Abstract
Background
Treatments for major internal bleeding after injury include permissive hypotension to decrease the rate of blood loss, intravenous infusion of plasma or clotting factors to improve clot formation, and rapid surgical haemostasis or arterial embolization to control bleeding vessels. Yet, little is known regarding major internal arterial haemostasis, or how these commonly-used treatments might influence haemostasis.
Objectives
(1) Use a swine model of femoral artery bleeding to understand the perivascular haemostatic response to contained arterial haemorrhage. (2) Directly confirm the association between hemodynamics and bleeding velocity. (3) Observe the feasibility of delivering an activated clotting factor directly to internal sites of bleeding using a simplified angiographic approach.
Methods
Ultrasound was used to measure bleeding velocity and in vivo clot formation by elastography in a swine model of contained femoral artery bleeding with fluid resuscitation. A swine model of internal pelvic and axillary artery haemorrhage was also used to demonstrate feasibility of local delivery of an activated clotting factor.
Results
In this model, clots formed slowly within the peri-wound hematoma , but eventually containing the bleeding. Central hemodynamics correlated positively with bleeding velocity. Infusion of recombinant human activated Factor VII into the injured artery nearby the site of major internal haemorrhage in the pelvis and axillae was feasible.
Conclusions
We rediscover that clot formation within the peri-wound haematoma is an integral component of haemostasis and a feasible target for treatment of major internal bleeding using activated clotting factors delivered using a simplified angiographic approach.
Keywords: Trauma, Hemorrhage, Hemostasis, Ultrasound, Angiography, Coagulopathy
Introduction
Bleeding from the anatomical junctions (e.g. groin and axillae where the limbs meet the body) and uncontrollable bleeding from internal arterial and pelvic bone injures are leading causes of mortality after severe trauma. These types of injury can be a result of isolated penetrating missiles in areas lacking body armour under combat conditions, high-velocity blunt trauma, or as a result of complex blast-type mechanisms. The fatality rate with junctional bleeding and complex pelvic injuries is estimated as high as 94% 1. The most common cause of death in the early stages of junctional bleeding and pelvic injuries is haemorrhagic shock. The main source of rapid bleeding is the femoral, axillary, or sacral arteries as well as the open spongy areas of the pelvic ring.
Historical studies of the native haemostatic response to major external arterial injury suggest that extravascular rather than intravascular clot formation is the dominant mechanism of haemostasis 2,3. This haemostatic response can be disrupted by aggressive transfusion restoring blood pressure to even “normal” levels 4. This is true mainly because high-velocity blood flow within large vessels in these areas limits vessel thrombus formation by rapidly washing away procoagulant clotting factors as they are generated at the site of vascular injury. Therefore, increasing the local concentration of coagulation activators in blood or decreasing local blood flow rate may allow threshold levels of coagulation activators to initiate and maintain haemostasis. These concepts underpin current trauma treatment approaches using damage control resuscitation with early blood product transfusion and permissive hypotension 5. However, direct evidence is lacking regarding their direct impact on major internal arterial haemorrhage.
To clarify the relationships between clot formation, blood flow/pressure, and major internal arterial bleeding, we use non-invasive ultrasound and ultrasound elastography to study bleeding and in vivo clot formation in a pig model of contained femoral artery bleeding. We hypothesize that; 1.) systemic blood pressure and flow correlate positively with velocity of bleeding at the wound site, and 2.) haemostatic clots form and organize within the perivascular wound haematoma. We also evaluate the feasibility of delivering human recombinant activated Factor VII (rFVIIa, NovoSeven®, NovoNordisk, Denmark) directly to the perivascular haematoma to induce clot formation and haemostasis using angiography in a second pig model.
Methods
Swine Femoral Artery Bleeding Model
This protocol was adapted from one used by the U.S. Army to evaluate topical hemostatic agents that was published by Kheirabadi et al., and includes femoral artery bleeding and Hextend™ (6% Hetastarch in a balanced salt solution, Hospira, Lake Forest Park, IL) for initial fluid resuscitation 6. Approval for all animal handling and procedures was obtained from University of Washington office of animal welfare. Eight immature female Yorkshire mix swine weighing 26-34 kg were fasted overnight with water ad libitum prior to the study. In the morning, they were sedated with ketamine (30 mg/kg IM) and anesthetized with a mixture of isoflurane (2-3%) and oxygen (33%) via nose cone. They were orotracheally intubated, and the isoflurane concentration was maintained at 1-1.5%. They were given a single dose of buprenorphine (0.01 mg/kg IM) for analgesia.
Animals were mechanically ventilated (Anesco Anesthesia Ventilator) to achieve normal pH, PCO2, and hemoglobin oxygen saturation. End-tidal CO2 was monitored continuously (Datex Capnomac Ultima, Datex Instrumentarium Corp). The right side of the neck and bilateral femoral sites were shaved and prepared with povidone-iodine solution. The right external jugular vein was isolated, an 8F introducer catheter was placed, and a 7F pulmonary artery catheter (Swan-Ganz, Edwards Lifesciences, Irvine, CA) was advanced into the pulmonary artery according to characteristic pressure wave form for measurement of cardiac output by thermodilution, and core body temperature monitoring. The left femoral artery and vein were isolated and cannulated for central blood pressure monitoring, blood sampling, and fluid and drug administration. The Biopac MP150 monitoring and data acquisition system (Biopac System, Inc., Santa Barbara, CA) was used to continuously record vital signs and hemodynamics.
