Abstract
In contrast to the regulation of calcium homeostasis, which has been extensively studied over the past several decades, relatively little is known about the regulation of phosphate homeostasis. Fibroblast growth factor 23 (FGF23) is part of a previously unrecognized hormonal bone-parathyroid-kidney axis, which is modulated by PTH, 1,25(OH)2-vitamin D (1,25(OH)2D), dietary and serum phosphorus levels. Synthesis and secretion of FGF23 by osteocytes are positively regulated by 1,25(OH)2D and serum phosphorus and negatively regulated, through yet unknown mechanisms, by the phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX) and by dentin matrix protein 1 (DMP1). In turn, FGF23 inhibits the synthesis of 1,25(OH)2D, and it may negatively regulate the secretion of parathyroid hormone (PTH) from the parathyroid glands. However, FGF23 synergizes with PTH to increase renal phosphate excretion by reducing expression of the renal sodium-phosphate cotransporters NaPi-IIa and NaPi-IIc in the proximal tubules. Most insights gained into the regulation of phosphate homeostasis by these factors are derived from human genetic disorders and genetically engineered mice, which are reviewed in this paper.
Keywords: PTH; 1,25(OH)2-vitamin D; FGF23; phosphate homeostasis
INTRODUCTION
Over the past decade, information derived from genetic analyses of familial disorders has led to the discovery of novel genes involved in the regulation of phosphate homeostasis (Figure 1), which in contrast to the regulation of calcium homeostasis (1) has been less well understood. Phosphate is taken up from the circulation into cells via type II and type III sodium-phosphate cotransporters. It is required for numerous cellular functions such as DNA and membrane lipid synthesis, generation of high-energy phosphate esters, and intracellular signaling. The serum phosphorus concentration is determined by the balance between intestinal absorption of phosphate from the diet (16 mg/kg/day), storage of phosphate in the skeleton (3 mg/kg/day), and excretion of phosphate through the urine (13 mg/kg/day) (2). Only 30% of intestinal phosphate absorption occurs in a regulated, 1,25(OH)2D-dependent manner (3). Consequently, reabsorption of phosphate from the urine in the renal proximal tubules via type II and type III sodium-phosphate cotransporters plays a key role in maintaining serum phosphate homeostasis (4). Renal phosphate reabsorption underlies tight hormonal control by parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23). Other hormones appear to contribute also to the regulation of phosphate homeostasis, but their actions are less well understood; these other regulators include insulin and hormones of the somatotropic pituitary axis (5), and possibly FGF7, matrix extracellular phosphoglycoprotein (MEPE), and secreted frizzled-related protein 4 (sFRP-4) (6).
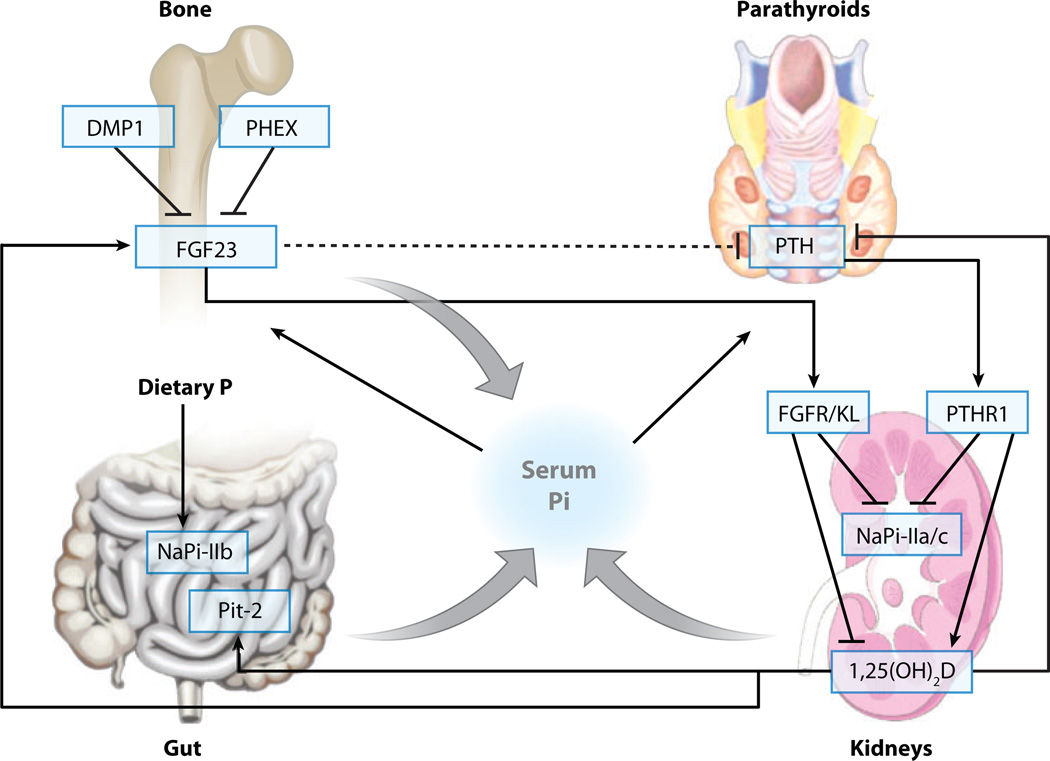
Figure 1.
Regulation of phosphate homeostasis. Phosphate is absorbed from the diet in the gut, stored in the skeleton, and excreted by the kidneys. 1,25(OH)2D stimulates absorption of phosphate from the diet. FGF23 increases renal phosphate clearance, suppresses synthesis of 1,25(OH)2D, and may decrease PTH (dashed line). PTH increases renal phosphate clearance and stimulates synthesis of 1,25(OH)2D.
This review discusses the regulation of phosphate homeostasis by PTH, 1,25(OH)2D, and FGF23 with focus on insights gained from human genetic disorders (see Table 1) and genetically modified mouse models (see Supplemental Table 1; follow the Supplemental Material link from the Annual Reviews home page at http://www.annualreviews.org). For a detailed discussion of the receptors and signal transduction cascades for PTH, 1,25(OH)2D, and FGF23 the reader is referred to several recent reviews (see Related Resources).
Table 1.
