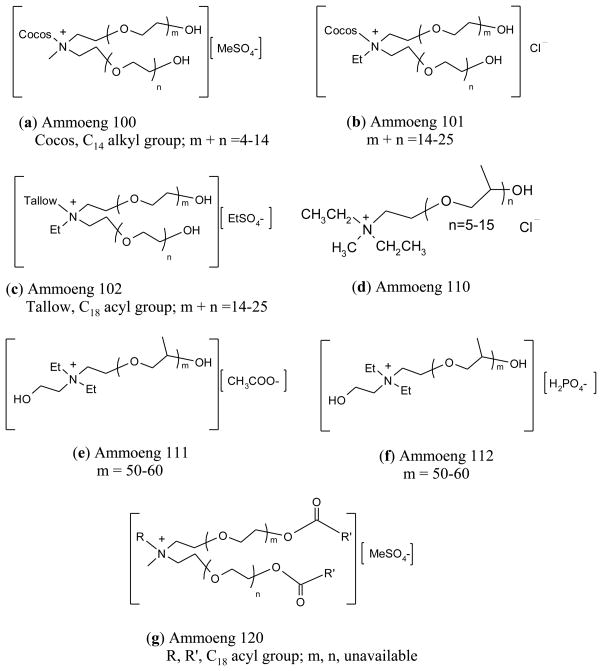
Abstract
There are still debates on whether the hydration of ions perturbs the water structure, and what is the degree of such disturbance; therefore, the origin of Hofmeister effect on protein stabilization continues being questioned. For this reason, it is suggested to use the ‘specific ion effect’ instead of other misleading terms such as Hofmeister effect, Hofmeister series, lyotropic effect, and lyotropic series. In this review, we firstly discuss the controversial aspect of inorganic ion effects on water structures, and several possible contributors to the specific ion effect of protein stability. Due to recent overwhelming attraction of ionic liquids (ILs) as benign solvents in many enzymatic reactions, we further evaluate the structural properties and molecular-level interactions in neat ILs and their aqueous solutions. Next, we systematically compare the specific ion effects of ILs on enzyme stability and activity, and conclude that (a) the specificity of many enzymatic systems in diluted aqueous IL solutions is roughly in line with the traditional Hofmeister series albeit some exceptions; (b) however, the specificity follows a different track in concentrated or neat ILs because other factors (such as hydrogen-bond basicity, nucelophilicity, and hydrophobicity, etc) are playing leading roles. In addition, we demonstrate some examples of biocatalytic reactions in IL systems that are guided by the empirical specificity rule.
Keywords: ionic liquid, specific ion effect, Hofmeister series, protein stabilization, biocatalysis
INTRODUCTION
As a new type of designable solvents, ionic liquids (ILs) have gained tremendous focus in biocatalysis, aiming to replace conventional volatile organic solvents and their solutions. A number of enzymatic systems have been evaluated in neat ILs or IL solutions; these enzymatic systems include various hydrolases (EC 3, e.g. lipases, proteases, thermolysin, α-chymotrypsin, lysozyme, β-glycosidase, cellulase, epoxide hydrolase and penicillin amidase), oxidoreductases (EC 1, e.g. horseradish peroxidase, alcohol dehydrogenase, laccase and lignin peroxidase), lyases (EC 4, e.g. oxynitrilase), and whole cells.1–4 To improve the stability and activity of enzymes, a variety of methods have been developed by different groups, such as enzyme immobilization (on solid support, sol–gel, or cross-linked enzyme aggregates), physical or covalent attachment to PEG, rinsing with n-propanol methods (PREP and EPRP), water-in-IL microemulsions, IL coating, and the design of enzyme-compatible ILs.5, 6
More importantly, several mechanistic overviews7–9 have highlighted some important properties of ILs that influence the enzyme’s behaviors in ionic solvents; these properties include IL polarity, hydrogen-bond (H-bond) basicity and nucleophilicity of anions, Hofmeister series, IL hydrophobicity, and IL viscosity, etc. However, there is a mixed understanding of the existence of specific ion effect of ILs on protein stabilization and enzyme activation, and how the specific ion effect is different in diluted solutions of ILs from that in concentrated or neat ILs. The present review aims to survey relevant literatures surrounding the theme of specific ion effect of ILs, and provide mechanistic insights into how different factors contribute to the specific ion effect at different IL concentrations. In particular, we discuss the specific ion effect of inorganic ions in aqueous solutions, followed by the structural properties of ILs and their aqueous solutions, and then the specific ion effects in aqueous ILs and concentrated/neat ILs, and lastly some examples of ion specificity-guided biocatalytic reactions.
SPECIFIC ION EFFECT OF INORGANIC IONS IN AQUEOUS SOLUTIONS
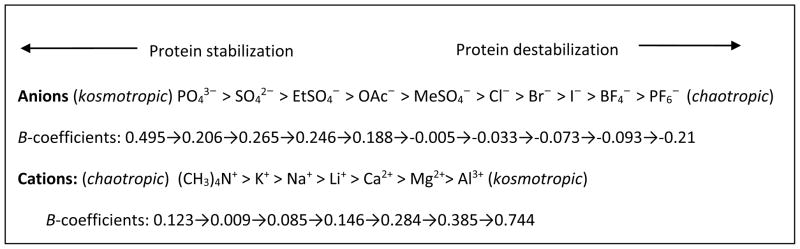
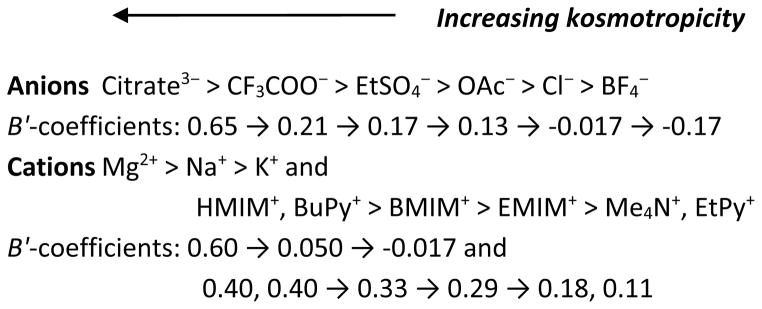
In 1888, Franz Hofmeister proposed the ion specificity based on his observation of ions exhibiting sequential capabilities in precipitating the proteins (globulins from blood serum and hen’s egg).10, 11 The order of these ions in salting out proteins is so called the ‘Hofmeister series’ (Fig 1) although later this concept also became associated with other phenomena in physical, colloid, polymer and surface chemistry.12, 13 At low concentrations (< 0.1 M), ions affect the protein stability and enzyme activity primarily through electrostatic interactions.14, 15 At higher salt concentrations (usually > 0.1–0.3 M,14, 15 but not too concentrated such as up to 3.0 M16), the Hofmeister ion effect becomes important when the ionic dispersion forces exceed the electrostatic forces. The Cremer group17 observed that at low salt concentrations (< 200–300 mM), the charge pairing between anions and the positively charged lysozyme surface (pH 9.4) is gradually reaching its saturation; at this stage, the liquid–liquid phase transition temperature of lysozyme is directly related to the size and hydration thermodynamics of the anions and thus follows an inverse Hofmeister series (ClO4− > SCN− > I− > NO3− > Br− > Cl−). Under higher salt conditions, the liquid–liquid phase transition is influenced by the polarizability of the anions and thus exhibits a direct Hofmeister series (Cl− > NO3− > Br− > ClO4− > I− > SCN−). On the other hand, the Falconer group18 suggested that anion and cation effects on the structural stability of lysozyme at pH 7 follow the Hofmeister series at high concentrations (> 20 mM), but fail to follow the Hofmeister (or inverse Hofmeister) series at low concentrations (< 5 mM).
Fig. 1.
The Hofmeister series as an order of the ion effect on protein stability22, 39 (viscosity B-coefficients in dm3·mol−1 at 25 ºC are taken from the Marcus collection40 except those of EtSO4− and MeSO4− were from Ref;41 the positions of EtSO4− and MeSO4− are based on the consideration of B-coefficients, NMR B′-coefficients42 and enzyme stability studies43–46).
Several theories have been developed to understand the Hofmeister series, including salt-in and salt-out interactions,19, 20 water-structure changes (low/high density water) and protein preferential hydration,14, 21–26 hydrophobic interactions,26–28 excluded volume,29–31 preferential interactions,32–34 electrostatic interactions,35, 36 ionic dispersion potentials,37, 38 etc. However, there is a lack of unified theory that can fully interpret the Hofmeister effect due to the complex nature of ion-water-protein interactions.
Early literatures often related the protein stability with the hydration behavior of ions.19, 37 Highly hydrated ions (e.g. Mg2+, Ca2+, Li+, CH3COO−, SO42−, and HPO42−) tend to interact strongly with water molecules and increase the ‘structuring of water’, resulting in a lower fluidity (or a higher viscosity) of the solution than that of pure water. For this reason, these ions are referred as ‘structure-makers’ or ‘kosmotropes’ (see Fig 1). In contrast, some other ions are poorly hydrated in aqueous solutions, such as SCN−, I−, NO3−, BF4−, Cs+, (NH2)3C+ (guanidinium), and (CH3)4N+ (tetramethylammonium). These ions have weak interactions with water molecules and reduce the ‘structuring of water’, leading to a higher fluidity of the solution. Thus, this effect is called the ‘negative hydration’,47, 48 and these ions are often denoted as ‘structure-breakers’ or ‘chaotropes’ (see Fig 1). Based on this theory, the capacity of an ion in strengthening the ‘water structure’, known as kosmotropicity (vs. chaotropicity), is directly associated with the degree of ion hydration. As discussed in our earlier review,49 the ion kosmotropicity can be quantified by various thermodynamic parameters including Jones-Dole viscosity B-coefficients, structural entropies, structural volumes, structural heat capacities, NMR B′-coefficients, and ion mobility, etc. These parameters provide valuable information of the interactions involved in the ion hydration from different aspects, and possibly reveal the mechanism behind some phenomena and properties. Jones-Dole viscosity B-coefficients are the most commonly used and widely available parameter for evaluating the ion kosmotropicity. The B-coefficients can be derived from the Jones-Dole empirical equation (eqn 1) of the relative viscosities of electrolyte solutions as functions of their concentrations,50
| (1) |
where η is the viscosity of the solution and η0 is the viscosity of the solvent (both of them have the same unit, for example Pa·s), while c is the molar concentration (mol·cm−3). The A-coefficient (also known as the Falkenhagen coefficient51), representing the solute-solute or electrostatic interactions, can be calculated theoretically. However, A-values are usually small and negligible for non-electrolytes;40 therefore, they are often neglected in the calculations. The B-coefficient represents the solute-solvent interactions (short-range dispersion forces), while D-coefficient indicates the solute-solute interactions as well as the solute-solvent interactions.52 For most salts at low concentrations [(< 0.5 M)40 or (< 0.1 M for binary strong electrolytes)53], the D or higher coefficients can be neglected although they are required at higher concentrations.40 Positive B-values typically indicate ions as kosmotropes since strongly hydrated ions exhibit a larger change in viscosity with concentration, while negative B-coefficients imply chaotropes for weakly hydrated ions.40 However, hydrophobic solutes tend to have unusually large B-coefficients due to so called ‘hydrophobic hydration’.49 For example, tetramethylammonium cation (Me4N+) has a positive B-value as high as 0.123,40 but this ion is considered as a structure-breaker.23, 54–58 Some groups40, 59–61 recommend the use of first derivatives of B-values over temperature because the sign of dB/dT could be more indicative in measuring the structure-making or breaking property than the sign or quantity of B-coefficients. The negative sign of dB/dT means structure-making (kosmotropic) while the positive sign suggests structure-breaking (chaotropic). In aqueous solutions of inorganic salts, many studies (see our earlier review16) have suggested that the ion effect on the enzyme activity follows the Hofmeister series: kosmotropic anions and chaotropic cations stabilize the enzyme, while chaotropic anions and kosmotropic cations destabilize it.
However, some contradictory experimental results argued whether the water structure is indeed influenced by the presence of ions.62, 63 Leberman and Soper64 found that some salts [e.g. 2 M Na2SO4 and 2 M (NH4)2SO4] disturbed more water H-bonding than 4 M NaCl and 4 M NH4Cl based on the water-water HH correlation functions obtained from the neutron diffraction using isotope substitution. Nucci and Vanderkooi65 examined the temperature excursion infrared response of the O–H stretch of aqueous salt solutions by a two-state H-bonding model, and found that ions do change the H-bond network of water and there is a strong correlation between salt effects on the Hofmeister series. They also noted that the specific ion–protein interactions cannot be excluded, and could be a co-factor along with the changes in bulk solvation properties. Thomas and Elcock66 conducted molecular dynamics (MD) simulations (1 μs) of the unbiased association of pairs of hydrophobic molecules (methane– methane and neopentane–neopentane) in different salt solutions, and found that the Hofmeister effects can be quantitatively predicted from the H-bond ratio from simulations of pure salt solutions containing no hydrophobic solute. Thus, they indicated that salt-induced changes in water structure is more important than preferential interactions between salt and hydrophobic solutes to the understanding of Hofmeister effects. On the other hand, the Saykally group67 measured the oxygen K-edge X-ray absorption spectrum (XAS) of aqueous sodium halide solutions (up to 4 M), and found ions greatly perturb the electronic structure of adjacent water molecules because of the direct perturbation of unoccupied orbitals on water by anions; however, such perturbation is not necessarily due to any significant distortion of the H-bond network beyond the first solvation shell. This group68 further confirmed monovalent cations (such as Li+, Na+, K+, NH4+ and C(NH2)3+) cause no considerable perturbation of the unoccupied molecular orbitals of water molecules in the vicinity of cations while the XAS spectral changes are mainly due to water-chloride interactions; however, they also observed that divalent cations (i.e. Mg2+ and Ca2+) induce a redistribution of charge among water molecules in the solvation shell and result in spectra changes. Krekeler and Delle Site69 conducted first-principle Car–Parrinello molecular dynamics of the hydration of monovalent and divalent ions, and suggested the preferential orientation of water molecules is only seen in the first shell and the water–water interaction plays a critical role in the first shell regardless of the size or the charge of ions. Based on the orientational correlation time of H2O molecules in 1–6 M solutions of three salts [i.e. Mg(ClO4)2, NaClO4, and Na2SO4] acquired from the femtosecond pump-probe spectroscopy, Omta et al70, 71 suggested that ions have negligible effect on the H-bond structure in liquid water. Their results indicate the anion interactions with water molecules (OH···ClO4- and OH···SO42−). Therefore, simple ions have no significant impact on the water structure, at least beyond the first hydration shell; even di- and tri-valent ions cause no appreciable change to the density or orientation of water more than two water molecules (5 Å) away.72
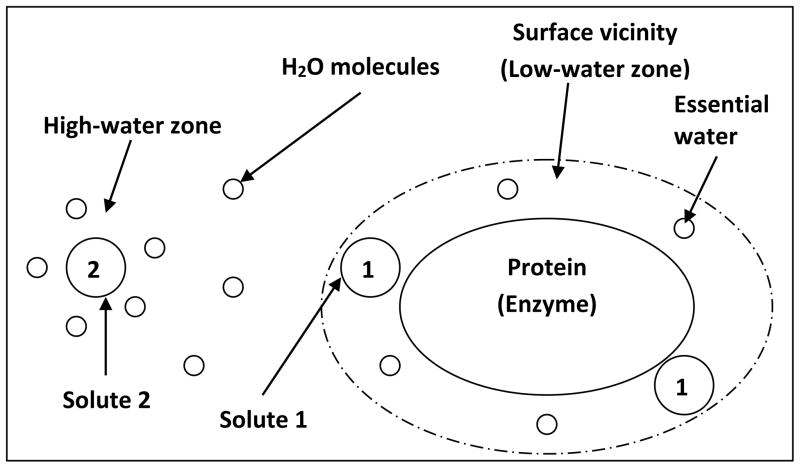
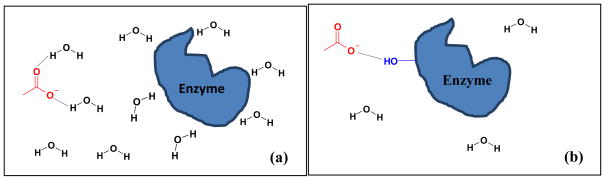
While the debate on the effect of ions on water structure continues, some groups suggest the bulk water structure is not greatly affected by ions and thus the kosmotropicity concept should be abandoned;73 instead, the Hofmeister series should be explained by the ion impact on protein hydration, and direct ion-protein interactions. Before we discuss other explanations of Hofmeister effect, it is necessary to outline how protein/enzyme molecules interact with solutes (denaturants or stabilizers). As shown in Fig 2, solute 1 is a denaturant that has a stronger interaction with protein molecules than with water molecules. Solute 1 excludes those co-factors of enzymes that are essential for the enzyme activity. This category (solute 1) includes chaotropic anions (because they are less hydrated), kosmotropic cations (such as Ca2+ strongly salts in the peptide group19), organic solutes (especially hydrophilic ones including urea33), and other ions (such as guanidinium GdnH+) that have strong interactions with protein surface. The strong interactions may expose the hydrophobic cores of the protein, causing its denaturation. Solute 2 is a stabilizer that has a stronger interaction with water molecules than with protein molecules. This category includes kosmotropic cations and anions (because they are strongly hydrated and salt out nonpolar groups;19 they ‘drag’ water molecules away from the protein which allows the protein to refold74), and organic solutes that have weak binding interactions with the protein.
Fig. 2.
Illustration of interactions between solutes and protein.
Several major alternative explanations of Hofmeister series are discussed below. The first theory is the preferential hydration of proteins.14, 21–26 Strongly hydrated anions tend to strongly interact with water molecules; as a result, they preferentially hydrated by water molecules instead of interacting directly with the enzyme surface. On the contrary, weakly hydrated anions have a low water affinity and a high polarizability, and therefore bind to the protein-water interface resulting in protein destabilization. Through examining the aqueous potassium salt solutions using femtosecond optical Kerr effect spectroscopy, Hou et al.75 found that the hyperpolarizability of six aqueous anions increased in the order: HPO42− < HSO4− < CO32− < CH3COO− < NO3− < SCN−, which correlates with the Hofmeister series (except CO32−). The role of cations is different. The presence of kosmotropic cations tends to minimize the effect of kosmotropic anions because a strong ion-pairing affinity between kosmotropic cations and anions decreases the amount of free anions in the solution. In aqueous solutions, ion pairs are easily formed between cations and anions with similar water affinity, such as kosmotrope-kosmotrope and chaotrope-chaotrope; the strengthen of these interactions (known as the ‘law of matching water affinity’) is in a decreasing order of kosmotrope-kosmotrope > kosmotrope-water > water-water > chaotrope-water > chaotrope-chaotrope.21, 53 Zhang et al.76 examined the hydration and interactions of a globular protein (bovine serum albumin, BSA) in concentrated salt solutions (up to 3.0 M) by small-angle neutron scattering (SANS). They suggested a hydration shell with a hydration level of ~0.30 g g−1 protein; they also indicated that the effective protein–protein interactions in concentrated salt solutions can be evaluated by the second virial coefficient, which follows the reverse order of the Hofmeister series: i.e. (NH4)2SO4 < Na2SO4 < NaOAc < NaCl < NaNO3 < NaSCN. To study the specific ion effect on interfacial water structure neighboring to a BSA monolayer adsorbed at the air/water interface, the Cremer group77 employed the vibrational sum frequency spectroscopy (VSFS) and suggested that specific anion effects are controlled by the charge state of the interfacial layer rather than its detailed chemical structure: for the positively charged protein layer at pH 2 and 3, more chaotropic anions induced more attenuation of water structure; for the protein layer at its isoelectric point (pH 5), more chaotropic anions lead to greater increase in water structure (although it’s weak); for the negatively charged protein layer (pH 9), no obvious effect could be detected.
A second important account for the Hofmeister series is the direct interactions between ions and protein. In aqueous solutions, protein molecules may interact with water molecules and ions via a variety of hydrophilic, polar, or charged moieties. In particular, the charge groups include dehydrated, chaotropic amide and amino groups, and the hydrated, kosmotropic carboxyl groups. Based on the ‘law of matching water affinity’, chaotropic anions have a greater affinity towards chaotropic amide of the peptide group, whilst the interaction between kosmotropic cations and the kosmotropic carboxyl moiety is weak due to the presence of water molecules in their nearest hydration shells.78 This explains the opposite trend of cations and anions in influencing the protein stability, and also the stronger effect of anions than cations. Sedlák et al.78 further pointed out that the hydration condition determines the direct interactions between the ions and the protein peptide bonds, which leads to Hofmeister effects of protein stability; the protein stability is more correlated with anion charge density than cation charge density. Gokarn et al.15 observed that anions selectively and preferentially accumulate at the surface of hen-egg white lysozyme even at low (< 0.1 M) salt concentrations. At a given ion normality of 50 mN, the protein’s effective charge (Q*) decreased in the order F− > Cl− > Br− > NO3− > I− > SCN− > ClO4− ≫ SO42−, which corresponds to the opposite order of anion association to the protein surface, and thus suggests that the SO42− anion interacts directly with the protein surface although it is highly hydrated. On the other hand, the cations have no apparent impact on the effective charge of the protein, which is almost unchanged for all the cations studied (Li+, Na+, K+, Rb+, Cs+, GdnH+, and Ca2+). On the contrary, the Jungwirth group79 suggested that the destabilizing effect of weakly hydrated Hofmeister anions (such as Br− or I−) is not caused by the direct interactions with the backbone amide groups, but rather due to the affinity of large soft ions toward hydrophobic groups and residues of proteins. A further study by this group and the Cremer group80 examined the specific binding sites of Hofmeister ions with an uncharged 600-residue elastin-like polypeptide, and suggested that the interaction between large soft anions (SCN− and I−) and the polypeptide backbone through a hybrid binding site comprising the amide nitrogen and the adjacent α-carbon. Cl− anions have a much weaker binding to this site, SO42− is excluded from the backbone as well as hydrophobic side chains of the polypeptide. The Gibb group81 found that chaotropic anions have a strong affinity towards the hydrophobic concavity, which surpasses the affinity between anions and amide groups; therefore, they implied that protein solubilization in solutions of chaotropes is mainly due to the direct binding of chaotropes to concavity in the molten globule state of a protein. Paterová et al.82 conducted the NMR and MD studies of ion interactions with capped and uncapped triglycine, and noted (a) a direct Hofmeister series for the capped peptide, which means that strongly hydrated ions (e.g., SO42−) are repelled from the peptide bond while weakly hydrated ions (e.g., I− and SCN−) interact with the peptide bond, and (b) a reversed Hofmeister series for the uncapped peptide due to anion interactions with the positively charged, uncapped N-terminus. It is also suggested that the same specific anion effect could be extrapolated for interactions with the positively charged side chains of lysine, arginine, and (protonated) histidine. Based on a two-scale MD simulation approach, Schwierz et al.83 observed a direct Hofmeister series for anions at the negatively charged hydrophobic surfaces or positive polar surfaces, but a reversal effect for the negative polar or positive nonpolar surfaces. As reviewed by Yang,9 the direct interactions between ions and enzyme may lead to several changes to the enzyme including surface pH, net charge, active site and catalytic mechanism.
