with copulation. Before copulatory behaviors take place, approach to a potential mate occurs as a consequence of the intensity of the level of sexual incentive motivation. The intensity of the behaviors for sexual approach has been conceptually characterized as an equivalent to sexual desire in humans (Uphouse, 2014).
Brain areas regulating sexual behaviors
A major contributing factor in sexual differentiation of the rodent brain is the gonadal secretion of testosterone during development. In brain, testosterone is converted to estradiol by the enzyme aromatase. Exposure to testosterone or estradiol masculinizes and defeminizes aspects of brain morphology and results in sex-specific behaviors. Brain sexual dimorphisms and concordant sex differences in physiology and behavior arise primarily due to these critical period influences (Bonthuis, et al., 2010). The neural control of sexual behavior in males and females involves different anatomical and neurochemical systems in the brain (Paredes, 2009), including the ubiquitous serotonergic pathways. The hypothalamus participates in the regulation of sexual behaviors and it is innervated by serotonergic fibers with the highest concentration in the lateral, medial and infundibular areas. The lateral area connects the hypothalamus with structures of the limbic brain, cortex and brainstem (Amstislavskaya and Popova, 2004). Likewise, other brain areas with serotonergic innervations participate in the modulation of these behaviors and they are discussed in more detail below (see Fig.1).
Figure 1.
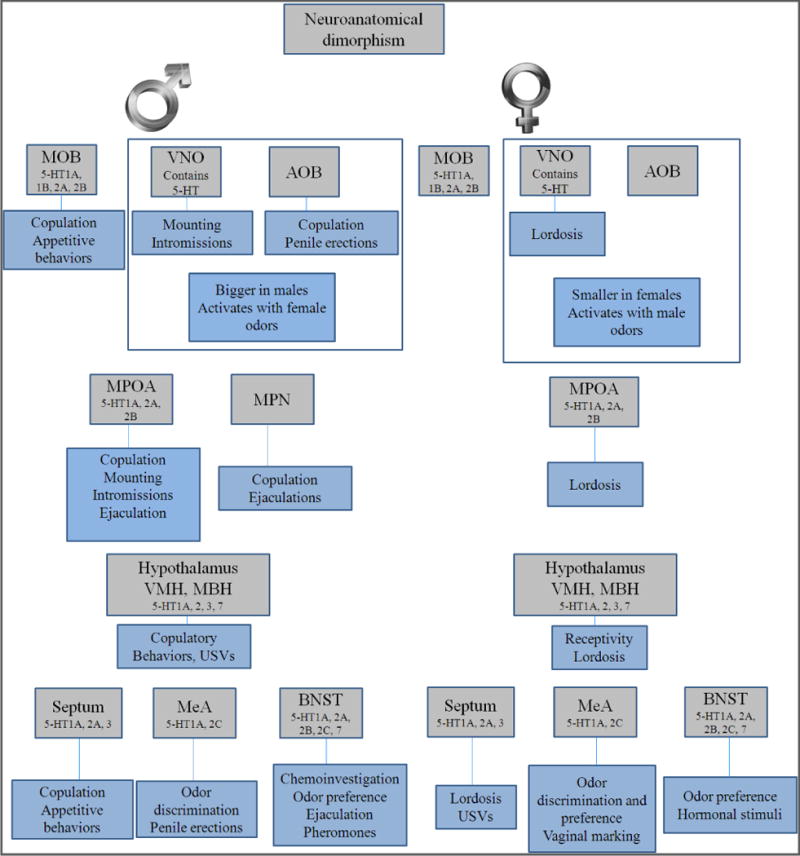
Components of sexual behavior are different in male and female rodents. Serotonin receptors are expressed virtually in every brain area involved in these behaviors. Neuroanatomical substrates for sexual behaviors are depicted in grey boxes, whereas the behaviors they influence are depicted in blue boxes.
Olfactory system
The rodent olfactory system has been divided into at least two, functionally distinct pathways: the main olfactory system that includes the olfactory epithelium and main olfactory bulb (MOB) and the accessory olfactory system, which includes the vomeronasal organ (VNO) and the accessory olfactory bulb (AOB) (Bonthuis, et al., 2010). The main olfactory system is thought to be devoted to detection of volatile odorants such as those from food, predators, and potential mates, whereas the accessory olfactory system is used to detect non-volatile odorants that influence reproductive and aggressive behaviors and also to aid in recognition of conspecifics (Bonthuis, et al., 2010). Chemosensory input from the main and vomeronasal systems is probably the most important stimulus for male rodent sexual behavior. Bilateral olfactory bulbectomy, which removes both the main and vomeronasal pathways, produced variable impairment of copulation and noncontact erections, with sexually naive males being more susceptible to impairment (Hull and Dominguez, 2007). Among male rodents, elimination of the VNO seems to have different consequences (Hernandez-Gonzalez, et al., 2008). In male rats, removal of the VNO caused a reduction in the mounts ending in intromission, suggesting deficiencies in erection and activity in the striated penile muscles. In sexually inexperienced male hamsters, removal of the VNO produced a reduction in copulatory behavior, but this effect was eliminated by exposing the males to vaginal fluids from receptive females. Finally, in male mice, removal of the VNO produced inconclusive effects on copulatory behaviors (Hernandez-Gonzalez, et al., 2008). In rats, most of the studies of sexual dimorphism in the olfactory pathway have been structural rather than functional and suggest that the VNO and the AOB are larger in males than in females. Studies in mice have also revealed sex differences in the activation of the VNO in response to both male and female bedding as well as the AOB in response to volatile odors. While opposite-sex odors activate the AOB of both males and females, same-sex odors have no effect (Bonthuis, et al., 2010). It appears that soluble chemicals produced and released by males may facilitate female lordosis via the VNO, while such chemicals emitted by females have only marginal effects on male copulatory behavior (Hernandez-Gonzalez, et al., 2008). In rats, olfactory stimuli seem to be of far less importance for the execution of copulatory behavior than for initial approach, but in mice, olfactory stimuli emitted by the female appear to be of substantial importance. Destruction of the nasal epithelium with ZnSO4 eliminated copulatory behavior both in experienced and inexperienced males (Hernandez-Gonzalez, et al., 2008). Neurons in the olfactory system express numerous serotonin receptors, including the 1A and 1B (Gao, et al., 2013), 2A (Liu, et al., 2012) and 2C (Petzold, et al., 2009) subtypes Biogenic amines including serotonin have been detected in the VNO (Zancanaro, et al., 1997) but the particular location of this neurotransmitter has not been described.