Human genetic disorders of phosphate homeostasis
| Disorder | Abbreviation | Inheritance | Gene | Mechanism | OMIM |
|---|---|---|---|---|---|
| Hyperphosphatemic disorders | |||||
| Hyperphosphatemic familial tumoral calcinosis type 1 and the allelic variant hyperphosphatemia syndrome |
HFTC HSS |
AR AR |
GALNT3 | FGF23-deficiency | #211900 #610233 |
| Hyperphosphatemic familial tumoral calcinosis type 2 |
HFTC | AR | FGF23 | FGF23-deficiency | #211900 |
| Hyperphosphatemic familial tumoral calcinosis type 3 |
HFTC | AR | KL | FGF23-resistance | #211900 |
| Pseudohypoparathyroidism | PHP1A PHP1B |
AD (impr.) AD (impr.) |
GNAS GNAS or up- stream regulatory region |
PTH-resistance; FGF23-independent |
#103580 #603233 |
| Familial isolated hypoparathyroidism |
FIH | AD or AR |
CaR GCMB PTH |
PTH-deficiency; FGF23-indepenent |
#146200 |
| Blomstrand disease | BOCD | AR | PTHR1 | PTH-resistance; FGF23-independent |
#215045 |
| Hypophosphatemic disorders | |||||
| X-linked hypophosphatemia | XLH | X-linked | PHEX | FGF23-dependent | #307800 |
| Autosomal dominant hypophosphatemic rickets |
ADHR | AD | FGF23 | FGF23-dependent | #193100 |
| Autosomal dominant hypophosphatemic rickets |
ADHR | AD | KL | FGF23-dependent | #612089 |
| Autosomal recessive hypophosphatemia |
ARHP | AR | DMP1 | FGF23-dependent | #241520 |
| Hereditary hypophosphatemic rickets with hypercalciuria |
HHRH | AR | SLC34A3 | Proximal tubular phosphate wasting, FGF23-independent |
#241530 |
| Vitamin-resistant rickets type 1 | VDDR1 | AR | CYP27B1 | 1,25(OH)2D deficiency, FGF23-independent |
#264700 |
| Vitamin-resistant rickets type 2 | VDDR2 | AR | VDR | 1,25(OH)2D-resistance, FGF23-independent |
#277440 |
| Familial hypocalciuric hypercalcemia/neonatal severe hyperparathyroidism |
FHH NSHPT |
AD/AR | CaR | PTH-excess, FGF23-independent |
#145980 #239200 |
| Jansen disease | AD | PTHR1 | Const. active PTHR1; FGF23-dependent |
#156400 | |
PARATHYROID HORMONE
The major physiological role of the parathyroid glands is to function as a “calciostat.” Consequently, parathyroid hormone (PTH) secretion by the parathyroid glands is tightly regulated on a transcriptional and posttranscriptional level dependent on the concentration of extracellular calcium (Cae) (7). In addition to calcium, it regulates serum phosphate levels through its actions at several organs, and elevated serum phosphate concentration in turn stimulates PTH secretion, presumably by lowering extracellular calcium and increasing stability of the PTH mRNA (8).
PTH is synthesized as prepropeptide containing a 25-amino-acid presequence (signal sequence) and a 6-amino-acid prosequence (1). Both are cleaved off in the endoplasmic reticulum, and mature full-length PTH(1–84) is stored in secretory vesicles (1). Activation of the calcium-sensor receptor (CaR) in the parathyroid cell membrane by Cae stimulates release of intracellular calcium via Gq/11/phospholipase C (PLC) and suppresses adenylate cyclase via the inhibitory G protein Gi, respectively (9). Mice with parathyroid-specific ablation of Gq combined with the global ablation of G11 have severe hyperparathyroidism similar to that present in mice homozygous for the deletion of the CaR, thus illustrating the important role of this signal transduction pathway for calcium sensing (10). Hydrolysis of PIP3 by PLC releases IP3, which stimulates release of intra-cellular calcium (Cai) and activates calcium-sensitive proteases in the secretory vesicles of the parathyroids, resulting in cleavage and inactivation of PTH (1). The activation of CaR also suppresses protein synthesis and release of PTH(1–84) into the circulation via mechanisms involving changes in the actin cytoskeleton (11). Heterozygous activating CaR mutations cause familial forms of hypoparathyroidism (12), whereas heterozygous and homozygous inactivating mutations lead to familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism, respectively (13). PTH gene expression is negatively regulated by 11,25(OH)2D (14). Parathyroid gland development is regulated by PAX9 (15), the transcription factor GCMB (16), and possibly several other, yet unknown factors. GCMB may also be involved in parathyroid cell differentiation and the regulation of hormone synthesis. FGF23 suppresses PTH mRNA synthesis and secretion in vitro and in vivo in an alpha klotho (KL)-dependent fashion (17). End organ resistance to FGF23 at the parathyroid may explain why one individual with hyperphosphatemic familial tumoral calcinosis (HFTC) due to a homozygous inactivating KL mutation developed parathyroid hyperplasia (18). KL may also have an FGF23-independent role by facilitating PTH-secretion through maintenance of membrane Na/K-ATPase activity in the setting of hypocalcemia, and KL-null mice are thus desensitized to hypocalcemic stress (19).
PTH and PTH-related peptide (PTHrP) act through a common G protein–coupled receptor, the PTH/PTHrP-receptor (PTHR1) (1). PTHR1 is coupled to the Gsα/PKA, the Gq/PLC/PKC, and the MAPK-pathways, but thus far it has been difficult to attribute individual functions of PTH to selected signaling pathways. Continuous exposure to PTH induces bone resorption by activation of osteoclasts indirectly through osteoblasts, while intermittent PTH increases bone formation by activation of osteoblasts. Osteoclasts do not have receptors for PTH and thus recruitment of osteoclast precursors and activation of bone resorption occur indirectly via osteoblasts, in which PTH enhances expression of receptor activator of NF-κB ligand (RANK-L), which in turn increases osteoclast number and activity in the presence of granulocyte monocyte colony stimulating factor (GMCSF). Conversely, intermittent PTH may lead to activation of the anabolic wnt-pathway by suppressing sclerostin and dickkopf 1 (DKK1) (20). At the level of the proximal renal tubule, PTH acts via the PTHR1 expressed at the basolateral and apical membrane to activate Gsα/PKA and Gq/PLC/PKC, respectively. Activation of both signaling pathways leads to internalization of the sodium-phosphate cotransporters NaPi-IIa and NaPi-IIc, resulting in renal phosphate wasting and hypophosphatemia (21). PTH also leads to proximal tubular bicarbonate wasting by inhibiting the amiloride-sensitive Na/H-exchanger and Na/K-ATPase (22). It also increases epithelial Cl− efflux, thereby hyperpolarizing the distal tubular cell, which leads to increased Ca-reabsorption via voltage-sensitive Ca-channels (23). The net effect of the PTH actions on bone and kidneys is an increase in serum calcium concentration and a decrease in serum phosphorus concentration, and prolonged elevations can lead to (mild) hyperchloremic metabolic acidosis.