Thirdly, it has been known that the Hofmeister ions affect the surface tension and surface potential at the air–water interface.22 Based on a surface-bulk partitioning model to assess the Hofmeister effect on the surface tension of water, Pegram and Record84, 85 suggested that those anions (such as SCN−) that interact favorably with protein surface exposing protein surface to water, tend to accumulate at the air–water interface; other anions (such as F−) that are excluded from protein surface and cause dehydration of protein surface, tend to be excluded from the air–water interface. A recent phenomenological theory developed by groups of Dér and Ramsden86, 87 indicates that the Hofmeister effect could be explained by the salt-induced changes of hydrophobic/hydrophilic properties of protein–water interfaces, quantitatively by the protein–water interfacial tension. This theory establishes the correlation between interfacial tension and protein structural stability, which is associated with protein conformational fluctuations. Therefore, this theory could interpret the salt effects on protein conformation, dynamics as well as stability, and could even explain the unusual observation of chaotropes stabilizing some proteins.
Due to the ongoing controversial discussion on the origin of Hofmeister series, Friedman88 suggested the use of term ‘specific ion effect’ instead of other misleading terms such as Hofmeister effect, Hofmeister series, lyotropic effect, and lyotropic series.
STRUCTURAL PROPERTIES OF ILS AND THEIR AQUEOUS SOLUTIONS
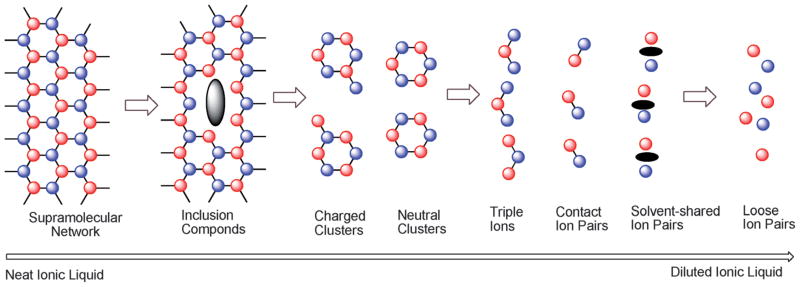
Imidazolium ILs could form H-bonded polymeric supramolecules, so-called organized ‘nano-structures’, with polar and non-polar regions in solid, liquid and solution states, or even in the gas phase.90, 91 For imidazolium-based ILs, each cation coordinates with at least three anions while each anion coordinates with three cations, resulting in a H-bonded polymeric network like [(R1R2Im)x(X)x-n]n+[(R1R2IM)x-n(X)x]n− (where R1R2IM represents 1,3-dialkylimidazolium cation, and X is the anion). As shown in Fig 3, upon the addition of more solvent molecules (such as acetonitrile, chloroform or water), the supramolecular network turns into various stages of structures such as aggregates and inclusion compounds, charged and neutral clusters, triple ions, contact ion pairs, solvent-shared ion pairs and loose ion pairs.89 Watanabe et al.92 probed the structures of protic and aprotic ILs ([MMIM][Tf2N], [MIM][Tf2N] and [Im][Tf2N]) by high-energy total scattering (HETS) experiments and MD simulations, and found that the closest cation–anion orientation varies without substantial longer range ordering of r > 12 Å by the N-methyl substitution to proton, resulting in the second layer consisting of ions of the same sign configuration changes. Additionally, they noticed that the O atoms of Tf2N− anions preferentially form H-bonds with the NH hydrogens of the protic imidazolium and the F atoms locate right above and below the imidazolium ring, and also the NH···O H-bond is short and linear while the C2H···O bond is long and bent. Very recently, the Ludwig group93 studied the H-bonding in [Cholinium][Tf2N] [Cholinium = (2-hydroxyethyl)-trimethylammonium] by far infrared spectra, and observed H-bonding between ions of like charge (in addition to H-bonding between cations and anions), i.e. forming cooperative H-bonds as OH···OH···O=S (O=S in Tf2N− anion) between hydroxyl groups of two choliniums resembling those in alcohol dimers. When comparing with [Me3NPr][Tf2N], the enhanced H-bond network in [Cholinium][Tf2N] leads to a higher melting temperature, a larger viscosity and a lower conductivity.
Fig. 3.
2D illustration of the structure of a neat IL to its infinite dilution in the presence of other solvent molecules. Most of these structures have been confirmed by experiments and/or simulations (red spheres = anions, blue spheres = cations, black spots = solvent molecules and the lines represent the hydrogen bonds and/or other weaker interactions) (Reproduced by permission from Ref,89 © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim).
Many ILs contain hydrophilic and lipophilic segments, which turn these ILs into amphiphilic compounds. The self-organization of amphiphilic ILs in solutions to form aggregates and micelles have been reported by both experimental and computer simulation methods, which has been reviewed by a number of literatures.94, 95 The aggregation property of dialkylimidazolium ILs has been shown to be similar to that of alkyltrimethylammonium salts (cationic surfactants) despite the higher self-organization ability and long-range ordering of ILs. Since the subject has been extensively reviewed, we only discuss a few recent examples herein. The Voth group96 conducted MD simulations of ILs, and found that OMIM+ cations are more prone to aggregate in water and form micelle-like structures than BMIM+ cations, while BMIM+ interacts stronger with water than OMIM+ leading the slower rotation of water at xw > 0.61 (xw is the mole fraction of water). Additionally, they noticed that changing the anion from BF4− to Cl− also slows the diffusion of cations and water molecules because Cl− anions tend to have a stronger electrostatic interaction with other particles in IL/water mixtures; since at low water mole fractions, the water structure depends on the strength of water–anion attractions, water molecules are more likely to form clusters in [OMIM][BF4]/water mixtures than in [OMIM]Cl/water mixtures at low concentrations. Greaves et al.97 examined the structures of aqueous protic ILs by small- and wide-angle X-ray scattering (SWAXS) and IR spectroscopy, and observed nanostructured aggregates in neat protic ILs; these aggregate structures are maintained upon dilution with minimal change in the size, and the water is present predominately as bulk water. Azadbakht et al.98 determined the critical micelle concentrations (cmc) of [C18MIM][BF4] and [C18MIM][PF6] as 0.04 mM and 0.02 mM respectively based on the tensiometry method; the smaller cmc value for the latter IL was explained as the PF6− ion has a larger size and more ability in forming H-bonds with water than BF4− does, and thus minimizes the surface charge of cations. On the contrary, based on 1H NMR chemical shift analysis, Inoue and Misono99 found that higher solvophilicity of polyoxyethylene (POE)-type nonionic surfactants in [BMIM][PF6] (vs [BMIM][BF4]) was due to weaker H-bond interaction between BMIM+ and PF6− than that between BMIM+ and BF4−.
It has been known that the hydration of organic cations is quite different from inorganic ions. Due to the hydrophobic nature of their alkyl groups, large organic cations (such as tetraalkylammoniums) in aqueous solutions are surrounded by water molecules forming “cagelike” structures, so called the ‘hydrophobic hydration’.100 The hydrophobic hydration results in a negative enthalpy change, due to multiple van der Waals interactions, and a negative entropy change due to the increased order in the surrounding water. As discussed by Wen,100 tetraalkylammonium cations are highly hydrated; for example, the hydration numbers of Me4N+, Et4N+, n-Pr4N+ and n-Bu4N+ are 16, 21, 27 and 32 respectively (or 25, 30, 35, and 40 respectively based on other studies). Despite these organic cations are large in size, single-charged, they are not necessarily chaotropes because of the hydrophobic hydration.28, 101, 102 Marcus54 indicated that Me4N+ is a chaotrope, Et4N+ is a borderline ion, n-Pr4N+, n-Bu4N+ and n-Pe4N+ are kosmotropes. A similar classification was confirmed by Kay et al55 and other groups.23, 56–58 In addition, these organic cations have larger B-coefficients than inorganic ions, even chaotropic Me4N+ ions have B-coefficients of 0.123.49, 103 As pointed out in a review by von Hippel and Schleich,104 Me4N+, Et4N+, n-Pr4N+, n-Bu4N+ and n-Pe4N+ ions exhibit an increasing order of destabilizing the ‘native’ form of collagen and ribonuclease; this is consistent with more kosmotropic cations destabilizing the protein. The Rogers group105 determined phase diagrams of kosmotropic inorganic salts (K3PO4, K2HPO4, K2CO3, KOH, and (NH4)2SO4) in salting out ILs, and further established the chaotropicity of ILs decreasing in the order of [Bu4P]Cl > [Bu4N]Cl ≫ [BuPy]Cl ≫ [BDMIM]Cl [BMIM]Cl.
The interactions between ILs and water molecules provide valuable insights into the IL hydration behavior. Typically, there is a strong H-bonding interaction between water molecules with basic anions of ILs (such as Cl−) as confirmed by negative excess chemical potentials of aqueous ILs.106 Mele et al.107 examined the cation–cation, cation–water, and cation–anion interactions in [BMIM][BF4] (with 0–0.52 mole fraction of water) by NMR spectroscopy through intermolecular nuclear Overhauser enhancements (NOEs), and found that increasing water content in IL progressively increases H-bonds between the cation and water (as H-bond acceptor) instead of C(sp2)–H···F interactions, and also increases the H-bonds between anion and water (as H-bond donor). In addition, they indicated that the presence of tight ion pairs in the neat IL even with a small amounts of water. The Koga group108 suggested that the influence of BMIM+ cation on water structure is similar to that of fructose or increased temperature, where water molecules interaction with the cation leading to the reduction of H-bonds of bulk water region. Xu et al.109 compared the relative chemical shifts of protons in [EMIM][BF4] upon dilution with water, and found the strength of H-bonds between water and three aromatic protons decreasing in the order of (C2)H···O > (C4)H···O > (C5)H···O; they also suggested that the ion pairs of this IL are dissociated rapidly when xwater > 0.9. Singh and Kumar110 compared the changes of OH (water) and CH (imidazoliums) vibrational stretching bands in aqueous mixtures of ILs using FT-IR spectroscopy, and observed that the blue shift of OH bands usually increases with the IL concentration and decreases in the order of different ILs: [BMIM][CH3SO4] > [BMIM][C8H17SO4] > [BMIM][BF4] > [OMIM]Cl. A higher blue shift of OH bands represents a stronger interruption of H-bonding network of water. In addition, the high hydration numbers of these ILs (14.3, 18.7, 12.7 and 12.8 respectively) along with 1H NMR spectra of aqueous ILs imply the significant interactions between water and alkyl chains, imidazolium rings and anions (i.e. hydrophobic hydration of cations and hydration of anions). The Mu group111 evaluated the H-bonding interactions between [EMIM][OAc] and several deuterated solvents including D2O in their whole concentrations by attenuated total reflectance infrared spectroscopy (ATR-IR) and 1H NMR, and reported that with the increase in deuterated solvent concentration, the H-bonding interaction among IL molecules decreases while that between IL and solvent molecules increases. Zhang et al.112 studied the H-bonding interactions between [EMIM][EtSO4] and water by ATR-IR, 1H NMR spectroscopy, and quantum chemical calculations, and noted that with the increase in water content, H-bonding of –SO3 group (in ethyl sulfate) and water is strengthened while H-bonding between C-H (in cations) and water is weakened; water preferentially interacts with ethyl sulfate anions. At high contents (xwater > 0.6), water molecules begin to interact with the hydrogen atoms on the imidazolium ring, yielding a stable new complex. They also suggested a decreasing order of interaction strength as EMIM+ – water – EtSO4− > EMIM+ –SO4− > EtSO4− – water > EMIM+ – water. Bernardes et al.113 investigated the aqueous solutions of [EMIM][EtSO4] by MD simulations, and obtained several interesting results: (1) Four distinct structural regimes were identified with four concentration ranges: isolated water molecules (xwater < 0.5); chain-like water aggregates (0.5 < xwater < 0.8); bicontinuous system (0.8 < xwater < 0.95); and isolated ions or small ion clusters (xwater > 0.95), respectively. (2) Two different percolation limits were identified: (a) that of water in the IL network (xwater 0.8), and (b) that of the IL in water (xwater 0.95), upon further dilution, the polar IL network begins to break into smaller aggregates and loses its continuous nature. (3) When xwater = 0.996, 60% of cations and anions become isolated, which implies that at this concentration the solvation energy of EMIM+ and EtSO4− by water obviously compensates the electrostatic interaction energy between the cation and anion, leading to their separation. Danten and coworkers114 examined the interactions of water in BMIM+ – based ILs (carrying anions of BF4−, PF6−, OTf−, and Tf2N−) using density functional theory (DFT) calculations as well as vibrational spectroscopic tools (IR absorption and Raman scattering), and found that water molecules preferentially interact with two distinct anions by forming associations of type (A···H–O–H···A) at low water concentrations, not in the form of H-bonding of water either with F-atoms (PF6− and BF4− anions) or with the O-atoms of the sulfonyl groups (OTf− and Tf2N− anions). The strength of water–anion interaction in water diluted in ILs decreases in the order of OTf− > Tf2N− BF4− > PF6−. Ficke and Brennecke115 determined the excess enthalpies of binary IL and water systems, and found several interesting interactions: (a) appending a hydroxyl group to the ethyl chain of EMIM+ cation increases IL/IL interactions; (b) electron-withdrawing fluorine groups on the OTf− anion lead to drastically increased weaker IL/water interactions when comparing with the MeSO3− anion; (c) increasing the cation’s alkyl chain length from ethyl to butyl reduces the cation/water interactions. The Castner group116 determined the diffusivity of water in [BMPyrr][Tf2N] and [BMPyrr][OTf] using the pulsed-gradient spin-echo NMR method, and reported that the ratio of water diffusivity to that of cation (Dwater/Dcation) is about 10–20, implying that hydrodynamic descriptions are not useful on the molecular scale, and this ratio decreases with increasing temperature for both ILs.
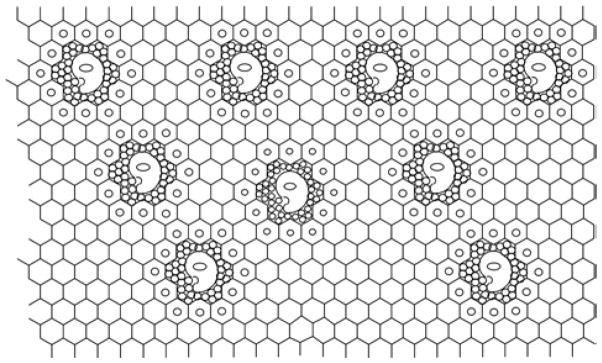
The Dupont group91 proposed that the inclusion of other molecules and macromolecules into the polymeric IL network results in the formation of polar and nonpolar regions; the aqueous solution of free enzymes could be surrounded by the IL network, which supports the retaining of native structures of proteins by preserving the essential water of proteins and the preferential solvophobic interactions. When the enzyme-in-water droplets are dissolved (or dispersed) into the IL network (in polar regions), the enzyme’s active conformation could be conserved by the network (see Fig 4).117 The inclusion of enzyme molecules in such highly ordered supramolecular structures of ILs prevents the protein from thermal unfolding.118 However, since enzymes are not soluble in most common ILs, enzyme molecules (in particular, immobilized enzymes) are practically suspended in reaction media with low or little water; as a result, the IL network theory is not always suitable for explaining the enzyme activity and stability. The impact of individual anions on enzyme inactivation also cannot be explained by the IL network.
Fig. 4.
Enzymes with a small amount of water are firmly trapped in the network of ILs (Reproduced by permission from Ref,117 © 2007 the Biochemical Society).
SPECIFIC ION EFFECT OF ILS ON PROTEIN STRUCTURES AND ENZYME ACTIVITIES
Aqueous IL solutions
In diluted aqueous solutions, hydrophilic ILs become (partially) dissociated and solvated individual ions, and these individual ions may interact with the enzyme directly. In aqueous solutions of inorganic salts, many studies (see our earlier review16) have concluded that the ion effect on the enzyme activity followed the ion kosmotropicity (Hofmeister series): kosmotropic anions and chaotropic cations stabilize the enzyme, while chaotropic anions and kosmotropic cations destabilize it. A list of studies are compiled in Table 1 (in terms of protein stability, and enzyme activity/stability) and some representative examples are discussed in details below. A series of studies42, 43, 49, 119–124 in our laboratory have demonstrated that the same principle is loosely applicable to the enzyme activity in diluted IL aqueous solutions. In our first study, the activities of Amano protease P6 (from Aspergillus melleus) in 0.7 M IL aqueous solutions were affected by anions in a decreasing order of CH3COO−, CF3COO− > Cl−, Br− > OTs− > BF4− (which is coherent with the decreasing order of anion’s kosmotropicity), and affected by cations in a decreasing order of EMIM+, BuPy+ > BMIM+ > EtPy+.119 In a second study,43 our group carried out the kinetic hydrolysis of enantiomeric phenylalanine methyl ester catalyzed by Bacillus licheniformis protease in aqueous solutions of several hydrophilic ILs (0.5 M). The protease enantioselectivity was in a decreasing order with these anions: PO43− > citrate3−, CH3COO−, EtSO4−, CF3COO− > Br− > OTs−, BF4− (decreasing kosmotropicity), and in the presence of these cations: EMIM+ > BMIM+ > HMIM+ (decreasing chaotropicity). The overall IL kosmotropicity can be measured by the δ value (difference in viscosity B-coefficients of anion and cation). In general, a high enzyme enantioselectivity was observed in the solution of IL with a high δ value. After measuring the NMR B′-coefficients of a number of ions (see Fig 5, which is consistent with Fig 1 in general), our group42 further found a linear correlation between the enzyme enantioselectivity in aqueous solution and the δ′ parameter (difference in NMR B′-coefficients of anion and cation) of ILs, suggesting that high enzyme enantiomeric ratios (E) could be achieved in solutions of ILs with high δ′ values. Other groups125–127 also reported low/no activities of β-glycosidase in aqueous solutions of [BMIM][BF4], which could be explained by the chaotropic nature of BF4− in solutions127 (Note: in neat or concentrated ILs containing anions of BF4−, the chaotropic property of anion may not influence the enzyme activity; therefore, many studies observed certain enzyme activities in BF4− based ILs). Our group120 also conducted the enzymatic hydrolysis of DL-phenylalanine methyl ester in aqueous solutions of ILs (0.5 M) containing anions of chiral- or ω-amino acids, and reported higher enantiomeric excess (ee) and yields in ILs containing anions of D-amino acids rather than in those containing anions of L-isomers. The likely explanation is that amino acid anions are more kosmotropic than zwitterionic amino acids,121 and D-amino acids are more kosmotropic than L-isomers.122 The use of ILs with kosmotropic anions (OAc− and CF3COO−) in activating hydrolases in aqueous solutions was further demonstrated in two of our studies.123, 124
Table 1.