A 4 cm longitudinal incision extending distally from the inguinal crease was made in the right femoral region, and the femoral artery was exposed. This length of the skin incision was chosen to allow for use of a single hemostatic clamp (iTClamp™, Innovative Trauma Care, Alberta CA) to close the skin over the wound, which has a quoted upper limit of wound size per clamp of 4.6 cm. The femoral artery was dissected from the surrounding tissue, and all small arterial branches were ligated. The exposed artery was then bathed in 5ml of 2% Lidocaine solution to dilate and paralyze the artery to prevent vasospasm after injury. This step was taken so that initial 30 second bleeding rate could be standardized and to isolate the direct effect of perivascular clot formation on bleeding velocity. We anticipate that using lidocaine to dilate the artery prior to bleeding likely increased bleeding velocity by inhibiting vasospasm.
Following a 30-minute period of equilibration, baseline measurements were obtained including ultrasound visualization and measurement of peak systolic and end diastolic blood flow velocity within the intact femoral artery. Proximal and distal artery clips were then placed on the isolated right femoral artery, and a 5mm diameter circular punch biopsy tool was used to injure one wall of the vessel with care taken not to transect the artery. The wound cavity was kept free of any pooling blood to prevent spontaneous clot formation. At the onset of hemorrhage, the artery clips were then removed, and the artery was allowed to bleed freely for 30 seconds. The clamp was then applied to the skin incision until effective wound seal was achieved and there was no visible external bleeding. Haematomas formed immediately after clamp application in all cases.
Fluid Resuscitation
At 3.5 minutes after onset of bleeding, all animals received one 15 ml/kg dose of Hextend™ (6% Hetastarch in a balanced salt solution, Hospira, Lake Forest Park, IL) over 15 minutes followed by 3 ml/kg/min Ringer's Lactate solution infused continuously to a maximum of 100 ml/kg as needed to maintain a goal mean arterial pressure (MAP) of 60 mmHg. Surviving animals were euthanized at 3 hours humanely while under anesthesia by intravenous pentobarbital overdose (Euthanasia III solution; Med-Pharmex, Inc, Pomona, CA, USA).
Ultrasound bleeding measurements
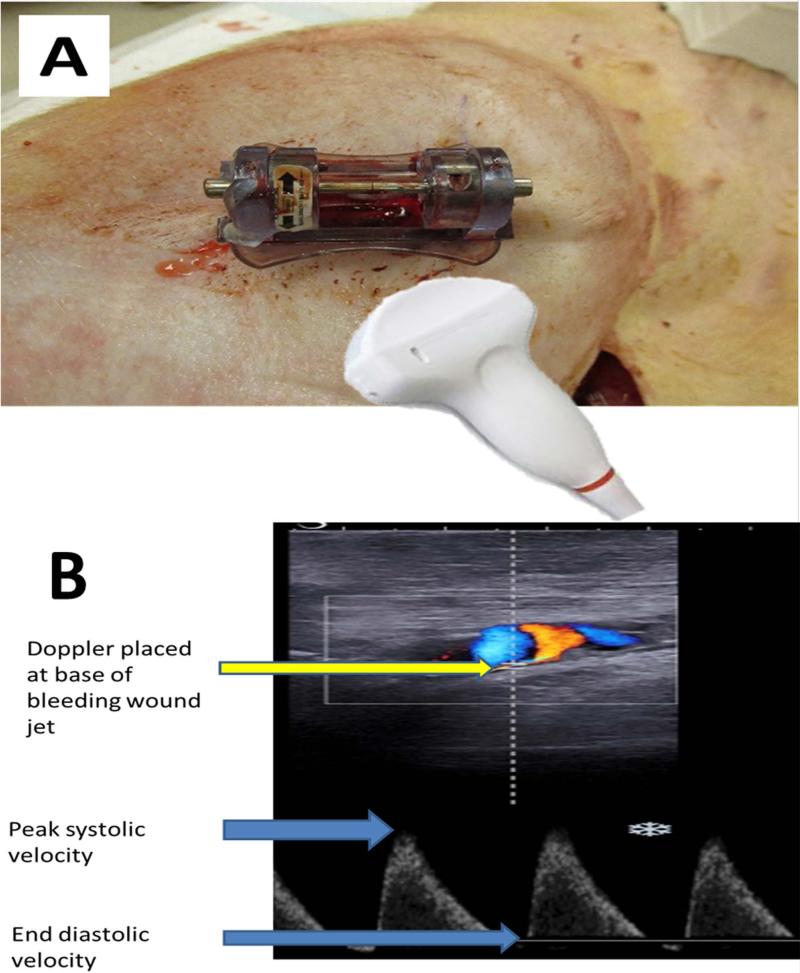
An ultrasound diagnostic imaging system (Aixplorer Multiwave Ultrasound System, SuperSonic Imagine, Aix-en-Provence, France) with both Doppler-shift and shear elastography capabilities was used to obtain measurements 7. A linear-array ultrasound probe (SL15-4 MHz transducer) was gently placed at the base of the clamp with care taken to prevent wound compression. The jet of blood emanating from the arterial punch wound was visualized using Doppler mode and peak systolic and end diastolic bleeding jet velocities were recorded at the site of exit of blood from the vessel. (Fig. 1) Measurements were taken at 5, 15, 30, 60, 90, 150, and 180 minutes after onset of bleeding. Ultrasound elastography was used to take shear modulus measurements within the hematoma at 30, 90, and 180 minutes. Of note, due to large hematoma formation, an Aixplorer curvilinear array probe (SuperCurved™ SC6-1) with the same ultrasound capabilities was substituted for the linear array probe for some measurements. Given that the wound bleeding jet was pulsatile, corresponding with the cardiac cycle, mean wound bleeding velocity (WBV) was calculated using the standard formula for MAP while substituting peak systolic bleeding velocity and end diastolic bleeding velocity into the equation. Clot formation within the internal hematoma surrounding the bleeding vessel was evaluated using ultrasound elastography shear modulus measurements following the methods in Xu et al 8. Clot shear modulus measurements for all animals were measured directly and combined at each measurement time for proximal, middle, and distal locations within the hematoma while using the origin of bleeding as the reference. For each time point at which animals were imaged, the location of the shear modulus data collection