Abstract
Neuroendocrine hormones such as growth hormone (GH) and prolactin (PRL) have been demonstrated to accelerate the recovery of the immune response after chemotherapy and bone marrow transplantation and to enhance the restoration of immunity in individuals infected with HIV and in normal individuals with compromised immune systems associated with aging. As the mechanism of action of these hormones has been elucidated, it has become clear that they are integral members of the immunological cytokine/chemokine network and share regulatory mechanisms with a wide variety of cytokines and chemokines. The members of this cytokine network induce and can be regulated by members of the suppressor of cytokine signaling (SOCS) family of intracellular proteins. In order to take advantage of the potential beneficial effects of hormones such as GH or PRL, it is essential to take into consideration the overall cytokine network and the regulatory effects of SOCS proteins.
Keywords: neuroendocrine hormone, growth hormone, prolactin, suppressors of cytokine signaling (SOCS) proteins, inflammation, aging, ghrelin, immune recovery, HIV/AIDS, bone marrow transplantation (BMT)
Introduction
This review focuses on the effects of GH, PRL and GH-related molecules on the immune system, particularly as these molecules are used to alter immune competency. The review will summarize some of the experimental model systems and clinical applications in which GH and GH-related molecules have been shown to have positive effects on the immune system. We will then summarize examinations of the mechanisms of GH activity. Recent studies of GH activity have clearly shown that GH must be considered in a broader context that includes cytokines and chemokines. All of these molecules share common regulatory mechanisms mediated by the suppressor of cytokine signaling (SOCS) family of molecules. We will also illustrate some of the ways in which GH-related molecules, cytokines and chemokines can interact with each other via the regulatory effects of SOCS proteins. Finally, we will summarize recent studies that have taken into account the interplay among these systems and then devised strategies to circumvent problems such as GH-resistance.
In vivo and in vitro findings demonstrating the utility of GH and PRL
Bone marrow transplantation
The pituitary-derived hormones GH, PRL and the GH-stimulated molecule insulin-like growth factor-1 (IGF-1) have all been demonstrated to accelerate recovery of the immune system following transplantation of various types of cells. For example, Murphy, et al., [1] transplanted murine thymocytes or human PBL into SCID mice and demonstrated that treatment with recombinant human GH (rhGH) stimulated increased engraftment of cells from both species. Other studies from this group showed that GH could exert effects both on hematopoiesis in the bone marrow (BM) [2] and on thymopoiesis [3]. Subsequently, this group [4] demonstrated that rhGH accelerated hematopoietic recovery after syngeneic BM transplantation (BMT). Shortly thereafter, this group [5] reviewed their previous studies and concluded that treatment with GH or PRL, although less potent than agents such as IL-2 or G-CSF, demonstrated real promise in accelerating recovery after BMT. More recent studies [5] have examined the effects of PRL in more detail and confirmed its effectiveness in accelerating recovery after congenic or syngeneic BMT.
The effects of GH and PRL can also be demonstrated in vitro in fetal thymocyte organ cultures (FTOC) under serum-free conditions in which one can carefully control hormone exposure. Carreno et al., [6] demonstrated that PRL functions in FTOC to increase thymocyte numbers. Similarly, we have compared GH and PRL and demonstrated they increase thymocyte numbers as illustrated in Figure 1. Other studies have extended the model systems to examine the effects of rhGH or its downstream effector molecule IGF-1 on allogeneic BMT in mice. In one study [7], T-cell depleted C57Bl/6 (H2b) BM cells were transplanted into lethally irradiated Balb/c (H2d) recipients that also received daily injections of saline (control) or 20 micrograms of rhGH for 4 weeks after transplantation. The rhGH-treated mice exhibited accelerated recovery of total thymocytes, B cells and both CD4+ and CD8+ T cells. Although the differences between rhGH-treated and controls essentially disappeared by day 28 after BMT, the treated mice showed faster rejection of third-party allografts.
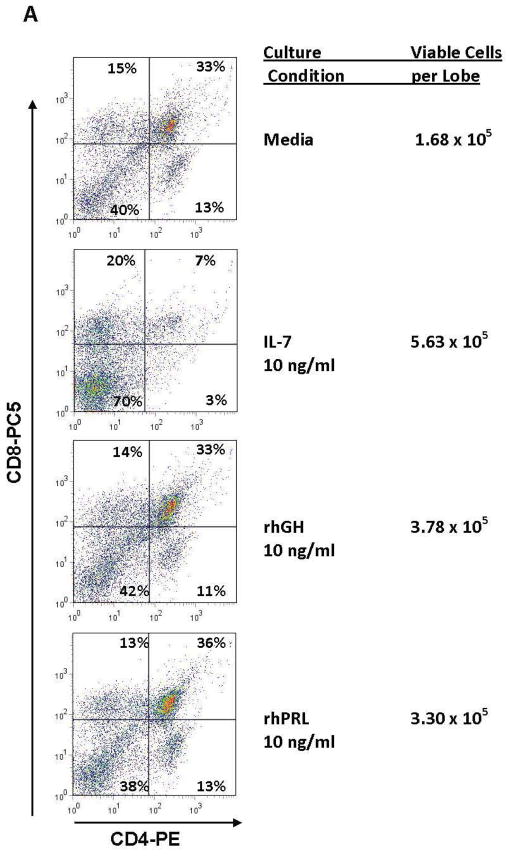
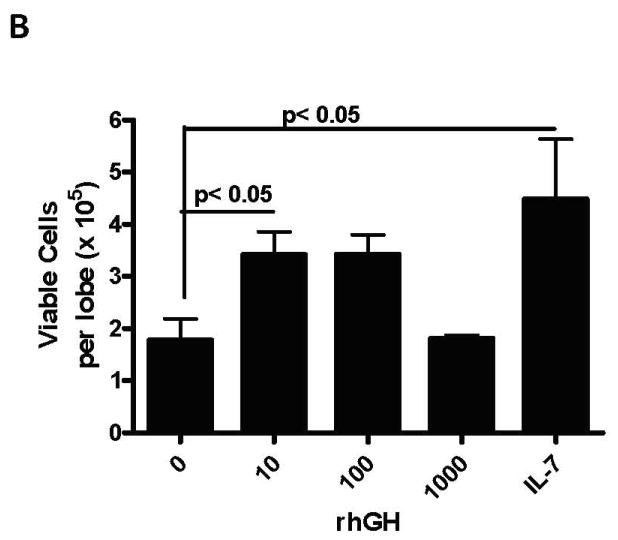
Figure 1. Promotion of Thymopoiesis by rhGH or rhPRL in Fetal Thymic Organ Cultures.