Medial preoptic area
The medial preoptic area (MPOA) contributes significantly to sexual motivation (Kondo, et al., 1990; Martinez and Petrulis, 2011) and it is one of the most critical sites for orchestrating male sexual behavior (Hull and Dominguez, 2007). The MPOA sends output to hypothalamic, midbrain and brain stem nuclei that regulate autonomic and somatomotor patterns and motivational states (Hull and Dominguez, 2007). The MPOA is generally believed to be one of the most important areas in the male rat forebrain involved in regulating masculine sexual behavior such as mounting, intromission, ejaculation (Kondo, et al., 1990) and copulation (Burns-Cusato, et al., 2004; Paredes and Vazquez, 1999). Destruction or lesions in this area effectively disrupted the display of masculine behavior (Kondo, et al., 1990) and produced impairment of copulation behaviors in male rats, and when these lesions are sufficiently large, copulation is completely abolished and the partner preference altered (Portillo, et al., 2003). In hamsters, lesions of the MPOA stop mating completely (Lau, et al., 2011). In contrast, facilitation of sexual behavior can be observed after electrical stimulation of the MPOA. Electrical stimulation of the MPOA in rats significantly increased their incidence of ejaculatory behavior, reduced the ejaculatory threshold in copula (Portillo, et al., 2003) and led to facilitation of copulation (Hull and Dominguez, 2007). Similarly to males, lesions of the MPOA also failed to facilitate feminine sexual behavior including lordosis in rats (Kondo, et al., 1990).
The medial preoptic nucleus (MPN) is a subnucleus within the MPOA extensively connected with the medial amygdala and the bed nucleus of the stria terminalis (BNST), both considered as convergence areas themselves because of their combined hormonal, genito-sensory and olfactory input (Veening and Coolen, 2014). The MPN is anatomically conspicuous but not homogeneous in its neuronal content. Functionally it is a diffuse nucleus that shows remarkable sexually dimorphic characteristics and plays a crucial role in the control of copulatory behavior in male rats. MPN is involved in removing the tonical inhibition of ejaculation responses by the brainstem (Veening and Coolen, 2014). Neurons in the MPOA have been found to express serotonin receptors (Gouveia and Franci, 2004), including the 1A, 2A and 2C subtypes. These two last types have been associated with facilitation of lordosis behaviors in females (Uphouse, 2014).
Hypothalamic nuclei
A limbic–hypothalamic circuit that regulates lordosis consists of the hypothalamus arcuate and ventromedial nucleus (VMH), as well as the MPN. Female sexual behaviour activates a distributed network within the brain, including the VMH, as demonstrated by behavioural studies performed in conjunction with neuroanatomical analyses of immediate early genes expression (Flanagan-Cato, et al., 2006). The VMH sends descending efferent projections to innervate the periaqueductal gray and the vestibular nucleus, which provide input to spinal motoneurons innervating muscles responsible for the lordotic posture (Christensen, et al., 2015). The mediobasal hypothalamus (MBH), a brain area containing the VMN is recognized to be critical for estradiol-facilitated female rat sexual receptivity (Blaustein, 2008). It has been shown that serotonin can inhibit, excite, or biphasically inhibit and excite individual neurons of VMN in female rats and several types of serotonin receptors are expressed in this nucleus (Kow, et al., 1992). Lesions of the VMN alter ultrasonic vocalization in female hamsters (Floody and Czipri, 2014). Unilateral lesions of the VMN disrupted lordosis in female rats in an essentially all-or-none fashion but comparable transections did not impair masculine sexual behavior in gonadally intact males (Yahr and Greene, 1992). Studies have shown that neurons in the VMH are preferentially activated after a bout of mating with no activation in näve animals. In addition, mating behavior produces short-term changes in structural proteins and long-term, selective changes in dendrite morphology in this area (Flanagan-Cato, et al., 2006). Serotonin receptor 1A, 2 and 3 subtypes are expressed in the VMN (Maswood, et al., 1997). In addition, it has been suggested that serotonin released after male ejaculation may promote the sexual quiescence of the PEI (Lorrain, et al., 1997), and the lateral hypothalamic area may be one of the sites responsible for this serotonergic inhibition of copulation during the PEI (Lorrain, et al., 1997).
Septum, amygdala, BNST and mesencephalon
Evidences suggest that the septal area is involved in mediating male sexual behavior (Gogate, et al., 1995; Kondo, et al., 1990). It was reported that the lateral septum may be involved in integrating cortical and subcortical influences for expression of masculine sexual behavior and reports indicate that the medial septum is also connected to the MPOA. While lesions in the lateral septum effectively suppressed the performance of mounts, intromissions, and ejaculations (Kondo, et al., 1990), lesions of the medial septal nuclei facilitate the male sexual arousal mechanism (Gogate, et al., 1995). Males with a serotonin depletion in the lateral septum displayed copulatory behavior more frequently than saline-treated lateral septum males, suggesting that this area plays an important role in regulating male sexual behavior (Kondo, et al., 1990). As mentioned above, lesions in the MPOA also suppress masculine sexual behavior but the behavior consequences were different from lateral septum disruptions. While MPOA lesioned males showed no interest in their female partners, lateral septum-lesioned males repeatedly pursued and courted the estrous females but failed to mount them. This failure to complete the mating sequence suggests that the lateral septum lesions result in the reiteration of one response in a sequence, and preclude the occurrence of behaviors that typically occur subsequent to that response (Kondo, et al., 1990).
For the regulation of feminine sexual behavior, the lateral septal area has been suggested to exert an inhibitory influence in the display of lordosis behavior. The lateral septum is thought to be a possible main source of neural efferents which inhibit the display of estrogen-induced lordosis behavior (Kondo, et al., 1990). Transections in the septum and preoptic area dramatically affected sexual behavior, including facilitation of lordosis and ultrasonic vocalizations in female hamsters. These lesions triggered disinhibitory changes in pathways that differ from those affected by VMN lesions (Floody and Czipri, 2014). In summary, the lateral septal area may be involved in regulating both masculine and feminine sexual behavior, facilitating masculine while inhibiting feminine (Kondo, et al., 1990).
The medial amygdala (MeA) is a sexually dimorphic structure, characterized by more neurons and larger neuronal soma in males compared to females (Hari Dass and Vyas, 2014). It plays an important role during male reproductive behavior in rodents through a unidirectional flow of chemosensory information through the anterior MeA to its downstream targets (Hari Dass, 2014). Lesions of the MeA reduce reproductive behavior in hamsters, rats and gerbils. In hamsters, the anterior MeA is involved in discrimination of conspecific odor from same-sex versus opposite-sex donors, while the posterodorsal MeA is selectively activated by opposite-sex conspecifics (Hari Dass and Vyas, 2014). Moreover, lesions of MeA eliminate opposite-sex odor preference and reduce overall levels of vaginal marking, in contrast to lesions of the MPOA, which decrease vaginal marking in response to male odors (Martinez and Petrulis, 2011). Observations in hamsters suggest that MeA involvement in male reproductive behavior is restricted to motivation and not to the initiation of mating as it has been seen in male rats. While MeA lesions drastically reduce penile erections in response to an inaccessible estrous female, they do not affect reflexive erections in response to penile sheath retraction (Hari Dass and Vyas, 2014).