The analysis of mice lacking PTH or PTHR1, knock-in mice carrying receptors deficient in the activation of Gq/PLC/PKC and lacking phosphorylation-sites important for desensitization, and of mice overexpressing a constitutively active PTHR1 under the control of either the DMP-1 or the Col1a1 (3.6 kb) promoter has provided important insights into the function of PTH and PTHrP in vivo (24–28). In this review only findings relevant for phosphate homeostasis are discussed. Mice lacking PTH are viable, yet newborns display diminished matrix mineralization, decreased neovascularization and reduced metaphyseal osteoblasts and trabecular bone (28). PTH-null animals suffer from renal calcium losses and phosphate retention, just like humans with hypoparathyroidism (29). Mice lacking the PTHR1 die at birth or shortly thereafter (30), an outcome similar to that in individuals with Blomstrand disease, a disorder caused by homozygous or compound heterozygous inactivating mutations in the PTHR1 (31). No data on phosphate homeostasis in the mice models are available. Mice null for PTHrP or PTHR1 but that express a constitutively active PTHR1 under the control of the col1a1 (3.6 kb) promoter show improved survival (32), but no data on their phosphate homeostasis are available. Conversely, mice in which the wild-type PTHR1 has been replaced by a receptor deficient in the activation of Gq/PLC lack hypophosphatemia when secondary hyperparathyroidism is induced by a calcium-deficient diet (27), whereas mice expressing a PTHR1, which lacks all phosphorylation-sites within the C-terminal receptor portion that are important for receptor desensitization, develop hypophosphatemia despite normal parathyroid function (33). These findings indicate that the effects of PTH on phosphate homeostasis are at least partly mediated through the Gq/PLC/PKC signaling pathway and are subject to desensitization at the level of PTHR1.
Healthy individuals injected with PTH(1–34) develop hypophosphatemia and elevated 1,25(OH)2D levels, along with an increase of serum FGF23 (34), suggesting that calcitriol is an important regulator of FGF23 synthesis, independent of the serum phosphate concentration. However, increased circulating FGF23 levels were likewise reported in Pth-null mice (29) as well as in some patients with hypoparathyroidism (35), indicating that the hyperphosphatemia observed under these conditions can modulate FGF23 despite low or normal calcitriol levels.
Phosphate-independent regulation of FGF23 by 1,25(OH)2D may also explain why FGF23 is unable to compensate in patients with hyper- or hypoparathyroidism, i.e., conditions in which 1,25(OH)2D is elevated to inappropriately stimulate or decreased to inappropriately reduce FGF23 levels, respectively.
No abnormality of phosphate homeostasis and FGF23 synthesis or secretion was observed in mice expressing the constitutively active PTHR1 expressed under the control of the DMP-1 or the col1a1(3.6-kb) promotor, or in mice lacking GNAS exon 1 in osteoblasts that leads to Gsα deficiency in this tissue (24–26, 36). However, some patients with McCune-Albright syndrome (37) and one individual with Jansen metaphyseal dysplasia (38) had increased serum FGF23 concentration; thus it remains unclear whether there is a direct role for PTH in the regulation of FGF23 synthesis and/or secretion.
1,25(OH)2-VITAMIN D
Cholecalciferol is generated from its precursors 7-dehydrocholesterol and ergosterol in the skin, subjected to 25-hydroxylation in the liver and converted to the active 1,25-dihydroxycholecalciferol [1,25(OH)2D] in the kidney (39). CYP27B1 encodes the enzyme responsible for 1-alpha-hydroxylation in the kidney and is induced by PTH, hypocalcemia and hypophosphatemia (39). FGF23, hypercalcemia, and hyperphosphatemia reduce CYP27B1 expression (40). Also, FGF7 and sFRP-4 appear to inhibit synthesis of 1,25(OH)2D, but MEPE lacks this inhibitory activity (6). FGF23 and 1,25(OH)2D also increase the activity of the renal CYP24 (40), which converts 25-OH-vitamin D and 1,25(OH)2D into the inactive metabolites.
Similar degrees of hypocalcemia and hypophosphatemia are seen in both the VDR-null mouse (41) and the CYP27B1-null mouse (42). These findings are similar to those observed in individuals with vitamin D-dependent rickets types 1 and 2 (VDDR1 and VDDR2), respectively, carrying loss-of-function mutations in either of these genes (43, 44), and indicate that the effects of vitamin D on calcium and phosphate homeostasis are mediated by the liganded VDR. Upon ligand binding the 1,25(OH)2D/VDR complex forms in the nucleus heterodimers with RXR to activate vitamin D-responsive elements (VREs). In enterocytes of the intestinal tract 1,25(OH)2D increases expression of the transient receptor potential cation channel, subfamily V, member 6 (TRPV6), and plasma membrane Ca2+ ATPase (PMCA) facilitates transcellular calcium uptake (39). It also increases phosphate uptake from the diet (39), possibly via upregulation of Pit-2 (45), whereas—at least in mice— transcellular phosphate uptake via NaPi-IIb appears to be regulated by dietary phosphate in a vitamin D-independent fashion (46). VDR is also expressed in osteoblasts, and 1,25(OH)2D was reported to increase bone formation and resorption. However, the complete rescue of rickets/osteomalacia of the Vdr-null mouse by a diet high in calcium and phosphate suggests that the major role of VDR is the delivery of calcium and phosphate to bone (47). 1,25(OH)2D stimulates the synthesis and secretion of FGF23 by osteoblasts and osteocytes (48). At the level of the parathyroid gland, 1,25(OH)2D acts to reduce PTH synthesis and secretion directly (49) and it increases CaR expression, thereby sensitizing the parathyroid gland to inhibition by calcium (50). At the renal distal tubules, 1,25(OH)2D increases the intracellular expression of calbindin-28 kDa, expression of TRPV5 at the apical membrane, and expression of the ATP-dependent calcium transporter at the basolateral membrane, thereby enhancing PTH-dependent calcium-reabsorption from the glomerular filtrate (51). The net effect of the actions of 1,25(OH)2D on parathyroid, gut, bone, and kidneys is an increase in serum calcium and phosphate level.