Ion specificity on the enzyme stabilities and activities in ILs.
| Enzyme | Condition | Activity or stability series | Key factor(s) | Ref |
|---|---|---|---|---|
| Aqueous IL solutions | ||||
| Protein stability (stability of native fold) | ||||
| Hen egg white lysozyme | Thermal unfolding transition temperatures Tm in 1.0 M ILs | [EMIM]Cl > [BMIM]Cl > [HMIM]Cl and [HO-EMIM]Cl > [HO-PMIM]Cl > [HO-HMIM]Cl | Hofmeister series and hydrophobic effect | 147 |
| Cytochrome c | Stability in ILs containing 20% (wt) water | Anions: H2PO4− > Bu2PO4− > OAc− > Lactate− > MeSO4−, Cations: Cholinium+ > BMPyrr+ > BMIM+ |
Hofmeister series | 45, 128, 129 |
| Ribonuclease A (RNase A) | Thermal stability of in aqueous solution of ILs (typically 0–2 M) | Anions: SO42− > HPO42− > Cl− > EtSO4− > BF4− ~ Br− > MeSO4− > OTf− > SCN− ~ dca− > Tf2N− Cations: K+ > Na+ ~ Me4N+ > Li+ > Et4N+ ~ EMIM+ > BMPyrr+ > BMIM+ ~ Pr4N+ > HMIM+ ~ Bu4N+ and K+ > Na+ ~ Me4N+ > Cholinium+ > EMIM+ ~ Guanidinium+ > BMIM+ |
Hofmeister series | 44, 132 |
| Cyclic dipeptides | Transfer free energies ΔG′tr (positive values) from water to aqueous ILs (30–70% v/v) | [Et3NH][HSO4] > [Et2NH2][HSO4] > [Et3NH][OAc] > [Et2NH2][OAc] > [Et3NH][H2PO4] > [Et2NH2][H2PO4] | Biocompatibility is reverse of ΔG′tr order, and follows the Hofmeister series | 138 |
| Stability of horseradish peroxidase (HRP) | 50 μM HRP incubated in up to 1.0 M ILs for 60 min | Anions: Cl− > Br− > NO3− > BF4− > OTf− > SCN− > dca− Cations: Me4N+ > Cholinium+ > EMIM+ > BMPip+ > BMPyrr+ > BMIM+ > BuPy+ > HMIM+ |
Hofmeister series | 139 |
| Enzyme activity and stability | ||||
| Chloroperoxidase from Caldariomyces fumago | Oxidation of 1,2-dihydronaphthalene in 10–30% (v/v) ILs | [MMIM][MeSO4] > [BMIM][MeSO4] ≫ [BMIM][BF4] | Hofmeister series | 148 |
| Amano protease P6 (from Aspergillus melleus) | Hydrolytic activity in 0.7 M IL aqueous solutions | Anions: CH3COO−, CF3COO− > Cl−, Br− > OTs− > BF4− Cations: EMIM+, BuPy+ > BMIM+ > EtPy+ | Hofmeister series in general | 119 |
| Bacillus licheniformis protease | Enantioselectivity in 0.5 M ILs | Anions: PO43− > citrate3−, CH3COO−, EtSO4−, CF3COO− > Br− > OTs−, BF4− Cations: EMIM+ > BMIM+ > HMIM+ |
Hofmeister series | 43 |
| Immobilized Candida antarctica lipase B (Novozym 435) | Enantioselective hydrolysis: initial rate in phosphate buffer containing 10–25% (v/v) ILs | Anions: BF4− > Cl−, Br− > NO3− > HSO4− (same BMIM+ cations) Cations: EMIM+ > PMIM+ > BMIM+ (same BF4− anions) |
Anions: possible H-bond basicity and nucleophilicity Cations: Hofmeister series |
149 |
| CALB | Hydrolytic activity in up to 0.06 M IL | Br− > Cl− > OTf− > OAc− > CH3SO3− > HSO4− (same [BMIM]+ cation); EMIM+ > BMIM+ > OMIM+ |
Thermodynamic water activity, H-bond basicity (anions), hydrophobic interaction (cations) | 150 |
| Penicillium expansum lipase | Activity, 4.14% (w/v) ILs | (a) cation effect: [MMIM][MeSO4] > [EMIM][MeSO4] > [BMIM][MeSO4], [Me4N][OAc] > [Bu4N][OAc], [Me3NH][MeSO3] > [Bu4N][MeSO3], and [Me3NH][H2PO4] > [Et3NH][H2PO4] > [Bu3NH][H2PO4]; (b) anion effect: [Cholinium][OAc] > [Cholinium][MeSO3] > [Cholinium][NO3], [Bu4N][OAc] > [Bu4N][MeSO3]. |
(a) Hofmeister series (b) Hofmeister series |
137 |
| Papain | Enantioselective hydrolysis: activity and enantioselectivity in phosphate buffer containing 15% (v/v) ILs | Anions: BF4− > OAc− > NO3− > Cl− > HSO4− (same BMIM+ cations) Cation: (activity) C2MIM+ > C3MIM+ > C4MIM+ > C5MIM+ > C6MIM+, (enantioselectivity) C2MIM+ < C3MIM+ < C4MIM+ < C5MIM+ < C6MIM+ (same BF4− anions) |
Anions: possible H-bond basicity Cations: Hofmeister series and hydrophobic effect |
151 |
| 3α-hydrosteroid dehydrogenase from Pseudomonas testosterone | Enzyme activity in 10% (v/v) ILs | [BMIM][Lactate] > [EMIM][OTf] > [BMIM][BF4] > [BMIM][OTf] | Hofmeister series | 152 |
| Alcohol dehydrogenase ADH-‘A’ from hodococcus ruber | Reduction conversion of acetophenone in 20% (v/v) ILs | [EMIM][OAc] > [BMIM][OAc] > [EMIM][MeSO3] | Hofmeister series | 153 |
| Mesophilic alcohol dehydrogenase from yeast | (a) Activity in up to 600 mM IL; (b) Thermal stability in 150 mM ILs |
(a) [BMIM]Cl > [BMIM][BF4] ≫ [MIm][BF4], [MIm]Cl (b) [MIm]Cl > [MIm][BF4] > [BMIM][BF4] > [BMIM]Cl |
(a) Competition with substrate a (b) Not strictly following Hofmeister series |
154 |
| Thermoanaerobacter brockii alcohol dehydrogenase | (a) Activity, up to ~700 mM IL; (b) Thermal stability in 150 mM ILs |
(a) [BMIM]Cl, [BMIM][BF4] ≫ [MIm][BF4], [MIm]Cl (b) [MIm]Cl > [MIm][BF4] > [BMIM]Cl > [BMIM][BF4] |
(a) Competition with substrate a (b) Hofmeister series |
155 |
| Yeast alcohol dehydrogenase | Enzymatic efficiency kcat/KM in 0.5 M ILs | (a) anion effect: Cl− > Br− > EtSO4− > OTf− > BF4− > dca− > SCN− (same EMIM+ cation) (b) cation effect: Na+ > Me4N+ > Cholinium+ > EMIM+ > Et4N+ > Bu4N+ > Guanidinium+ > BMIM+ (same Cl− anion) |
Hofmeister series and hydrophobic interaction | 140 |
| β-glucosidases, xylanase E2, arabinofuranosidase F1 | Hydrolytic activity; up to 20% (v/v) IL | Me2PO4− > OAc− > Et2PO4− (same [EMIM]+ cation) | Not following Hofmeister series | 156 |
| Laccase from Aspergillus | Activity in 10% (v/v) ILs at pH 9.0 | [C4MIM]Cl > [C8MIM]Cl > [C10MIM]Cl | Hofmeister series and hydrophobic interaction | 157 |
| Mushroom tyrosinase | Oxidation activity in up to 10% (v/v) ILs Stability in up to 2% (v/v) ILs |
[BMIM][PF6] > [BMIM][BF4] > [BMIM][MeSO4] [BMIM][BF4] > [BMIM][PF6] > [BMIM][MeSO4] |
Not following Hofmeister series | 46 |
| Mushroom tyrosinase | Activity, 5.85% (w/v) ILs Stability, 5% (w/v) ILs |
(a) cation effect: [MMIM][MeSO4] > [EMIM][MeSO4] > [BMIM][MeSO4], [Me4N][OAc] > [Bu4N][OAc], and [Me3NH][H2PO4] > [Et3NH][H2PO4] (b) anion effect: [Cholinium][OAc] < [Cholinium][MeSO3] < [Cholinium][NO3] and [Bu4N][OAc] < [Bu4N][MeSO3] Stability: [MMIM][MeSO4] > [EMIM][MeSO4] > [BMIM][MeSO4] |
Activity: (a) Hofmeister series (b) Reverse Hofmeister series (kosmotropic anions interact with Cu2+ of metalloenzyme Stability: Hofmeister series |
137 |
| Lysozyme from chicken egg white | Residual activity after incubation in 0–1.0 M ILs at 25 °C for 30 min | Cl−, BF4− > > OTf− (same EMIM+ cation) (however, above 1.0 M, lysozyme is more stable in BF4− than in Cl−) | Hofmeister series | 158 |
| Concentrated or neat ILs | ||||
| Protein stability (stability of native fold) | ||||
| Aβ16–22 peptide | Amyloid fibrilization in 90% (v/v) ILs | Rate of amyloid fibrilization (protein destabilization rate): HSO4−, H2PO4− > CF3COO− > lactate− > OTf− > CH3SO3− (same Et3NH+ cation) | Reverse Hofmeister series | 159 |
| Enzyme activity and stability | ||||
| Candida antarctica lipase B (CALB) | Enantioselectivity of resolution of 1-phenylethanol via transesterification | Anions: Tf2N−, CF3SO3− ≫ PF6−, BF4− (same BMIM+ cation) Cations: OMIM+ > HMIM+ > BMIM+ (same BF4− anion) |
Anions: unknown Cations:hydrophobicity |
160 |
| Free CALB | Transesterification activity in ILs with 2% v/v water | Anions: BF4− > Tf2N− (same EMIM+ cation) PF6− > Tf2N− (same BMIM+ cation) Cations: EMIM+ > BMIM+ (Tf2N− anion) |
unknown | 161 |
| Novozym 435 (Immobilized CALB) | Stability in ILs at 30 °C | Anions: OAc− > PF6− > NO3− (same BMIM+ cation) OAc− > CH3SO3− > NO3− (same MMEP+ cation) Cations: MMEP+ > BMIM+ (OAc− or NO3− anions) |
unknown | 162 |
| Novozym 435 | Transesterification activity | BF4 > PF6 ≫ Lactate > NO3 (same BMIM+ cation) | unknown | 163 |
| Novozym 435 | Asymmetric ammonolysis: initial rate and enantioselectivity | C3MIM+ < C4MIM+ < C5MIM+ < C6MIM+ > C7MIM+ > C8MIM+ (same BF4− anions) | Hydrophobicity | 164 |
| Novozym 435 | Initial rate and enantioselectivity of acylation with controlled water activity | [C4MIM][PF6] > [C8MIM][BF4] > [C7MIM][BF4] > [C6MIM][BF4] > [C5MIM][BF4] > [C4MIM][BF4] | Hydrophobicity | 165 |
| Novozym 435 | Transesterification activity in neat ILs | Tf2N− > PF6− > BF4− > dca− (same BMIM+ cation) | Hydrophobicity and H-bond basicity | 166 |
| Novozym 435 | Transesterification of triolein and methanol | Anions: Tf2N− > PF6− > BF4− Cations: C18MIM+ > C12MIM+ > C8MIM+ > C4MIM+ |
Hydrophobicity | 167 |
| α-chymotrypsin | Stability in ILs (2% v/v water and 50 °C) | PF6− > BF4− (same BMIM+ cation) | Hydrophobicity | 168 |
| PEG complex of lipase PS from Pseudomonas cepacia | Initial rate of alcoholysis in ILs containing 1% (v/v) water | OMIM+ > HMIM+ > BMIM+ (same PF6− anion) | Hydrophobicity | 169, 170 |
| Bacillus stearothermophilus esterase immobilized on Celite | Transesterification activity at aw = 0.11 | Tf2N− > BF4− > PF6− (same BMIM+ cation) | unknown | 171 |
| Bacillus subtilis esterase immobilized on Celite | Transesterification activity at aw = 0.11 | Tf2N− > PF6− > BF4− (same BMIM+ cation) | Hydrophobicity | 171 |
| Candida rugosa lipase | Esterification of 2-substituted-propanoic acids and 1-butanol | Reaction rate: [BMIM][PF6] > [ONIM][PF6] > [BMIM][BF4] Enantioselectivity: [ONIM][PF6] > [BMIM][PF6] > [BMIM][BF4] |
Hydrophobicity and H-bond basicity | 172 |
| Penicillin G amidase (PGA) | (a) Stability in ILs with 1–20% water (aw = 0.66–0.80) (b) Activity in ILs (aw = ~0.80) |
(a) Stability: BF4− > PF6− > MeSO4; BMIM+ > OMIM+ (b) Activity: [BMIM][PF6] > [BMIM][BF4] > [BMIM][MeSO4] (PGA in ILs requires an optimal hydration aw = ~0.80) |
(a) anions: H-bond basicity and hydrophobic interaction (b) H-bond basicity |
173 |
| Alcohol dehydrogenase from Rhodococcus erythropolis | Initial rates in 10% (v/v) ILs Half-time stability in 10% (v/v) ILs |
(a) reduction of 4′-Br-2,2,2-trifluoroacetophenone [BMIM][PF6] > [EMIM][OTs] > [BMIM][BF4] (b) reduction of 6-Br-β-tetralone [BMIM][PF6] > [BMIM][BF4] > [EMIM][OTs] Stability: [EMIM][EtSO4] > [BMIM][PF6] > [EMIM][OTs] > [BMIM][BF4] |
H-bond basicity and hydrophobic interaction unknown |
174 |
| Glucose dehydrogenase 103 Lipase from Burkholderia cepacia | Half-time stability in 10% (v/v) ILs Transesterification activity | [EMIM][EtSO4] > [BMIM][BF4] > [BMIM][PF6] > [EMIM][OTs] PF6− > Tf2N− > OTf− > BF4− > CH3SO3− ~ Cl− (same [BMIM]+ cation) |
unknown H-bond basicity and Hydrophobicity |
174 175 |
| Feruloyl esterase A from Aspergillus niger | Esterification activity (15% v/v aqueous buffer) | PF6− > BF4− (same [BMIM]+ cation) | H-bond basicity | 176 |
| Naringinase from Penicilliun decumbens | Hydrolytic activity catalyzed by the enzyme immobilized on IL sol–gel matrices | OMIM+ > BMIM+ > EMIM+ > C2OHMIM+ > BIM+ | Hydrophobicity | 177 |
| Endo-1,4-β-D-glucanase from Aspergillus niger | Hydrolysis of cellulose azure | [(HOCH2CH2)3MeN][MeSO4] > [BMIM]Cl ≫ [BMIM][MeSO4] (neat ILs); [(HOCH2CH2)3MeN][MeSO4] ≫ [BMIM][MeSO4] > [BMIM]Cl (1.0 M) | H-bond basicity and anion nucleophilicity (neat ILs); Hofmeister series (1.0 M ILs) | 178 |
Note:
Due to structural similarity between MIm (1-methylimidazolium) and substrate adenine moiety (NADP+).
Fig. 5.
NMR B′-coefficients of some ions.42
Recently, Fujita et al.45, 128, 129 evaluated the stability of cytochrome c in ILs containing 20% (wt) water and its relevance to the kosmotropicity of individual ions; the cation’s effect on the protein stability followed a decreasing order of Cholinium+ > BMPyrr+ > BMIM+, which is also a decreasing order of cation chaotropicity; the anion’s effect on the protein stability followed a decreasing order of H2PO4− > Bu2PO4− > OAc− > lactate− > MeSO4−, which is the decreasing order of anion kosmotropicity (B-coefficients at 25 °C: H2PO4− = 0.340,40 OAc− = 0.246,40 MeSO4− = 0.18841; lactate might be considered as a kosmotropic anion130). This group131 further dissolved various metallo proteins (cytochrome c, peroxidase, ascorbate oxidase, azurin, pseudoazurin and D-fructose dehydrogenase) in hydrated [Cholinium][H2PO4] (with 30 wt% water), and observed that proteins maintained their active sites and secondary structures in the ionic medium. In addition, they found that some proteins retained their activities in hydrated [Cholinium][H2PO4] and D-fructose dehydrogenase showed substantially improved thermal stability in the ionic solution.
Constantinescu et al.44, 132 concluded that the thermal stability of ribonuclease A (RNase A) in aqueous solution of ILs (typically 0–2 M) follows the Hofmeister series. In their study, differential scanning calorimetry (DSC) was employed to measure the effect of ILs on the thermal denaturation of RNase A near 60 °C. In terms of decreasing protein stability, the cation series are
K+ > Na+ ~ Me4N+ > Li+ > Et4N+ ~ EMIM+ > BMPyrr+ > BMIM+~Pr4N+ > HMIM+ ~ Bu4N+ and K+ > Na+ ~ Me4N+ > Cholinium+ > EMIM+ ~ Guanidinium+ > BMIM+
and the anion series follows
SO42− > HPO42− > Cl− > EtSO4− > BF4− ~ Br− > MeSO4− > OTf− > SCN− ~ dca− > Tf2N−
The cation series suggests the higher the cation’s hydrophobicity, the higher the cation’s kosmotropicity, and the lower the protein stability in general. The anion series offers the opposite: the higher the anion’s kosmotropicity, the higher the protein stability in general (with small differences in the position of neighboring ions from our earlier discussion). Constatinescu et al.132 also indicated ILs could improve the stability of the native state, accelerate refolding, and suppress irreversible aggregation; in addition, all ILs evaluated could suppress protein aggregation under certain conditions, regardless of their protein stabilizing/destabilizing effect. Yang et al.46 found that mushroom tyrosinase is more active in aqueous [BMIM][BF4] than in aqueous [BMIM][MeSO4]; however, the enzyme stability follows a decreasing order of KMeSO4 > NaBF4 > KPF6. Yang et al.133 determined the activity and stability of alkaline phosphatase in up to 1.0 M inorganic salt solutions; they found the initial reaction rate or Vmax/Km exhibits a bell-shaped relationship with the (B− –B+) values of the salts, where B− and B+ are Jones–Dole viscosity B-coefficients for anions and cations respectively, and the highest activities are obtained by salts (such as NaCl, KCl, and KNO3) where the anion and cation have similar kosmotropic/chaotropic properties. This effect is likely due to the influence of cations and anions on the enzyme’s surface pH, active site, and catalytic mechanism. The enzyme’s thermal stability increases with the B− or (B− – B+) values, where anions seem to be more essential to the enzyme stabilization. Such a correlation may be explained by the ion effect on the enzyme surface solvation, as well as the ion interaction with surface and internal structure of the enzyme.
The Hinderberger group134 probed the impact of ILs on the tertiary structure of human serum albumin (HSA) by using continuous wave electron paramagnetic resonance (EPR) spectroscopy and nanoscale distance measurements with double electron–electron resonance (DEER) spectroscopy. They observed that the protein begins to unfold in 15% (v/v) [BMIM][BF4] and more hydrophobic alkyl chains promote strong protein-IL interactions; however, the binding capacity and the tertiary structure of HSA is mostly maintained in 25% (v/v) [Cholinium][H2PO4]. This can be explained by the Hofmeister series: [BMIM][BF4] contains a kosmotropic cation and a chaotropic anion while [Cholinium][H2PO4] consists of a chaotropic cation and a kosmotropic anion. Urea is a non-ionic chaotrope, and is a known protein denaturant that preferentially interacts with the protein surface and interrupts H-bonds of proteins.135 Attri et al.136 observed that [Et3NH][OAc] reduces the denaturing property of urea on α-chymotrypsin in aqueous solutions based on studies using circular dichroism (CD), fluorescence and NMR methods; the likely reason is that kosmotropic acetate ion interacts with urea and water via H-bonds, minimizing the urea-enzyme interactions. The Yang group137 found that the activity of Penicillium expansum lipase in 4.14% (w/v) ILs follows the Hofmeister series: for cations [MMIM][MeSO4] > [EMIM][MeSO4] > [BMIM][MeSO4], [Me4N][OAc] > [Bu4N][OAc], [Me3NH][MeSO3] > [Bu4N][MeSO3], and [Me3NH][H2PO4] > [Et3NH][H2PO4] > [Bu3NH][H2PO4]; for anions [Cholinium][OAc] > [Cholinium][MeSO3] > [Cholinium][NO3], [Bu4N][OAc] > [Bu4N][MeSO3]. They also observed a similar Hofmeister cation effect on mushroom tyrosinase, for activity in 5.85%, (w/v) ILs: [MMIM][MeSO4] > [EMIM][MeSO4] > [BMIM][MeSO4], [Me4N][OAc] > [Bu4N][OAc], and [Me3NH][H2PO4] > [Et3NH][H2PO4]; for stability in 5% (w/v) ILs: [MMIM][MeSO4] > [EMIM][MeSO4] > [BMIM][MeSO4].
Attri and Venkatesu138 determined the transfer free energies (ΔG′tr) of a homologous series of cyclic dipeptides from water to aqueous protic ILs (30%, 50% and 70%, v/v) from solubility measurements at 25 °C under atmospheric pressure. They observed that ΔG′tr values are positive in all cases studied, and decrease in the order of [Et3NH][HSO4] > [Et2NH2][HSO4] > [Et3NH][OAc] > [Et2NH2][OAc] > [Et3NH][H2PO4] > [Et2NH2][H2PO4]. A higher ΔG′tr value indicates a stronger unfavorable interaction between an IL and cyclic dipeptide; therefore, the biocompatibility of these ILs is the reverse order of the above ΔG′tr sequence, which follows the Hofmeister series: kosmotropic anions and chaotropic cations stabilize proteins [viscosity B-coefficients (dm3 mol−1 at 25 °C):40 H2PO4− (0.340) > OAc− (0.246) > HSO4− (0.127), Et3NH+ (0.385) > Et2NH2+ (0.293)]. Lu et al.139 found that anodic peak current of horseradish peroxidase (HRP) at bare glassy carbon electrode (GCE) can be correlated with the catalytic activity and the secondary structure of HRP. Therefore, the current signals in the presence of ILs could quantify the impact of ions on the structural stability of HRP. The effect of cations and anions (up to 1.0 M) on the HRP structural stability seems to follow the Hofmeister series:
(Cations) Me4N+ > Cholinium+ > EMIM+ > BMPip+ > BMPyrr+ > BMIM+ > BuPy+ > HMIM+,
(Anions) Cl− > Br− > NO3− > BF4− > OTf− > SCN− > dca−
Weibels et al.140 evaluated the activities of yeast alcohol dehydrogenase in 0.5 M ILs, and noticed that all ILs studied lower the turnover number (kcat) when comparing with the reaction in buffer whilst the apparent dissociation constant of the substrate (KM) varies. Overall, the enzymatic efficiency kcat/KM follows the series below:
(Anion) Cl− > Br− > EtSO4− > OTf− > BF4− > dca− > SCN− (same EMIM+ cation)
(Cation) Na+ > Me4N+ > Cholinium+ > EMIM+ > Et4N+ > Bu4N+ > Guanidinium+ > BMIM+ (same Cl− anion)
This group argued that the observed Hofmeister series could be explained by the hydrophobic interactions as a controlling factor for ion-specific effects on the enzymatic activity.
Yan et al.141 studied the interaction bovine serum albumin (BSA) and [CnMIM]Br (n = 4, 6, 8, 10) (up to 8.0 mM) by fluorescence, UV–Vis and FT-IR spectroscopy, as well as the density functional theory (DFT). Their data suggest that these ILs bind with BSA through two types of interactions: (a) H-bonding between cationic headgroups and Asp/Glu amino acid residues at the BSA surface, and hydrophobic interaction between cationic hydrocarbon chains and hydrophobic amino acid residues in the core of BSA. Since the hydrophobic interaction increases with the alkyl chain length, it is the predominated interaction of [C10MIM]Br with BSA; on the other hand, H-bonding and van der Waals force are primary interactions between [CnMIM]Br (n = 4, 6, 8) with BSA. An excellent review by Yang9 systematically discussed the possible mechanisms of Hofmeister effects of ILs on the enzyme activity and stability. The above experimental studies have shown that the kosmotropic effect of ILs on enzymes may be applicable to diluted aqueous solutions of ILs,16, 43, 119 as well as some concentrated ILs (such as 20 wt% water45). However, it is not quite clear if such an effect exists in neat or concentrated ILs, and how the IL hydrophobicity may influence the kosmotropicity. For example, PF6− is a chaotropic anion,49 and denatures enzymes when dissolved in aqueous solutions as Na+ or K+ salt (more denaturing than BF4− and MeSO4− for mushroom tyrosinase46). However, PF6− based ILs (such as [BMIM][PF6]) are hydrophobic, and thus the solubility and degree of dissociation of ILs in water become limited. Meanwhile, it is also known PF6− based ILs containing low water contents are usually enzyme stabilizing.1 Therefore, the Hofmeister effect may not be suitable for explaining the enzyme’s behaviors in these hydrophobic ILs or their mixtures with water. Without sufficient water to hydrate them, kosmotropic or borderline anions (such as acetate, lactate and chloride) of ILs bearing high H-bond basicities tend to interact strongly with enzymes causing their inactivation (see a later section H-bond basicity and nucleophilicity of anions). Consequently, the enzyme stabilization/activation kosmotropic anions (such as OAc− and Cl−) in diluted aqueous solutions become enzyme-inactivating agents in ILs with low water contents (see a simple illustration in Fig 6). For example, several papers43, 119, 123, 142 have reported the enzyme activation at low-concentrations of chloride-based ILs in water, but inactivation at high concentrations.