The MeA interconnects with the MPOA and the BNST to coordinate the expression of both sexual odor preference and vaginal marking. These three areas are broadly involved in processing conspecific odor information detected by the main and accessory olfactory systems. This odor information is initially processed by MeA and relayed to MPOA, either directly or via BNST (Martinez and Petrulis, 2013). Electrolytic lesion of the BNST in male hamsters abolishes chemoinvestigation of females (Lau, et al., 2011). The BNST and MeA also process olfactory and hormonal stimuli to provide excitatory effects on mating activities and are involved in the expression of ejaculation-induced neural activation. Both the main and accessory olfactory systems have direct projections to the anterior subdivisions of the MeA and the BNST. Lesions of the MeA or BNST decrease the preference for opposite-sex odors and reduce chemosensory investigation without altering copulatory activities. BNST and MeA process olfactory information involved in the initiation of mating, as well as ejaculation-related information relevant for inhibition of mating. Recently, inhibitory pheromonal effects were observed upon release of an ‘alarm pheromone’ by other male rats, which affected neural activation patterns in several parts of the BNST and MeA (Veening and Coolen, 2014). In addition, BNST and MeA contain steroid receptors and are involved in processing of hormonal stimuli (Veening and Coolen, 2014). Multiple serotonin receptor subtypes have been found to be expressed in the BNST, to include 1A, 2A, 1B, 2C and 7 subtypes (Hazra, et al., 2012). Expression of serotonin receptors 1A (Koenig, et al., 2011), 2A (de Paula, et al., 2012) and type 3 (Urzedo-Rodrigues, et al., 2014) has been reported in the septum, while the subtypes 1A (Sun, et al., 2015) and 2C (Scopinho, et al., 2012) are expressed in the MeA.
Lesions in the median raphé facilitated male sexual behavior and stimulated locomotor activity (Albinsson, et al., 1996). Infusion of serotonin receptor agonists into the raphé nuclei of rats induced changes in the mating pattern that resembled lesions in the same structure, including a decrease in intromissions and shortening of the ejaculation latency (Albinsson, et al., 1996).
Serotonin effectors and their relationship with sexual behavior
Pharmacological and genetic manipulations of several serotonin effectors have contributed to elucidate the involvement of this neurotransmitter in the regulation of sexual behaviors. Serotonin acts on multiple receptor families which vary in their amino acid sequence, pharmacology, and intracellular mechanisms (Uphouse, 2000). Besides the studies of these receptors, more information has been obtained with the use of transgenic animals with genetic mutations in the serotonin transporter or the neurotransmitter synthesizing enzymes.
Serotonin 5-HT1A receptors
Serotonin 1A (5-HT1A) receptors are G-protein coupled primarily to inhibition of adenylate cyclase and/or lead to opening of a K+ channel. These receptors are expressed on soma and dendrites of both serotonergic neurons and neurons that are postsynaptic to serotonin terminals (Uphouse, 2014). Drugs acting on 5-HT1A receptor may influence female precopulatory as well as copulatory behaviors as evidenced by reductions in lordosis behavior, proceptivity and receptivity (Aubert, et al., 2013) after treatment with agonists for 5-HT1A receptors (Uphouse, 2014). The most effective intracranial site for 5-HT1A receptor agonist effects on lordosis may be the MBH, a brain area containing the VMN that is recognized to be critical for estradiol-facilitated female rat sexual receptivity (Blaustein, 2008). Likewise, other hormones such as progesterone can alter the 5-HT1A receptor modulation of lordosis behavior (Truitt, et al., 2003). Infusion of several 5-HT1A receptor agonists into the MBH region rapidly inhibited lordosis behavior. Infusion of 5-HT1A receptor agonists into the vicinity of serotonin cell bodies in the dorsal raphé nucleus inhibited lordosis behavior but only at higher concentrations than required to reduce lordosis behavior in the MBH (Uphouse, 2014). While MBH 5-HT1A receptors may be especially relevant for consummatory responses, other brain areas may be involved in the appetitive/precopulatory effects of 5-HT1A receptor agonists (Uphouse, 2014).
In males, a facilitatory role on ejaculation has been ascribed to 5-HT1A receptors possibly through activation of their presynaptic type, which would lead to an inhibition of serotonergic neuronal firing and consequently, to a stimulation of sexual behavior (Olivier, et al., 2011). Administration of 5-HT1A receptor agonists produced a pronounced decrease in ejaculation latency in the male rat (Ahlenius and Larsson, 1997). While the majority of data have implicated 5-HT1A receptors agonists in the facilitation of sexual behavior in male rats (Hillegaart and Ahlenius, 1998), they inhibit female sexual activity (Snoeren, et al., 2014). Antagonists of 5-HT1A receptors increased lordosis in non-receptive rats indicating that endogenous serotonin acting on these receptors has a tonic inhibitory effect on receptivity (Siddiqui, et al., 2007).
Serotonin 5-HT1B/1D receptors
Serotonin 1B and 1D (5-HT1B/1D) receptors are negatively coupled to cAMP and are located on terminals of serotonergic (autoreceptors) and non serotonergic (heteroceptors) neurons and reduce the depolarization-dependent release of serotonin from nerve terminals (Uphouse, 2014). The role of 5-HT1B/1D receptors in the display of sexual behavior has remained elusive. While initial studies using drugs with relatively selective agonist action at 5-HT1B/1D receptors implicated them in female rat lordosis behavior, studies with more selective agonists showed no effect on appetitive/precopulatory behaviors in females or mounts, ejaculations and intromissions in males (Kaspersen and Agmo, 2012).
The pharmacological manipulation of male sexual activity with 5-HT1B agonists inhibited male copulatory behavior in mice (Fernandez-Guasti and Rodriguez-Manzo, 1992), resulting in a prolongation of the ejaculation latency, an increase in the number of mounts preceding ejaculation, and a tendency for a similar effect on the number of intromissions (Hillegaart and Ahlenius, 1998). Likewise, administration of 5-HT1B receptor agonists had no influence on the levels of testosterone induced by the presence of a female and reduced the amount of time males spent near the females in a partition chamber (Popova and Amstislavskaya, 2002). This suggests that these receptors subtype participate in the inhibitory actions of serotonin. The absence of the 5-HT1B receptor affected both components of mouse masculine sexual behavior, motivation and execution (Rodriguez-Manzo, et al., 2002). Male mice lacking the 5-HT1B receptor become interested earlier in sexual behavior, but require more stimulation to achieve ejaculation (Rodriguez-Manzo, et al., 2002).