varied. Because the Aixplorer imaging device does not allow the elastography window to cover the entirety of the forming clot due to processing limitations, multiple images were combined to create a representative map of the entire clot during a selected time interval. A MATLAB™ algorithm (The MathWorks, Inc. Natick, MA, USA) was written which converted the color elastography area obtained from the Aixplorer into an averaged shear modulus value for a hand selected region. For every elastography image collected, the analysis window was divided into a maximum of 16 regions, allowing a much finer set of data to be extracted from the elastography image. The data from each imaging location was projected onto a master grid spanning a normalized, 2-D representational image of the general hematoma. Using 2 adjacent pixels in every direction, an averaging/smoothing algorithm and imputation was then used to create a shear modulus heat map of the hematoma for each time point, which was then placed over a normalized b-mode ultrasound image.
Fig. 1.
Placement of ultrasound probe (A) and measurements of wound bleeding velocity (B).
Outcome Measurements
The liquid blood and clot was removed from within the wound cavity after euthanasia and measured using pre-weighed gauze sponges. MAP was recorded from the arterial catheter in the distal aorta. Cardiac output was measured at the same time as ultrasound measurements by thermodilution via the Swan-Ganz catheter. Blood was also sampled from the contralateral femoral artery catheter into standard citrated vacutainers for thrombelastography (TEG, TEG-5000, Haemonetics, Niles, IL) and activated to coagulate by simple recalcification.
Pelvic Bleeding Model
A total of 4 immature domestic swine weighing 40-45 kg were used for these experiments following local animal ethics committee approval. Animals were premedicated with Azaperone 4mg/kg IM, (Stresnil ™, Janssen, Vienna, Austria) and Atropin (0.1mg/kg IM) one hour before the start of testing. The Anesthesia was induced and maintained using Propofol (1-2mg/kg iv) and Piritramid (30mg, Opioid with ~4 to 8 hour HWD, Dipidolor™, Janssen, Vienna, Austria). For muscle relaxation, Pancuronium was injected after intubation by 0.2mg*kg-1*h-1.
Preparation of both Aa. femorales as well as the V. femoralis or the V. subclavia was performed. Crystalloid fluid (4mg/kg) was infused during animal preparation and experiment to meet basic fluid requirements. Subsequently the following invasive catheters were inserted into the vessels: large bore (large single lumen venous line with a length of 15-20cm), invasive arterial pressure measurement and sluice for the insertion of the angiography probe.
The A. iliaca int. within the pelvis was located under fluoroscopy and the vessel ruptured using balloon dilatation (n=3). Similarly, balloon dilatation with rupture of the A. axillaris (n=1) was also performed. In each case, the tip of the balloon catheter was then drawn back from the rupture site by 3-5cm and a dosage of 100 μg/kg rFVIIa (n=3, total dose range 4,000-4,500 μg) or saline (n=1) was administered as a bolus through the catheter. Animals were monitored for haemostasis for 60 minutes following balloon dilation and survivors euthanized humanely under anaesthesia.
Analysis
Mean and standard deviation were used to describe continuous normally-distributed data. Aggregate shear maps were created for all ultrasound measurements in all animals obtained during the first 30 minutes of bleeding, at 90 minutes of bleeding, and at 180 minutes of bleeding for surviving animals. MAP and CO were compared over time using one way ANOVA with Tukey adjustment for individual differences. Two-way ANOVA was used to test the effects of protocol time (30, 90, 180 minutes) and location within the hematoma (proximal, middle, distal) on ultrasound elastic modulus. WBV, MAP, and CO was correlated using simple Pearson rank sums correlation. Ultrasound elastic modulus measurements were correlated with protocol time and TEG measurements taken from 60-180 minutes after bleeding onset were also pooled and correlated with ultrasound elastic modulus using simple Spearman rank sums correlation.