Intravenous injection of calcitriol increases FGF23 levels in humans (52) and mice (48). Mice that are null for Vdr (41) or Cyp27b1 (42) exhibit hypophosphatemia and have accordingly low FGF23 levels (53). Vdr-null animals on a rescue diet, which corrects secondary hyperparathyroidism and hypophosphatemia, and thus improves bone mineralization, are able to normalize circulating FGF23 levels despite absence of 1,25(OH)2D action, suggesting a vitamin D-independent role of Pi (53). Findings from these animal models suggest that phosphate and 1,25(OH)2D are independent regulators of the circulating FGF23 levels. FGF23 synthesis and secretion are decreased when VDR is selectively ablated in chondrocytes using Vdrfl/fl mice that were mated with col2a1-cre animals to target ablation of the VDR to proliferating chondrocytes (54). These data suggest that a VDR-dependent regulator of FGF23 is present in the growth plate.
FIBROBLAST GROWTH FACTOR 23
FGF23 was first identified in mouse embryos by homology-based PCR (55). However, its importance for phosphate homeostasis became apparent only when FGF23 loss-of-function mutations were shown to cause autosomal dominant hypophosphatemic rickets (ADHR) (56). Furthermore, clones encoding FGF23 were isolated from cDNA libraries of tumors causing oncogenic osteomalacia (OOM) and recombinant FGF23 was shown to induce renal phosphate-wasting leading to hypophosphatemia and osteomalacia (57). The human FGF23 gene on chromosome 12p13.3 encodes for a glycoprotein comprising 251 amino acids. The main sources of FGF23 are osteocytes and osteoblasts in the skeleton, but low levels of unclear significance can be detected in the ventrolateral thalamic nucleus, the thymus, small intestine, and heart (55). The phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX) and dentin matrix protein 1 (DMP1) suppress expression of FGF23 in bone, most likely through indirect mechanisms (58). In contrast, dietary phosphate (59) and serum 1,25(OH)2D stimulate FGF23 synthesis (52). After cleavage of the signal sequence comprising 24 amino acids and O-glycosylation by UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyl-transferase 3 (GALNT3), the mature protein, FGF23(25–251), is secreted into the circulation. O-glycosylation of FGF23 occurs in the 162–228 region (60) and this posttranslational modification appears to protect FGF23 from cleavage by subtilisin-like proprotein convertases when using recombinant peptides in vitro (61). Mutations identified in individuals affected by hypophosphatemic familial tumoral calcinosis (HFTC2) reside in the N-terminal portion of FGF23 (62). One mutation, S71G, leads to an accumulation of mutant FGF23 in the Golgi apparatus (63), and the impaired secretion of Flag-tagged versions of [G71]hFGF23 and [F129]hFGF23 by HEK293 cells can be rescued by lowering the culture temperature to 25°C or by compounding the mutant FGF23 with R176Q (64). The R176Q mutation, which was identified in a patient with ADHR, appears to stabilize hFGF23 by protecting a subtilisinfurin cleavage site (61). We have recently shown that the HFTC2 mutations S129P, S71G, and S129F impair O-glycosylation of FGF23, resulting in the intracellular accumulation of intact FGF23 (65). Loss-of-function mutations in GALNT3 cause hypophosphatemic familial tumoral calcinosis (HFTC1) in humans; equivalent findings are made in mice that are null for Galnt3 (66). O-glycosylation is essential for secretion of FGF23 by CHO cells (67) and for secretion of FGF7 by human embryonic kidney cells (HEK293) (68). Furthermore, expression of GALNT3 is stimulated by extracellular phosphate and suppressed by extracellular calcium and 1,25(OH)2D in HEK293 cells (68). This suggests that GALNT3 may be an important component of the regulatory mechanism of FGF23 secretion in vivo, which is disrupted by HFTC2 mutations.
FGF23 lacks heparan-sulfate (HS) binding-motifs, otherwise characteristic for the fibro-blast growth factor family. Lack of HS binding-motifs reduces matrix-binding and enables this ligand to function in an endocrine fashion (69). Four distinct genes encode FGF receptors (FGFR1–FGFR4).All FGFRs share a similar domain structure. The extracellular domain is made up of three immunoglobulin-like domains (D1–D3), followed by a single-pass transmembrane domain and an intracellular domain, which contains tyrosine kinase activity (70). A major tissue-specific alternative splicing event in the second half of D3 of FGFR1-3 creates epithelial lineage-specific “b” (FGFR1b-FGFR3b) and mesenchymal lineage-specific “c” (FGFR1c-FGFR3c) isoforms with distinct ligand-binding specificities (71). FGF23 requires KL as a coreceptor. Immunoprecipitation experiments, surface plasmon resonance (SPR) spectroscopy, and functional assays measuring the mitogenic response of BaF3 cells or activation of the MAPK-pathway in HEK293 cells have shown that KL forms a ternary complex with FGF23 and either FGFR1c, FGFR2c, FGFR3c, or FGFR4 (72). Recent work using neutralizing anti-FGF23 antibodies indicates that the N-terminal portion of FGF23 interacts with FGFR1c, whereas the C-terminus binds to KL, and both interactions appear to be important for bioactiv-ity in vitro and in vitro (73). Deletion of FGFR3 or FGFR4 in Hyp mice, a mouse model of human X-linked hypophosphatemia (XLH), did not correct the hypophosphatemia in those mice (74). Based on these data, it was concluded that neither FGFR3 nor FGFR4 mediates the phosphaturic activity of FGF23. A second study using mice with kidney-specific deletion of FGFR1 (75) suggests that FGFR1 mediates the phosphaturic effects of FGF23, although lack of suppression of 1,25(OH)2D in these animals may suggest that deletion of FGFR1 in the kidney was incomplete.
The site of action of FGF23 in the kidney is still controversial. Although FGF23 decreases expression of NPT2a and NPT2c and CYP27B1 activity in the proximal tubules (76), its coreceptor KL is expressed mainly in the distal tubules. Mice injected with FGF23 show phosphorylation of MAPK and expression of the early growth-response gene 1 (Egr-1) in the distal tubules (77). These findings suggest that either FGF23 uses a noncanonical signal-transduction pathway at the proximal tubules or it induces the secretion of an intermediary phosphatonin in the distal tubules, which acts in a paracrine fashion on the proximal tubules. KL may also have ligand-independent actions by regulating the expression of calcium channels (TRPV5) (78) and potassium channels (ROMK) at the distal tubules (79).