Serotonin 5-HT2 receptors
The serotonin type-2 (5-HT2) receptors, including 5-HT2A and 5-HT2B, activate signaling pathways of the phospholipase C second messenger (Uphouse, 2014). These receptors have been implicated in serotonergic modulation of female sexual behavior. Most drugs that have been used in the study of female sexual behavior have some affinity for both 5-HT2A and 5-HT2C receptors. Inhibition of lordosis behavior by drugs with antagonist action at 5-HT2 receptors, initially led to assumptions that 5-HT2 receptors might facilitate lordosis behavior. Systemic treatment with 5-HT2 receptor antagonists has been unequivocally associated with a decline in lordosis behavior. Interestingly, the SSRI, fluoxetine also acts as a 5-HT2 receptor antagonist so that 5-HT2 receptor effects may contribute to this antidepressant’s ability to reduce lordosis behavior (Uphouse, 2014). Administration of 5-HT2A/2C receptor agonists to sexually receptive females increased proceptivity but not receptivity (Rossler, et al., 2006), caused a decline in partner preference (Kaspersen and Agmo, 2012), and was related to an increase in sexual motivation on paced mating behaviors (Nedergaard, et al., 2004). Similarly, 5-HT2A/2C receptors within the VMN have been suggested to participate in the modulation of lordosis behaviors (Wolf, et al., 1999). Infusion of 5-HT2 receptor agonists into the MBH facilitated lordosis behavior in rats with low sexual receptivity while antagonists of these receptors reversed the effect (Uphouse, 2014). In receptive females, an increase in both turnover of serotonin and 5-HT2A receptors has been found in the preoptic area and the median eminence and a stimulatory role of the 5-HT2 system on sexual behavior has been suggested (Gonzalez, et al., 1997).
In males, stimulation of 5-HT2C receptors has been consistently associated with increases in penile erections but their role on ejaculation remains inconclusive. While some studies have reported that activation of these receptors inhibits ejaculation (Hull, et al., 2004), others suggest they facilitate it (Yonezawa, et al., 2008). In addition, 5-HT2A receptors have been associated with a proejaculatory role, while 5-HT1B receptors have the opposite effects and possess a rather inhibitory role on ejaculation (Yonezawa, et al., 2008).
Serotonin 5-HT3 receptors
Serotonin type 3 (5-HT3) receptors are cation-selective, ligand-gated ion channels (Barnes, et al., 2009). In male mice, the 5-HT3 receptors participate in the activation of the hypothalamus-hypophyseal-testicular complex that takes place in the presence of a receptive female (Amstislavskaya and Popova, 2004). In female rats, systemic treatment with 5-HT3 receptor agonists had only minor effects on lordosis behavior (Tanco, et al., 1994). However, a significant decline in lordosis behavior occurred when the 5-HT3 receptor antagonist was infused into the VMN of hormonally primed ovariectomized rats, but not in naturally cycling proestrous rats (Maswood, et al., 1997). Peripheral administration of non-selective 5-HT3 agonists resulted in facilitation of lordosis behavior in female rats while inhibiting copulatory behavior in males (Mendelson and Gorzalka, 1990). It has been suggested that the blockade of 5-HT3 receptors activity mediates these sexual behaviors (Mendelson and Gorzalka, 1990). It has been suggested that the activation of 5-HT3 receptors may facilitate the somatosensory modulation of lordosis behavior through an increase in the sensitivity of peripheral sensory fibers (Uphouse, 2014).
Serotonin 5-HT7 receptors
The serotonin type 7 (5-HT7) receptor is the most recently identified receptor for serotonin and a member of the G-protein-coupled receptor family (Hedlund, 2009). With regard to sexual behavior, only a few studies have been devoted to these receptors and the focus has been on females. Reports indicate that this receptor subtype is involved in the inhibitory effect of serotonin on luteinizing hormone release (Siddiqui, et al., 2004) and is therefore related to sexual behavior (Siddiqui, et al., 2007). Administration of 5-HT7 receptor agonists significantly reduced lordotic activity in receptive female rats, while these inhibitory effects were blocked by 5-HT7 receptor antagonists (Siddiqui, et al., 2007). Additionally, administration of 5-HT7 receptor antagonists increased lordosis in non-receptive rats, indicating that endogenous serotonin acting on 5-HT7 receptors may have a tonic inhibitory effect on female receptivity (Siddiqui, et al., 2007).
Serotonin transporter
One of the major side effects of specific serotonin reuptake inhibitors (SSRIs) is sexual dysfunction (Kaspersen and Agmo, 2012). In humans, SSRIs are commonly associated with delayed ejaculation and absent or delayed orgasm, while a specific association with libido (i.e. sexual motivation) has not been consistently demonstrated (Barrett, et al., 2006). Studies have reported that SSRIs, including fluoxetine, paroxetine and sertraline are able to delay ejaculation in subjects with premature ejaculation (Olivier, et al., 2011).
Chronic administration of fluoxetine inhibited ear wiggling and hopping/darting, but did not modify lordosis or its intensity in female rats (Ventura-Aquino and Fernandez-Guasti, 2013). However, acute fluoxetine administered systemically reduced lordosis behavior in both female rats and hamsters. Chronic treatment of female rats with paroxetine did not cause sexual side effects (Snoeren, et al., 2011), and did not consistently affect lordosis or any other behavior in a paced mating procedure (Kaspersen and Agmo, 2012). Thus, the effects of SSRIs on lordosis in female rodents seem to be related to the individual component of sexual behavior and the length of drug administration. In male rats, acute administration of citalopram, paroxetine, fluoxetine and sertraline did not or marginally delayed ejaculation whereas chronic administration of fluoxetine and paroxetine delayed ejaculation more effectively and inhibited sexual behavior (Olivier, et al., 2011). In addition, acute administration of different SSRIs including fluoxetine, paroxetine and sertraline during an exhaustion paradigm (rats get more “sluggish’ when they have had multiple ejaculations) also showed no major effects on inhibition of male sexual behavior (Mos, et al., 1999). Similarly, chronic administration of fluoxetine decreased the ability of male rats to achieve ejaculations but did not affect their propensity to pursue the female (Frank, et al., 2000), indicating that only the consummatory components of male sexual behavior were altered by this drug. Interestingly, these inhibitory effects of fluoxetine on copulatory behaviors, particularly on ejaculation, seem to be exerted directly at the spinal level (Hueletl-Soto, et al., 2012). Other SSRIs administered chronically to male rats produced differential effects, with paroxetine being able to delay ejaculation, while fluvoxamine was ineffective (Waldinger, et al., 2002). These effects were explained through different SSRIs potencies in altering 5-HT1A receptors and their inhibitory actions on ejaculation (de Jong, et al., 2005). Besides the use of SSRIs, other serotonin transporter inhibitors (i.e. DA-8031, developed for the treatment of premature ejaculation) have been shown to increase ejaculation latency and reduction of the number of ejaculations in male rats (Kang, et al., 2014).