Fig. 6.
Illustration of interactions of enzyme in acetate-containing IL: (a) between acetate anion with water molecules in diluted IL solution, and (b) between acetate anion and enzyme molecule in concentrated IL.
On the other hand, there are a number of studies that indicate enzyme activities in aqueous ILs do not follow Hofmeister series or even follow a reverse order (see Table 1). A few selected examples are discussed below. The Yang group137 found that the activity of mushroom tyrosinase in 5.85% (w/v) ILs follows a reverse Hofmeister series: [Cholinium][OAc] < [Cholinium][MeSO3] < [Cholinium][NO3] and [Bu4N][OAc] < [Bu4N][MeSO3]; the likely explanation is that kosmotropic anions interact with Cu2+ of the metalloenzyme, resulting in lower activities. Curto et al.143 observed the activity of lactate oxidase in 0.5 M choline-based ILs follows a decreasing order with anions as Cl− > H2PO4− > NO3− > Levulinate− > HCOO−. The viscosity B-coefficients for these anions at 25 °C (in dm3 mol−1) are: Cl− (−0.005), H2PO4− (0.340), NO3− (−0.043) and HCOO− (0.052).40 The B-coefficient for Levulinate− is unknown, but is estimated to be greater than that of butanoate (0.419).40 Therefore, the kosmotropicity of these anions based on B-coefficients and known Hofmeister series16 can be listed in a decreasing order of Levulinate− > H2PO4− > HCOO− > Cl− > NO3−, which is not in agreement with the order of lactate oxidase activities. This group143 also measured the secondary structure of lactate oxidase by CD spectroscopy, and found a considerable decrease of α-helices and increase of β-sheets in hydrated [Cholinium][H2PO4] (25%, w/w). However, the changes in its secondary structure lead no appreciable impact on the activity and stability of lactate oxidase. Baker et al.144 examined the equilibrium unfolding behavior of site-specific tetramethylrhodamine-labelled yeast cytochrome c in aqueous ILs (up to 2.5 M), and found the protein denaturation is highly anion-dependent. However, they noted that Hofmeister theory seems inadequate for providing reason explanations, and more complex factors (such as H-bonding and other specific solvent–solute interactions) should be considered. Kumar and Venkatesu145 observed the transition temperature (Tm) of myoglobin decreasing in 0.01 – 0.04 M [BMIM]+ – based ILs in the order of anions as Br− > Cl−> HSO4− > SCN− > CH3COO− > I−; this sequence is not consistent with the known Hofmeister series. Similarly, this group146 further determined the Tm values of α-chymotrypsin from fluorescent measurements in 0.01 M salt solutions, which decrease for the sodium salts in the order of SO42− > Br− > I− > SCN− > CH3COO− > Cl−, and for [BMIM]+ –based ILs in the order of CH3COO− > Br− > Cl− > HSO4− > SCN− > I−. These sequences do not seem to follow the Hofmeister series. [BMIM]+-based ILs carrying anions of CH3COO−, Cl− and Br− enhance the thermal stability of α-chymotrypsin, while HSO4−, SCN− and I− containing ILs act as protein denaturants.
Enzyme activation by low concentrations of ILs
A number of studies reported that enzymes are activated/stabilized by low concentrations of ILs. The Rogers group142 observed that the cellulase’s fluorescence intensity associated with tryptophan increased in low concentrations of [BMIM]Cl (up to ~10%) and then drastically decreased at higher salt concentrations. Our group observed that proteases could be activated by a low concentration (e.g. 0.5 M) of [EMIM][EtSO4]43 or [BMIM][CF3COO].123 Baker and Heller179 studied the structures of human serum albumin (HSA) and equine heart cytochrome c in aqueous [BMIM]Cl by CD spectroscopy and small-angle neutron scattering measurements. They found that both proteins maintain most of their higher-order structures in up to 25% (v/v) [BMIM]Cl, and become highly denatured in 50% (v/v) [BMIM]Cl; in addition, HSA dimerizes at high concentrations of [BMIM]Cl, while cytochrome c exclusively retains the monomeric form. Domínguez et al.180 found that laccase from Trametes versicolor could be activated by 10% (v/v) [BMIM]Cl, but inactivated by the same concentration of [EMIM][EtSO4] (slight inactivation) or [HMIM]Br (substantial inactivation).
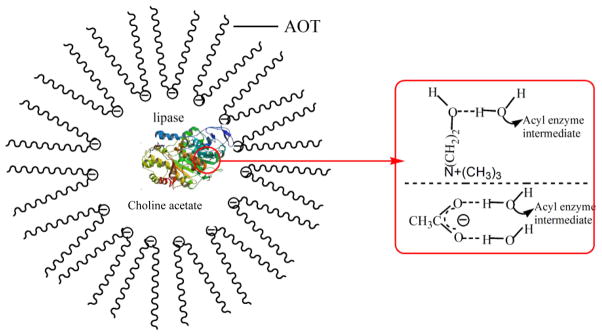
Yang et al.46 reported that the activity of mushroom tyrosinase increases with IL concentration at up to 5% (v/v) for [BMIM][BF4] and 2% (v/v) for [BMIM][MeSO4], and then declines with a higher IL concentration. The catalytic efficiency (Vmax/Km) increases initially with IL content and then decreases, showing a bell-shaped relationship with the IL concentration. Choline acetate is an IL consisting of a kosmotropic anion and a chaotropic cation. The Huang group181 found that at low concentrations (up to 5 mM), choline acetate could improve the hydrolytic activity of Candida rogusa lipase in AOT/water/isooctane reverse micelles (Fig 7), and cause no lipase conformational changes as evidenced by fluorescence spectra. Infrared spectra suggest stronger H-bonds between choline acetate and water than those between water molecules; as a result, the addition of a low content of choline acetate improves the nucleophilicity of water, accelerating the attack of water molecules on the acyl enzyme intermediate and increasing the lipase’s catalytic efficiency.
Fig. 7.
Illustration of choline acetate influencing the nucleophilicity of water molecules near the lipase in AOT reverse micelles (Adapted from Ref,181 with permission from Elsevier).
Li et al.182 examined the hydrolytic activity of Candida rugosa lipase in aqueous solutions of a serious ILs [CnMIM]X (n = 2, 4, 6, 8, 10, or 12; X = Cl−, Br−, BF4− or PF6−), and found that the lipase activities increase with the IL contents to optimum concentrations and then decline with higher IL concentrations. In general, the optimum concentrations decrease with the alkyl chain length of cations, and are several-fold lower than their corresponding critical micelle concentration (CMC). Filice et al.183 studied the activities of immobilized lipases in a low concentration (0.01 M) of ILs (based on BF4−, PF6−, NO3−, and MeSO4−), and observed that some IL solutions could activate the lipases. For example, an engineered variant (σ-L230C) of Geobacillus thermocatenolatus lipase (GTL) showed seven-fold improvement of activity in [EMIM][PF6] and five-fold improvement of activity in [EMIM][MeSO4] during the monodeacetylation of peracetylated glucal; this lipase variant also exhibited a higher regioselectivity in the hydrolysis of peracetylated glucal (from 78% to 96% yield of the C-3 monodeprotected product) in the [BDMIM][PF6] solution. In addition, the addition of [EMIM][PF6] improved the regioselectivity of Candida rugosa lipase in the hydrolysis of peracetylated thymidine (from 72% to 81% yield of C-5 monodeprotected product), however, the use of BF4− –based ILs generally led to lower enzyme activities. CD and fluorescence measurements suggested that a low concentration of ILs could cause conformational changes in the tertiary structure of the lipase.
Concentrated or neat ILs
As discussed earlier, cations and anions of concentrated or neat ILs form complex polymeric network through interactions like electrostatic attractions and H-bonding (Fig 3). Therefore, several key properties, such as H-bond basicity and nucleophilicity of anions and IL hydrophobicity, begin to play critical roles in enzyme stabilization and activation. Some examples are listed in Table 1 and a few representative studies are discussed in-depth below.
H-bond basicity and nucleophilicity of anions
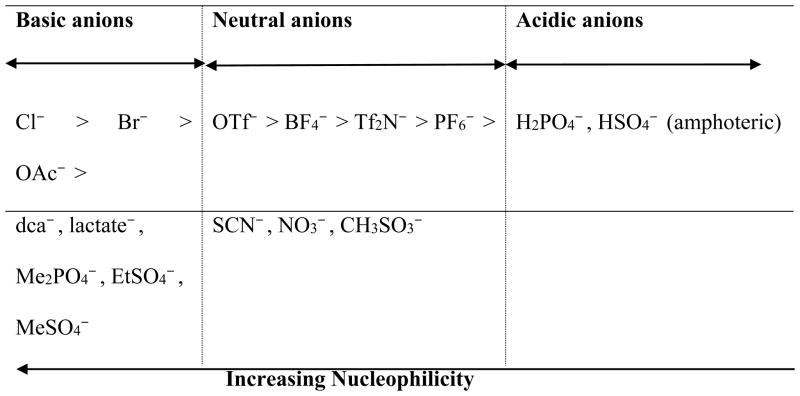
H-bond basicity and nucleophilicity are two different concepts,† but are often closely related. For molecules containing the same nucleophilic atoms of the same charge, the stronger base is usually the stronger nucleophile in aprotic solvents. Relying on the solvatochromic measurements, several studies have suggested the order of anion’s basicity as the following (in decreasing orders):
Basicity series #1:184 OTf− (CF3SO3−) > Tf2N− > PF6−
Basicity series #2:185 Cl− > Br− > SCN− > OAc− > I− > NO3− > OTf− > ClO4− > BF4
Basicity series #3:186 Cl− > Br− > OAc− > OTf− > ClO4− > BF4−
Basicity series #4:187 Cl− > Br− > CH3OSO3− > SCN− > BF4− ~ OTf− > PF6−
Basicity series #5:188 OAc−, Me2PO4−, MeHPO3− > EtSO4− > MeSO4− > BF4− > Tf2N− > PF6−
Based on the above series and other discussions in literatures,189, 190 a summary of the basicity of selected anions is illustrated in Fig 8. These anions are divided into three categories (basic, neutral and acidic), and some of them are ranked in the order of basicity. Basic anions include halides, acetate, dicyanamide (dca−), lactate and methyl sulfate; these anions are good H-bond donors and tend to form H-bonds with proteins resulting in enzyme denaturation and/or inactivation at high salt concentrations. Neutral anions include those tending to form hydrophobic ILs (Tf2N− and PF6−) and others tending to form hydrophilic ILs (BF4−, OTf−, SCN−, NO3− and CH3SO3−). These anions have weak abilities in forming H-bonds; i.e., if enzymes are inactivated in ILs containing neutral anions, the H-bond basicity is unlikely the main reason. Acidic anions (such as amphoteric H2PO4− and HSO4−) are not common anions in ILs for biocatalysis. However, the Ohno group45, 128 found that choline dihydrogen phosphate (m.p. 119°C) containing 20% (wt) water could dissolve and stabilize cytochrome c.
Fig. 8.
Comparison of H-bond basicity of selected anions in ILs.
Bernson and Lindgren191 dissolved lithium salts LiX in poly(propylene glycol) (MW = 3000) with hydroxy end-groups. Using IR spectroscopy, they observed that the shifts of –– OH stretching band depend on the strength of H-bond formed between the – OH group and the anion, as well as the coordination of cations with the -OH group. The strength of anion coordination is further dependent on the H-bond basicity of the anion, and is summarized from the IR band shifts as (in an increasing order),
PF6− < BF4− < ClO4− < OTf− < I− < Br− < Cl−
In general, this basicity series is consistent with the basicity order from solvatochromic measurements (Fig 8). From experimental data of IR and ESI-MS, Dupont91 suggested the strength of H-bond basicity in a similar increasing order of
BPh4− < PF6− < BF4− < CF3COO−
On the other hand, the ionic association strength of LiX salts was also examined in a variety of aprotic solvents including glymes (see a short review in the Supporting Information of Ref192). The approximate ionic association strength in aprotic solvents is listed below in an increasing order:192, 193
beti−, Tf2N− < PF6− < ClO4−, I− < SCN− < BF4− < CF3SO3− < Br− < NO3− < CF3COO− < Cl−
This order represents the strength of an anion in interacting with solvated cations through ionic attraction, or could be implied to represent the strength of interactions between the anions and charged regions of macromolecules (such as proteins). This ionic association strength series resembles the anion’s H-bond basicity order in Fig 8.
In the following sections, a number of enzymatic reactions in ILs demonstrate how the nucleophilicity and basicity of anions contribute to the enzyme activity and stability. The first group of examples focused on the effect of anion’s nucleophilicity. Kaar et al162 observed that free Candida rugosa lipase was only active in hydrophobic [BMIM][PF6], but inactive in all hydrophilic ILs based on NO3−, OAc− and CF3COO− during the transesterification of methylmethacrylate with 2-ethyl-1-hexanol. They indicated that the latter three anions are more nucleophilic than PF6−, and thus could interact with the enzyme causing the protein conformation changes. In this example, the solvent hydrophobicity is another important factor in influencing the enzyme activity (see a later section ‘Hydrophobicity’). Hernández-Fernández et al194 reported that the stability of CALB (lipase B from Candida antarctica) in ILs was in the following order: [HMIM][PF6] > [HMIM][Tf2N] > [HMIM][BF4], and [BMIM][PF6] > [BMIM][dca], and the stability of Penicillin G acylase was in a similar order of [BMIM][Tf2N] > [BMIM][PF6] > [BMIM][BF4]. They explained the decreasing stability were in general consistent with the increasing order of nucleophilicity in Fig 8 (PF6− < BF4− < Tf2N− < dca−), where the more nucleophilic anions tend to interact with the positively charged sites on enzymes and to modify the enzyme’s conformation. On the other hand, they also pointed out that the enzyme stability was in agreement with the hydrophobicity of ILs: both enzymes were more stable in hydrophobic ILs than in hydrophilic ones. However, in another study, a contradictory result was reported. Irimescu and Kato195 carried out the CALB-catalyzed enantioselective acylation of 1-phenylethylamine with 4-pentenoic acid, and found that the reaction rates relied on the type of IL anions (reaction rates in a decreasing order of OTf− > BF4− > PF6−, same cations). Thus, this example implies a higher anion nucleophilicity leading to a higher enzymatic activity. In a second acylation reaction of 2-phenyl-1-propylamine with 4-pentenoic acid, however, Irimescu and Kato195 observed that PF6− based ILs afforded fastest reaction rates, followed by OTf− and BF4− based ILs. The rather confusion findings may be due to the fact that the enzymatic reaction is affected by multiple factors of ILs such as nucleophilicity, hydrophobicity, viscosity and impurity. Lee et al196 measured the initial transesterification rates of three lipases (Novozym® 435, Rhizomucor miehei lipase, and Candida rugosa lipase) in different ILs under the same water activity (aw), and observed the anion effect on the initial rates followed a decreasing order of Tf2N− > PF6− > OTf− > SbF6− ~ BF4−. They explained that OTf− and BF4− are more nucleophilic than PF6−. The second factor could the IL hydrophobicity because lipases seemed more active in hydrophobic ILs than in hydrophilic ones.
The second group of examples focused on the effect of the anion’s H-bond basicity. [BMIM]Cl could effectively dissolve cellulose197, 198 because chloride ions (as H-acceptors) interact with the cellulose -OH group and break the H-bonding network of cellulose.199 Because of the same reason, this IL induced the inactivation of cellulase (from Trichoderma reesei).142 Similarly, Lee et al200 observed a dramatic decrease of the lipase activity in [OMIM][Tf2N] in a higher concentration of [OMIM]Cl. Based on the multiple salvation interactions, [BMIM]Cl showed the largest H-bond basicity among ILs considered in a study by Anderson et al,201 and thus could dissolve complex polar molecules such as cyclodextrins and antibiotics.202 Lou et al164 reported that Novozym® 435 showed no ammonolysis activity towards (R,S)-p-hydroxyphenylglycine methyl ester in [BMIM]Br and [BMIM][NO3], implying the denaturing nature of these two ILs. Lau et al163 suggested that the low CALB activity in [BMIM][Lactate] was caused by the secondary structure changes of the protein, which was further triggered by the H-bonding interaction between lactate anions and peptide chains. Dicyanamide (dca−) based ILs such as [BMIM][dca] are capable of dissolving carbohydrates,203, 204 however, [BMIM][dca] is an enzyme-denaturing IL166, 205, 206 probably due to the high H-bond basicity of the anion. Fujita et al45 detected a low stability of cytochrome c in [BMIM][MeSO4], [BMIM][Lactate] and [BMIM][OAc] all containing 20 wt% water, implying the high H-bond basicity and enzyme-denaturing nature of MeSO4−, lactate and OAc−. Our group207 also suggested both free and immobilized CALB in [EMIM][OTf] were about as inactive as in [BMIM][dca]. Bermejo et al208 observed that free CALB lost 35% of its initial activity once being dissolved in [HOPMIm][NO3], but maintained 80% of the remaining activity after 3 months of incubation in this IL. The CALB activity loss in [HOPMIm][NO3] was primarily due to the denaturing effect of NO3− as discussed earlier. On the other hand, the less denaturing property of this IL (vs. [BMIM][NO3]) may be explained by two reasons: (1) the HOPMIm+ cation is larger than BMIM+, and as a result, the molar concentration of NO3− in [HOPMIm][NO3] is lower than that in [BMIM][NO3]; (2) [HOPMIm][NO3] contains a hydroxyl group, which may favorably interact with NO3− and thus reduce the interaction between NO3− and the lipase. Zeuner et al.176 carried out the esterification of glycerol with sinapic acid catalyzed by Feruloyl esterase A from Aspergillus niger (15% v/v aqueous buffer, 18% v/v glycerol, and 67% v/v IL), and found the enzyme is active in PF6− –based ILs ([BMIM][PF6] and [(HOCH2CH2)MIM][PF6] but inactive in BF4− –based ILs. The COSMO-RS simulations suggest that BF4− is a stronger H-bond acceptor than PF6−, disrupting the H-bond based enzyme structure. The Yang group209 obtained up to 86% conversion of corn oil to biodiesel in [BMIM][PF6] catalyzed by Penicillium expansum lipase; however, they obtained no enzymatic activity in other ILs containing anions of MeSO4−, OAc−, NO3−, and H2PO4−. Bekhouche et al.210 examined the activity and stability of formate dehydrogenase from Candida boidinii (FDH, EC: 1.2.1.2) in three ILs (i.e. [MMIM][Me2PO4], [BMIM][OAc], [MMIM][CH3HPO2(OCH3)]) by activity assays and steady-state fluorescence spectroscopy (using iodide as the dynamic quencher or acrylamide as the static quencher). They found the third IL is more denaturing than the first two and each IL induces a different denaturation mechanism. The enzymatic activity was reduced in the presence of 30% (v/v) [MMIM][Me2PO4], 10% [BMIM][OAc] or 10% [MMIM][CH3HPO2(OCH3)], and was totally inactivated in 70% (v/v) [MMIM][Me2PO4], 30% [BMIM][OAc] or 20% [MMIM][CH3HPO2(OCH3)].