The effects of FGF23 on renal function lead to hypophosphatemia, decreased 1,25(OH)2D synthesis, and suppressed or inappropriately normal parathyroid function. Regulation of PTH secretion by the serum calcium independent of the effects of phosphate, FGF23, and KL at the parathyroid gland may explain why PTH is unable to compensate in states of FGF23 excess or deficiency, conditions in which serum calcium is decreased to inappropriately stimulate, or increased to inappropriately reduce, PTH secretion, respectively.
Much has been learned about the regulation of phosphate homeostasis through the discovery of genetic causes of human hypo-and hyperphosphatemic disorders. These findings were extended by the generation of a number of different animal models. Individuals suffering from familial hyperphosphatemic tumoral calcinosis (HFTC) have homozygous loss-of-function mutations in GALNT3 (80), FGF23 (63), or KL (18). Like mice that are null for Fgf23 (81), Galnt3 (66), or Kl (82), affected individuals suffer from hyperphosphatemia and increased 1,25(OH)2D levels due to the loss of FGF23 activity or end-organ resistance to FGF23, respectively. Consistent with an increased intestinal absorption of calcium from the gut, patients and mice with these genetic modifications display mild hypercalcemia, suppressed PTH levels, and hypercalciuria. The increased calcium-phosphorus product in these disorders is thought to cause the characteristic tissue calcifications. Ablation of Npt2a (83), Vdr (84), or Cyp27B1 (85) rescues the serum-biochemical abnormalities of Fgf23-null mice, and ablation of Cyp27B1 was also shown to rescue the Kl-null mice (86). Likewise, low-phosphate/low-vitamin D diets (87) can normalize the changes in mineral ion homeostasis of Fgf23- and Kl-null animals, although mineralization defects in the skeleton may persist (83). Conversely, increased FGF23 levels in most individuals affected by X-linked hypophosphatemia (88), autosomal dominant hypophosphatemic rickets (56), or autosomal recessive hypophosphatemia (89, 90) leads to renal phosphate wasting. More prominent elevations in FGF23 are observed in mice lacking PHEX (hyp mice) (91) or overexpressing FGF23 (92). Along with hypophosphatemia, these mice show low 1,25(OH)2D concentrations and low-normal serum calcium concentrations, which lead to the development of secondary hyperparathyroidism (92). Overexpression of PHEX systemically or targeted to osteoblasts of Hyp mice (93, 94) rescued the skeletal phenotype, while the serum-biochemical abnormalities of these animals persisted [with the exception of secondary hyperparathyroidism, which persisted with systemic overexpression of PHEX (94)]. This is in contrast to studies of Hyp mice crossed with mice carrying a reporter expressed under the control of the FGF23 promoter; these mice show elevated FGF23 gene transcription in bone. These findings suggest that the FGF23 excess in mice with an abnormal Phex function is originating from the skeleton (91). Furthermore, the FGF23-null mice and mice lacking PHEX and FGF23 (91), as well as mice lacking DMP1 and FGF23 (95), show indistinguishable phenotypes, suggesting that the regulatory actions of PHEX and DMP1 reside upstream of FGF23. Distinct from the FGF23-dependent forms of renal phosphate wasting, hereditary hypophosphatemic rickets with hypercalciuria (HHRH) (96, 97) leads to appropriate upregulation of CYP27B1, thereby increasing 1,25(OH)2D levels, resulting in absorptive hypercalciuria due to loss of SLC34A3/NaPi-IIc in the kidney. Mice lacking SLC34A1/NaPi-IIa also display FGF23-independent renal phosphate wasting and absorptive hypercalciuria (98). In contrast, mice lacking SLC34A3/NaPi-IIc show only mild hypercalcemia and absorptive hypercalciuria at an early age probably due to increased 1,25(OH)2D levels, but these animals do not develop hypophosphatemia, and thus have a milder phenotype than human individuals with HHRH (99).
Supplementary Material
SUMMARY POINTS.
PTH synthesis and secretion are up-regulated by low serum calcium and increased serum phosphate levels, and down-regulated by increased serum calcium and 1,25(OH)2D levels, and possibly by increased FGF23 levels. PTH acts through a G protein–coupled receptor, the PTHR1, to increase osteoblast activity (and indirectly osteoclast activity), to inhibit renal phosphate reabsorption, and to stimulate the synthesis of 1,25(OH)2D. The net effect of these actions is an increase of serum calcium levels and a decrease in serum phosphate levels.
FGF23 expression is up-regulated by increased serum phosphate and 1,25(OH)2D levels, and down-regulated, through yet unknown mechanisms, by PHEX and DMP1. FGF23 acts through one or more FGF-receptors, with Klotho as co-receptor, to inhibit renal phosphate reabsorption and to decrease circulating 1,25(OH)2D levels, and possibly to inhibit PTH secretion by the parathyroid glands. Its net effect is a reduction in serum phosphate and 1,25(OH)2D levels, which may result in hypocalcemia.
1,25(OH)2D expression is up-regulated by PTH and down-regulated by increased serum calcium and phosphate levels, and by increased FGF23 levels. 1,25(OH)2D acts through VDR/RXR dimers to stimulate the intestinal absorption of phosphate and to stimulate FGF23 synthesis and secretion by osteocytes, and possibly to inhibit PTH secretion by the parathyroid glands. Its net effect is an increase in serum calcium and phosphorus level.
FUTURE ISSUES.
How do dietary and serum phosphate regulate PTH, 1,25(OH)2D, and FGF23 secretion?
How do PHEX and DMP1 affect FGF23 gene expression and secretion?
How do FGF23 mutations impair negative feedback regulation of FGF23 synthesis and secretion?
Which FGF-receptors mediate the action of FGF23 in the parathyroids and in the kidney?
What is the physiological role of the sFRP-4, FGF7, and the sibling proteins MEPE and DMP1 in the regulation of phosphate homeostasis?