Male rats with a life-long absence of the serotonin transporter (SERT) have altered sexual behaviors and 5-HT1A receptor functioning (Chan, et al., 2011). SERT knockout males had lower basal ejaculation frequencies and took longer to achieve the first ejaculation than their wild-type counterparts (Chan, et al., 2011). However, in females, the depletion of the SERT had no effects when tested for proceptive and receptive behaviors under normal conditions (Snoeren, et al., 2010).
Another layer of complexity arises from the fact that SSRIs seem to cause desensitization of 5-HT1A autoreceptors and this may underlie the delayed ejaculation induced by these antidepressants (de Jong, et al., 2007). A difference appears to exist between individual SSRIs and their ability to desensitize 5-HT1A receptors as well as to delay ejaculation (de Jong, et al., 2007). Paroxetine and fluoxetine seem more potent in desensitizing 5-HT1A receptors and also more prone to delay ejaculation than citalopram and fluvoxamine (de Jong, et al., 2007).
Sexual behaviors in the absence of central serotonin
The consequences of lowered or absent neuronal serotonin have long been associated with hypersexuality (Ferguson, et al., 1970; van de Poll, et al., 1977) and less conclusively with sexual preference (Angoa-Perez, et al., 2015; Liu, et al., 2011). Early studies showed that pharmacological depletion of brain serotonin induced mounting behavior in female rats, suggesting that brain serotonin had a direct influence on the neutral substrate underlying masculine sexual behavior (van de Poll, et al., 1977). However, more recent studies using transgenic mouse models with specific genetic depletions of serotonin (e.g. tryptophan hydroxylase-2, knockout mice that lack the rate limiting enzyme in the synthesis of serotonin; or Lmx-1b knockout mice that are devoid of serotonergic neurons) have not confirmed a clear increase in sexual activity in the absence of this neurotransmitter (Angoa-Perez, et al., 2015; Beis, et al., 2015; Liu, et al., 2011; Zhang, et al., 2013).
Serotonin relationship with sexual hormones and other sexually-associated molecules
There is abundant evidence to show that in most mammals, sexual behavior depends upon hormonal (estrogen, progesterone, testosterone) stimulation (Beach, 1976). In addition, evidence is accumulating for an involvement of estrogens in the modulation of neural systems that are thought to play important roles in male reproductive functioning. Specifically, the serotonergic system is implicated in diverse autonomic functions, most or all of which are sensitive to estradiol as well (Barrett, et al., 2006). It has been reported that estrogens modulate 5-HT1A receptors (Le Saux and Di Paolo, 2005; Uphouse, 2000). Serotonin relationship with sexual hormones also includes a modulatory action on the secretion of godanotropins (Gouveia and Franci, 2004; Siddiqui, et al., 2004).
In female rats, ovariectomy, which models hormonal withdrawal in menopause, leads to a state of 5-HT1A inhibitory autoreceptor hyperactivity in the dorsal raphe. In the dorsal raphe, estrogen receptor (ER) β is expressed in serotonergic neurons, whereas ERα is expressed in non-serotonergic neurons. It is likely that ERβ mediates the estradiol decrease in 5-HT1A receptor density and coupling (Le Saux and Di Paolo, 2005). Besides estrogen, progesterone receptors are also expressed in the dorsal raphé (Alves, et al., 1998).
In male rats, both testosterone and estradiol increase the density of the SERT and 5-HT2A receptor mRNA and binding sites in brain (Barrett, et al., 2006). Likewise, there is some evidence for an association between serotonergic function and male potency. A large percentage of men suffer from sexual dysfunction, which is often comorbid with disorders of mood and is affected by psychotropic medications, including SSRIs (Barrett, et al., 2006). The current notion that male sexual behavior has been emancipated from activational hormonal control in higher primates has changed after studies finding that male sexual behavior is regulated by estrogen modulation of the serotonergic system in intact male Japanese macaques. Analysis revealed that while estradiol and whole blood tryptophan had additive and independent effects on male potency over a range of concentrations, testosterone or 5alpha-dihydrotestosterone were confirmed to be the primary determinants of sexual motivation (Barrett, et al., 2006).
Early studies have indicated that oxytocin facilitates penile responses and contributes to postejaculatory refractoriness (Bitran and Hull, 1987). While systemic administration of oxytocin in young adult rats reduced the number of intromissions, it shortened the mount, intromission and ejaculation latencies in older animals (de Jong and Neumann, 2015). Serotonin modulates oxytocin release from oxytocinergic neurons (de Jong and Neumann, 2015). It has been hypothesized that an increase in serotonin levels (i.e. after SSRIs administration) leads to oxytocin release, which might be able to activate sexual reflexes such as erection and ejaculation. Desensitization of the 5-HT1A receptors expressed on oxytocinergic neurons has been suggested to induce a delay in ejaculation and injection of oxytocin would reinstall normal behavior (de Jong and Neumann, 2015).
Conclusions
The neurotransmitter serotonin has remarkable modulatory actions on different components of sexual behaviors in both genders. Facilitation or inhibition of these behaviors seems to be dependent upon factors that include the neuroanatomical regions affected, the type of signaling pathway (e.g. different pools of receptors, transporter) and whether the subject is a male or a female. Because serotonin receptors show unique but overlapping expression within the brain, activation of the same receptor subtype can lead to an increase or decrease of a particular behavior. However, despite its long history, the study of serotonergic regulation of sexual behaviors has been done with pharmacological manipulations that often involve the use of non specific agents, and thus, achieving controversial results. The more recent use of specific mutant models has opened other venues but more studies are necessary to close the gaps in our understanding of these important behaviors.