Thymic lobes from day 14.5 gestation age C57BL/6 fetal mice were cultured for 13 days over transwell membranes containing serum-free media (Stemspan Expansion Medium, StemCell Technologies, Vancouver, CA) with the indicated concentration of rhGH (Serono Inc, Rockland, MA), rhPRL (kindly provided by Genzyme Corp., Cambridge, MA) or 10 ng/ml rhIL-7. At the end of the culture, cells were dissociated from the lobes, viable cells numbers were assessed using the Viacount method (Guava Technologies, Hayward, CA), labeled with CD45-FITC, CD4-PE and CD8-PC5 and analyzed by flow cytometry. A. Representative dotplots demonstrating normal distribution of thymocytes in lobes cultured with rhGH or rhPRL. Cells were gated on CD45+ expression. B. Significant increases in total viable cells in FTOC cultured with 10 ng/ml of rhGH (ANOVA with Dunnett’s post-hoc test p< 0.05).
GH has also been shown to work in allogeneic settings. For example, the van den Brink laboratory [8] examined the effect of rhIGF-1 that was continuously given (4mg/kg/day) from days 14–28 after three models of allogeneic BMT, namely, MHC matched/background disparate and two models of parent into F1. In these more clinically relevant models, rhIGF-1 accelerated recovery of lymphoid and myeloid (granulocytes) cells, but did not exacerbate GvHD. Thus, in some murine models, GH, or its principal effector molecule IGF-1, can hasten the recovery of T and B cells after allo-BMT without increasing GvHD.
Another area in which GH treatment has been examined is recovery of immune function after depletions caused by chemotherapy. Murphy, et al., [2] performed an early study examining the effects of GH on recovery following azidothymidine (AZT), a reverse transcriptase inhibitor that was used as the first anti-HIV therapy. In the study, normal and SCID Balb/c mice were first treated with rhGH alone, which increased the content of hematopoietic progenitor cells. Mice were then treated with AZT followed by rhGH, which can also bind to the murine PRL-receptor [9], or ovine GH (ovGH) which does not bind the PRL-R in mice. Both types of GH accelerated recovery from AZT toxicity demonstrating that the effect was mediated through the GH receptor. In clinical settings, GH therapy after chemotherapy raises many concerns due to the possible growth-promoting effects on tumor cells. In a more recent study [10], patients with hematological malignancies who had received intensive chemotherapy were given rhGH (500 micrograms/day) or placebo for 6 weeks. There was no difference in the time to relapse between groups and the GH-treated patients had accelerated platelet recovery. Thus, there may be applications in which GH can be used to treat immune depletion following chemotherapy. However, significant questions remain concerning the possible effects of GH on tumor cells although the cited studies detected no such effects. Although it can be noted that in one clinical trial [11] in which patients were given rhGH or placebo after surgery for gastrointestinal malignancies, there was no evidence that GH had adverse effects and, in fact, the GH-treated patients has slightly increased survival times and time to relapse.
Treating HIV/AIDS
There has been considerable interest in using GH to treat HIV-infected patients, both to reverse the wasting that can occur and to attempt to restore the immune system. GH received FDA approval for the treatment of HIV patients in 1995 [12] and a number of studies have been performed since that time. Many of the early studies had limited or mixed results. For example, Nguyen et al., [13] gave rhGH or rhIGF-1 to patients with CD4 counts of 100–400/microliter for 12 weeks. The treated patients did show modest weight gains (~4kg), but there was no significant increase in CD4+ T cell numbers. One patient demonstrated a significant increase in IL-2 production elicited in vitro by antigens or polyclonal activators. These early studies of GH in HIV patients were summarized in 2004 by Kelley [14]. As noted in that review, HIV patients typically required 5–10 fold higher “pharmacological” amounts of GH to achieve results that could be obtained in non-HIV infected GH-deficient patients by “physiological” amounts of GH. Krentz, et al., [15] demonstrated that AIDS patients did not show elevations in IGF-1 if given physiologic amounts of GH, but required 5–10 fold higher amounts. It was subsequently reported that GH resistance in AIDS was related to ongoing inflammation [16]. Other studies [17, 18] showed similar resistance to IGF-1 indicating that even if sufficient GH could be given to induce IGF-1 that resistance could still occur. More recent studies have elucidated some of the connections between inflammation and GH-mediated effects and these connections will be more fully discussed below.
Since those earlier studies, highly aggressive anti-retroviral therapy (HAART) has resulted in much more effective control of HIV infection and this improved viral control has almost certainly reduced the level of inflammation. Since the widespread use of HAART, there have been more studies showing that GH can improve the immune status of HIV patients. For example, an elegant study by Napolitano, et al., [19] showed by imaging that GH treatment increased thymic mass and this increase was accompanied by increase in circulating CD4 cells. More recent studies by this same group have not only demonstrated increased T cell numbers but also improved T cell function (personal communication). Pires et al [20] have also shown that rhGH given to patients also receiving HAART resulted in increased T cell numbers and increased their in vitro antigen-specific responsiveness. The potential restorative effects of GH have not been limited to T cells. The same group [21] also demonstrated that GH could increase the number and function of CD56Bright NK cells, which are typically depleted in patients receiving HAART alone. Thus, under current conditions in which more effective anti-retroviral therapy has lowered the baseline state of inflammation in HIV-infected individuals, it appears that GH therapy could be effective in restoring the immune response.