Hydrophobicity
‘Hydrophobicity’ could be considered as a subset concept of ‘polarity’. However, it is practically important to differentiate ‘hydrophobicity’ from ‘polarity’ because the former one is often related to the miscibility with water.211 The hydrophobicity of ILs can be quantified by the log P scale, a concept derived from the partition coefficient of ILs between 1-octanol and water. The partition coefficient (KOW or P) is a ratio of concentrations of un-ionized compound between the two phases. The log P is defined as the partition coefficient at the unlimited dilution concentration of solute,
| (2) |
where Co is the IL concentration in the octanol phase and Cw is the IL concentration in the aqueous phase. For the simplicity, it is common to use extremely low concentrations of IL in the experiment instead of extrapolating the IL concentration to zero (eqn 2). However, since ILs dissociate into ions in water and current KOW values were reported as the ratio of concentrations of both undissociated and dissociated ILs in two phases, most log P values of ILs (Table 2) should be strictly called log D, where D is the distribution coefficient, the ratio of the total concentrations of all forms of IL (ionized and un-ionized) between two phases. Alternatively, the intrinsic partition coefficients of ILs should be calculated from the apparent partition coefficients (D).212
Table 2.
log P (or log KOW at low concentrations a) values of ILs at 25 °C
| Solvent | log P/log KOW | Reference | |
|---|---|---|---|
| 1 | dichloromethane | 1.25 | selected value by Ref 213 |
| 2 | THF | 0.46 | selected value by Ref 213 |
| 3 | t-butanol | 0.35 | selected value by Ref 213 |
| 4 | acetone | −0.24 | selected value by Ref 213 |
| 5 | acetonitrile | −0.34 | selected value by Ref 213 |
| 6 | [EMIM][Tf2N] | −1.18 | 214 |
| log KOW (−1.05 to −0.96) (0.28−2.8 mM) | calculated from Ref 215 | ||
| 7 | [BMIM][Tf2N] | 0.11 | 166 |
| log KOW (−0.96 to −0.21) (0.15−2.2 mM) | calculated from Ref 215 | ||
| 0.33 | 216 | ||
| −1.74 | 212 | ||
| 8 | [HMIM][Tf2N] | 0.64 | 166 |
| log KOW (0.15 to 0.22) (0.32−0.38 mM) | calculated from Ref 215 | ||
| 0.65 | 216 | ||
| 9 | [OMIM][Tf2N] | 0.79 | 214 |
| log KOW (0.80−1.05) (0.099−0.21 mM) | calculated from Ref 215 | ||
| 10 | [EMMIM][Tf2N] | log KOW (−1.15 to −0.92) (0.32−2.9 mM) | calculated from Ref 215 |
| 11 | [PMMIM][Tf2N] | log KOW (−0.92 to −0.62) (1.4−2.8 mM) | calculated from Ref 215 |
| 12 | [HMMIM][Tf2N] | log KOW (0.13 to 0.25) (0.36−0.49 mM) | calculated from Ref 215 |
| 13 | [BMIM][PF6] | −1.66 | calculated from Ref 215 |
| −2.39 | 162, 216 | ||
| −2.38 | 172, 212 | ||
| −2.06 | 214 | ||
| −2.35 | 165 | ||
| 14 | [HMIM][PF6] | −1.86 | 216 |
| 15 | [OMIM][PF6] | −0.35 | 214 |
| −1.33 | 216 | ||
| 16 | [ONIM][PF6] | −2.19 | 172 |
| 17 | [BMIM]Cl | −2.40 | calculated from Ref 215 |
| 18 | [BMIM]Br | −2.48 | calculated from Ref 215 |
| 19 | [EMIM][OAc] | −2.53 | 166 |
| 20 | [BMIM][OAc] | −2.77 | 162 |
| 21 | [EMIM][CF3COO] | −2.75 | 166 |
| 22 | [HMIM][ CF3COO] | −2.30 | 166 |
| 23 | [BMIM][NO3] | −2.90 | 162 |
| −2.42 | calculated from Ref 215 | ||
| 24 | [BMIM][dca] | −2.32 | 166 |
| 25 | [EMIM][BF4] | −2.57 | 166 |
| 26 | [BMIM][BF4] | −2.51 | 166 |
| −2.44 | 165, 172 | ||
| −2.52 | calculated from Ref 215 | ||
| 27 | [OMIM][BF4] | −1.34 | 166 |
| −1.14 | 214 | ||
| 28 | [EtPy][ CF3COO] | −2.57 | 166 |
| 29 | [EtPy][Tf2N] | −0.90 | 166 |
| 30 | [BuPy][Tf2N] | −0.26 | 166 |
| 31 | [Cholinium][Tf2N] | log KOW = −0.57 (calculated value) | 217b |
Note:
log KOW values calculated from Ref215 were converted from initial values of KOW measured at room temperature (24 ± 2 °C), and the concentration range given for each log KOW was the IL concentration range in water phase;
This reference also provides KOW values for a number of pyridinium and imidazolium ILs based on Tf2N− and B(CN)4−.
From a practical point of view, the log P values (or log KOW at low concentrations) of ILs in Table 2 are valuable for comparing the hydrophobicity of ILs with conventional organic solvents. In general, ILs are very hydrophilic in nature based on the negative log P values (or log KOW) for most ILs (including water-immiscible Tf2N− and PF6− ones); however, by convention, we usually refer those ILs that are poorly miscible with water (e.g. Tf2N− and PF6− types) as hydrophobic ILs. The discrepancy between different measurements of the same ILs might be caused by different initial concentrations of ILs (as high concentrations leading to higher KOW values212, 215), and different experimental techniques.
The Russell group162 measured the log P values (< −2.0) of several ILs, and suggested that they are very hydrophilic in nature based on the Laane’s scale;218–220 they also observed that free lipase (Candida rugosa) was only active in hydrophobic [BMIM][PF6] (log P = −2.39), but inactive in other hydrophilic ILs including [BMIM][CH3COO] (log P = −2.77), [BMIM][NO3] (log P = −2.90) and [BMIM][CF3COO].162 Similarly, Nara et al221 achieved higher transesterification activities of lipases in [BMIM][PF6] than in [BMIM][BF4]. The Goto group also reported higher activities of PEG-modified lipase222 and subtilisin223 in more hydrophobic ILs such as [EMIM][Tf2N]. Zhang et al224 reported low penicillin acylase stabilities in [BMIM][BF4] and [BMIM][dca]. Lou and Zong165 studied the enantioselective acylation of (R,S)-1-trimethylsilylethanol with vinyl acetate catalyzed by lipases in several ILs, and indicated the activity, enantioselectivity and thermostability of Novozym® 435 increasing with the IL hydrophobicity ([BMIM][PF6] > [OMIM][BF4] > [C7MIM][BF4] > [HMIM][BF4] > [C5MIM][BF4] > [BMIM][BF4]). Paljevac et al225 reported that the cellulase activity decreased in the order of IL hydrophobicity: [BMIM][PF6] > [BMIM][BF4] > [BMIM]Cl. The Víllora group226 observed a lower stability of penicillin G acylase in [BMIM][BF4] than in hydrophobic ILs (Tf2N− and PF6−), particularly in the absence of substrate. A recent study227 on the alcoholysis of vinyl butyrate and 1-butanol by free CALB suggested that the lipase activities were generally much lower in water-miscible ILs (such as BF4−, dca−, NO3− and OAc−, etc.) than in water-immiscible ones (PF6− and Tf2N−), and the enzyme’s activities increased with the cation’s hydrophobicity (EMIM+ < BMIM+ < HMIM+ < OMIM+). Ha et al228 also found Novozym® 435 was less active and stable in hydrophilic ILs (BF4− and OTf−) than in other hydrophobic ILs (Tf2N− and PF6−). Lee et al196 reported that Novozym® 435 was more thermally stable in hydrophobic ILs than in hydrophilic ones following the order of [BMIM][Tf2N] > [BMIM][PF6] > [BMIM][OTf] > [BMIM][BF4] > [BMIM][SbF6]. Shen et al229 noticed that during the kinetic resolution of racemic cyanohydrins, Amano lipase PS showed a high enantioselectivity (80% eep) in hydrophobic [OMIM][PF6], but poor enantioselectivities (< 5% eep) in hydrophilic [HMIM][BF4] and [HMIM]Cl. Hernández-Fernández et al194 concluded that both free CALB and penicillin G acylase (PGA) were more stable in hydrophobic ILs than in hydrophilic ones: in the case of CALB, the stability was in a decreasing order of [HMIM][PF6] > [HMIM][Tf2N] > [HMIM][BF4], and [BMIM][PF6] > [BMIM][dca], as well as [OMIM][PF6] > [HMIM][PF6] > [BMIM][PF6]; in the case of PGA, the stability was in a decreasing order of [BMIM][Tf2N] > [BMIM][PF6] > [BMIM][BF4]. However, the hydrophobic cations showed an adverse effect on the PGA stability: [EMIM][Tf2N] > [BMIM][Tf2N], and [BMIM][PF6] > [OMIM][PF6]. The effect of nucleophilicity of these anions has been discussed previously. These examples implied that the high hydrophobicity (large log P) of ILs could be beneficial to the enzyme stabilization.
Through a systematic investigation of Novozym® 435-catalyzed transesterification in over 20 ILs, our group166 observed that the lipase activity increased with the log P value of ILs to a maximum, and then declined with a further increase in log P (a bell shape). Our previous discussion implied that the enzyme is active in hydrophobic solvents (with a high log P). However, a higher log P of the solvent also means a more thermodynamic ground-state stabilization of substrates,230 which might reduce the conversion of substrates. This could explain the decreasing reaction rate in very hydrophobic ILs. Similarly, Lou et al164 found the initial rates of Novozym® 435-catalyzed ammonolysis of (R,S)-p-hydroxyphenylglycine methyl ester increased with the hydrophobicity of BF4− based ILs to a maximum (C3MIM+ < C4MIM+ < C5MIM+ < C6MIM+), and then decreased with a further increase in the IL hydrophobicity (C6MIM+ > C7MIM+ > C8MIM+).
As discussed previously, the stabilization of substrates could be one reason. But the possibility of hydrophobic interactions between large IL molecules and the enzyme cannot be fully excluded. For example, the Atkin group231 investigated the stability and activity of hen’s egg white lysozyme in aqueous solutions of four protic ILs (25–75 wt%); the protein denaturing-renaturing CD experiments and the activity measurements of lysozyme indicated that the highest catalytic activity and most complete refolding was achieved in solutions of [(EtOH)NH3][HCOO], followed by [PrNH3][HCOO], and then [EtNH3][HCOO] and [(MeOEt)NH3][HCOO]. It is believed that the protein-IL interactions include the electrostatic interaction of IL cations with negatively charged residues in the protein, H-bonds between amine protons and the protein, as well as the hydrophobic interactions between alkyl chains in ILs and hydrophobic regions of the protein. Since electrostatic interactions between [(EtOH)NH3]+ and lysozyme is about the same as for [EtNH3]+, the hydroxyl group in [(EtOH)NH3]+ probably reduces the strength of hydrophobic interactions with the protein. Another possibility is that the hydroxyl group interacts with the anion formate via H-bonds, reducing the interaction of formate with the protein. The IL viscosity-induced mass transport was not a limiting factor in the study because [(EtOH)NH3][HCOO] is several times more viscous that other three ILs. In summary, the hydrophobicity factor of ILs is a combination effect of anion’s H-bond basicity and cation’s hydrophobic effect.
Klähn et al.232, 233 carried out MD simulations of CALB in imidazolium or guanidinium–based ILs containing anions of NO3−, BF4− or PF6−. They confirmed that the CALB stability is mainly influenced by anions and follows a decreasing order of PF6− > BF4− ≫ NO3−, and long decyl side chains, polar methoxy groups and guanidinium-based cations induce more CALB destabilization than short methyl groups, other non-polar groups and imidazolium-based cations. Two destabilization mechanisms are identified: (a) Destabilization of protein surface by Coulomb interactions with anions carrying a localized charge and strong polarization, or with polar cations. This type of destabilization shows a roughening of the protein surface, loss of compactness, and unraveling of α-helices. Smaller anions and a high anion surface charge lead to stronger Coulomb interactions. (b) Destabilization of protein core by direct hydrophobic interactions of protein core with long alkyl chains or hydrophobic ILs, which leads to a disintegration of β-sheets, diffusion of ions into CAL-B and increasing protein–IL van der Waals interactions. Due to van der Waals interactions with the aliphatic residues at the active site entrance, the butyl group of [BMIM]+ cations can easily diffuse into the active site of CALB; this could affect the binding between substrate molecules and active sites.
Other factors
Since hydrophobicity is not the only factor in controlling the hydrolase activity, complications arose in interpreting some biocatalytic reactions. De Diego et al234 conducted the transesterification of vinyl propionate and 1-butanol catalyzed by free and immobilized lipases from Candida antarctica (CALA and CALB), Thermomyces lanuginosus (TLL) and Rhizomuncor miehei (RML). Most of the enzyme preparations (except free CALA) showed higher activities in more hydrophobic [OMIM][PF6] than in [BMIM][PF6], but lower activities in other more hydrophobic based ILs ([OMIM][BF4] < [HMIM][BF4] < [BMIM][BF4], and [BDMIM][PF6] < [BDMIM][BF4]). Another study by Irimescu and Kato195 on the lipase-catalyzed acylation of primary amines indicated lower reaction rates in ILs with longer alkyl chains in cations, and the water miscibility of ILs was not a main factor in influencing the reaction rate. Some studies also obtained relatively high enzyme activities in hydrophilic ILs (such as [BMIM][BF4], [EMIM][BF4], [BMIM][OTf] and [MMIM][MeSO4]).163, 235–239 The Bruce group240 evaluated the activities of proteases (chymotrypsin and subtilisin) dissolved in several protic hydroxylalkylammonium-based ILs (containing ~1–2 wt% water), and found that subtilisin was only active in diethanolammonium chloride and chymotrypsin was inactive in these protic ILs. They further indicated that subtilisin retained its secondary and tertiary structures in diethanolammonium chloride as confirmed by the far and near UV CD spectra. Therefore, multiple factors must be considered when explaining the enzymatic systems like these.6
Amyloid fibrilization represents a process where the peptide assembles from monomers to oligomers and then into fibrils; this process is associated with the protein destabilization since the development of amyloid fibrils results from the formation of intramolecular H-bonds. The
Byrne group159 determined the rates of amyloid fibrilization of Aβ16–22 peptide in 90% (v/v) protic triethylammonium-based ILs, and found these rates decrease with IL anions in the order of HSO4−, H2PO4− > CF3COO− > lactate− > OTf− > CH3SO3−. The reverse of this series is the protein stabilization order, which is roughly a reverse Hofmeister series. The competitive H-bonding between the anion and water contributes to the self-assembly of Aβ16–22 peptide into amyloid fibrils; kosmotropic anions leads to faster amyloid fibrilization (“salt-in”) while chaotropic anions such as mesylate) suppress the formation of amyloid fibrilization (“salt-out”).
ION SPECIFICITY-GUIDED BIOCATALYTIC APPLICATIONS
The empirical ion specificity rules at different concentrations of ILs could provide some general guidance for designing/selecting enzyme-compatible ionic solvents. [EMIM][OAc] contains a chaotropic cation and kosmotropic anion, a unique combination for enzyme stabilization when it is used at low concentrations.43, 49, 119 Our group124 carried out the enzymatic chiral hydrolysis of amino acid esters catalyzed by Bacillus licheniformis protease in [EMIM][OAc] solutions, and obtained high enantioselectivities in up to 4.0 M [EMIM][OAc] despite lower yields of L-amino acid beyond 2.0 M IL concentrations. In addition, Wang et al.241 found that aqueous solutions of [BMIM][OAc] are highly compatible with cellulases. At first, they observed a high stability of a mixture of cellulases and β-glucosidase in [EMIM][OAc] solutions; after incubated in 15% and 20% (w/v) [EMIM][OAc] aqueous solutions at 50°C for 3 h, the enzyme mixture still retained 77% and 65% of its original activity respectively. In addition, the cellulase mixture exhibited a high activity in 15% [EMIM][OAc], leading to 91% conversion of Avicel® cellulose and up to 54% conversion of yellow poplar biomass into reducing sugars.
Bekhouche et al.242 suggested that [MMIM][Me2PO4] consists of a chaotropic cation and a kosmotropic anion, and found that formate dehydrogenase (FDH) from Candida boidinii maintained 76% of its activity in 20% (v/v) [MMIM][Me2PO4] (vs 100% activity in carbonate buffer). This group also indicated that FDH grafted with ILs (e.g. [Cholinium]Cl, [HO-EMIM]Cl and [HO-PrMIM]Cl) via covalent coupling exhibited a more tolerance to ionic media (such as in 70% (v/v) [MMIM][Me2PO4], the modified enzymes retained ca. 30–45% of their activity in aqueous buffer). They also concluded that more chaotropic grafted cation (such as cholinium) leads to a higher stabilizing effect on the enzyme in aqueous media. Thomas et al.156 found that xylanase and the arabinofurosidases maintained high or even enhanced hydrolytic activities in up to 20% (v/v) aqueous solutions of [MMIM][Me2PO4], [EMIM][Me2PO4] and [EMIM][OAc]. Yamamoto et al.243 evaluated aqueous solutions of N-alkylpyridinium chlorides and N-alkyl-N-methylpyrrolidinium chlorides for the refolding of denatured lysozyme, and found that less hydrophobic and chaotropic ILs (i.e. N-ethyl, N-butyl and N-hexylpyridinium chlorides, and N-butyl-N-methylpyrrolidinium chloride) could suppress protein aggregation and thus are effective for refolding the protein (46–69% yields). On the other hand, although more hydrophobic ILs such as N-octylpyridinium chloride and N-dodecylpyridinium chloride could fully prevent aggregation at lower concentrations, these salts interact directly with the protein via hydrophobic interactions and are not effective in improving refolding yields.
Hydroxyl- or ether-functionalized cations tend to be less hydrophobic and more chaotropic; as a result, such a modification often leads to more enzyme-compatible ILs. As shown in Fig 9, each of the Ammoeng family ILs is an ionic mixture containing multiple alkyloxy groups, which have both hydrophilic and hydrophobic properties like polyethylene glycols (PEGs). The Xu group244–249 judiciously selected a group of commercial tetraammonium-based ILs as reaction media for the enzymatic glycerolysis. In particular, Ammoeng 100 (also known as [CPMA][MeSO4]‡) and 102 are capable of dissolving triglycerides and have shown to be lipase-compatible during the glycerolysis reaction;245, 246 trioctylmethylammonium bis(trifluoromethylsulfonyl)imide ([TOMA][Tf2N]) and its mixture with Ammoeng 102 have also been evaluated as suitable solvents for the enzymatic glycerolysis.248–250 De Diego et al234 have further confirmed higher transesterification activities of both free and immobilized CALB in [CPMA][MeSO4] than in several PF6− and BF4− based ILs; however, the other two lipases from Thermomyces lanuginosus (TLL) and Rhizomuncor miehei (RML) seemed less active in [CPMA][MeSO4] than in PF6− and BF4− based ILs. Xu and co-workers245, 247 utilized the Conductor-like Screening Model for Real Solvents (COSMS-RS) to derive various parameters (such as misfit, H-bonding and van der Waals interaction energy) to understand the multiple interactions in ILs; the model also provides guidance in designing the structures of cations and anions.251 Similarly, the Kroutil group153 found that alcohol dehydrogenase is more active in hydroxyl-functionalized ILs than ordinary ILs, even at 50–90% (v/v) IL concentrations; the enzyme activity decreased in the order of [(HO-Et)3MeN][MeSO4] > Ammoeng 101 > Ammoeng 100 > Ammoeng 102. The Kragl group252 found an IL in the Ammoeng family - Ammoeng 110 (Fig 9d) –is quite effective in forming aqueous two-phase (ATP) for the purification of active enzymes (two different alcohol dehydrogenases); the IL is capable of stabilizing the enzymes and enhancing the solubility of hydrophobic substrates. It is interesting to mention that oxygen-containing ILs (such as Ammoeng series, and [C2OHmim]Cl) were used as additives in the enantioselective hydrolysis of diester malonates by pig liver esterase (PLE), and less than 1% of these ILs and 10% isopropanol/water were sufficient to improve the activity of PLE (up to four times) as well as the enantioselectivity.253
Fig. 9.
Structures of tetraammonium-based ILs (AmmoengTM series).
Based on the lyoprotectant effect of tris(hydroxymethyl)aminomethane (Tris) as excipient in horseradish peroxidase lyophilization,254 Das et al255 mimicked the structure of Tris and rationally designed a new IL known as tetrakis(2-hydroxyethyl)ammonium triflouromethanesulfonate (Fig 10); they reported that horseradish peroxidase in this new IL was 10 times more active than in methanol and at least 30–240-fold more active than in conventional ILs. Abe et al256 synthesized an alkyloxy-containing hydrophobic IL named 2-methoxyethyl(tri-n-butyl)phosphonium bis(trifluoromethane)sulfonamide ([MeOCH2CH2-Bu3P][Tf2N]), and observed a faster reaction rate (lipase PS-catalyzed transesterification of secondary alcohols) in this IL than in diisopropyl ether. Vafiadi et al257 employed two functionalized ILs [C2OHmim][PF6] and [C5O2mim][PF6] as solvents for the feruloyl esterase-catalyzed esterification of glycerol with sinapic acid, and achieved high conversion yields (72.5% and 76.7% respectively in two ILs under optimal conditions). These two ILs are considered as amphiphilic (hydrophilic cation and hydrophobic anion), and have relatively low viscosities.
Fig. 10.
Structure of tetrakis(2-hydroxyethyl)ammonium triflouromethanesulfonate.
Li et al.258 covalently attached ether-functionalized ILs (containing carboxylic acid group) to Candida rugosa lipase (CRL) by using the coupling reagent N,N′-carbodiimide (see Fig 11). The modified lipase showed improved catalytic activity, thermostability, organic solvent tolerance, and adaptability to temperature and pH changes in olive oil hydrolysis reaction. In particular, a higher CRL activity is associated with more kosmotropic anions of ILs (H2PO4− > Cl− > BF4−), and the use of a small glycol molecule (PEG 350 vs PEG 750) leads to a more active enzyme. The CD spectra suggest that the chemical modification by ILs resulted in an increase in β-sheet and a decrease in α-helix content of secondary structures of CRL.
Fig. 11.
Candida rugosa lipase (CRL) modified by covalent linkage to a glycol-functionalized IL (Reproduced with permission from Ref.258 Copyright (2015) American Chemical Society).
SUMMARY
Molecular level structures of ILs and their solutions are controlled by complex interactions of electrostatic attraction, H-bonds and dispersion forces depending on the concentration of ILs. Clearly, there is a need for more experimental and simulation studies to further visualize the microstructures of ILs and IL solutions. The interactions between proteins and IL solutions depend on some microscopic properties such as ion hydration, ion effect on protein hydration, and direct interactions between ions and proteins, and could be influenced by some macroscopic parameters such as viscosity B-coefficients of ions, H-bond basicity, and hydrophobicity. In diluted aqueous IL solutions, the ion specificity of many enzymatic systems is in line with the traditional Hofmeister series/kosmotropicity despite a number of exceptions, however, the specificity in concentrated or neat ILs is determined by H-bond basicity and nucelophilicity of anions, IL hydrophobicity and other factors. Due to the complex nature of many enzymatic systems, the specific ion effect may provide some empirical guidelines but not universal rules. Hopefully, these simple guidelines could lead to more custom design of enzyme-compatible ILs and biocatalytic systems.
Acknowledgments
The author acknowledges the supports by the Henry Dreyfus Teacher-Scholar Award (2012), NIH MBRS-RISE grant (1R25GM096956), NIH NIBIB contract award (HHSN268201200011C), and the National Natural Science Foundation of China (21328601).
NOTATION
IL Cations
- EMIM+
1-ethyl-3-methylimidazolium
- BMIM+
1-butyl-3-methylimidazolium
- BIM+
1-butylimidazolium
- BDMIM+
1-butyl-2,3-dimethylimidazolium
- PMIM+
1-methyl-3-propylimidazolium
- HMIM+
1-hexyl-3-methylimidazolium
- OMIM+
1-octyl-3-methylimidazolium
- ONIM+
1-nonyl-3-octylimidazolium
- C18MIM+
1-methyl-3-octadecylimidazolium
- BMPip+
1-butyl-1-methylpiperidinium
- BMPyrr+
1-butyl-1-methylpyrrolidinium
- EtPy+
1-ethylpyridinium
- BuPy+
1-butylpyridinium
- Me3NPr+
N,N,N-trimethyl-N-propylammonium
IL Anions
- BF4−
tetrafluoroborate
- PF6−
hexafluorophosphate
- OAc−
acetate
- Tf2N−
bis(trifluoromethane)sulfonamide, (CF3SO2)2N−
- beti–
bis(perfluoroethylsulfonyl)imide, (C2F5SO2)2N−
- OTf−
triflate (i.e. trifluoromethanesulfonate)
- dca−
dicyanamide
- MeSO4−
methyl sulfate
- EtSO4−
ethyl sulfate
- OTs−
tosylate
Footnotes
Basicity refers to the ability of a base to accept a proton, and is a matter of equilibrium. Nucleophilicity of a Lewis base refers to the relative reaction rate of different nucleophilic reagents towards a common substrate, most usually involving the formation of a bond to carbon; nucleophilicity is a matter of kinetics (rate).