Results
Femoral Artery Bleeding Model
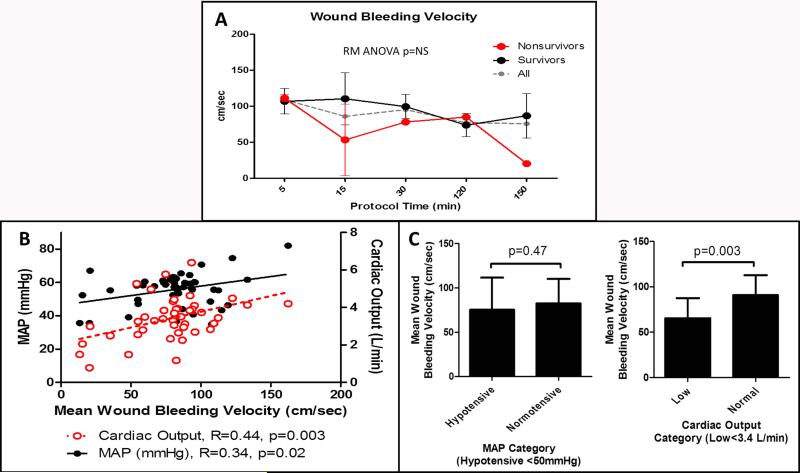
Five of eight animals (62%) survived to 3 hours with a mean overall survival time of 174 (11) minutes for the group. Mean hematoma volume was 21.2 (6.0) ml/kg and was positively correlated with survival time (R=0.66, p=0.01). Mean (SD) fluids infused for all animals was 15.4(0.76) ml/kg for Hextend and 86.5(23.4) ml/kg for Ringers Lactate. Mean (SD) hemoglobin was 11.3(1.2) g/dl at baseline and fell to 5.9(2.0) g/dl after 60 minutes of fluid resuscitation (T-test p<0.001). Platelet count was 328(95) ×10^9/L at baseline and fell to 174(53) ×10^9/L after 60 minutes of fluid resuscitation (T-test p=0.001). Fibrinogen concentration was 181.7(66.0) at baseline and decreased to 107.8(41.9) mg/dl after 60 minutes of fluid resuscitation (T-test p=0.02). All animals were still alive after 60 minutes of fluid resuscitation. These results indicate that dilutional coagulopathy was induced by the Hextend bolus and early Ringers Lactate infusion. Mean WBV was maintained at relatively high velocities throughout the protocol regardless of survival. (Fig. 2A) Mean WBV was positively correlated with both MAP and CO. (Fig. 2B) There was no difference in WBV when MAP was stratified into hypotensive or normal categories defined by MAP <50 mmHg. However, WBV was significantly decreased when CO was low vs. normal after stratification by the median CO value for the cohort. (Fig. 2C)
Fig. 2.
Mean wound bleeding velocity measurements in all animals, survivors (n = 5) and non-survivors (n = 3). High-velocity bleeding into the hematoma was present throughout the protocol, suggesting a lack of occlusive intravascular thrombus formation (A). Simple correlation between mean wound jet velocity and mean arterial pressure (MAP) and cardiac output measured centrally (B). Significant positive correlations exist between bleeding velocity and systemic blood pressure and flow. Wound bleeding velocity was not significantly different when MAP was categorized to hypotensive or normal values; however, it was significantly lower when cardiac output was decreased (C).
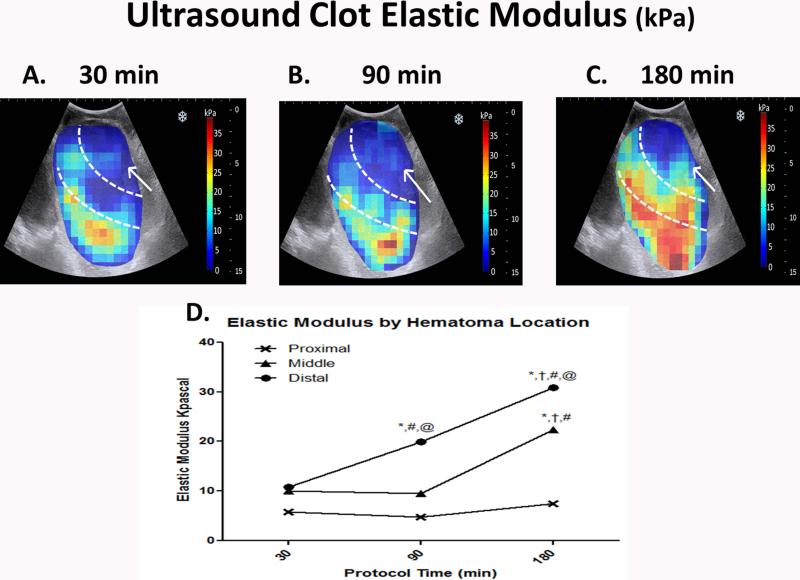
Color-coded maps of elastic modulus within the hematomas over time revealed a distal to proximal progression of clot formation resulting in containment of arterial haemorrhage within the extravascular clot. Modulus increased progressively within the distal and middle portions of the hematoma over time and correlated positively with increasing protocol time (distal R=0.61, p<0.001, middle R=0.42, p<0.001), indicating increasingly more solid clot formation over time. (Fig. 3A-C) The area most proximal to the origin of bleeding remained liquid, and did not correlate with protocol time (R=0.1, p=0.16). (Fig. 3D)
Fig. 3.