References
- Ahlenius S, Larsson K. Specific involvement of central 5-HT1A receptors in the mediation of male rat ejaculatory behavior. Neurochem Res. 1997;22:1065–1070. doi: 10.1023/a:1022443413745. [DOI] [PubMed] [Google Scholar]
- Albinsson A, et al. The effects of lesions in the mesencephalic raphe systems on male rat sexual behavior and locomotor activity. Behav Brain Res. 1996;80:57–63. doi: 10.1016/0166-4328(96)00020-4. [DOI] [PubMed] [Google Scholar]
- Alves SE, et al. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. J Comp Neurol. 1998;391:322–334. [PubMed] [Google Scholar]
- Amstislavskaya TG, Popova NK. The roles of different types of serotonin receptors in activation of the hypophyseal-testicular complex induced in mice by the presence of a female. Neurosci Behav Physiol. 2004;34:833–837. doi: 10.1023/b:neab.0000038136.27999.3d. [DOI] [PubMed] [Google Scholar]
- Angoa-Perez M, et al. Brain serotonin signaling does not determine sexual preference in male mice. PLoS One. 2015;10:e0118603. doi: 10.1371/journal.pone.0118603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert Y, et al. Brain region-specific transcriptomic markers of serotonin-1A receptor agonist action mediating sexual rejection and aggression in female marmoset monkeys. J Sex Med. 2013;10:1461–1475. doi: 10.1111/jsm.12131. [DOI] [PubMed] [Google Scholar]
- Barnes NM, et al. The 5-HT3 receptor–the relationship between structure and function. Neuropharmacology. 2009;56:273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett GM, et al. Regulation of sexual behaviour in male macaques by sex steroid modulation of the serotonergic system. Exp Physiol. 2006;91:445–456. doi: 10.1113/expphysiol.2005.032193. [DOI] [PubMed] [Google Scholar]
- Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Beis D, et al. Brain serotonin deficiency leads to social communication deficits in mice. Biol Lett. 2015;11 doi: 10.1098/rsbl.2015.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Hull EM. Pharmacological analysis of male rat sexual behavior. Neurosci Biobehav Rev. 1987;11:365–389. doi: 10.1016/s0149-7634(87)80008-8. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Neuroendocrine regulation of feminine sexual behavior: lessons from rodent models and thoughts about humans. Annu Rev Psychol. 2008;59:93–118. doi: 10.1146/annurev.psych.59.103006.093556. [DOI] [PubMed] [Google Scholar]
- Bonthuis PJ, et al. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Front Neuroendocrinol. 2010;31:341–358. doi: 10.1016/j.yfrne.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns-Cusato M, Scordalakes EM, Rissman EF. Of mice and missing data: what we know (and need to learn) about male sexual behavior. Physiol Behav. 2004;83:217–232. doi: 10.1016/j.physbeh.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Chan JS, et al. The serotonin transporter plays an important role in male sexual behavior: a study in serotonin transporter knockout rats. J Sex Med. 2011;8:97–108. doi: 10.1111/j.1743-6109.2010.01961.x. [DOI] [PubMed] [Google Scholar]
- Christensen A, Dewing P, Micevych P. Immediate early gene activity-regulated cytoskeletal-associated protein regulates estradiol-induced lordosis behavior in female rats. J Neurosci Res. 2015;93:67–74. doi: 10.1002/jnr.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu X, Agmo A. Sociosexual behaviors during the transition from non-receptivity to receptivity in rats housed in a seminatural environment. Behav Processes. 2015;113C:24–34. doi: 10.1016/j.beproc.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Corona R, Larriva-Sahd J, Paredes RG. Paced-mating increases the number of adult new born cells in the internal cellular (granular) layer of the accessory olfactory bulb. PLoS One. 2011;6:e19380. doi: 10.1371/journal.pone.0019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong TR, Neumann ID. Moderate Role of Oxytocin in the Pro-Ejaculatory Effect of the 5-HT1A Receptor Agonist 8-OH-DPAT. J Sex Med. 2015;12:17–28. doi: 10.1111/jsm.12742. [DOI] [PubMed] [Google Scholar]
- de Jong TR, et al. Effects of chronic selective serotonin reuptake inhibitors on 8-OH-DPAT-induced facilitation of ejaculation in rats: comparison of fluvoxamine and paroxetine. Psychopharmacology (Berl) 2005;179:509–515. doi: 10.1007/s00213-005-2186-6. [DOI] [PubMed] [Google Scholar]
- de Jong TR, et al. Oxytocin involvement in SSRI-induced delayed ejaculation: a review of animal studies. J Sex Med. 2007;4:14–28. doi: 10.1111/j.1743-6109.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- de Paula DC, et al. 5-HT(2A) receptor activation in the dorsolateral septum facilitates inhibitory avoidance in the elevated T-maze. Behav Brain Res. 2012;226:50–55. doi: 10.1016/j.bbr.2011.08.044. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Solicitation behavior in the estrous female rat: a review. Horm Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Fabre-Nys C, et al. Biphasic role of dopamine on female sexual behaviour via D2 receptors in the mediobasal hypothalamus. Neuropharmacology. 2003;44:354–366. doi: 10.1016/s0028-3908(02)00410-0. [DOI] [PubMed] [Google Scholar]
- Ferguson J, et al. “Hypersexuality” and behavioral changes in cats caused by administration of p-chlorophenylalanine. Science. 1970;168:499–501. doi: 10.1126/science.168.3930.499. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Rodriguez-Manzo G. Further evidence showing that the inhibitory action of serotonin on rat masculine sexual behavior is mediated after the stimulation of 5-HT1B receptors. Pharmacol Biochem Behav. 1992;42:529–533. doi: 10.1016/0091-3057(92)90150-e. [DOI] [PubMed] [Google Scholar]
- Flanagan-Cato LM, Lee BJ, Calizo LH. Co-localization of midbrain projections, progestin receptors, and mating-induced fos in the hypothalamic ventromedial nucleus of the female rat. Horm Behav. 2006;50:52–60. doi: 10.1016/j.yhbeh.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Floody OR, Czipri SL. Rapid facilitation of ultrasound production and lordosis in female hamsters by horizontal cuts between the septum and preoptic area. Physiol Behav. 2014;123:33–40. doi: 10.1016/j.physbeh.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Frank JL, Hendricks SE, Olson CH. Multiple ejaculations and chronic fluoxetine: effects on male rat copulatory behavior. Pharmacology, biochemistry, and behavior. 2000;66:337–342. doi: 10.1016/s0091-3057(00)00191-x. [DOI] [PubMed] [Google Scholar]
- Gao S, et al. Serotonin modulates outward potassium currents in mouse olfactory receptor neurons. Physiol Res. 2013;62:455–462. doi: 10.33549/physiolres.932413. [DOI] [PubMed] [Google Scholar]