Aging
Decreased levels of GH and thymic involution occur with increasing age leading to experiments to test whether GH could reverse the adverse effects on the immune system. Kelley, et al [22] examined the effect of GH in an interesting model in which pituitary tumor cells that produced GH and PRL were implanted subcutaneously into 16 and 22 month old rats. When the rats were sacrificed and examined two months later, the treated rats had readily detectable thymus glands whereas thymus tissue was essentially undetectable in the untreated animals. Treated rats had more T cells and more helper type T cells and their splenocytes had substantially higher responses to lectin stimulation. More recently, the Kelley group [23] performed a similar but more extensive set of studies on 24 month old rats that received the same type of GH-producing tumor cells or rhGH. In addition to the effects on GH on thymic tissue, they also observed that GH increased hematopoietic precursor cells and reduced adipocytes in the bone marrow and resulted in more extensive extramedullary hematopoiesis. The Dorshkind laboratory [24] has examined the ability of IGF-1 to improve the effectiveness of BMT into 18 month old mice. In their model system, 18 month old BALB/c mice were given IGF-1 for 2 weeks or were sublethally irradiated and transplanted with syngeneic young BM cells alone or with IGF-1. IGF-1 given alone increased thymic cellularity by approximately two-fold. However, there was a much greater increase in thymic function in mice that were irradiated, given two injections of BM cells 4 hr and 7 days post irradiation, followed by implantation of a minipump loaded with saline or IGF-1 three weeks after the second dose of cells. A group at Merck [25] has extended these types of studies by examining the effects of a non-peptide agent that stimulates GH secretion. This drug was given for three weeks to 5–6 week old (young) mice or 16–24 month old (aged) mice. In the young mice, the drug stimulated increased numbers of blood lymphocytes, but had no detectable effect on T or B cell proliferative responses. On the other hand, the treated aged mice did not have increased numbers of blood lymphocytes but did have increased thymic cellularity and displayed increased resistance to a transplantable tumor (EL4). Thus, these studies have shown that treatment with a drug that stimulates GH secretion, treatment with GH itself or treatment with IGF-1, the downstream effector of GH, can all have positive effects in restoring aspects of the aged immune system.
Mechanism of GH actions and Regulation
While detailed accounts of the mechanisms by which GH and its upstream and downstream related molecules act on cells and how these signaling pathways are regulated are well beyond the scope of this review, it is important to emphasize that GH stimulated effects share mechanisms with a large family of cytokines and chemokines. The shared pathways suggest that these molecules have common regulatory mechanisms. In fact, there are shared regulatory components and these components exhibit a high degree of cross-talk such that feedback mechanisms stimulated by inflammatory cytokines, e.g., IL-6 or TNFα, can also exert their inhibitory effects on other pathways including GH. This section will summarize these regulatory processes and point out observed biological effects in which these processes may be directly involved.
GH-mediated stimulation
Detailed studies of GH action have been possible because GH itself was cloned over 20 years ago [26], [27] [28] and the GH receptor (GHR) was cloned shortly thereafter [29], [30]. The human GHR (hGHR) is a type I transmembrane protein of 687 residues that has no sequence homology with known kinases in its cytoplasmic region. The most widely accepted model of GH-stimulated GHR activation involves GH binding initiating dimerization of the GHR [31]. However, a more recent model is based upon the GHR being in a constitutive dimeric state. In this model, the binding of GH to the existing GHR dimer causes a rotation within the complex that creates the sites for kinase binding discussed below [32].
Regardless of whether GH-stimulated GHR dimerization is an essential step, there continues to be agreement that GH-GHR interaction leads to the creation of a binding site for a member of the Janus kinase family (JAK2) of tyrosine kinases [33]. JAK2 phosphorylates itself and the GHR creating in turn a binding site for members of the signal transducers and activators of transcription (STAT) family of transcription factors via their SH2 domains. Activated JAK2 is known to phosphorylate at least four members of the STAT family, namely STAT 1, 3, 5a and 5b [34]. Phosphorylated STATs dimerize, move to the nucleus and become activators of transcription factors. Activated JAK2 can also mediate effects via the Ras-mitogen-activated protein (Ras-MAP) kinase pathway leading to expression of several transcription factors including fos, jun and egr1 [35] [36].
The possibility of cross-talk among GH and various cytokines is partially based on the fact that the JAK-STAT pathway is involved in numerous receptor-mediated signaling processes as summarized by Imada and Leonard [37]. In Table 1 of that review, it is noted that JAK2 is involved in signaling by GH, PRL, erythropoietin (EPO), IFN-γ, IL-13, IL-3, IL-5, IL-6, IL-12 and GM-CSF among others. Other members of the JAK family, (i.e., JAK1, JAK3, TYK2), are involved in signaling by other cytokines including IL-2, IL-4, IL-7 and IL-15 that are central players in many aspects of the immune response. Thus, regulatory mechanisms that affect signaling by one of these molecules could also affect others.
Suppressor of cytokine signaling (SOCS) proteins
In the late 1990’s, a group of molecules now known as the suppressor of cytokine signaling (SOCS) family was described and these molecules comprise a common regulatory mechanism for GH, cytokines and chemokines. The SOCS literature is voluminous consisting of more than 1000 papers including more than 150 reviews. Thus, our goal here is to point out some of the ways in SOCS proteins can regulate and cross-regulate GH relevant processes in immunity.