Color-coded heat maps of in vivo clot elastic modulus of perivascular hematomas measured using ultrasound elastography in a swine model of femoral artery injury. Measurements were taken serially at 30, 90 and 180 min after onset of bleeding (A–C). Separate measurements were taken closest to the origin of bleeding (Proximal), in the middle portion (Middle) and at the furthest distance away (Distal) and these regions are demarcated by the white interrupted lines. Mean measurements were compared using two-way ANOVA with Tukey adjustment for multiple comparisons (D). *P < 0.001 vs. the same location at 30 min. †P < 0.001 vs. the same location at 90 min. #P < 0.001 vs. Proximal location at the same protocol time. @P <0.001 vs. Middle location at the same protocol time. Origin of bleeding is marked by the white arrow.Elastic modulus within the hematoma progressively increased in the distal and middle portions of the hematoma over time relative to the origin of bleeding (arrow).
Clot elastic modulus measurements measured within the mid and distal portions of the hematoma clot correlated with several TEG measurements of blood sampled at the same time as the ultrasound measurements. TEG parameters reflective of thrombin generation, including split point (SP), onset time (R), and time to maximal amplitude (TMA), were the most closely associated with in vivo elastic modulus. (Table 1)
| US Elastic Modulus |
||
|---|---|---|
| TEG Parameter | Correlation Coefficient | P value |
| SP(min) | −0.24 | 0.062 |
| R(min) | −0.27 | 0.033 |
| K(min) | −0.20 | 0.118 |
| Angle(deg) | 0.33 | 0.008 |
| MA(mm) | 0.15 | 0.256 |
| TMA(min) | −0.27 | 0.031 |
| G(d/sc) | 0.19 | 0.145 |
Spearman correlation of pooled measurements taken 60-180 minutes after injury
SP-split point, R-clot onset time, K-clot formation time, Angle – rate of clot formation, MA- maximal clot amplitude, TMA- time to maximal amplitude, G-maximal shear elastic modulus.
Visual inspection of the hematoma after euthanasia typically revealed the presence of a single large and highly-organized clot containing multiple internal channels allowing for circulation of blood from the injured vessel within the clot. (Supplemental Fig. 1)
Pelvic Bleeding Model
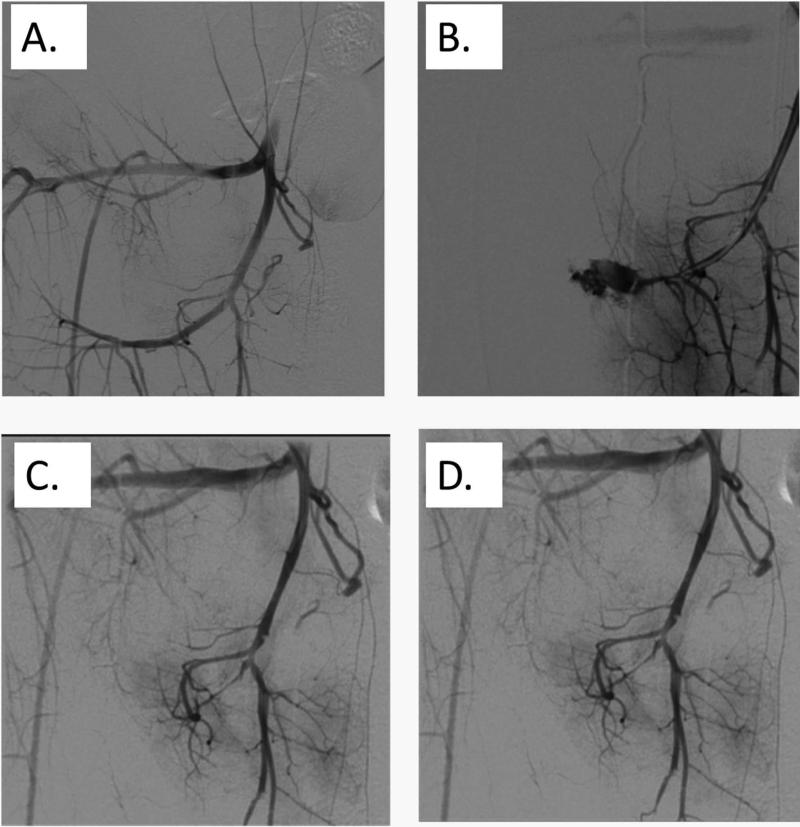
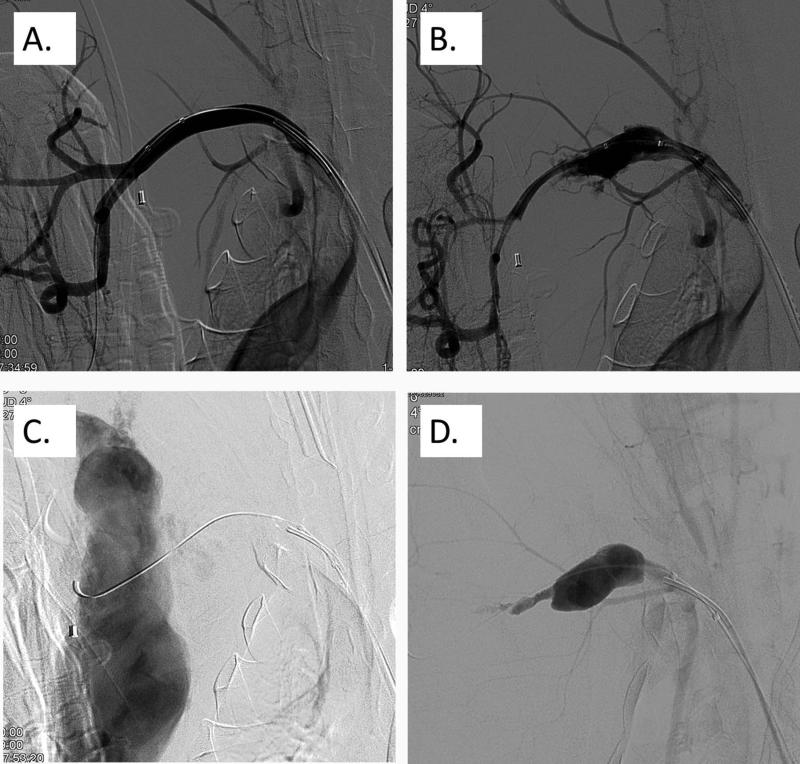
N=4 pigs were studied to demonstrate feasibility for this angiographic method. For Pig #1, saline was administered to the injured A. iliaca vessel through the drawn back angiography catheter. Arterial bleeding was visualized to continue after saline injection and the pig died after 40 minutes. For pig #2 a single dose of 100 μg/kg rFVIIa was given to the injured and bleeding A. iliaca through the drawn-back angiography catheter, no active bleeding was visualized one minute after the infusion ended, and the pig