From the name of cocosalkyl pentaethoxy methylammonium methylsulfate.
References
- 1.van Rantwijk F, Sheldon RA. Biocatalysis in ionic liquids. Chem Rev. 2007;107:2757–2785. doi: 10.1021/cr050946x. [DOI] [PubMed] [Google Scholar]
- 2.Moniruzzaman M, Nakashima K, Kamiya N, Goto M. Recent advances of enzymatic reactions in ionic liquids. Biochem Eng J. 2010;48:295–314. [Google Scholar]
- 3.Domínguez de María P, editor. Ionic Liquids in Biotransformations and Organocatalysis: Solvents and Beyond. Wiley; New York: 2012. [Google Scholar]
- 4.Yang Z, Pan W. Ionic liquids: Green solvents for nonaqueous biocatalysis. Enzyme Microbial Technol. 2005;37:19–28. [Google Scholar]
- 5.Zhao H. Methods for stabilizing and activating enzymes in ionic liquids — A review. J Chem Tech Biotechnol. 2010;85:891–907. [Google Scholar]
- 6.Moniruzzaman M, Kamiya N, Goto M. Activation and stabilization of enzymes in ionic liquids. Org Biomol Chem. 2010;8:2887–2899. doi: 10.1039/b926130c. [DOI] [PubMed] [Google Scholar]
- 7.Zhao H. Ionic liquids as (co-)solvents for hydrolytic enzymes. In: Dominguez de Maria P, editor. Ionic Liquids in Biotransformations & Organocatalysis: Solvents and Beyond. John Wiley & Sons; New York: 2012. [Google Scholar]
- 8.Weingärtner H, Cabrele C, Herrmann C. How ionic liquids can help to stabilize native proteins. Phys Chem Chem Phys. 2012;14:415–426. doi: 10.1039/c1cp21947b. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z. Hofmeister effects: an explanation for the impact of ionic liquids on biocatalysis. J Biotechnol. 2009;144:12–22. doi: 10.1016/j.jbiotec.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Hofmeister F. Zur Lehre von der Wirkung der Salze. Arch Exp Pathol Pharmakol. 1888;24:247–260. [Google Scholar]
- 11.Kunz W, Henle J, Ninham BW. ‘Zur Lehre von der Wirkung der Salze’ (about the science of the effect of salts: Franz Hofmeister’s historical papers) Curr Opin Colloid Interface Sci. 2004;9:19–37. [Google Scholar]
- 12.Kunz W, Lo Nostro P, Ninham BW. The present state of affairs with Hofmeister effects. Curr Opin Colloid Interface Sci. 2004;9:1–18. [Google Scholar]
- 13.Pinna MC, Salis A, Monduzzi M, Ninham BW. Hofmeister series: the hydrolytic activity of Aspergillus niger lipase depends on specific anion effects. J Phys Chem B. 2005;109:5406–5408. doi: 10.1021/jp050574w. [DOI] [PubMed] [Google Scholar]
- 14.Bauduin P, Renoncourt A, Touraud D, Kunz W, Ninham BW. Hofmeister effect on enzymatic catalysis and colloidal structures. Curr Opin Colloid Interface Sci. 2004;9:43–47. [Google Scholar]
- 15.Gokarn YR, Fesinmeyer RM, AS, Razinkov V, Chase SF, Laue TM, Brems DN. Effective charge measurements reveal selective and preferential accumulation of anions, but not cations, at the protein surface in dilute salt solutions. Protein Sci. 2011;20:580–587. doi: 10.1002/pro.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao H. Effect of ions and other compatible solutes on enzyme activity, and its implication for biocatalysis using ionic liquids. J Mol Catal B: Enzym. 2005;37:16–25. [Google Scholar]
- 17.Zhang Y, Cremer PS. The inverse and direct Hofmeister series for lysozyme. Proc Natd Acad Sci USA. 2009;106:15249–15253. doi: 10.1073/pnas.0907616106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bye JW, Falconer RJ. Thermal stability of lysozyme as a function of ion concentration: A reappraisal of the relationship between the Hofmeister series and protein stability. Protein Sci. 2013;22:1563–1570. doi: 10.1002/pro.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldwin RL. How Hofmeister ion interactions affect protein stability. Biophys J. 1996;71:2056–2063. doi: 10.1016/S0006-3495(96)79404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arakawa T, Timasheff SN. Mechanism of protein salting in and salting out by divalent cation salts: balance between hydration and salt binding. Biochem. 1984;23:5912–5923. doi: 10.1021/bi00320a004. [DOI] [PubMed] [Google Scholar]
- 21.Collins KD. Ions from the Hofmeister series and osmolytes: effects on proteins in solution and in the crystallization process. Methods. 2004;34:300–311. doi: 10.1016/j.ymeth.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Collins KD, Washabaugh MW. The Hofmeister effect and the behaviour of water at interfaces. Quart Rev Biophys. 1985;18:323–422. doi: 10.1017/s0033583500005369. [DOI] [PubMed] [Google Scholar]
- 23.Frank HS, Wen W-Y. Ion-solvent interaction. Structural aspects of ion-solvent interaction in aqueous solutions: a suggested picture of water structure. Discuss Faraday Soc. 1957;24:133–140. [Google Scholar]
- 24.Franks F. Water, a Comprehensive Treatise. Volume 3. Aqueous Solutions of Simple Electrolytes. Plenum Press; New York: 1973. [Google Scholar]
- 25.von Hippel PH, Wong K-Y. On the conformational stability of globular proteins. J Biol Chem. 1965;240:3909–3923. [PubMed] [Google Scholar]
- 26.Wiggins PM. Hydrophobic hydration, hydrophobic forces and protein folding. Physica A. 1997;238:113–128. [Google Scholar]
- 27.Tanford C. The hydrophobic effect and the organization of living matter. Sci. 1978;200:1012–1018. doi: 10.1126/science.653353. [DOI] [PubMed] [Google Scholar]
- 28.Némethy G. Hydrophobic interactions. Angew Chem Int Ed Engl. 1967;6:195–206. doi: 10.1002/anie.196701951. [DOI] [PubMed] [Google Scholar]
- 29.Minton AP. Excluded volume as a determinant of macromolecular structure and reactivity. Biopolymers. 1981;20:2093–2120. [Google Scholar]
- 30.Davis-Searles PR, Saunders AJ, Erie DA, Winzor DJ, Pielak GJ. Interpreting the effects of small uncharged solutes on protein-folding equilibria. Annu Rev Biophys Biomol Struct. 2001;30:271–306. doi: 10.1146/annurev.biophys.30.1.271. [DOI] [PubMed] [Google Scholar]
- 31.Schellman JA. Protein stability in mixed solvents: A balance of contact interaction and excluded volume. Biophys J. 2003;85:108–125. doi: 10.1016/S0006-3495(03)74459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson DR, Jencks WP. The effect of compounds of the urea-guanidinium class on the activity coefficient of acetyltetraglycine ethyl ester and related compounds. J Am Chem Soc. 1965;87:2462–2470. doi: 10.1021/ja01089a028. [DOI] [PubMed] [Google Scholar]
- 33.Courtenay ES, Capp MW, Record MTJ. Thermodynamics of interactions of urea and guanidinium salts with protein surface: Relationship between solute effects on protein processes and changes in water-accessible surface area. Protein Sci. 2001;10:2485–2497. doi: 10.1110/ps.ps.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers JK, Pace CN, Scholtz JM. Denaturant m values and heat capacity changes: Relation to changes in accessible surface areas of protein unfolding. Protein Sci. 1995;4:2138–2148. doi: 10.1002/pro.5560041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honig B, Nicholls A. Classical electrostatics in biology and chemistry. Sci. 1995;268:1144–1149. doi: 10.1126/science.7761829. [DOI] [PubMed] [Google Scholar]
- 36.Perutz MF. Electrostatic effects in proteins. Sci. 1978;201:1187–1191. doi: 10.1126/science.694508. [DOI] [PubMed] [Google Scholar]
- 37.Boström M, Williams DRM, Ninham BW. Why the properties of proteins in salt solutions follow a Hofmeister series. Curr Opin Colloid Interface Sci. 2004;9:48–52. [Google Scholar]
- 38.Kunz W, Belloni L, Bernard O, Ninham BW. Osmotic coefficients and surface tensions of aqueous electrolyte solutions: Role of dispersion forces. J Phys Chem B. 2004;108:2398–2404. [Google Scholar]
- 39.von Hippel PH, Schleich T. Ion effects on the solution structure of biological macromolecules. Accounts Chem Res. 1969;2:257–265. [Google Scholar]
- 40.Jenkins HDB, Marcus Y. Viscosity B-coefficients of ions in solution. Chem Rev. 1995;95:2695–2724. [Google Scholar]
- 41.Tamaki K, Ohara Y, Isomura Y. Viscosity B coefficients for some alkyl sulfates in aqueous solutions. Bull Chem Soc Jpn. 1973;46:1551–1552. [Google Scholar]
- 42.Zhao H, Song Z. Nuclear magnetic relaxation of water in ionic-liquid solutions: Determining the kosmotropicity of ionic liquids and its relationship with the enzyme enantioselectivity. J Chem Tech Biotechnol. 2007;82:304–312. [Google Scholar]
- 43.Zhao H, Campbell S, Jackson L, Song Z, Olubajo O. Hofmeister series of ionic liquids: kosmotropic effect of ionic liquids on the enzymatic hydrolysis of enantiomeric phenylalanine methyl ester. Tetrahedron: Asymmetry. 2006;17:377–383. [Google Scholar]
- 44.Constantinescu D, Weingärtner H, Herrmann C. Protein denaturation by ionic liquids and the Hofmeister series: A case study of aqueous solutions of ribonuclease A. Angew Chem Int Ed. 2007;46:8887–8889. doi: 10.1002/anie.200702295. [DOI] [PubMed] [Google Scholar]
- 45.Fujita K, MacFarlane DR, Forsyth M, Yoshizawa-Fujita M, Murata K, Nakamura N, Ohno H. Solubility and stability of cytochrome c in hydrated ionic liquids: effect of oxo acid residues and kosmotropicity. Biomacromolecules. 2007;8:2080–2086. doi: 10.1021/bm070041o. [DOI] [PubMed] [Google Scholar]
- 46.Yang Z, Yue Y-J, Huang W-C, Zhuang X-M, Chen Z-T, Xing M. Importance of the ionic nature of ionic liquids in affecting enzyme performance. J Biochem. 2009;145:355–364. doi: 10.1093/jb/mvn173. [DOI] [PubMed] [Google Scholar]
- 47.Samoilov OY. A new approach to the study of hydration of ions in aqueous solutions. Discuss Faraday Soc. 1957;24:141–146. [Google Scholar]
- 48.Samoilov OY. Hydration of ions in aqueous solution. Bull Acad Sci USSR, Div Chem Sci. 1953;2:219–225. [Google Scholar]
- 49.Zhao H. Are ionic liquids kosmotropic or chaotropic? An evaluation of available thermodynamic parameters for quantifying the ion kosmotropicity of ionic liquids. J Chem Technol Biotechnol. 2006;81:877–891. [Google Scholar]
- 50.Jones G, Dole M. The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J Am Chem Soc. 1929;51:2950–2964. [Google Scholar]
- 51.Ali A, Hyder S, Akhtar Y. Viscometric studies of α-amino acid in aqueous NaCl and MgCl2 at 303 K. Indian J Phys. 2005;79:157–160. [Google Scholar]
- 52.Belibagli KB, Ayranci E. Viscosities and apparent molar volumes of some amino acids in water and in 6 M guanidine hydrochloride at 25 °C. J Solution Chem. 1990;19:867–882. [Google Scholar]
- 53.Collins KD. Charge density-dependent strength of hydration and biological structure. Biophys Chem. 1997;72:65–76. doi: 10.1016/S0006-3495(97)78647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marcus Y. Viscosity B-coefficients, structural entropies and heat capacities, and the effects of ions on the structure of water. J Solution Chem. 1994;23:831–848. [Google Scholar]
- 55.Kay RL, Vituccio T, Zawoyski C, Evans DF. Viscosity B coefficients for the tetraalkylammonium halides. J Phys Chem. 1966;70:2336–2341. [Google Scholar]
- 56.Frank HS, Evans MW. Free volume and entropy in condensed systems III Entropy in binary liquid mixtures; partial molal entropy in dilute solutions; structure and thermodynamics in aqueous electrolytes. J Chem Phys. 1945;13:507–532. [Google Scholar]
- 57.Nightingale ER. Viscosity of aqueous solutions. III Tetramethylammonium bromide and the role of the tetraalkylammonium ions. J Phys Chem. 1962;66:894–897. [Google Scholar]
- 58.Frank HS. Single ion activities and ion-solvent interaction in dilute aqueous solutions. J Phys Chem. 1963;67:1554–1558. [Google Scholar]
- 59.Sarma TS, Ahluwalia JC. Experimental studies on the structure of aqueous solutions of hydrophobic solutes. Chem Soc Rev. 1973;2:203–232. [Google Scholar]
- 60.Tsangaris JM, Martin RB. Viscosities of aqueous solutions of dipolar ions. Arch Biochem Biophys. 1965;112:267–272. [Google Scholar]
- 61.Devine W, Lowe BM. Viscosity B-coefficients at 15 and 25 °C for glycine, β-alanine, 4-amino-n-butyric acid, and 6-amino-n-hexanoic acid in aqueous solution. J Chem Soc A: Inorg Phys Theor. 1971:2113–2116. [Google Scholar]
- 62.Tobias DJ, Hemminger JC. Getting specific about specific ion effects. Science. 2008;319:1197–1198. doi: 10.1126/science.1152799. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Cremer PS. Interactions between macromolecules and ions: the Hofmeister series. Curr Opin Chem Biol. 2006;10:658–663. doi: 10.1016/j.cbpa.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 64.Leberman R, Soper AK. Effect of high salt concentration on water structure. Nature. 1995;378:364–366. doi: 10.1038/378364a0. [DOI] [PubMed] [Google Scholar]
- 65.Nucci NV, Vanderkooi JM. Effects of salts of the Hofmeister series on the hydrogen bond network of water. J Mol Liq. 2008;143:160–170. doi: 10.1016/j.molliq.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas AS, Elcock AH. Molecular dynamics simulations of hydrophobic associations in aqueous salt solutions indicate a connection between water hydrogen bonding and the Hofmeister effect. J Am Chem Soc. 2007;129:14887–14898. doi: 10.1021/ja073097z. [DOI] [PubMed] [Google Scholar]
- 67.Cappa CD, Smith JD, Wilson KR, Messer BM, Gilles MK, Cohen RC, Saykally RJ. Effects of alkali metal halide salts on the hydrogen bond network of liquid water. J Phys Chem B. 2005;109:7046–7052. doi: 10.1021/jp0445324. [DOI] [PubMed] [Google Scholar]
- 68.Cappa CD, Smith JD, Messer BM, Cohen RC, Saykally RJ. Effects of cations on the hydrogen bond network of liquid water: New results from x-ray absorption spectroscopy of liquid microjets. J Phys Chem B. 2006;110:5301–5309. doi: 10.1021/jp054699c. [DOI] [PubMed] [Google Scholar]
- 69.Krekeler C, Delle Site L. Solvation of positive ions in water: the dominant role of water–water interaction. J Phys: Condens Matter. 2007;19:192101. [Google Scholar]
- 70.Omta AW, Kropman MF, Woutersen S, Bakker HJ. Negligible effect of ions on the hydrogen-bond structure in liquid water. Science. 2003;301:347–349. doi: 10.1126/science.1084801. [DOI] [PubMed] [Google Scholar]
- 71.Omta AW, Kropman MF, Woutersen S, Bakker HJ. Influence of ions on the hydrogen-bond structure in liquid water. J Chem Phys. 2003;119:12457–12461. doi: 10.1126/science.1084801. [DOI] [PubMed] [Google Scholar]
- 72.Collins KD, Neilson GW, Enderby JE. Ions in water: Characterizing the forces that control chemical processes and biological structure. Biophys Chem. 2007;128:95–104. doi: 10.1016/j.bpc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Ball P. Water as a biomolecule. ChemPhysChem. 2008;9:2677–2685. doi: 10.1002/cphc.200800515. [DOI] [PubMed] [Google Scholar]
- 74.Schiffer CA, Dotsch V. The role of protein-solvent interactions in protein unfolding. Curr Opin Biotechnol. 1996;7:428–432. doi: 10.1016/s0958-1669(96)80119-4. [DOI] [PubMed] [Google Scholar]
- 75.Hou M, Lu R, Yu A. Polarizability series of aqueous polyatomic anions revealed by femtosecond Kerr effect spectroscopy. RSC Adv. 2014;4:23078–23083. [Google Scholar]
- 76.Zhang F, Roosen-Runge F, Skoda MWA, Jacobs RMJ, Wolf M, Callow P, Frielinghaus H, Pipich V, Prévost S, Schreiber F. Hydration and interactions in protein solutions containing concentrated electrolytes studied by small-angle scattering. Phys Chem Chem Phys. 2012;14:2483–2493. doi: 10.1039/c2cp23460b. [DOI] [PubMed] [Google Scholar]
- 77.Chen X, Flores SC, Lim S-M, Zhang Y, Yang T, Kherb J, Cremer PS. Specific anion effects on water structure adjacent to protein monolayers. Langmuir. 2010;26:16447–16454. doi: 10.1021/la1015862. [DOI] [PubMed] [Google Scholar]
- 78.Sedlák E, Stagg L, Wittung-Stafshede P. Effect of Hofmeister ions on protein thermal stability: Roles of ion hydration and peptide groups? Arch Biochem Biophys. 2008;479:69–73. doi: 10.1016/j.abb.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 79.Heyda J, Vincent JC, Tobias DJ, Dzubiella J, Jungwirth P. Ion specificity at the peptide bond: Molecular dynamics simulations of N-methylacetamide in aqueous salt solutions. J Phys Chem B. 2010;114:1213–1220. doi: 10.1021/jp910953w. [DOI] [PubMed] [Google Scholar]
- 80.Rembert KB, Paterová J, Heyda J, Hilty C, Jungwirth P, Cremer PS. Molecular mechanisms of ion-specific effects on proteins. J Am Chem Soc. 2012;134:10039–10046. doi: 10.1021/ja301297g. [DOI] [PubMed] [Google Scholar]
- 81.Gibb CLD, Gibb BC. Anion binding to hydrophobic concavity is central to the salting-in effects of Hofmeister chaotropes. J Am Chem Soc. 2011;133:7344–7347. doi: 10.1021/ja202308n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paterová J, Rembert KB, Heyda J, Kurra Y, Okur HI, Liu WR, Hilty C, Cremer PS, Jungwirth P. Reversal of the Hofmeister series: Specific ion effects on peptides. J Phys Chem B. 2013;117:8150–8158. doi: 10.1021/jp405683s. [DOI] [PubMed] [Google Scholar]
- 83.Schwierz N, Horinek D, Netz RR. Reversed anionic Hofmeister series: The interplay of surface charge and surface polarity. Langmuir. 2010;26:7370–7379. doi: 10.1021/la904397v. [DOI] [PubMed] [Google Scholar]
- 84.Pegram LM, Record MT., Jr Partitioning of atmospherically relevant ions between bulk water and the water/vapor interface. Proc Natd Acad Sci USA. 2006;103:14278–14281. doi: 10.1073/pnas.0606256103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pegram LM, Record MT., Jr Hofmeister salt effects on surface tension arise from partitioning of anions and cations between bulk water and the air–water interface. J Phys Chem B. 2007;111:5411–5417. doi: 10.1021/jp070245z. [DOI] [PubMed] [Google Scholar]
- 86.Dér A, Kelemen L, Fábián L, Taneva SG, Fodor E, Páli T, Cupane A, Cacace MG, Ramsden JJ. Interfacial water structure controls protein conformation. J Phys Chem B. 2007;111:5344–5350. doi: 10.1021/jp066206p. [DOI] [PubMed] [Google Scholar]
- 87.Szalontai B, Nagy G, Krumova S, Fodor E, Páli T, Taneva SG, Garab G, Peters J, Dér A. Hofmeister ions control protein dynamics. Biochim Biophys Acta - Gen Sub. 2013;1830:4564–4572. doi: 10.1016/j.bbagen.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 88.Friedman R. Electrolyte solutions and specific ion effects on interfaces. J Chem Educ. 2013;90:1018–1023. [Google Scholar]
- 89.Stassen HK, Ludwig R, Wulf A, Dupont J. Imidazolium salt ion pairs in solution. Chem Eur J. 2015;21:8324–8335. doi: 10.1002/chem.201500239. [DOI] [PubMed] [Google Scholar]
- 90.Schröder U, Wadhawan JD, Compton RG, Marken F, Suarez PAZ, Consorti CS, de Souza RF, Dupont J. Water-induced accelerated ion diffusion: voltammetric studies in 1-methyl-3-[2,6-(S)-dimethylocten-2-yl]imidazolium tetrafluoroborate, 1-butyl-3-methylimidazolium tetrafluoroborate and hexafluorophosphate ionic liquids. New J Chem. 2000;24:1009–1015. [Google Scholar]
- 91.Dupont J. On the solid, liquid and solution structural organization of imidazolium ionic liquids. J Braz Chem Soc. 2004;15:341–350. [Google Scholar]