Glossary
- FGF23
fibroblast growth factor 23
- MEPE
matrix extracellular phosphoglycoprotein
- HFTC
hyperphosphatemic familial tumoral calcinosis
- CYP27B1
1-alpha-hydroyxylase, a vitamin D-activating enzyme expressed in the renal proximal tubule
- VDR
vitamin D receptor, forms heterodimer with RXR
- TRPV
transient receptor potential cation channel, subfamily V, members 5 and 6 are calcium-selective
- Pit-2
solute carrier family 20 (sodium-phosphate cotransporter), members 2, expressed ubiquitously
- PHEX
phosphate-regulating gene with homologies to endopeptidases on the X chromosome
- DMP1
dentin matrix protein 1
- GALNT3
UDP-N-Acetyl-α-D-galactosamine: polypeptide N-acetylgalactosaminyl-transferase, isoform 3 appears to be important for O-glycosylation of FGF23
- XLH
X-linked hypophosphatemia
- SLC34
solute carrier family 34 (sodium-phosphate cotransporter), members 1 and 3 are expressed in the proximal renal tubule, member 2 is expressed in the intestine
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Jüppner H, Gardella TJ, Brown EM, et al. Parathyroid hormone and parathyroid hormone-related peptide in the regulation of calcium homeostasis and bone development. 2006. pp. 1377–1417. See Ref. 100. [Google Scholar]
- 2.Berndt T, Kumar R. Novel mechanisms in the regulation of phosphorus homeostasis. Physiology. 2009;24:17–25. doi: 10.1152/physiol.00034.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilz DR, Gray RW, Dominguez JH, Lemann J., Jr Plasma 1,25-(OH)2-vitamin D concentrations and net intestinal calcium, phosphate, and magnesium absorption in humans. Am. J. Clin. Nutr. 1979;32:2052–2060. doi: 10.1093/ajcn/32.10.2052. [DOI] [PubMed] [Google Scholar]
- 4.Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflugers Arch. 2004;447:763–767. doi: 10.1007/s00424-003-1072-5. [DOI] [PubMed] [Google Scholar]
- 5.Bringhurst FR, Leder BZ. Regulation of calcium and phosphate homeostasis. 2006. pp. 805–843. See Ref. 100. [Google Scholar]
- 6.Sommer S, Berndt T, Craig T, Kumar R. The phosphatonins and the regulation of phosphate transport and vitamin D metabolism. J. Steroid Biochem. Mol. Biol. 2007;103:497–503. doi: 10.1016/j.jsbmb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Kemper B, Habener JF, Rich A, Potts JT., Jr Parathyroid secretion: discovery of a major calcium-dependent protein. Science. 1974;184:167–169. doi: 10.1126/science.184.4133.167. [DOI] [PubMed] [Google Scholar]
- 8.Moallem E, Kilav R, Silver J, Naveh-Many T. RNA-Protein binding and post-transcriptional regulation of parathyroid hormone gene expression by calcium and phosphate. J. Biol. Chem. 1998;273:5253–5259. doi: 10.1074/jbc.273.9.5253. [DOI] [PubMed] [Google Scholar]
- 9.Diaz R, Brown EM. Familial hypocalciuric hypercalcemia and other disorders due to calcium-sensing receptor mutations. 2006. pp. 1595–1609. See Ref. 100. [Google Scholar]
- 10.Wettschureck N, Lee E, Libutti SK, et al. Parathyroid-specific double knockout of Gq and G11 alpha-subunits leads to a phenotype resembling germline knockout of the extracellular Ca2+-sensing receptor. Mol. Endocrinol. 2007;21:274–280. doi: 10.1210/me.2006-0110. [DOI] [PubMed] [Google Scholar]
- 11.Quinn SJ, Kifor O, Kifor I, et al. Role of the cytoskeleton in extracellular calcium-regulated PTH release. Biochem. Biophys. Res. Commun. 2007;354:8–13. doi: 10.1016/j.bbrc.2006.12.160. [DOI] [PubMed] [Google Scholar]
- 12.Pollak MR, Brown EM, Estep HL, et al. Autosomal dominant hypocalcaemia caused by a Ca2+-sensing receptor gene mutation. Nat. Genet. 1994;8:303–307. doi: 10.1038/ng1194-303. [DOI] [PubMed] [Google Scholar]
- 13.Pollak MR, Brown EM, Wu Chou YH, et al. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993;75:1297–1303. doi: 10.1016/0092-8674(93)90617-y. [DOI] [PubMed] [Google Scholar]
- 14.Silver J, Russell J, Sherwood LM. Regulation by vitamin D metabolites of messenger ribronucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells. Proc. Natl. Acad. Sci. USA. 1985;82:4270–4273. doi: 10.1073/pnas.82.12.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters H, Neubuser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12:2735–2747. doi: 10.1101/gad.12.17.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding C, Buckingham B, Levine MA. Familial isolated hypoparathyroidism caused by a mutation in the gene for the transcription factor GCMB. J. Clin. Invest. 2001;108:1215–1220. doi: 10.1172/JCI13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, et al. The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichikawa S, Imel EA, Kreiter ML, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imura A, Tsuji Y, Murata M, et al. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 20.Kousteni S, Bilezikian JP. The cell biology of parathyroid hormone in osteoblasts. Curr. Osteoporos. Rep. 2008;6:72–76. doi: 10.1007/s11914-008-0013-9. [DOI] [PubMed] [Google Scholar]
- 21.Forster IC, Hernando N, Biber J, Murer H. Proximal tubular handling of phosphate: A molecular perspective. Kidney Int. 2006;70:1548–1559. doi: 10.1038/sj.ki.5001813. [DOI] [PubMed] [Google Scholar]
- 22.Alpern RJ. Cell mechanisms of proximal tubule acidification. Physiol. Rev. 1990;70:79–114. doi: 10.1152/physrev.1990.70.1.79. [DOI] [PubMed] [Google Scholar]
- 23.Friedman PA, Gesek FA. Calcium transport in renal epithelial cells. Am. J. Physiol. Ren. Physiol. 1993;264:F181–F198. doi: 10.1152/ajprenal.1993.264.2.F181. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien CA, Plotkin LI, Galli C, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One. 2008;3:e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvi L, Sims N, Hunzelman J, et al. Activation of the PTH/PTHrP receptor in osteoblastic cells has differential effects on cortical and trabecular bone. J. Clin. Invest. 2001;107:277–286. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schipani E, Lanske B, Hunzelman J, et al. Targeted expression of constitutively active PTH/PTHrP receptors delays endochondral bone formation and rescues PTHrP-less mice. Proc. Natl. Acad. Sci. USA. 1997;94:13689–13694. doi: 10.1073/pnas.94.25.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo J, Chung U-i, Kondo H, et al. The PTH/PTHrP receptor can delay chondrocyte hypertrophy in vivo without activating phospholipase C. Mol. Cell. 2002;3:183–194. doi: 10.1016/s1534-5807(02)00218-6. [DOI] [PubMed] [Google Scholar]
- 28.Miao D, He B, Karaplis A, Goltzman D. Parathyroid hormone is essential for normal fetal bone formation. J. Clin. Invest. 2002;109:1173–1182. doi: 10.1172/JCI14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai X, Miao D, Goltzman D, Karaplis AC. Early lethality in Hyp mice with targeted deletion of Pth gene. Endocrinology. 2007;148:4974–4983. doi: 10.1210/en.2007-0243. [DOI] [PubMed] [Google Scholar]