survived to 60 minutes. (Fig. 4) For pig #3 a single dose of 100 μg/kg rFVIIa was given to the injured A. iliaca through the drawn-back angiography catheter, no active bleeding was visualized 3 minutes after the infusion ended, and the pig survived to 60 minutes. For pig #4, the A. Axillaris was injured and active bleeding was visualized. A single dose of 100 μg/kg rFVIIa was then given to the injured A. Axillaris through the drawn-back angiography catheter. No active bleeding could be visualized seventeen minutes after the infusion ended, and the pig survived to 60 minutes. (Fig. 5) The arteries into which rFVIIa was infused were visualized to remain patent after infusion.
Fig. 4.
Baseline angiographic scan of the right A. iliaca (A). Disruption of the right A. iliaca interna using balloon dilation (B). Angiographic scan 1 min after the administration of rFVIIa through the drawn back angiography catheter, demonstrating complete resolution of bleeding (C). Angiographic scan 5 min after the administration of rFVIIa through the drawn back angiography catheter (D).
Fig. 5.
Baseline angiographic scan of the right A. axillaris (A). Dis-ruption of the right A. axillaris using a balloon dilation (B). Further disruption of the right A. axillaris using balloon dilation to increase blood loss dynamics (C). Angiographic scan 17 min after the admin-istration of rFVIIa through the drawn back angiography catheter,demonstrating a contained hematoma without active extravasation of blood (D).
Discussion
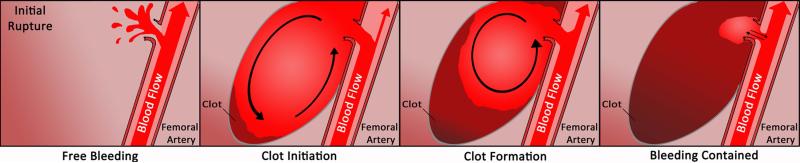
This study contributes to general knowledge regarding haemostasis of contained major arterial haemorrhage. Our data indicates that formation of a robust extravascular clot contributes to haemostasis for major arterial haemorrhage. Moreover, our data demonstrate for the first time how the extravascular clot forms more quickly distal to the origin of bleeding and then grows towards the origin. (Fig. 6) This finding supports the current suite of therapeutic manoeuvres in the case of internal or junctional arterial bleeding: rapid recognition and direct compression of the wound. Wound compression would reduce the perivascular wound space and slow flow within the haematoma to encourage clot formation. However, by definition internal injuries and junctional anatomical injuries may be only partially-compressible because tourniquets cannot typically be placed proximally. The clot also appears to form under highly dynamic swirling conditions which can potentially delay fibrin formation 9. Therefore, without direct compression these clots may require higher thresholds of clot activation to quickly form fibrin under such conditions. In fact, TEG parameters associated with thrombin generation correlated the strongest with in vivo clot formation in our model.
Fig. 6.