- Gogate MG, et al. Septal regulation of male sexual behavior in rats. Physiol Behav. 1995;57:1205–1207. doi: 10.1016/0031-9384(94)00302-l. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, et al. Comparison of serotonin receptor numbers and activity in specific hypothalamic areas of sexually active and inactive female rats. Neuroendocrinology. 1997;66:384–392. doi: 10.1159/000127277. [DOI] [PubMed] [Google Scholar]
- Gouveia EM, Franci CR. Involvement of serotonin 5HT1 and 5HT2 receptors and nitric oxide synthase in the medial preoptic area on gonadotropin secretion. Brain Res Bull. 2004;63:243–251. doi: 10.1016/j.brainresbull.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Hari Dass SA, Vyas A. Copulation or sensory cues from the female augment Fos expression in arginine vasopressin neurons of the posterodorsal medial amygdala of male rats. Front Zool. 2014;11:42. doi: 10.1186/1742-9994-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra R, et al. Differential distribution of serotonin receptor subtypes in BNST(ALG) neurons: modulation by unpredictable shock stress. Neuroscience. 2012;225:9–21. doi: 10.1016/j.neuroscience.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB. The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology. 2009;206:345–354. doi: 10.1007/s00213-009-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Gonzalez M, Guevara MA, Agmo A. Motivational influences on the degree and direction of sexual attraction. Ann N Y Acad Sci. 2008;1129:61–87. doi: 10.1196/annals.1417.010. [DOI] [PubMed] [Google Scholar]
- Hillegaart V, Ahlenius S. Facilitation and inhibition of male rat ejaculatory behaviour by the respective 5-HT1A and 5-HT1B receptor agonists 8-OH-DPAT and anpirtoline, as evidenced by use of the corresponding new and selective receptor antagonists NAD-299 and NAS-181. British journal of pharmacology. 1998;125:1733–1743. doi: 10.1038/sj.bjp.0702239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlinak Z. Precopulatory behaviour of male rats: developmental aspects and dependence on female’s solicitation. Act Nerv Super (Praha) 1990;32:264–282. [PubMed] [Google Scholar]
- Hueletl-Soto ME, Carro-Juarez M, Rodriguez-Manzo G. Fluoxetine chronic treatment inhibits male rat sexual behavior by affecting both copulatory behavior and the genital motor pattern of ejaculation. The journal of sexual medicine. 2012;9:1015–1026. doi: 10.1111/j.1743-6109.2011.02339.x. [DOI] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Sexual behavior in male rodents. Horm Behav. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiol Behav. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Kang KK, et al. Effect of DA-8031, a novel oral compound for premature ejaculation, on male rat sexual behavior. Int J Urol. 2014;21:325–329. doi: 10.1111/iju.12256. [DOI] [PubMed] [Google Scholar]
- Kaspersen H, Agmo A. Paroxetine-induced reduction of sexual incentive motivation in female rats is not modified by 5-HT1B or 5-HT2C antagonists. Psychopharmacology (Berl) 2012;220:269–280. doi: 10.1007/s00213-011-2475-1. [DOI] [PubMed] [Google Scholar]
- Koenig J, et al. Spatial memory alterations by activation of septal 5HT 1A receptors: no implication of cholinergic septohippocampal neurons. Psychopharmacology (Berl) 2011;214:437–454. doi: 10.1007/s00213-010-2049-7. [DOI] [PubMed] [Google Scholar]
- Kondo Y, et al. Role of septum and preoptic area in regulating masculine and feminine sexual behavior in male rats. Horm Behav. 1990;24:421–434. doi: 10.1016/0018-506x(90)90019-t. [DOI] [PubMed] [Google Scholar]
- Kow LM, et al. Electrophysiological analyses of serotonergic actions on neurons in hypothalamic ventromedial nucleus in vitro: receptor subtypes involved and implications for regulation of feeding and lordosis behaviors. Chin J Physiol. 1992;35:105–121. [PubMed] [Google Scholar]
- Lau BW, et al. Effect of corticosterone and paroxetine on masculine mating behavior: possible involvement of neurogenesis. J Sex Med. 2011;8:1390–1403. doi: 10.1111/j.1743-6109.2010.02081.x. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T. Changes in 5-HT1A receptor binding and G-protein activation in the rat brain after estrogen treatment: comparison with tamoxifen and raloxifene. J Psychiatry Neurosci. 2005;30:110–117. [PMC free article] [PubMed] [Google Scholar]
- Liu S, et al. Serotonin modulates the population activity profile of olfactory bulb external tufted cells. J Neurophysiol. 2012;107:473–483. doi: 10.1152/jn.00741.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature. 2011;472:95–99. doi: 10.1038/nature09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, et al. Extracellular serotonin in the lateral hypothalamic area is increased during the postejaculatory interval and impairs copulation in male rats. J Neurosci. 1997;17:9361–9366. doi: 10.1523/JNEUROSCI.17-23-09361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Petrulis A. The bed nucleus of the stria terminalis is critical for sexual solicitation, but not for opposite-sex odor preference, in female Syrian hamsters. Horm Behav. 2011;60:651–659. doi: 10.1016/j.yhbeh.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Petrulis A. The medial preoptic area is necessary for sexual odor preference, but not sexual solicitation, in female Syrian hamsters. Horm Behav. 2013;63:606–614. doi: 10.1016/j.yhbeh.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maswood N, Caldarola-Pastuszka M, Uphouse L. 5-HT3 receptors in the ventromedial nucleus of the hypothalamus and female sexual behavior. Brain Res. 1997;769:13–20. doi: 10.1016/s0006-8993(97)00670-7. [DOI] [PubMed] [Google Scholar]
- Mendelson SD, Gorzalka BB. Sex differences in the effects of 1-(m-trifluoromethylphenyl) piperazine and 1-(m-chlorophenyl) piperazine on copulatory behavior in the rat. Neuropharmacology. 1990;29:783–786. doi: 10.1016/0028-3908(90)90133-c. [DOI] [PubMed] [Google Scholar]
- Mos J, et al. A comparison of the effects of different serotonin reuptake blockers on sexual behaviour of the male rat. Eur Neuropsychopharmacol. 1999;9:123–135. doi: 10.1016/s0924-977x(98)00015-7. [DOI] [PubMed] [Google Scholar]
- Nedergaard P, Sanchez C, Mellerup E. Different roles of 5-HT2A and 5-HT2C receptors in regulation of female rat paced mating behaviour. Behav Brain Res. 2004;149:151–157. doi: 10.1016/s0166-4328(03)00215-8. [DOI] [PubMed] [Google Scholar]
- Oboti L, et al. A wide range of pheromone-stimulated sexual and reproductive behaviors in female mice depend on G protein Galphao. BMC Biol. 2014;12:31. doi: 10.1186/1741-7007-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier B. Serotonin: A never-ending story. Eur J Pharmacol. 2014 doi: 10.1016/j.ejphar.2014.10.031. [DOI] [PubMed] [Google Scholar]
- Olivier B, et al. Differences in sexual behaviour in male and female rodents: role of serotonin. Curr Top Behav Neurosci. 2011;8:15–36. doi: 10.1007/7854_2010_116. [DOI] [PubMed] [Google Scholar]