The SOCS family consists of 8 members, i.e., SOCS1-7 and CIS, that all share two structural features, namely an SH2 domain and a region known as the SOCS box [38]. Production of the SOCS proteins is induced by cytokine signaling via the JAK-STAT pathway and these proteins can then mediate feedback inhibition of the JAK-STAT pathway via several mechanisms. In addition to the common domains, SOCS1 and SOCS3 uniquely contain a kinase inhibitory region (KIR) that can bind to JAKs and directly inhibit JAK activity [39], [40]. The SH2 domains of the SOCS proteins mediate binding to phosphorylated JAKs and other intermediates and in turn interfere with STAT binding and activation. Finally, the SOCS box domain is involved in the ubiquitin pathway that leads to degradation via proteasomes [41]. With that broad overview in mind, we can now point out some specific examples of how SOCS family members can be involved in GH relevant processes in immune cells.
It is first important to note that GH interaction with the GHR stimulates the production of SOCS2, SOCS3 and CIS [42] which in turn inhibit GH stimulation. The mechanisms of negative regulation of GH stimulations have recently been reviewed in detail [43]. In addition to the JAK inhibition mediated by the KIR domains, the SOCS box domains can down-regulate GHR expression by ubiquitination and targeting to proteasomes for degradation. Gene knockout mice have helped to clarify the relative importance of the SOCS proteins in GH mediated effects. For example, it is clear that SOCS2 is a key player in normal GH feedback inhibition since Socs2−/− mice exhibit gigantism and are 30–40% larger than normal littermates [44] [45]. However, there are other layers of complexity since SOCS proteins can also target SOCS proteins for degradation [46]. Thus, Socs2Tg mice that over express SOCS2 are not dwarf mice but instead are also larger than normal littermates [47] [48]. It has been interpreted that SOCS2 over expression leads to accelerated degradation of one or more other members of the SOCS family that can also provide feedback inhibition to GH signaling. SOCS3 is a likely target since it has been demonstrated that GH leads to SOCS3 upregulation which in turn renders cells GH refractory by blocking JAK2 activation [49]. It should also be noted that the response to PRL, which utilizes the JAK-STAT pathway, can also be regulated by SOCS proteins [50]. Thus, the SOCS proteins, while not the only mechanisms of GH regulated effects, are clearly important. Furthermore, if SOCS proteins are upregulated by activation through other cytokine systems, then the result can be an effect on GH signaling. These commonalities in signaling were recognized several years ago to place both GH and PRL in the “cytokine network” [51].
SOCS effects on receptor tyrosine kinases
The network of regulatory effects mediated via SOCS proteins can also extend to receptor tyrosine kinases including the IGF-1 receptor that mediates the effects stimulated by IGF-1 produced as the result of GH signaling. It was recognized several years ago that SOCS2 could interact with and inhibit the IGF-1R [52]. It has been subsequently demonstrated that not only could IGF-1R be affected by SOCS proteins, but that IGF-1R signaling could upregulate the expression of SOCS3 directly [53]. Stem cell factor (SCF) receptor (kit) is another receptor tyrosine kinase that is relevant to hematopoietic recovery since it is expressed on a variety of stem cells. SOCS1 can block signaling through kit [54] and this group [55] later showed that SOCS6 may be specific for receptor tyrosine kinases. Therefore, SOCS proteins can be involved in cell signaling in hematopoietic cells from the earliest stage of HSCs through cells expressing GH or PRL receptors to cells responding to IGF-1, the downstream effector molecule of GHR stimulation.
Distribution of GH and PRL receptors
Bearing in mind that GH-related effects on the immune system can be affected by and can affect many other systems, how might GH and/or PRL mediate the biological effects summarized above? One important consideration in determining whether the effects are direct and/or indirect is the distribution of receptors for GH, PRL and IGF-1. These receptors are widely expressed on many tissues including cells of the immune system. For example, Badolato et al. [56] used fluorescent antibody to GHR and fluorescent hGH and flow cytometry to show that GHR and GHR binding activity was present on human T, NK and B cells with higher levels of expression on B cells. Likewise, Gagnerault et al. [57] used antibody to murine GHR and flow cytometry to examine their expression on lymphoid cells. GHR expression was found on all lineages of hematopoietic cells in the bone marrow and on several subsets in the thymus. In the spleen and lymph nodes, substantial fractions of both T and B cells expressed GHR molecules. T cell activation with Con-A or anti-CD3 caused increased expression. This group [58] subsequently reported that cells expressing GHR also expressed receptors for PRL. Similarly, GHR mRNA was found to be widely distributed in the lymphoid tissues of fowl [59]. Chen, et al., [60] examined cells from bovine fetal spleen and thymus tissue and found GHR expression in early to mid-gestational age with expression diminishing in later gestation. In vitro experiments showed that incubation of GHR+ cells with GH resulted in downregulation of GHR expression. GHR expression has also been shown on human lymphoid and myeloid cell lines [61]. Pankov [62] reviewed these and other studies that showed that lymphoid tissues including the spleen, thymus, lymph nodes and peripheral blood cells expressed GH, GHR and IGF-1, the downstream effector of GH. Thus, GH and PRL can act directly on hematopoietic cells including lymphocytes to mediate effects such as enhanced proliferation [63] [64] and/or inhibition of apoptosis, e.g., [65], [66], [67], [68].