Schematic representation of clot formation in the setting of contained major arterial hemorrhage. 10
We also confirm a direct relationship between blood pressure/flow and rate of bleeding in this model. Current damage-control resuscitation guidelines suggest that permissive hypotension be used as a strategy to limit blood loss 5. Studies in animals measuring blood loss during fluid resuscitation support the concept of hypotensive resuscitation and cautious fluid resuscitation to reduce bleeding 10,11. However, direct in vivo measurements of bleeding rate during fluid resuscitation have not been previously reported. We used ultrasound to measure the velocity of bleeding from a major arterial vessel into a contained hematoma in vivo and confirm that there exists a positive association between systemic hemodynamics and velocity of bleeding. We found that both MAP and CO measured centrally are positively associated with the velocity of blood loss from a bleeding femoral artery. Moreover, we found that CO may be a better correlate when compared to MAP. Current clinical guidelines rely upon MAP to manage hypotensive resuscitation due to its non-invasive ease of measure. However, our data suggest that perhaps direct measurement of flow may be a more relevant surrogate of bleeding rate during major uncontrolled arterial bleeding.
It may be feasible to enhance haemostasis of major internal bleeding by direct delivery of activated clotting activators to the haematoma. This approach is currently used quite successfully when administering thrombin directly to femoral artery pseudoaneurysms arising after cardiac catheterization 12. Using angiography, we found that delivery of rFVIIa delivered directly to the bleeding artery was feasible. Many experts are of the opinion that rFVIIa should be used in potentially fatal haemorrhage cases which cannot be resolved by surgery or angiography. In recent years rFVIIa was protocolized for successful “off-label” use as a last resort in many cases of trauma- and surgery-related bleeding 13. However, the largest randomized trial of intravenous rFVIIa in trauma patients met with mixed results, having reduced the transfusion burden without conferring a mortality benefit 14. Our data indicate that it may be feasible to deliver rFVIIa directly to a major artery to control arterial bleeding. This simplified approach may save time and reduce overall blood loss because current angiographic approaches require a high degree of skill on the part of the operator to identify, cannulate, and selectively embolize each bleeding vessel. Even in the hands of an experienced person it can be quite time consuming. Despite its increasing availability in many hospitals, angiography usually takes place five to seventeen hours after admission and requires ongoing transfusion to support circulation during the procedure 15. The average requirement of red blood cell concentrate (RBC) is 1.2 U/h, while patients who are being treated with pelvic binding or tamponade require an average RBC amount of 2.8-8 U/h 16. Our data suggest that perhaps intra-arterial delivery of clotting activators such as rFVIIa might be studied as an alternative to current approaches using selective embolization. This approach may greatly simplify the procedure making it more widely available not only in very high specialized hospitals and centres but also in small hospitals, or even in mobile army surgical hospitals.
Limitations
This study is limited in several ways. First, lidocaine was used to prevent vasospasm of the bleeding femoral artery. This was done to standardize the initial blood loss during the first 30 seconds of bleeding and to limit the confounding influence of vasospasm on bleeding velocity. However, vasospasm is a critical component of haemostasis. Bleeding velocity would likely have been lower if lidocaine was not used. Further studies should attempt to quantify and include the contribution of both vasospasm and clot formation to arterial haemostasis. Future studies should attempt to quantify and include the contribution of vasospasm in addition to clot formation to arterial haemostasis. The use of Hextend during resuscitation may have also negatively impacted clot formation and haemostasis because of its negative effects on fibrin polymerization and dilutional effects. We are also unable to demonstrate efficacy for intra-arterial delivery of activated clotting factors to stop internal haemorrhage. We did demonstrate that this simplified angiographic approach may be feasible and deserving of further scientific study.
Conclusion
In this model of contained femoral artery haemorrhage, haemostasis was achievable by formation a robust extravascular clot. Bleeding velocity was associated with systemic hemodynamics and intra-arterial delivery of activated clotting factors to the peri-wound haematoma may be a feasible way to treat major internal haemorrhage.
Supplementary Material
Acknowledgments
We would like to acknowledge the contributions of M. Freund from the Department of Radiology, Medical Clinic Innsbruck, Austria for his angiography assistance. The femoral artery haemorrhage model was supported by a grant from iTrauma Care. Inc., Alberta Canada. N.J. White is supported in part, by the National Center for Advancing Translational Sciences (NCATS) (grant KL2 TR000421), a component of the National Institutes of Health (NIH). P.D. Mourad and E. Mehic were supported by the University of Washington's Entrepreneurial Faculty Fellowship. The views expressed in this article are those of the authors and do not necessarily represent the official view of NCATS or NIH.
Footnotes
The work was carried out at the University of Washington Seattle, WA USA, and Medical Clinic Innsbruck, Austria.
Addendum
N. J. White was responsible for initial study concepts, femoral haemorrhage experiments, data analysis, and manuscript creation. E. Mehic and P. D. Mourad performed ultrasound measurements, performed analysis of ultrasound data, and provided critical manuscript revisions. X. Wang, D. Chien, E. Lim, A. E. St John, and S. Stern performed junctional haemorrhage experiments and contributed to critical manuscript revisions. D. Fries, M. Rieger, and U. Martinowitz were primarily responsible for conducting the pelvic angiography haemorrhage model, manuscript creation, and provided critical revisions. All authors have reviewed and approved the final manuscript.
Disclosure
X. Wang, D. Chien, A. St John, E. Mehic, E. Lim, S. Stern report grants from iTrauma Care Inc. during the conduct of the study.
M. Rieger reports grants from the Department of Radiology, Medical Clinic Innsbruck, Austria, during the conduct of the study.