- Paredes RG. Evaluating the neurobiology of sexual reward. ILAR J. 2009;50:15–27. doi: 10.1093/ilar.50.1.15. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Vazquez B. What do female rats like about sex? Paced mating. Behav Brain Res. 1999;105:117–127. doi: 10.1016/s0166-4328(99)00087-x. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Hagiwara A, Murthy VN. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nature neuroscience. 2009;12:784–791. doi: 10.1038/nn.2335. [DOI] [PubMed] [Google Scholar]
- Popova NK, Amstislavskaya TG. Involvement of the 5-HT(1A) and 5-HT(1B) serotonergic receptor subtypes in sexual arousal in male mice. Psychoneuroendocrinology. 2002;27:609–618. doi: 10.1016/s0306-4530(01)00097-x. [DOI] [PubMed] [Google Scholar]
- Portillo W, Basanez E, Paredes RG. Permanent changes in sexual behavior induced by medial preoptic area kindling-like stimulation. Brain Res. 2003;961:10–14. doi: 10.1016/s0006-8993(02)03827-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzo G, et al. Participation of 5-HT(1B) receptors in the inhibitory actions of serotonin on masculine sexual behaviour of mice: pharmacological analysis in 5-HT(1B) receptor knockout mice. Br J Pharmacol. 2002;136:1127–1134. doi: 10.1038/sj.bjp.0704827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossler AS, et al. Effect of the 5-HT receptor agonist DOI on female rat sexual behavior. J Sex Med. 2006;3:432–441. doi: 10.1111/j.1743-6109.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- Scopinho AA, et al. Medial amygdaloid nucleus 5-HT(2)c receptors are involved in the hypophagic effect caused by zimelidine in rats. Neuropharmacology. 2012;63:301–309. doi: 10.1016/j.neuropharm.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Siddiqui A, et al. 5-HT7 receptor subtype as a mediator of the serotonergic regulation of luteinizing hormone release in the zona incerta. Eur J Pharmacol. 2004;491:77–84. doi: 10.1016/j.ejphar.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Siddiqui A, et al. The 5HT(7) receptor subtype is involved in the regulation of female sexual behaviour in the rat. Pharmacol Biochem Behav. 2007;87:386–392. doi: 10.1016/j.pbb.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Snoeren E, et al. Serotonin transporter null mutation and sexual behavior in female rats: 5-HT1A receptor desensitization. J Sex Med. 2010;7:2424–2434. doi: 10.1111/j.1743-6109.2010.01829.x. [DOI] [PubMed] [Google Scholar]
- Snoeren EM, et al. Chronic paroxetine treatment does not affect sexual behavior in hormonally sub-primed female rats despite 5-HT(1)(A) receptor desensitization. J Sex Med. 2011;8:976–988. doi: 10.1111/j.1743-6109.2010.02192.x. [DOI] [PubMed] [Google Scholar]
- Snoeren EM, et al. Serotonin 1A receptors and sexual behavior in female rats: a review. Pharmacol Biochem Behav. 2014;121:43–52. doi: 10.1016/j.pbb.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Sun YN, et al. Activation of 5-HT receptors in the medial subdivision of the central nucleus of the amygdala produces anxiolytic effects in a rat model of Parkinson’s disease. Neuropharmacology. 2015;95:181–191. doi: 10.1016/j.neuropharm.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Tagliamonte A, et al. Compulsive sexual activity induced by p-chlorophenylalanine in normal and pinealectomized male rats. Science. 1969;166:1433–1435. doi: 10.1126/science.166.3911.1433. [DOI] [PubMed] [Google Scholar]
- Tanco SA, Watson NV, Gorzalka BB. Effects of 5-HT3 agonists on reproductive behaviors in rats. Psychopharmacology (Berl) 1994;115:245–248. doi: 10.1007/BF02244778. [DOI] [PubMed] [Google Scholar]
- Truitt W, et al. Progesterone attenuates the effect of the 5-HT1A receptor agonist, 8-OH-DPAT, and of mild restraint on lordosis behavior. Brain research. 2003;974:202–211. doi: 10.1016/s0006-8993(03)02581-2. [DOI] [PubMed] [Google Scholar]
- Uphouse L. Female gonadal hormones, serotonin, and sexual receptivity. Brain Res Brain Res Rev. 2000;33:242–257. doi: 10.1016/s0165-0173(00)00032-1. [DOI] [PubMed] [Google Scholar]
- Uphouse L. Pharmacology of serotonin and female sexual behavior. Pharmacol Biochem Behav. 2014;121:31–42. doi: 10.1016/j.pbb.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzedo-Rodrigues LS, et al. Blockade of 5-Ht3 receptors in the septal area increases Fos expression in selected brain areas. Auton Neurosci. 2014;181:55–68. doi: 10.1016/j.autneu.2014.01.003. [DOI] [PubMed] [Google Scholar]
- van de Poll NE, van Dis H, Bermond B. The induction of mounting behavior in female rats by p-chlorophenylalanine. Eur J Pharmacol. 1977;41:225–229. doi: 10.1016/0014-2999(77)90214-x. [DOI] [PubMed] [Google Scholar]
- Veening JG, Coolen LM. Neural mechanisms of sexual behavior in the male rat: emphasis on ejaculation-related circuits. Pharmacol Biochem Behav. 2014;121:170–183. doi: 10.1016/j.pbb.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Ventura-Aquino E, Fernandez-Guasti A. The antidepressants fluoxetine and bupropion differentially affect proceptive behavior in the naturally cycling female rat. J Sex Med. 2013;10:2679–2687. doi: 10.1111/jsm.12280. [DOI] [PubMed] [Google Scholar]
- Waldinger MD, et al. The selective serotonin re-uptake inhibitors fluvoxamine and paroxetine differ in sexual inhibitory effects after chronic treatment. Psychopharmacology (Berl) 2002;160:283–289. doi: 10.1007/s00213-001-0980-3. [DOI] [PubMed] [Google Scholar]
- Wallen K, Zehr JL. Hormones and history: the evolution and development of primate female sexuality. J Sex Res. 2004;41:101–112. doi: 10.1080/00224490409552218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A, et al. 5-HT2C receptor involvement in female rat lordosis behavior. Brain research. 1999;825:146–151. doi: 10.1016/s0006-8993(99)01159-2. [DOI] [PubMed] [Google Scholar]
- Yahr P, Greene SB. Effects of unilateral hypothalamic manipulations on the sexual behaviors of rats. Behav Neurosci. 1992;106:698–709. doi: 10.1037//0735-7044.106.4.698. [DOI] [PubMed] [Google Scholar]
- Yonezawa A, et al. Ejaculatory response induced by a 5-HT2 receptor agonist m-CPP in rats: differential roles of 5-HT2 receptor subtypes. Pharmacology, biochemistry, and behavior. 2008;88:367–373. doi: 10.1016/j.pbb.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Zancanaro C, et al. Biogenic amines in the vomeronasal organ. Chem Senses. 1997;22:439–445. doi: 10.1093/chemse/22.4.439. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu Y, Rao Y. Serotonin signaling in the brain of adult female mice is required for sexual preference. Proc Natl Acad Sci U S A. 2013;110:9968–9973. doi: 10.1073/pnas.1220712110. [DOI] [PMC free article] [PubMed] [Google Scholar]