- 30.Lanske B, Karaplis AC, Luz A, et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 31.Zhang P, Jobert AS, Couvineau A, Silve C. A homozygous inactivating mutation in the parathyroid hormone/parathyroid hormone-related peptide receptor causing Blomstrand chondrodysplasia. J. Clin. Endocrinol. Metab. 1998;83:3365–3368. doi: 10.1210/jcem.83.9.5245. [DOI] [PubMed] [Google Scholar]
- 32.Soegiarto D, Kiachopoulos S, Schipani E, et al. Partial rescue of PTH/PTHrP receptor knockout mice by targeted expression of the Jansen transgene. Endocrinology. 2001;142:5303–5310. doi: 10.1210/endo.142.12.8553. [DOI] [PubMed] [Google Scholar]
- 33.Bounoutas GS, Tawfeek H, Frohlich LF, et al. Impact of impaired receptor internalization on calcium homeostasis in knock-in mice expressing a phosphorylation-deficient parathyroid hormone (PTH)/PTH-related peptide receptor. Endocrinology. 2006;147:4674–4679. doi: 10.1210/en.2006-0301. [DOI] [PubMed] [Google Scholar]
- 34.Burnett-Bowie SA, Henao MP, Dere ME, et al. Effects of hPTH(1-34) infusion on circulating serum phosphate, 1,25-dihydroxyvitamin D and FGF23 levels in healthy men. J. Bone Miner. Res. 2009;24:1681–1685. doi: 10.1359/JBMR.090406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta A, Winer K, Econs M, et al. FGF-23 is elevated by chronic hyperphosphatemia. J. Clin. Endocrinol. Metab. 2004;89:4489–4492. doi: 10.1210/jc.2004-0724. [DOI] [PubMed] [Google Scholar]
- 36.Wu JY, Purton LE, Rodda SJ, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc. Natl. Acad. Sci. USA. 2008;105:16976–16981. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins MT, Chebli C, Jones J, et al. Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J. Bone Miner. Res. 2001;16:806–813. doi: 10.1359/jbmr.2001.16.5.806. [DOI] [PubMed] [Google Scholar]
- 38.Brown WW, Jüppner H, Langman CB, et al. Hypophosphatemia with elevations in serum FGF23 in a child with Jansen’s metaphyseal chondrodysplasia. J. Clin. Endocrinol. Metab. 2008;94:17–20. doi: 10.1210/jc.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boullion R. Vitamin D: From photosynthesis, metabolism, and action to clinical application. 2006. pp. 1435–1463. See Ref. 100. [Google Scholar]
- 40.Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J. Biol. Chem. 2003;278:9843–9849. doi: 10.1074/jbc.M210490200. [DOI] [PubMed] [Google Scholar]
- 41.Li YC, Pirro AE, Amling M, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc. Natl. Acad. Sci. USA. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panda DK, Miao D, Tremblay ML, et al. Targeted ablation of the 25-hydroxyvitamin D 1 alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc. Natl. Acad. Sci. USA. 2001;98:7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitanaka S, Takeyama K, Murayama A, et al. Inactivating mutations in the 25-hydroxyvitamin D3 1alpha-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. N. Engl. J. Med. 1998;338:653–661. doi: 10.1056/NEJM199803053381004. [DOI] [PubMed] [Google Scholar]
- 44.Hughes MR, Malloy PJ, Kieback DG, et al. Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science. 1988;242:1702–1705. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- 45.Katai K, Miyamoto K, Kishida S, et al. Regulation of intestinal Na+-dependent phosphate co-transporters by a low-phosphate diet and 1,25-dihydroxyvitamin D3. Biochem. J. 1999;343(Pt. 3):705–712. [PMC free article] [PubMed] [Google Scholar]
- 46.Capuano P, Radanovic T, Wagner CA, et al. Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1alphaOHase-deficient mice. Am. J. Physiol. Cell Physiol. 2005;288:C429–C434. doi: 10.1152/ajpcell.00331.2004. [DOI] [PubMed] [Google Scholar]
- 47.Amling M, Priemel M, Holzmann T, et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140:4982–4987. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- 48.Kolek OI, Hines ER, Jones MD, et al. 1alpha, 25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in arenal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 49.Demay MB, Kiernan MS, DeLuca HF, Kronenberg HM. Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA. 1992;89:8097–8101. doi: 10.1073/pnas.89.17.8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Canaff L, Hendy GN. Human calcium-sensing receptor gene. Vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. J. Biol. Chem. 2002;277:30337–30350. doi: 10.1074/jbc.M201804200. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki Y, Landowski CP, Hediger MA. Mechanisms and regulation of epithelial Ca2+ absorption in health and disease. Annu. Rev. Physiol. 2008;70:257–271. doi: 10.1146/annurev.physiol.69.031905.161003. [DOI] [PubMed] [Google Scholar]
- 52.Nishi H, Nii-Kono T, Nakanishi S, et al. Intravenous calcitriol therapy increases serum concentrations of fibroblast growth factor-23 in dialysis patients with secondary hyperparathyroidism. Nephron Clin. Pract. 2005;101:c94–c99. doi: 10.1159/000086347. [DOI] [PubMed] [Google Scholar]
- 53.Yu X, Sabbagh Y, Davis SI, et al. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone. 2005;36:971–977. doi: 10.1016/j.bone.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Masuyama R, Stockmans I, Torrekens S, et al. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J. Clin. Invest. 2006;116:3150–3159. doi: 10.1172/JCI29463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem. Biophys. Res. Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 56.ADHR Consort. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 57.Shimada T, Mizutani S, Muto T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl. Acad. Sci. USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J. Clin. Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burnett SM, Gunawardene SC, Bringhurst FR, et al. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J. Bone Miner. Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 60.Shimada T, Muto T, Urakawa I, et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–3182. doi: 10.1210/endo.143.8.8795. [DOI] [PubMed] [Google Scholar]
- 61.Kato K, Jeanneau C, Tarp MA, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem. 2006;281:18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 62.Garringer HJ, Malekpour M, Esteghamat F, et al. Molecular genetic and biochemical analyses of FGF23 mutations in familial tumoral calcinosis. Am. J. Physiol. Endocrinol. Metab. 2008;295:E929–E937. doi: 10.1152/ajpendo.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benet-Pagès A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum. Mol. Genet. 2005;14:385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 64.Larsson T, Davis SI, Garringer HJ, et al. Fibroblast growth factor-23 mutants causing familial tumoral calcinosis are differentially processed. Endocrinology. 2005;146:3883–3891. doi: 10.1210/en.2005-0431. [DOI] [PubMed] [Google Scholar]