GH effects on the thymus
Because of the great interest in the thymic-dependent generation of T cells, there has been particular attention devoted to examining the ways in which GH, PRL and IGF-1 could mediate effects on the thymus. As we illustrated above (Fig. 1), GH and PRL can act directly on isolated fetal thymic tissue to expand the number of thymocytes. From studies of the mechanism(s) responsible, it has emerged that these molecules could act on thymic function in several ways. First, as noted above, GH, PRL and/or IGF-1 can directly expand the number of thymocytes by enhancing their proliferation [63] [64]. Second, it was recognized several years ago that GH and IGF-1 could stimulate the growth of thymic epithelial cells (TEC) which are needed to support thymic function, e.g., [69], [70]. Expanded numbers of TEC produce increased amounts of extracellular matrix material such as laminin that are essential for thymocyte adherence [71]. Third, PRL can act indirectly to support the proliferation of thymocytes by directly stimulating the production of IL-2. Carreno, et al., [6] demonstrated that thymocytes expressed PRL receptors and then showed that PRL-stimulated proliferation could be blocked by an antibody to the IL-2R alpha chain (CD25) indicating that PRL stimulated a process involving IL-2/IL-2R interactions. Fourth, GH can enhance the production of the chemokine CXCL12 (formerly known as stromal derived factor-1 or SDF-1) by TEC leading to increased chemotaxis of CXCR4 expressing thymocytes [72,73]. It should be noted that CXCR4 is a JAK/STAT dependent receptor that is also affected by SOCS proteins [74] and some of the resulting implications will be further discussed below. Thus, there are multiple mechanisms by which PRL, GH and its downstream effector IGF-1 can increase thymic mass and improve thymic function. These mechanisms can help explain the biological effects that were summarized above.
CXCR4 and CXCL12 interactions
The effects mediated by the interaction of CXCL12 with its receptor CXCR4 are important in thymic function, as noted above, and equally important in the interactions of stem cells with bone marrow stromal cells. In the context of HIV infection, CXCR4 is also an important molecule since it is utilized as a binding protein by HIV along with CD4 and CCR5 leading to impaired thymic function, e.g., [75]. The interaction of CXCL12 with CXCR4 is mediated through the JAK/STAT pathway [76] and is inhibited by SOCS3 [77]. The inhibition is not associated with a loss of CXCR4 expression but SOCS3 acts in this system primarily to block JAK binding and activation [77]. These observations are of importance because conditions that elevate SOCS3 expression, such as inflammatory cytokines or GH itself, could lead to impaired CXCL12-CXCR4 mediated functions illustrating yet another way in which inflammation can interfere with T cell generation and activity. However, these activities can also be utilized to good effect in some situations. For example, Pello et al. [78] has recently described the use of GH to mobilize stem cells from the bone marrow into the circulation. In their system, GH was shown to increase both SOCS1 and SOCS3 expression which in turn inhibited the chemotactic activity normally stimulated by the binding of CXCL12 to its receptor CXCR4 leading to the release of stem cells from the bone marrow. SOCS-mediated effects on CXCR4 signaling are likely also involved in G-CSF stimulated stem cell mobilization since the G-CSF receptor also acts through the JAK/STAT pathway [79].
Ghrelin
Although the discussion to this point has focused primarily on the direct and downstream effects of PRL and GH and how these effects interact with regulate and can be regulated by cytokines and chemokines, upstream molecules that stimulate GH production have interesting and somewhat unexpected effects that should also be noted. In some cases, upstream effector molecules produce exactly the sorts of effects that one would expect as illustrated by two types of findings. The production of GH by pituitary cells is stimulated by another pituitary product known as growth hormone releasing hormone (GHRH), e.g., reviewed by Mayo, et al. [80]. Subsequent studies examining transgenic mice over-expressing either GH or GHRH [81] have shown that both types of mice had similar phenotypes including enlarged spleens and lymph nodes. Similarly, it was noted above that a synthetic agent that stimulated GH production independently of the GHRH receptor produced effects resembling those produced by GH [25]. In these cases, upstream stimulation of the GH system produced predictable and expected results. However, additional studies with the synthetic GH secretagogue led to characterization of a new receptor and its natural ligand, i.e., ghrelin, that have produced unexpected results and that have opened new areas of study that will be briefly summarized.
Howard, et al. [82] in 1996 described how small molecule GH secretagogues were used to clone and characterize a receptor in pituitary cells in addition to the GHRH receptor that stimulated GH secretion. Subsequently, Kojima, et al. [83] isolated a peptide of 28 residues from rat stomach that bound the GHS-R which they named ghrelin. The peptide is unusual in that the serine residue in position 3 is n-octanoylated and this acyl group is required for activity. Ghrelin was shown to function in humans also and to be a more powerful stimulus for GH production that GHRH [84]. Although ghrelin was initially isolated from rat and human stomach tissue, later studies showed that tissues other than the stomach could also produce it. For example, mRNA for ghrelin and for its receptor were found in all human tissues that were examined including lymph nodes, circulating lymphocytes and spleen [85]. Ghrelin has generated tremendous interest since its initial description and has been recently reviewed, e.g., [86]. Despite the extensive studies, there are basic questions remaining concerning ghrelin and its receptor. For example, adenosine is a partial agonist for the ghrelin receptor that binds to the receptor via a different site [87] and that mediates signals through different pathways [88]. In addition, while the acyl group on ghrelin is essential for it to stimulate GH production, both acyl and des-acyl ghrelin have anti-apoptotic effects on cardiac myocytes [89]. This finding demonstrating biological activity for des-acyl ghrelin supports the speculation that an additional receptor may exist for ghrelin. Attempting to discuss the full range of studies concerning ghrelin is beyond the scope of this review (see Patel and Taub in this review series), so we will focus on unexpected findings concerning the ability of ghrelin to suppress inflammatory responses and promote thymopoiesis.