- 92.Watanabe H, Doi H, Saito S, Matsugami M, Fujii K, Kanzaki R, Kameda Y, Umebayashi Y. Hydrogen bond in imidazolium based protic and aprotic ionic liquids. J Mol Liq. 2015 doi: 10.1016/j.molliq.2015.08.005. [DOI] [Google Scholar]
- 93.Knorr A, Fumino K, Bonsa A-M, Ludwig R. Spectroscopic evidence of ‘jumping and pecking’ of cholinium and H-bond enhanced cation–cation interaction in ionic liquids. Phys Chem Chem Phys. 2015 doi: 10.1039/c5cp03412d. [DOI] [PubMed] [Google Scholar]
- 94.Smirnova NA, Safonova EA. Micellization in solutions of ionic liquids. Colloid J. 2012;74:254–265. [Google Scholar]
- 95.Wang J, Wang H. Aggregation in systems of ionic liquids. In: Zhang S, Wang J, Lu X, Zhou Q, editors. Structures and Interactions of Ionic Liquids. Springer; Berlin Heidelberg, New York: 2014. pp. 39–77. [Google Scholar]
- 96.Feng S, Voth GA. Molecular dynamics simulations of imidazolium-based ionic liquid/water mixtures: Alkyl side chain length and anion effects. Fluid Phase Equilib. 2010;294:148–156. [Google Scholar]
- 97.Greaves TL, Kennedy DF, Weerawardena A, Tse NMK, Kirby N, Drummond CJ. Nanostructured protic ionic liquids retain nanoscale features in aqueous solution while precursor Brønsted acids and bases exhibit different behavior. J Phys Chem B. 2011;115:2055–2066. doi: 10.1021/jp1112203. [DOI] [PubMed] [Google Scholar]
- 98.Azadbakht R, Sohrabi B, Sharifi A. The ionic liquids counterion effect on their aggregation behavior. Proc 17th Int Electron Conf Synth Org Chem. 2013;17:f005. [Google Scholar]
- 99.Inoue T, Misono T. Cloud point phenomena for POE-type nonionic surfactants in imidazolium-based ionic liquids: Effect of anion species of ionic liquids on the cloud point. J Colloid Interface Sci. 2009;337:247–253. doi: 10.1016/j.jcis.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 100.Wen W-Y. Aqueous solutions of symmetrical tetraalkylammonium salts. In: Horne RA, editor. Water and Aqueous Solutions. Wiley Interscience; New York: 1972. pp. 613–661. [Google Scholar]
- 101.Yaminsky VV, Vogler EA. Hydrophobic hydration. Curr Opin Colloid Interface Sci. 2001;6:342–349. [Google Scholar]
- 102.Widom B, Bhimalapuram P, Koga K. The hydrophobic effect. Phys Chem Chem Phys. 2003;5:3085–3093. [Google Scholar]
- 103.Zhao H. Erratum: are ionic liquids kosmotropic or chaotropic? An evaluation of available thermodynamic parameters for quantifying the ion kosmotropicity of ionic liquids. J Chem Technol Biotechnol. 2006;81:1723. [Google Scholar]
- 104.von Hippel PH, Schleich T. The effects of neutral salts on the structure and conformational stability of macromolecules in solution. In: Timasheff SN, Fasman GD, editors. Structure and Stability of Biological Macromolecules. Marcel Dekker, Inc; New York: 1969. pp. 417–574. [Google Scholar]
- 105.Bridges NJ, Gutowski KE, Rogers RD. Investigation of aqueous biphasic systems formed from solutions of chaotropic salts with kosmotropic salts (salt–salt ABS) Green Chem. 2007;9:177–183. [Google Scholar]
- 106.Lynden-Bell RM, Atamas NA, Vasilyuk A, Hanke CG. Chemical potentials of water and organic solutes in imidazolium ionic liquids: a simulation study. Mol Phys. 2002;100:3225–3229. [Google Scholar]
- 107.Mele A, Tran CD, De Paoli Lacerda SH. The structure of a room-temperature ionic liquid with and without trace amounts of water: The role of C-H···O and C-H···F interactions in 1-n-butyl-3-methylimidazolium tetrafluoroborate. Angew Chem Int Ed. 2003;42:4364–4366. doi: 10.1002/anie.200351783. [DOI] [PubMed] [Google Scholar]
- 108.Miki K, Westh P, Nishikawa K, Koga K. Effect of an “ionic liquid” cation, 1-butyl-3-methylimidazolium, on the molecular organization of H2O. J Phys Chem B. 2005;109:9014–9019. doi: 10.1021/jp046309c. [DOI] [PubMed] [Google Scholar]
- 109.Xu Y, Gao Y, Zhang L, Yao J, Wang C, Li H. Microscopic structures of ionic liquids 1-ethyl-3-methylimidazolium tetrafluoroborate in water probed by the relative chemical shift. Sci China Chem. 2010;53:1561–1565. [Google Scholar]
- 110.Singh T, Kumar A. Cation–anion–water interactions in aqueous mixtures of imidazolium based ionic liquids. Vib Spectrosc. 2011;55:119–125. [Google Scholar]
- 111.Chen Y, Cao Y, Sun X, Mu T. Hydrogen bonding interaction between acetate-based ionic liquid 1-ethyl-3-methylimidazolium acetate and common solvents. J Mol Liq. 2014;190:151–158. [Google Scholar]
- 112.Zhang Q, Wang N, Yu Z. The hydrogen bonding interactions between the ionic liquid 1-ethyl-3-methylimidazolium ethyl sulfate and water. J Phys Chem B. 2010;114:4747–4754. doi: 10.1021/jp1009498. [DOI] [PubMed] [Google Scholar]
- 113.Bernardes CES, Minas da Piedade ME, Canongia Lopes JN. The structure of aqueous solutions of a hydrophilic ionic liquid: The full concentration range of 1-ethyl-3-methylimidazolium ethylsulfate and water. J Phys Chem B. 2011;115:2067–2074. doi: 10.1021/jp1113202. [DOI] [PubMed] [Google Scholar]
- 114.Danten Y, Cabaço MI, Besnard M. Interaction of water diluted in 1-butyl-3-methyl imidazolium ionic liquids by vibrational spectroscopy modeling. J Mol Liq. 2010;153:57–66. [Google Scholar]
- 115.Ficke LE, Brennecke JF. Interactions of ionic liquids and water. J Phys Chem B. 2010;114:10496–10501. doi: 10.1021/jp1012736. [DOI] [PubMed] [Google Scholar]
- 116.Fadeeva TA, Husson P, DeVine JA, Gomes MFC, Greenbaum SG, Castner EWJ. Interactions between water and 1-butyl-1-methylpyrrolidinium ionic liquids. J Chem Phys. 2015;143:064503. doi: 10.1063/1.4928065. [DOI] [PubMed] [Google Scholar]
- 117.Fehér E, Major B, Bélafi-Bakó K, Gubicza L. On the background of enhanced stability and reusability of enzymes in ionic liquids. Biochem Soc Trans. 2007;35:1624–1627. doi: 10.1042/BST0351624. [DOI] [PubMed] [Google Scholar]
- 118.Lozano P, De Diego T, Gmouh S, Vaultier M, Iborra JL. Dynamic structure–function relationships in enzyme stabilization by ionic liquids. Biocatalysis Biotransform. 2005;23:169–176. [Google Scholar]
- 119.Zhao H, Olubajo O, Song Z, Sims AL, Person TE, Lawal RA, Holley LA. Effect of kosmotropicity of ionic liquids on the enzyme stability in aqueous solutions. Bioorg Chem. 2006;34:15–25. doi: 10.1016/j.bioorg.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 120.Zhao H, Jackson L, Song Z, Olubajo O. Enhancing protease enantioselectivity by ionic liquids based on chiral- or ω-amino acids. Tetrahedron: Asymmetry. 2006;17:1549–1553. [Google Scholar]
- 121.Zhao H. Viscosity B-coefficients and standard partial molar volumes of amino acids, and their roles in interpreting the protein (enzyme) stabilization. Biophys Chem. 2006;122:157–183. doi: 10.1016/j.bpc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 122.Wiggins PM. Methods for the separation of isomers. 6638360. US Patent. 2003
- 123.Zhao H, Campbell S, Solomon J, Song Z, Olubajo O. Improving the enzyme catalytic efficiency using ionic liquids with kosmotropic anions. Chinese J Chem. 2006;24:580–584. [Google Scholar]
- 124.Zhao H, Jackson L, Song Z, Olubajo O. Using ionic liquid [EMIM][CH3COO] as an enzyme-’friendly’ co-solvent for resolution of amino acids. Tetrahedron: Asymmetry. 2006;17:2491–2498. [Google Scholar]
- 125.Husum TL, Jorgensen CT, Christensen MW, Kirk O. Enzyme catalysed synthesis in ambient temperature ionic liquids. Biocatalysis Biotransform. 2001;19:331–338. [Google Scholar]
- 126.Kaftzik N, Wasserscheid P, Kragl U. Use of ionic liquids to increase the yield and enzyme stability in the β-galactosidase catalysed synthesis of N-acetyllactosamine. Org Proc Res Dev. 2002;6:553–557. [Google Scholar]
- 127.Lang M, Kamrat T, Nidetzky B. Influence of ionic liquid cosolvent on transgalactosylation reactions catalyzed by thermostable β-glycosylhydrolase CelB from Pyrococcus furiosus. Biotechnol Bioeng. 2006;95:1093–1100. doi: 10.1002/bit.21068. [DOI] [PubMed] [Google Scholar]
- 128.Fujita K, MacFarlane DR, Forsyth M. Protein solubilising and stabilising ionic liquids. Chem Commun. 2005:4804–4806. doi: 10.1039/b508238b. [DOI] [PubMed] [Google Scholar]
- 129.Fujita K, Forsyth M, MacFarlane DR, Reid RW, Elliott GD. Unexpected improvement in stability and utility of cytochrome c by solution in biocompatible ionic liquids. Biotechnol Bioeng. 2006;94:1209–1213. doi: 10.1002/bit.20928. [DOI] [PubMed] [Google Scholar]
- 130.Tanaka H, Oka Y. Chaotropic ions and multivalent ions activate sperm in the viviparous fish guppy Poecilia reticulata. Biochim Biophys Acta, Gen Subj. 2005;1724:173–180. doi: 10.1016/j.bbagen.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 131.Fujita K, Ohno H. Enzymatic activity and thermal stability of metallo proteins in hydrated ionic liquids. Biopolymers. 2010;93:1093–1099. doi: 10.1002/bip.21526. [DOI] [PubMed] [Google Scholar]
- 132.Constatinescu D, Herrmann C, Weingärtner H. Patterns of protein unfolding and protein aggregation in ionic liquids. Phys Chem Chem Phys. 2010;12:1756–1763. doi: 10.1039/b921037g. [DOI] [PubMed] [Google Scholar]
- 133.Yang Z, Liu X-J, Chen C, Halling PJ. Hofmeister effects on activity and stability of alkaline phosphatase. Biochim Biophys Acta. 2010;1804:821–828. doi: 10.1016/j.bbapap.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 134.Akdogan Y, Junk MJN, Hinderberger D. Effect of ionic liquids on the solution structure of human serum albumin. Biomacromolecules. 2011;12:1072–1079. doi: 10.1021/bm1014156. [DOI] [PubMed] [Google Scholar]
- 135.Venkatesu P, Lee M-J, Lin H. Osmolyte counteracts urea-induced denaturation of α-chymotrypsin. J Phys Chem B. 2009;113:5327–5338. doi: 10.1021/jp8113013. [DOI] [PubMed] [Google Scholar]
- 136.Attri P, Venkatesu P, Kumar A, Byrne N. A protic ionic liquid attenuates the deleterious actions of urea on α-chymotrypsin. Phys Chem Chem Phys. 2011;13:17023–17026. doi: 10.1039/c1cp22195g. [DOI] [PubMed] [Google Scholar]
- 137.Lai J-Q, Li Z, Lü Y-H, Yang Z. Specific ion effects of ionic liquids on enzyme activity and stability. Green Chem. 2011;13:1860–1868. [Google Scholar]
- 138.Attri P, Venkatesu P. Thermodynamic characterization of the biocompatible ionic liquid effects on protein model compounds and their functional groups. Phys Chem Chem Phys. 2011;13:6566–6575. doi: 10.1039/c0cp02768e. [DOI] [PubMed] [Google Scholar]
- 139.Lu L, Hu Y, Huang X, Qu Y. A bioelectrochemical method for the quantitative description of the Hofmeister effect of ionic liquids in aqueous solution. J Phys Chem B. 2012;116:11075–11080. doi: 10.1021/jp3054263. [DOI] [PubMed] [Google Scholar]
- 140.Weibels S, Syguda A, Herrmann C, Weingärtner H. Steering the enzymatic activity of proteins by ionic liquids. A case study of the enzyme kinetics of yeast alcohol dehydrogenase. Phys Chem Chem Phys. 2012;14:4635–4639. doi: 10.1039/c2cp24041f. [DOI] [PubMed] [Google Scholar]
- 141.Yan H, Wu J, Dai G, Zhong A, Chen H, Yang J, Han D. Interaction mechanisms of ionic liquids [Cnmim]Br (n=4, 6, 8, 10) with bovine serum albumin. J Lumin. 2012;132:622–628. [Google Scholar]
- 142.Turner MB, Spear SK, Huddleston JG, Holbrey JD, Rogers RD. Ionic liquid salt-induced inactivation and unfolding of cellulase from Trichoderma reesei. Green Chem. 2003;5:443–447. [Google Scholar]
- 143.Curto VF, Scheuermann S, Owens RM, Ranganathan V, MacFarlane DR, Benito-Lopez F, Diamond D. Probing the specific ion effects of biocompatible hydrated choline ionic liquids on lactate oxidase biofunctionality in sensor applications. Phys Chem Chem Phys. 2014;16:1841–1849. doi: 10.1039/c3cp52845f. [DOI] [PubMed] [Google Scholar]
- 144.Baker SN, Zhao H, Pandey S, Heller WT, Bright FV, Baker GA. Fluorescence energy transfer efficiency in labeled yeast cytochrome c: A rapid screen for ion biocompatibility in aqueous ionic liquids. Phys Chem Chem Phys. 2011;13:3642–3644. doi: 10.1039/c0cp02345k. [DOI] [PubMed] [Google Scholar]
- 145.Kumar A, Venkatesu P. A comparative study of myoglobin stability in the presence of Hofmeister anions of ionic liquids and ionic salts. Process Biochem. 2014;49:2158–2169. [Google Scholar]
- 146.Kumar A, Rani A, Venkatesu P. A comparative study of the effects of the Hofmeister series anions of the ionic salts and ionic liquids on the stability of α-chymotrypsin. New J Chem. 2015 doi: 10.1039/C4NJ01596G. [DOI] [Google Scholar]
- 147.Lange C, Patil G, Rudolph R. Ionic liquids as refolding additives: N′-alkyl and N′-(ω-hydroxyalkyl) N-methylimidazolium chlorides. Protein Sci. 2005;14:2693–2701. doi: 10.1110/ps.051596605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sanfilippo C, D’Antona N, Nicolosi G. Chloroperoxidase from Caldariomyces fumago is active in the presence of an ionic liquid as co-solvent. Biotechnol Lett. 2004;26:1815–1819. doi: 10.1007/s10529-004-5087-6. [DOI] [PubMed] [Google Scholar]
- 149.Lou W-Y, Zong M-H, Liu Y-Y, Wang J-F. Efficient enantioselective hydrolysis of D,L-phenylglycine methyl ester catalyzed by immobilized Candida antarctica lipase B in ionic liquid containing systems. J Biotechnol. 2006;125:64–74. doi: 10.1016/j.jbiotec.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 150.Ventura SPM, Santos LDF, Saraiva JA, Coutinho JAP. Concentration effect of hydrophilic ionic liquids on the enzymatic activity of Candida antarctica lipase B. World JMicrobiol Biotechnol. 2012;28:2303–2310. doi: 10.1007/s11274-012-1037-y. [DOI] [PubMed] [Google Scholar]
- 151.Lou W-Y, Zong M-H, Smith TJ, Wu H, Wang J-F. Impact of ionic liquids on papain: an investigation of structure-function relationship. Green Chem. 2006;8:509–512. [Google Scholar]
- 152.Okochi M, Nakagawa I, Kobayashi T, Hayashi S, Furusaki S, Honda H. Enhanced activity of 3α-hydroxysteroid dehydrogenase by addition of the co-solvent 1-butyl-3-methylimidazolium (L)-lactate in aqueous phase of biphasic systems for reductive production of steroids. J Biotechnol. 2007;128:376–382. doi: 10.1016/j.jbiotec.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 153.de Gonzalo G, Lavandera I, Durchschein K, Wurm D, Faber K, Kroutil W. Asymmetric biocatalytic reduction of ketones using hydroxy-functionalised water-miscible ionic liquids as solvents. Tetrahedron: Asymmetry. 2007;18:2541–2546. [Google Scholar]
- 154.Dabirmanesh B, Khajeh K, Akbari J, Falahati H, Daneshjoo S, Heydari A. Mesophilic alcohol dehydrogenase behavior in imidazolium based ionic liquids. J Mol Liq. 2011;161:139–143. [Google Scholar]
- 155.Dabirmanesh B, Khajeh K, Ranjbar B, Ghazi F, Heydari A. Inhibition mediated stabilization effect of imidazolium based ionic liquids on alcohol dehydrogenase. J Mol Liq. 2012;170:66–71. [Google Scholar]
- 156.Thomas MF, Li L-L, Handley-Pendleton JM, van der Lelie D, Dunn JJ, Wishart JF. Enzyme activity in dialkyl phosphate ionic liquids. Bioresour Technol. 2011;102:11200–11203. doi: 10.1016/j.biortech.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 157.Rodríguez O, Cristóvão RO, Tavares APM, Macedo EA. Study of the alkyl chain length on laccase stability and enzymatic kinetic with imidazolium ionic liquids. Appl Biochem Biotechnol. 2011;164:524–533. doi: 10.1007/s12010-010-9154-2. [DOI] [PubMed] [Google Scholar]
- 158.Noritomi H, Minamisawa K, Kamiya R, Kato S. Thermal stability of proteins in the presence of aprotic ionic liquids. J Biomed Sci Eng. 2011;4:94–99. [Google Scholar]
- 159.Debeljuh N, Barrow CJ, Byrne N. The impact of ionic liquids on amyloid fibrilization of Aβ16-22: tuning the rate of fibrilization using a reverse Hofmeister strategy. Phys Chem Chem Phys. 2011;13:16534–16536. doi: 10.1039/c1cp22256b. [DOI] [PubMed] [Google Scholar]
- 160.Schöfer SH, Kaftzik N, Wasserscheid P, Kragl U. Enzyme catalysis in ionic liquids: lipase catalysed kinetic resolution of 1-phenylethanol with improved enantioselectivity. Chem Commun. 2001:425–426. [Google Scholar]
- 161.Lozano P, De Diego T, Carrié D, Vaultier M, Iborra JL. Over-stabilization of Candida antarctica lipase B by ionic liquids in ester synthesis. Biotechnol Lett. 2001;23:1529–1533. [Google Scholar]
- 162.Kaar JL, Jesionowski AM, Berberich JA, Moulton R, Russell AJ. Impact of ionic liquid physical properties on lipase activity and stability. J Am Chem Soc. 2003;125:4125–4131. doi: 10.1021/ja028557x. [DOI] [PubMed] [Google Scholar]
- 163.Lau RM, Sorgedrager MJ, Carrea G, van Rantwijk F, Secundo F, Sheldon RA. Dissolution of Candida antarctica lipase B in ionic liquids: effects on structure and activity. Green Chem. 2004;6:483–487. [Google Scholar]
- 164.Lou W-Y, Zong M-H, Wu H, Xu R, Wang J-F. Markedly improving lipase-mediated asymmetric ammonolysis of D,L-p-hydroxyphenylglycine methyl ester by using an ionic liquid as the reaction medium. Green Chem. 2005;7:500–506. [Google Scholar]
- 165.Lou W-Y, Zong M-H. Efficient kinetic resolution of (R,S)-1-trimethylsilylethanol via lipase-mediated enantioselective acylation in ionic liquids. Chirality. 2006;18:814–821. doi: 10.1002/chir.20307. [DOI] [PubMed] [Google Scholar]
- 166.Zhao H, Baker GA, Song Z, Olubajo O, Zanders L, Campbell SM. Effect of ionic liquid properties on lipase stabilization under microwave irradiation. J Mol Catal B: Enzym. 2009;57:149–157. [Google Scholar]
- 167.Lozano P, Bernal JM, Vaultier M. Towards continuous sustainable processes for enzymatic synthesis of biodiesel in hydrophobic ionic liquids/supercritical carbon dioxide biphasic systems. Fuel. 2011;90:3461–3467. [Google Scholar]
- 168.Lozano P, de Diego T, Guegan J-P, Vaultier M, Iborra JL. Stabilization of α-chymotrypsin by ionic liquids in transesterification reactions. Biotechnol Bioeng. 2001;75:563–569. doi: 10.1002/bit.10089. [DOI] [PubMed] [Google Scholar]
- 169.Maruyama T, Nagasawa S, Goto M. Poly(ethylene glycol)-lipase complex that is catalytically active for alcoholysis reactions in ionic liquids. Biotechnol Lett. 2002;24:1341–1345. [Google Scholar]
- 170.Maruyama T, Yamamura H, Kotani T, Kamiya N, Goto M. Poly(ethylene glycol)-lipase complexes that are highly active and enantioselective in ionic liquids. Org Biomol Chem. 2004;2:1239–1244. doi: 10.1039/b401012d. [DOI] [PubMed] [Google Scholar]
- 171.Persson M, Bornscheuer UT. Increased stability of an esterase from Bacillus stearothermophilus in ionic liquids as compared to organic solvents. J Mol Catalysis B: Enzym. 2003;22:21–27. [Google Scholar]
- 172.Ulbert O, Frater T, Belafi-Bako K, Gubicza L. Enhanced enantioselectivity of Candida rugosa lipase in ionic liquids as compared to organic solvents. J Mol Catalysis B: Enzym. 2004;31:39–45. [Google Scholar]
- 173.Basso A, Cantone S, Linda P, Ebert C. Stability and activity of immobilised penicillin G amidase in ionic liquids at controlled aw. Green Chem. 2005;7:671–676. [Google Scholar]