N. White reports grants from iTrauma Care Inc. and NIH NCATS KL2 TR000421 during the conduct of the study.
References
- 1.Burkhardt M, Nienaber U, Krause J, Pizanis A, Moersdorf P, Culemann U, Aghayev E, Paffrath T, Pohlemann T, Holstein JH, Beckenregister DGU, TraumaRegister DGU . Complex pelvic traumas: Data linkage of the German Pelvic Injury Register and the Trauma Register. DGU Unfallchirurg; 2014. Epub. [DOI] [PubMed] [Google Scholar]
- 2.Shaftan GW, Chiu CJ, Grosz CS, Dennis C. The Fundamentals of Control of Physiologic Control of Arterial Hemorrhage. Surgery. 1964;58:851–856. [PubMed] [Google Scholar]
- 3.Tocantins L. The Mechanism of Hemostasis. Annals of Surgery. 1947;125:292–310. doi: 10.1097/00000658-194703000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaftan GW, Chiu CJ, Grosz CS, Dennis C. The Effect of Transfusion and of Certain Hemodynamic Factors on the Spontaneous Control of Arterial Hemorrhage. The Journal of Cardiovascular Surgery. 1964;5:251–256. [PubMed] [Google Scholar]
- 5.Duchesne JC, Barbeau JM, Islam TM, Wahl G, Greiffenstein P, McSwain NE., Jr. Damage control resuscitation: from emergency department to the operating room. Am Surg. 2011;77:201–206. doi: 10.1177/000313481107700222. [DOI] [PubMed] [Google Scholar]
- 6.Kheirabadi BS, Arnaud F, McCarron R, Murdock AD, Hodge DL, Ritter B, Dubick MA, Blackbourne LH. Development of a standard swine hemorrhage model for efficacy assessment of topical hemostatic agents. J Trauma. 2011;711:S139–146. doi: 10.1097/TA.0b013e318221931e. [DOI] [PubMed] [Google Scholar]
- 7.Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: principles and techniques. Diagn Interv Imaging. 2013;94:487–95. doi: 10.1016/j.diii.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Xu ZS, Lee RJ, Chu SS, Yao A, Paun MK, Murphy SP, Mourad PD. Evidence of Changes in Brain Tissue Stiffness After Ischemic Stroke Derived From Ultrasound-Based Elastography. J Ultrasound Med. 2013;32:485–494. doi: 10.7863/jum.2013.32.3.485. [DOI] [PubMed] [Google Scholar]
- 9.Onasoga-Jarvis AA, Puls TJ, O'Brien SK, Kuang L, Liang HJ, Neeves KB. Thrombin generation and fibrin formation under flow on biomimetic tissue factor-rich surfaces. J Thromb Haemost. 2014;12:373–82. doi: 10.1111/jth.12491. [DOI] [PubMed] [Google Scholar]
- 10.Stern SA, Dronen SC, Birrer P, Wang X. Effect of blood pressure on hemorrhage volume and survival in a near-fatal hemorrhage model incorporating a vascular injury. Ann Emerg Med. 1993;22:155–163. doi: 10.1016/s0196-0644(05)80195-7. [DOI] [PubMed] [Google Scholar]
- 11.Stern SA, Kowalenko T, Younger J, Wang X, Dronen SC. Comparison of the effects of bolus vs. slow infusion of 7.5% NaCl/6% dextran-70 in a model of near-lethal uncontrolled hemorrhage. Shock. 2000;14:616–22. doi: 10.1097/00024382-200014060-00008. [DOI] [PubMed] [Google Scholar]
- 12.Chen DH, Sammel AM, Jain P, Jepson NS. Cardiologist Operated Ultrasound Guided Thrombin Injection as a Safe and Efficacious First Line Treatment for Iatrogenic Femoral Artery Pseudoaneurysms. Heart Lung Circ. 2015;24:165–72. doi: 10.1016/j.hlc.2014.07.066. [DOI] [PubMed] [Google Scholar]
- 13.Bain J, Lewis D, Bernard A, Hatton K, Reda H, Flynn J. Implementation of an off-label recombinant factor VIIa protocol for patients with critical bleeding at an academic medical center. J Thromb Thrombolysis. 2014;38:447–52. doi: 10.1007/s11239-014-1107-0. [DOI] [PubMed] [Google Scholar]
- 14.Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, Leppaniemi A, Parr M, Vincent JL, Tortella BJ, Dimsits J, Bouillon B, CONTROL Study Group Results of the CONTROL trial: efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69:489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 15.Hornez E, Maurin O, Bourgouin S, Cotte J, Monchal T, de Roulhac J, Meyrat L, Platel JP, Delort G, Meaudre E, Thouard H. Management of exsanguinating pelvic trauma: Do we still need the radiologist? J Visc Surg. 2011;148:e379–84. doi: 10.1016/j.jviscsurg.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Osborn PM, Smith WR, Moore EE, Cothren CC, Morgan SJ, Williams AE, Stahel PF. Direct retroperitoneal pelvic packing versus pelvic angiography: A comparison of two management protocols for haemodynamically unstable pelvic fractures. Injury. 2009;40:54–60. doi: 10.1016/j.injury.2008.08.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.