One of the most interesting activities of ghrelin is its ability to inhibit inflammatory responses both in vitro and in vivo. The occurrence of ghrelin receptors on lymphoid tissue was implied by the presence of mRNA in those tissues [85] and has been subsequently confirmed at the level of protein [90]. After finding ghrelin receptor expression on human lymphocytes and monocytes, the Taub laboratory showed that ghrelin blocked the production of the inflammatory cytokines IL-1α, IL-1β, IL-6 and TNFα in vitro and also blocked the generation of inflammatory processes in an in vivo mouse model [90]. The anti-inflammatory effects are not limited to lymphocytes, monocytes and dendritic cells (D. Taub, personal observation) since ghrelin can also inhibit the production of inflammatory cytokines from endothelial cells by blocking the activation of NFκB [91]. The anti-inflammatory effects have been confirmed in other in vivo models. For example, in a model of adjuvant-induced arthritis in rats, a ghrelin agonist significantly reduced the symptoms [92]. Ghrelin also significantly reduced the inflammatory gastrointestinal damage in a murine model of colitis [93]. These studies clearly have shown that ghrelin can reduce inflammation; however, it is unclear if ghrelin also mediates its effects via SOCS family members that inhibit beneficial immune responses. It has been demonstrated that a cell-permeate recombinant form of SOCS3 could protect animals from LPS-induced damage in vivo presumably by blocking the response to IL-6, TNFα, etc., [94]. It has also been reported that SOCS proteins could inhibit Toll-like receptor (TLR) signaling that lead to the production of inflammatory cytokines [95]. However, more detailed studies showed that SOCS proteins did not directly regulate the activation of NFκB leading to cytokine production [96]. The effect of SOCS proteins was indirect by blocking downstream autocrine/paracrine responses from type I interferons that were stimulated by the TLR signaling. Thus, the anti-inflammatory effects of ghrelin and ghrelin agonists appear to be mediated by processes independent of SOCS proteins.
Based on the ability of GHS-R agonists to stimulate the GH-IGF-1 axis, Koo and colleagues [25] treated 14-month old mice with a synthetic GHS-R agonist for three weeks and examined the effects on the thymus and bone marrow. As discussed earlier in this text, their results revealed that oral GHS administration resulted in a significant increase in thymic cellularity in old mice along with improved bone marrow engraftment using a SCID model. These investigators proposed that the observed effects were being mediated through GH induction in response to the GHS administration. However, the precise role for GH in mediating these effects remains to be defined. More recently, Taub and colleagues [97, 98] have demonstrated that administration of acylated but not des-acylated ghrelin into aged (6-, 14- and 24-month old) but not young (2- and 4-month old) mice resulted in a partial reversal of thymic involution. Infusion of ghrelin also significantly improved the age-associated changes in thymic architecture and thymocyte counts along with an increase in recent thymic emigrants, an improvement of TCR diversity of peripheral T cell subsets and increased numbers of early thymocyte progenitors (ETP), common lymphoid progenitors (CLP) and bone marrow-derived pluripotential stem cells [97]. Furthermore, the ghrelin and GHS-R deficient mice displayed enhanced age-associated thymic involution with reduced thymopoiesis, contraction of stem cells and major perturbations in the TCR repertoire of peripheral T lymphocytes [97]. These findings demonstrate a novel role for ghrelin and its receptor in thymic biology and T cell development. The overall interplay between these hormones and their receptors in the thymic and BM compartments appear to play an important role in rejuvenation of thymic output in old animals [98]. Furthermore, it should be noted that the expression of ghrelin and GHS-R as well as GH appear to be significantly diminished with age within specific immune subsets and lymphoid organs, including the thymus. While the relevance of immune-derived hormone deficiency with age remains to be defined, we believe that this loss of GH and ghrelin secretion may be associated with a loss of control of cytokine expression resulting in increased levels of circulating and tissue-associated IL-6 and other proinflammatory cytokines (commonly observed in older subjects), possibly playing a role in thymic loss and immune dysfunction. Moreover, loss of lymphoid-associated ghrelin and GH may also result in significant changes in the thymic anatomy and the mediators playing a role in the maintenance of thymic structure and thymocyte development [98]. Thus, the loss of ghrelin or GHS-R (and GH) expression in aging subjects may actually influence the rate of thymic involution as well as the diminished hematopoiesis and thymopoiesis observed with advancing age.
Future Directions
We began this review by noting some of the many studies demonstrating that neuroendocrine hormones such as GH and PRL can have beneficial effects on the immune system, particularly when it has been compromised by disease, therapy or aging. However, these hormones use the same pathways and are subject to the same feedback regulatory effects of SOCS proteins as do inflammatory cytokines and others required for normal immune responses. Elevated levels of SOCS proteins can be protective by inhibiting the deleterious effects of inflammatory cytokines such as TNFα or IL-6 but can also reduce overall immune capabilities by blunting the development of effective immunity as the result of blocking the actions of cytokines such as IL-2, IL-4, IL-7 and IL-15. Therefore, combination treatments of anti-inflammatory agents and hormones such as GH would appear to be much better than simply administering agents such as GH to restore damaged immune systems. As noted above, ghrelin or ghrelin agonists could be particularly effective by combining their ability to stimulate GH production with their inherent anti-inflammatory effects. This approach is currently being used clinically to treat inflammatory bowel disease (IBD) by combining an antibody to TNFα, i.e., infliximab, with GH as recently reviewed [98]. IBD is not an immunodeficiency disease but depletion of TNFα overcomes the resistance to GH permitting it to mediate effects to stimulate the repair of damaged tissue. In the future, it will be of interest to determine if anti-inflammatory therapy can improve the effectiveness of GH treatments in conditions such as aging that are accompanied by increased levels of inflammatory cytokines. Moreover, the potential therapeutic use of ghrelin and GHS-R agonists in the management of acute and chronic inflammation and cancer and in restoration of thymic function in aged and immunocompromised individuals needs to be explored. Such GH-inducing agents may be quite valuable in the patients undergoing bone marrow transplantation where it is necessary to engraft and replenish the host after radiation treatment with donor cells.