- 174.Hussain W, Pollard DJ, Truppo M, Lye GJ. Enzymatic ketone reductions with cofactor recycling: Improved reactions with ionic liquid co-solvents. J Mol Catal B: Enzym. 2008;55:19–29. [Google Scholar]
- 175.Liu Y, Chen D, Yan Y, Peng C, Xu L. Biodiesel synthesis and conformation of lipase from Burkholderia cepacia in room temperature ionic liquids and organic solvents. Biores Technol. 2011;102:10414–10418. doi: 10.1016/j.biortech.2011.08.056. [DOI] [PubMed] [Google Scholar]
- 176.Zeuner B, Ståhlberg T, Nguyen van Buu O, Kunov-Kruse AJ, Riisager A, Meyer AS. Dependency of the hydrogen bonding capacity of the solvent anion on the thermal stability of feruloyl esterases in ionic liquid systems. Green Chem. 2011;13:1550–1557. [Google Scholar]
- 177.Vila-Real H, Alfaia AJ, Rosa JN, Gois PMP, Rosa ME, Calado ART, Ribeiro MH. α-Rhamnosidase and β-glucosidase expressed by naringinase immobilized on new ionic liquid sol–gel matrices: Activity and stability studies. J Biotechnol. 2011;152:147–158. doi: 10.1016/j.jbiotec.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 178.Bose S, Barnes CA, Petrich JW. Enhanced stability and activity of cellulase in an ionic liquid and the effect of pretreatment on cellulose hydrolysis. Biotechnol Bioeng. 2012;109:434–443. doi: 10.1002/bit.23352. [DOI] [PubMed] [Google Scholar]
- 179.Baker GA, Heller WT. Small-angle neutron scattering studies of model protein denaturation in aqueous solutions of the ionic liquid 1-butyl-3-methylimidazolium chloride. Chem Eng J. 2009;147:6–12. [Google Scholar]
- 180.Domínguez A, Rodríguez O, Tavares APM, Macedo EA, Longo MA, Sanromán MA. Studies of laccase from Trametes versicolor in aqueous solutions of several methylimidazolium ionic liquids. Bioresour Technol. 2011;102:7494–7499. doi: 10.1016/j.biortech.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 181.Xue L, Zhao Y, Yu L, Sun Y, Yan K, Li Y, Huang X, Qu Y. Choline acetate enhanced the catalytic performance of Candida rogusa lipase in AOT reverse micelles. Colloids Surf B. 2013;105:81–86. doi: 10.1016/j.colsurfb.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 182.Li N, Du W, Huang Z, Zhao W, Wang S. Effect of imidazolium ionic liquids on the hydrolytic activity of lipase. Chin J Catal. 2013;34:769–780. [Google Scholar]
- 183.Filice M, Romero O, Abian O, de las Rivas B, Palomo JM. Low ionic liquid concentration in water: a green and simple approach to improve activity and selectivity of lipases. RSC Adv. 2014;4:49115–49122. [Google Scholar]
- 184.Muldoon MJ, Gordon GM, Dunkin IR. Investigation of solvent-solute interaction in room temperature ionic liquids using solvatochromic dyes. J Chem Soc, Perkin Trans. 2001;2:433–435. [Google Scholar]
- 185.Linert W, Jameson RF, Taha A. Donor numbers of anions in solution: the use of solvatochromic Lewis acid–base indicators. J Chem Soc, Dalton Trans. 1993:3181–3186. [Google Scholar]
- 186.Camard A, Ihara Y, Murata F, Mereiter K, Fukuda Y, Linert W. The use of solvatochromic mixed copper(II) chelates with N,N,N′,N′-tetramethylethylenediamine and tropolonato or hinokitiolato ligands, [Cu(trop/hino)(tmen)]B(C6H5)4, as indicator for Lewis basicity of solvents and low-coordinating anions. Inorg Chim Acta. 2005;358:409–414. [Google Scholar]
- 187.Oehlke A, Hofmann K, Spange S. New aspects on polarity of 1-alkyl-3-methylimidazolium salts as measured by solvatochromic probes. New J Chem. 2006;30:533–536. [Google Scholar]
- 188.Palgunadi J, Hong SY, Lee JK, Lee H, Lee SD, Cheong M, Kim HS. Correlation between hydrogen bond basicity and acetylene solubility in room temperature ionic liquids. J Phys Chem B. 2011;115:1067–1074. doi: 10.1021/jp108351f. [DOI] [PubMed] [Google Scholar]
- 189.MacFarlane DR, Pringle JM, Johansson KM, Forsyth SA, Forsyth M. Lewis base ionic liquids. Chem Commun. 2006:1905–1917. doi: 10.1039/b516961p. [DOI] [PubMed] [Google Scholar]
- 190.Pagni RM. Ionic liquids as alternatives to traditional organic and inorganic solvents. In: Rogers RD, Seddon KR, Volkov S, editors. Green Industrial Applications of Ionic Liquids. Kluwer Academic Publishers; Dordrecht: 2002. pp. 105–127. [Google Scholar]
- 191.Bernson A, Lindgren J. Coordination of OH end-groups in the polymer electrolyte system LiX-PPG for X = PF6−, BF4−, ClO4−, CF3SO3−, I−, Br− and Cl−. Polymer. 1994;35:4848–4851. [Google Scholar]
- 192.Henderson WA. Glyme-lithium salt phase behavior. J Phys Chem B. 2006;110:13177–13183. doi: 10.1021/jp061516t. [DOI] [PubMed] [Google Scholar]
- 193.Henderson WA. Crystallization kinetics of glyme-LiX and PEO-LiX polymer electrolytes. Macromolecules. 2007;40:4963–4971. [Google Scholar]
- 194.Hernández-Fernández FJ, de los Ríos AP, Tomás-Alonso F, Gómez D, Víllora G. Stability of hydrolase enzymes in ionic liquids. Can J Chem Eng. 2009;87:910–914. [Google Scholar]
- 195.Irimescu R, Kato K. Investigation of ionic liquids as reaction media for enzymatic enantioselective acylation of amines. J Mol Catal B: Enzym. 2004;30:189–194. [Google Scholar]
- 196.Lee SH, Koo Y-M, Ha SH. Influence of ionic liquids under controlled water activity and low halide content on lipase activity. Korean J Chem Eng. 2008;25:1456–1462. [Google Scholar]
- 197.Swatloski RP, Spear SK, Holbrey JD, Rogers RD. Dissolution of cellulose with ionic liquids. J Am Chem Soc. 2002;124:4974–4975. doi: 10.1021/ja025790m. [DOI] [PubMed] [Google Scholar]
- 198.Zhu S, Wu Y, Chen Q, Yu Z, Wang C, Jin S, Ding Y, Wu G. Dissolution of cellulose with ionic liquids and its application: a mini-review. Green Chem. 2006;8:325–327. [Google Scholar]
- 199.Remsing RC, Swatloski RP, Rogers RD, Moyna G. Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3-methylimidazolium chloride: a 13C and 35/37Cl NMR relaxation study on model systems. Chem Commun. 2006:1271–1273. doi: 10.1039/b600586c. [DOI] [PubMed] [Google Scholar]
- 200.Lee SH, Ha SH, Lee SB, Koo Y-M. Adverse effect of chloride impurities on lipase-catalyzed transesterifications in ionic liquids. Biotechnol Lett. 2006;28:1335–1339. doi: 10.1007/s10529-006-9095-6. [DOI] [PubMed] [Google Scholar]
- 201.Anderson JL, Ding J, Welton T, Armstrong DW. Characterizing ionic liquids on the basis of multiple solvation interactions. J Am Chem Soc. 2002;124:14247–14254. doi: 10.1021/ja028156h. [DOI] [PubMed] [Google Scholar]
- 202.Armstrong DW, He L, Liu YS. Examination of ionic liquids and their interaction with molecules, when used as stationary phases in gas chromatography. Anal Chem. 1999;71:3873–3876. doi: 10.1021/ac990443p. [DOI] [PubMed] [Google Scholar]
- 203.Forsyth SA, MacFarlane DR, Thomson RJ, von Itzstein M. Rapid, clean, and mild O-acetylation of alcohols and carbohydrates in an ionic liquid. Chem Commun. 2002:714–715. doi: 10.1039/b200306f. [DOI] [PubMed] [Google Scholar]
- 204.Liu Q, Janssen MHA, van Rantwijk F, Sheldon RA. Room-temperature ionic liquids that dissolve carbohydrates in high concentrations. Green Chem. 2005;7:39–42. [Google Scholar]
- 205.Toral AR, de los Ríos AP, Hernández FJ, Janssen MHA, Schoevaart R, van Rantwijk F, Sheldon RA. Cross-linked Candida antarctica lipase B is active in denaturing ionic liquids. Enzyme Microb Technol. 2007;40:1095–1099. [Google Scholar]
- 206.Zhao H, Baker GA, Song Z, Olubajo O, Crittle T, Peters D. Designing enzyme-compatible ionic liquids that can dissolve carbohydrates. Green Chem. 2008;10:696–705. [Google Scholar]
- 207.Zhao H, Jones CL, Cowins JV. Lipase dissolution and stabilization in ether-functionalized ionic liquids. Green Chem. 2009;11:1128–1138. [Google Scholar]
- 208.Bermejo MD, Kotlewska AJ, Florusse LJ, Cocero MJ, van Rantwijk F, Peters CJ. Influence of the enzyme concentration on the phase behaviour for developing a homogeneous enzymatic reaction in ionic liquid–CO2 media. Green Chem. 2008;10:1049–1054. [Google Scholar]
- 209.Zhang K-P, Lai J-Q, Huang Z-L, Yang Z. Penicillium expansum lipase-catalyzed production of biodiesel in ionic liquids. Bioresour Technol. 2011;102:2767–2772. doi: 10.1016/j.biortech.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 210.Bekhouche M, Blum LJ, Doumèche B. Contribution of dynamic and static quenchers for the study of protein conformation in ionic liquids by steady-state fluorescence spectroscopy. J Phys Chem B. 2012;116:413–423. doi: 10.1021/jp205094c. [DOI] [PubMed] [Google Scholar]
- 211.van Rantwijk F, Madeira Lau R, Sheldon RA. Biocatalytic transformations in ionic liquids. Trends Biotechnol. 2003;21:131–138. doi: 10.1016/S0167-7799(03)00008-8. [DOI] [PubMed] [Google Scholar]
- 212.Lee SH, Lee SB. Octanol/water partition coefficients of ionic liquids. J Chem Tech Biotechnol. 2009;84:202–207. [Google Scholar]
- 213.Sangster J. Octanol-water partition coefficients of simple organic compounds. J Phys Chem Ref Data. 1989;18:1111–1229. [Google Scholar]
- 214.Lee SH, Doan TTN, Ha SH, Koo Y-M. Using ionic liquids to stabilize lipase within sol–gel derived silica. J Mol Catal B: Enzym. 2007;45:57–61. [Google Scholar]
- 215.Ropel L, Belvèze LS, Aki SNVK, Stadtherr MA, Brennecke JF. Octanol–water partition coefficients of imidazolium-based ionic liquids. Green Chem. 2005;7:83–90. [Google Scholar]
- 216.Lee S-M, Chang W-J, Choi A-R, Koo Y-M. Influence of ionic liquids on the growth of Escherichia coli. Korean J Chem Eng. 2005;22:687–690. [Google Scholar]
- 217.Chapeaux A, Simoni LD, Stadtherr MA, Brennecke JF. Liquid phase behavior of ionic liquids with water and 1-octanol and modeling of 1-octanol/water partition coefficients. J Chem Eng Data. 2007;52:2462–2467. [Google Scholar]
- 218.Hilhorst R, Spruijt R, Laane C, Veeger C. Rules for the regulation of enzyme activity in reserved micelles as illustrated by the conversion of apolar steroids by 20 β-hydroxysteroid dehydrogenase. Eur J Biochem. 1984;144:459–466. doi: 10.1111/j.1432-1033.1984.tb08488.x. [DOI] [PubMed] [Google Scholar]
- 219.Laane C, Boeren S, Vos K. On optimizing organic solvents in multi-liquid-phase biocatalysis. Trends Biotechnol. 1985;3:251–252. [Google Scholar]
- 220.Laane C, Boeren S, Vos K, Veeger C. Rules for optimization of biocatalysis in organic solvents. Biotechnol Bioeng. 1987;30:81–87. doi: 10.1002/bit.260300112. [DOI] [PubMed] [Google Scholar]
- 221.Nara SJ, Harjani JR, Salunkhe MM. Lipase-catalysed transesterification in ionic liquids and organic solvents: a comparative study. Tetrahedron Lett. 2002;43:2979–2982. [Google Scholar]
- 222.Nakashima K, Okada J, Maruyama T, Kamiya N, Goto M. Activation of lipase in ionic liquids by modification with comb-shaped poly(ethylene glycol) Sci Technol Adv Mat. 2006;7:692–698. [Google Scholar]
- 223.Nakashima K, Maruyama T, Kamiya N, Goto M. Homogeneous enzymatic reactions in ionic liquids with poly(ethylene glycol)-modified subtilisin. Org Biomol Chem. 2006;4:3462–3467. doi: 10.1039/b608920h. [DOI] [PubMed] [Google Scholar]
- 224.Zhang W-G, Wei D-Z, Yang X-P, Song Q-X. Penicillin acylase catalysis in the presence of ionic liquids. Bioprocess Biosyst Eng. 2006;29:379–383. doi: 10.1007/s00449-006-0085-9. [DOI] [PubMed] [Google Scholar]
- 225.Paljevac M, Habulin M, Knez Z. Ionic liquids as (co)solvents for enzymatic reactions. Chem Ind Chem Eng Q. 2006;12:181–186. [Google Scholar]
- 226.de los Ríos AP, Hernández-Fernández FJ, Rubio M, Gómez D, Víllora G. Stabilization of native penicillin G acylase by ionic liquids. J Chem Tech Biotechnol. 2007;82:190–195. [Google Scholar]
- 227.de los Ríos AP, Hernández-Fernández FJ, Martínez FA, Rubio M, Víllora G. The effect of ionic liquid media on activity, selectivity and stability of Candida antarctica lipase B in transesterification reactions. Biocatalysis Biotransform. 2007;25:151–156. [Google Scholar]
- 228.Ha SH, Lee SH, Dang DT, Kwon MS, Chang W-J, Yu YJ, Byun IS, Koo Y-M. Enhanced stability of Candida antarctica lipase B in ionic liquids. Korean J Chem Eng. 2008;25:291–294. [Google Scholar]
- 229.Shen Z-L, Zhou W-J, Liu Y-T, Ji S-J, Loh T-P. One-pot chemoenzymatic syntheses of enantiomerically-enriched O-acetyl cyanohydrins from aldehydes in ionic liquid. Green Chem. 2008;10:283–286. [Google Scholar]
- 230.Ryu K, Dordick JS. How do organic solvents affect peroxidase structure and function? Biochemistry. 1992;31:2588–2598. doi: 10.1021/bi00124a020. [DOI] [PubMed] [Google Scholar]
- 231.Mann JP, McCluskey A, Atkin R. Activity and thermal stability of lysozyme in alkylammonium formate ionic liquids—influence of cation modification. Green Chem. 2009;11:785–792. [Google Scholar]
- 232.Klähn M, Lim GS, Seduraman A, Wu P. On the different roles of anions and cations in the solvation of enzymes in ionic liquids. Phys Chem Chem Phys. 2011;13:1649–1662. doi: 10.1039/c0cp01509a. [DOI] [PubMed] [Google Scholar]
- 233.Klähn M, Lim GS, Wu P. How ion properties determine the stability of a lipase enzyme in ionic liquids: A molecular dynamics study. Phys Chem Chem Phys. 2011;13:18647–18660. doi: 10.1039/c1cp22056j. [DOI] [PubMed] [Google Scholar]
- 234.De Diego T, Lozano P, Abad MA, Steffensky K, Vaultier M, Iborra JL. On the nature of ionic liquids and their effects on lipases that catalyze ester synthesis. J Biotechnol. 2009;140:234–241. doi: 10.1016/j.jbiotec.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 235.Park S, Kazlauskas RJ. Improved preparation and use of room-temperature ionic liquids in lipase-catalyzed enantio- and regioselective acylations. J Org Chem. 2001;66:8395–8401. doi: 10.1021/jo015761e. [DOI] [PubMed] [Google Scholar]
- 236.Lau RM, van Rantwijk F, Seddon KR, Sheldon RA. Lipase-catalyzed reactions in ionic liquids. Org Lett. 2000;2:4189–4191. doi: 10.1021/ol006732d. [DOI] [PubMed] [Google Scholar]
- 237.Itoh T, Nishimura Y, Kashiwagi M, Onaka M. Efficient lipase-catalyzed enantioselective acylation in an ionic liquid solvent system. In: Rogers RD, Seddon KR, editors. Ionic Liquids as Green Solvents: Progress and Prospects. American Chemical Society; Washington D.C: 2003. pp. 251–261. [Google Scholar]
- 238.Kaftzik N, Neumann S, Kula M-R, Kragl U. Enzymatic condension reactions in ionic liquids. In: Rogers RD, Seddon KR, editors. Ionic Liquids as Green Solvents: Progress and Prospects. American Chemical Society; Washington D.C: 2003. pp. 206–211. [Google Scholar]
- 239.Lozano P, de Diego T, Carrié D, Vaultier M, Iborra JL. Enzymatic catalysis in ionic liquids and supercritical carbon dioxide. In: Rogers RD, Seddon KR, editors. Ionic Liquids as Green Solvents: Progress and Prospects. American Chemical Society; Washington D.C: 2003. pp. 239–250. [Google Scholar]
- 240.Falcioni F, Housden HR, Ling Z, Shimizu S, Walker AJ, Bruce NC. Soluble, folded and active subtilisin in a protic ionic liquid. Chem Commun. 2010;46:749–751. doi: 10.1039/b916497a. [DOI] [PubMed] [Google Scholar]
- 241.Wang Y, Radosevich M, Hayes D, Labbé N. Compatible Ionic liquid-cellulases system for hydrolysis of lignocellulosic biomass. Biotechnol Bioeng. 2011;108:1042–1048. doi: 10.1002/bit.23045. [DOI] [PubMed] [Google Scholar]
- 242.Bekhouche M, Doumèche B, Blum LJ. Chemical modifications by ionic liquid-inspired cations improve the activity and the stability of formate dehydrogenase in [MMIm][Me2PO4] J Mol Catal B: Enzym. 2010;65:73–78. [Google Scholar]
- 243.Yamamoto E, Yamaguchi S, Nagamune T. Protein refolding by N-alkylpyridinium and N-alkyl-N-methylpyrrolidinium ionic liquids. Appl Biochem Biotechnol. 2011;164:957–967. doi: 10.1007/s12010-011-9187-1. [DOI] [PubMed] [Google Scholar]
- 244.Guo Z, Xu X. New opportunity for enzymatic modification of fats and oils with industrial potentials. Org Biomol Chem. 2005;3:2615–2619. doi: 10.1039/b506763d. [DOI] [PubMed] [Google Scholar]
- 245.Guo Z, Chen B, Murillo RL, Tan T, Xu X. Functional dependency of structures of ionic liquids: do substituents govern the selectivity of enzymatic glycerolysis? Org Biomol Chem. 2006;4:2772–2776. doi: 10.1039/b606900b. [DOI] [PubMed] [Google Scholar]
- 246.Guo Z, Xu X. Lipase-catalyzed glycerolysis of fats and oils in ionic liquids: a further study on the reaction system. Green Chem. 2006;8:54–62. [Google Scholar]
- 247.Chen B, Guo Z, Tan T, Xu X. Structures of ionic liquids dictate the conversion and selectivity of enzymatic glycerolysis: Theoretical characterization by COSMO-RS. Biotechnol Bioeng. 2008;99:18–29. doi: 10.1002/bit.21520. [DOI] [PubMed] [Google Scholar]
- 248.Kahveci D, Guo Z, Özçelik B, Xu X. Lipase-catalyzed glycerolysis in ionic liquids directed towards diglyceride synthesis. Process Biochem. 2009;44:1358–1365. [Google Scholar]
- 249.Guo Z, Kahveci D, Özçelik B, Xu X. Improving enzymatic production of diglycerides by engineering binary ionic liquid medium system. New Biotechnol. 2009;26:37–43. doi: 10.1016/j.nbt.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 250.Kahveci D, Guo Z, Özçelik B, Xu X. Optimisation of enzymatic synthesis of diacylglycerols in binary medium systems containing ionic liquids. Food Chem. 2010;119:880–885. [Google Scholar]
- 251.Guo Z, Lue B-M, Thomasen K, Meyer AS, Xu X. Predictions of flavonoid solubility in ionic liquids by COSMO-RS: experimental verification, structural elucidation, and solvation characterization. Green Chem. 2007;9:1362–1373. [Google Scholar]
- 252.Dreyer S, Kragl U. Ionic liquids for aqueous two-phase extraction and stabilization of enzymes. Biotechnol Bioeng. 2008;99:1416–1424. doi: 10.1002/bit.21720. [DOI] [PubMed] [Google Scholar]
- 253.Wallert S, Drauz K, Grayson I, Gröger H, Dominguez de Maria P, Bolm C. Ionic liquids as additives in the pig liver esterase (PLE) catalysed synthesis of chiral disubstituted malonates. Green Chem. 2005;7:602–605. [Google Scholar]
- 254.Dai L, Klibanov AM. Striking activation of oxidative enzymes suspended in nonaqueous media. Proc Natl Acad Sci USA. 1999;96:9475–9478. doi: 10.1073/pnas.96.17.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Das D, Dasgupta A, Das PK. Improved activity of horseradish peroxidase (HRP) in ‘specifically designed’ ionic liquid. Tetrahedron Lett. 2007;48:5635–5639. [Google Scholar]
- 256.Abe Y, Kude K, Hayase S, Kawatsura M, Tsunashima K, Itoh T. Design of phosphonium ionic liquids for lipase-catalyzed transesterification. J Mol Catal B: Enzym. 2008;51:81–85. [Google Scholar]
- 257.Vafiadi C, Topakas E, Nahmias VR, Faulds CB, Christakopoulos P. Feruloyl esterase-catalysed synthesis of glycerol sinapate using ionic liquids mixtures. J Biotechnol. 2009;139:124–129. doi: 10.1016/j.jbiotec.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 258.Li X, Zhang C, Li S, Huang H, Hu Y. Improving catalytic performance of Candida rugosa lipase by chemical modification with polyethylene glycol functional ionic liquids. Ind Eng Chem Res. 2015;54:8072–8079. [Google Scholar]