- 65.Bergwitz C, Banerjee S, Abu-Zahra H, et al. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack human fibroblast growth factor 23 secretion and tumoral calcinosis. J. Clin. Endocrinol. Metab. 2009;94(11):4267–4274. doi: 10.1210/jc.2009-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ichikawa S, Sorenson AH, Austin AM, et al. Ablation of the Galnt3 gene leads to low circulating intact Fgf23 concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology. 2009;150:2543–2550. doi: 10.1210/en.2008-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frishberg Y, Ito N, Rinat C, et al. Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J. Bone Miner. Res. 2007;22:235–242. doi: 10.1359/jbmr.061105. [DOI] [PubMed] [Google Scholar]
- 68.Chefetz I, Kohno K, Izumi H, et al. GALNT3, a gene associated with hyperphosphatemic familial tumoral calcinosis, is transcriptionally regulated by extracellular phosphate and modulates matrix metalloproteinase activity. Biochim. Biophys. Acta. 2009;1792:61–67. doi: 10.1016/j.bbadis.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 71.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu X, White KE. Fibroblast growth factor 23 and its receptors. Ther. Apher. Dial. 2005;9:308–312. doi: 10.1111/j.1744-9987.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 73.Yamazaki Y, Tamada T, Kasai N, et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J. Bone Miner. Res. 2008;23:1509–1518. doi: 10.1359/jbmr.080417. [DOI] [PubMed] [Google Scholar]
- 74.Liu S, Vierthaler L, Tang W, et al. FGFR3 and FGFR4 do not mediate renal effects of FGF23. J. Am. Soc. Nephrol. 2008;19:2342–2350. doi: 10.1681/ASN.2007121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gattineni J, Bates C, Twombley K, et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am. J. Physiol. Renal. Physiol. 2009;297:F282–F291. doi: 10.1152/ajprenal.90742.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Strom TM, Jüppner H. PHEX, FGF23, DMP1 and beyond. Curr. Opin. Nephrol. Hypertens. 2008;17:357–362. doi: 10.1097/MNH.0b013e3282fd6e5b. [DOI] [PubMed] [Google Scholar]
- 77.Farrow EG, Davis SI, Summers LJ, White KE. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J. Am. Soc. Nephrol. 2009;20:955–960. doi: 10.1681/ASN.2008070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang Q, Hoefs S, van der Kemp AW, et al. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 79.Cha SK, Hu MC, Kurosu H, et al. Regulation of renal outer medullary potassium channel and renal K+ excretion by Klotho. Mol. Pharmacol. 2009;76:38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Topaz O, Shurman D, Bergman R, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat. Genet. 2004;36:579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 81.Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 83.Sitara D, Kim S, Razzaque MS, et al. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 2008;4:e1000154. doi: 10.1371/journal.pgen.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hesse M, Frohlich LF, Zeitz U, et al. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26:75–84. doi: 10.1016/j.matbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 85.Razzaque MS, Sitara D, Taguchi T, et al. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009;75:1166–1172. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stubbs JR, Liu S, Tang W, et al. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality infibroblastic growth factor 23 null mice. J. Am. Soc. Nephrol. 2007;18:2116–2124. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 88.HYP Consort. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat. Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 89.Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat. Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feng JQ, Ward LM, Liu S, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu S, Zhou J, Tang W, et al. Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 2006;291:E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 92.Larsson T, Marsell R, Schipani E, et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 93.Liu S, Guo R, Tu Q, Quarles LD. Overexpression of PHEX in osteoblasts fails to rescue the Hyp mouse phenotype. J. Biol. Chem. 2002;277:3686–3697. doi: 10.1074/jbc.M107707200. [DOI] [PubMed] [Google Scholar]
- 94.Erben RG, Mayer D, Weber K, et al. Overexpression of human PHEX under the human beta-actin promoter does not fully rescue the Hyp mouse phenotype. J. Bone Miner. Res. 2005;20:1149–1160. doi: 10.1359/JBMR.050212. [DOI] [PubMed] [Google Scholar]
- 95.Liu S, Zhou J, Tang W, et al. Pathogenic role of Fgf23 in Dmp1-null mice. Am. J. Physiol. Endocrinol. Metab. 2008;295:E254–E261. doi: 10.1152/ajpendo.90201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lorenz-Depiereux B, Benet-Pagès A, Eckstein G, et al. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am. J. Hum. Genet. 2006;78:193–201. doi: 10.1086/499410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bergwitz C, Roslin NM, Tieder M, et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am. J. Hum. Genet. 2006;78:179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beck L, Karaplis AC, Amizuka N, et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl. Acad. Sci. USA. 1998;95:5372–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Segawa H, Onitsuka A, Kuwahata M, et al. Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J. Am. Soc. Nephrol. 2009;20:104–113. doi: 10.1681/ASN.2008020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.DeGroot LJ, Jameson JL, editors. Endocrinology. Philadelphia: Elsevier Saunders; 2006. [Google Scholar]
RELATED RESOURCES
For a detailed discussion of the receptors and signal transduction cascades for PTH, 1,25(OH)2D, and FGF23 the reader is referred to several recent reviews:
- 1.Strom TM, Jüppner H. PHEX, FGF23, DMP1 and beyond. Curr. Opin. Nephrol. Hypertens. 2008;17:357–362. doi: 10.1097/MNH.0b013e3282fd6e5b. [DOI] [PubMed] [Google Scholar]
- 2.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J. Clin. Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White KE, Larsson TE, Econs MJ. The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis: frizzled related protein-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23. Endocr. Rev. 2006;27:221–241. doi: 10.1210/er.2005-0019. [DOI] [PubMed] [Google Scholar]
- 4.Kuro-o M. Endocrine FGFs and Klothos: emerging concepts. Trends Endocrinol. Metab. 2008;19:239–245. doi: 10.1016/j.tem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Gardella TJ. Mimetic ligands for the PTHR1: approaches, developments, and considerations. IBMS BoneKEy. 2009;6:71–85. [Google Scholar]
- 6.Kronenberg HM. PTH regulates the hematopoietic stem cell niche in bone. Adv. Exp. Med. Biol. 2007;602:57–60. doi: 10.1007/978-0-387-72009-8_7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.