Acknowledgments
Supported in part by NIH 1 R01 AG022661 and the UNR Cytometry Center is supported in part by the Nevada INBRE NIH 2 P20 RR016464 and the Intramural Research Program of the National Institute on Aging, National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy WJ, Durum SK, Longo DL. Human growth hormone promotes engraftment of murine or human T cells in severe combined immunodeficient mice. Proc Natl Acad Sci U S A. 1992;89:4481–5. doi: 10.1073/pnas.89.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy WJ, Tsarfaty G, Longo DL. Growth hormone exerts hematopoietic growth-promoting effects in vivo and partially counteracts the myelosuppressive effects of azidothymidine. Blood. 1992;80:1443–7. [PubMed] [Google Scholar]
- 3.Murphy WJ, Durum SK, Longo DL. Role of neuroendocrine hormones in murine T cell development. Growth hormone exerts thymopoietic effects in vivo. J Immunol. 1992;149:3851–7. [PubMed] [Google Scholar]
- 4.Tian ZG, Woody MA, Sun R, Welniak LA, Raziuddin A, Funakoshi S, Tsarfaty G, Longo DL, Murphy WJ. Recombinant human growth hormone promotes hematopoietic reconstitution after syngeneic bone marrow transplantation in mice. Stem Cells. 1998;16:193–9. doi: 10.1002/stem.160193. [DOI] [PubMed] [Google Scholar]
- 5.Woody MA, Welniak LA, Richards S, Taub DD, Tian Z, Sun R, Longo DL, Murphy WJ. Use of neuroendocrine hormones to promote reconstitution after bone marrow transplantation. Neuroimmunomodulation. 1999;6:69–80. doi: 10.1159/000026366. [DOI] [PubMed] [Google Scholar]
- 6.Carreno PC, Sacedon R, Jimenez E, Vicente A, Zapata AG. Prolactin affects both survival and differentiation of T-cell progenitors. J Neuroimmunol. 2005;160:135–45. doi: 10.1016/j.jneuroim.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Chen BJ, Cui X, Sempowski GD, Chao NJ. Growth hormone accelerates immune recovery following allogeneic T-cell-depleted bone marrow transplantation in mice. Exp Hematol. 2003;31:953–8. doi: 10.1016/s0301-472x(03)00196-6. [DOI] [PubMed] [Google Scholar]
- 8.Alpdogan O, Muriglan SJ, Kappel BJ, Doubrovina E, Schmaltz C, Schiro R, Eng JM, Greenberg AS, Willis LM, Rotolo JA, O’Reilly RJ, van den Brink MR. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation. 2003;75:1977–83. doi: 10.1097/01.TP.0000070167.81584.A2. [DOI] [PubMed] [Google Scholar]
- 9.Nicoll CS, Mayer GL, Russell SM. Structural features of prolactins and growth hormones that can be related to their biological properties. Endocr Rev. 1986;7:169–203. doi: 10.1210/edrv-7-2-169. [DOI] [PubMed] [Google Scholar]
- 10.Sirohi B, Powles R, Morgan G, Treleaven J, Kulkarni S, Horton C, Saso R, Rolfe D, Cook G, Shaw C, Wass J. Use of physiological doses of human growth hormone in haematological patients receiving intensive chemotherapy promotes haematopoietic recovery: a double-blind randomized, placebo-controlled study. Bone Marrow Transplant. 2007;39:115–20. doi: 10.1038/sj.bmt.1705545. [DOI] [PubMed] [Google Scholar]
- 11.Tacke J, Bolder U, Herrmann A, Berger G, Jauch KW. Long-term risk of gastrointestinal tumor recurrence after postoperative treatment with recombinant human growth hormone. JPEN J Parenter Enteral Nutr. 2000;24:140–4. doi: 10.1177/0148607100024003140. [DOI] [PubMed] [Google Scholar]
- 12.Nightingale SL. From the Food and Drug Administration. Jama. 1995;273:982. doi: 10.1001/jama.273.13.982. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen BY, Clerici M, Venzon DJ, Bauza S, Murphy WJ, Longo DL, Baseler M, Gesundheit N, Broder S, Shearer G, Yarchoan R. Pilot study of the immunologic effects of recombinant human growth hormone and recombinant insulin-like growth factor in HIV-infected patients. Aids. 1998;12:895–904. doi: 10.1097/00002030-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Kelley KW. From hormones to immunity: the physiology of immunology. Brain Behav Immun. 2004;18:95–113. doi: 10.1016/j.bbi.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Krentz AJ, Koster FT, Crist DM, Finn K, Johnson LZ, Boyle PJ, Schade DS. Anthropometric, metabolic, and immunological effects of recombinant human growth hormone in AIDS and AIDS-related complex. J Acquir Immune Defic Syndr. 1993;6:245–51. [PubMed] [Google Scholar]
- 16.Lieberman SA, Butterfield GE, Harrison D, Hoffman AR. Anabolic effects of recombinant insulin-like growth factor-I in cachectic patients with the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1994;78:404–10. doi: 10.1210/jcem.78.2.7508949. [DOI] [PubMed] [Google Scholar]
- 17.Ellis KJ, Lee PD, Pivarnik JM, Bukar JG, Gesundheit N. Changes in body composition of human immunodeficiency virus-infected males receiving insulin-like growth factor I and growth hormone. J Clin Endocrinol Metab. 1996;81:3033–8. doi: 10.1210/jcem.81.8.8768870. [DOI] [PubMed] [Google Scholar]
- 18.Lee PD, Pivarnik JM, Bukar JG, Muurahainen N, Berry PS, Skolnik PR, Nerad JL, Kudsk KA, Jackson L, Ellis KJ, Gesundheit N. A randomized, placebo-controlled trial of combined insulin-like growth factor I and low dose growth hormone therapy for wasting associated with human immunodeficiency virus infection. J Clin Endocrinol Metab. 1996;81:2968–75. doi: 10.1210/jcem.81.8.8768860. [DOI] [PubMed] [Google Scholar]
- 19.Napolitano LA, Lo JC, Gotway MB, Mulligan K, Barbour JD, Schmidt D, Grant RM, Halvorsen RA, Schambelan M, McCune JM. Increased thymic mass and circulating naive CD4 T cells in HIV-1-infected adults treated with growth hormone. Aids. 2002;16:1103–11. doi: 10.1097/00002030-200205240-00003. [DOI] [PubMed] [Google Scholar]
- 20.Pires A, Pido-Lopez J, Moyle G, Gazzard B, Gotch F, Imami N. Enhanced T-cell maturation, differentiation and function in HIV-1-infected individuals after growth hormone and highly active antiretroviral therapy. Antivir Ther. 2004;9:67–75. [PubMed] [Google Scholar]
- 21.Goodier MR, Imami N, Moyle G, Gazzard B, Gotch F. Loss of the CD56hiCD16- NK cell subset and NK cell interferon-gamma production during antiretroviral therapy for HIV-1: partial recovery by human growth hormone. Clin Exp Immunol. 2003;134:470–6. doi: 10.1111/j.1365-2249.2003.02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley KW, Brief S, Westly HJ, Novakofski J, Bechtel PJ, Simon J, Walker EB. GH3 pituitary adenoma cells can reverse thymic aging in rats. Proc Natl Acad Sci U S A. 1986;83:5663–7. doi: 10.1073/pnas.83.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.French RA, Broussard SR, Meier WA, Minshall C, Arkins S, Zachary JF, Dantzer R, Kelley KW. Age-associated loss of bone marrow hematopoietic cells is reversed by GH and accompanies thymic reconstitution. Endocrinology. 2002;143:690–9. doi: 10.1210/endo.143.2.8612. [DOI] [PubMed] [Google Scholar]
- 24.Montecino-Rodriguez E, Clark R, Dorshkind K. Effects of insulin-like growth factor administration and bone marrow transplantation on thymopoiesis in aged mice. Endocrinology. 1998;139:4120–6. doi: 10.1210/endo.139.10.6263. [DOI] [PubMed] [Google Scholar]
- 25.Koo GC, Huang C, Camacho R, Trainor C, Blake JT, Sirotina-Meisher A, Schleim KD, Wu TJ, Cheng K, Nargund R, McKissick G. Immune enhancing effect of a growth hormone secretagogue. J Immunol. 2001;166:4195–201. doi: 10.4049/jimmunol.166.6.4195. [DOI] [PubMed] [Google Scholar]
- 26.Martial JA, Hallewell RA, Baxter JD, Goodman HM. Human growth hormone: complementary DNA cloning and expression in bacteria. Science. 1979;205:602–7. doi: 10.1126/science.377496. [DOI] [PubMed] [Google Scholar]
- 27.Roskam WG, Rougeon F. Molecular cloning and nucleotide sequence of the human growth hormone structural gene. Nucleic Acids Res. 1979;7:305–20. doi: 10.1093/nar/7.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikehara M, Ohtsuka E, Tokunaga T, Taniyama Y, Iwai S, Kitano K, Miyamoto S, Ohgi T, Sakuragawa Y, Fujiyama K, et al. Synthesis of a gene for human growth hormone and its expression in Escherichia coli. Proc Natl Acad Sci U S A. 1984;81:5956–60. doi: 10.1073/pnas.81.19.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung DW, Spencer SA, Cachianes G, Hammonds RG, Collins C, Henzel WJ, Barnard R, Waters MJ, Wood WI. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature. 1987;330:537–43. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- 30.Godowski PJ, Leung DW, Meacham LR, Galgani JP, Hellmiss R, Keret R, Rotwein PS, Parks JS, Laron Z, Wood WI. Characterization of the human growth hormone receptor gene and demonstration of a partial gene deletion in two patients with Laron-type dwarfism. Proc Natl Acad Sci U S A. 1989;86:8083–7. doi: 10.1073/pnas.86.20.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrington J, Carter-Su C. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol Metab. 2001;12:252–7. doi: 10.1016/s1043-2760(01)00423-4. [DOI] [PubMed] [Google Scholar]
- 32.Waters MJ, Hoang HN, Fairlie DP, Pelekanos RA, Brown RJ. New insights into growth hormone action. J Mol Endocrinol. 2006;36:1–7. doi: 10.1677/jme.1.01933. [DOI] [PubMed] [Google Scholar]
- 33.Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993;74:237–44. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- 34.Herrington J, Smit LS, Schwartz J, Carter-Su C. The role of STAT proteins in growth hormone signaling. Oncogene. 2000;19:2585–97. doi: 10.1038/sj.onc.1203526. [DOI] [PubMed] [Google Scholar]
- 35.Liao J, Hodge C, Meyer D, Ho PS, Rosenspire K, Schwartz J. Growth hormone regulates ternary complex factors and serum response factor associated with the c-fos serum response element. J Biol Chem. 1997;272:25951–8. doi: 10.1074/jbc.272.41.25951. [DOI] [PubMed] [Google Scholar]
- 36.Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J. Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem. 1998;273:31327–36. doi: 10.1074/jbc.273.47.31327. [DOI] [PubMed] [Google Scholar]
- 37.Imada K, Leonard WJ. The Jak-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 38.Elliott J, Johnston JA. SOCS: role in inflammation, allergy and homeostasis. Trends Immunol. 2004;25:434–40. doi: 10.1016/j.it.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Narazaki M, Fujimoto M, Matsumoto T, Morita Y, Saito H, Kajita T, Yoshizaki K, Naka T, Kishimoto T. Three distinct domains of SSI-1/SOCS-1/JAB protein are required for its suppression of interleukin 6 signaling. Proc Natl Acad Sci U S A. 1998;95:13130–4. doi: 10.1073/pnas.95.22.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T, Ohtsuka S, Imaizumi T, Matsuda T, Ihle JN, Yoshimura A. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. Embo J. 1999;18:1309–20. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamura T, Sato S, Haque D, Liu L, Kaelin WG, Jr, Conaway RC, Conaway JW. The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 1998;12:3872–81. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tollet-Egnell P, Flores-Morales A, Stavreus-Evers A, Sahlin L, Norstedt G. Growth hormone regulation of SOCS-2, SOCS-3, and CIS messenger ribonucleic acid expression in the rat. Endocrinology. 1999;140:3693–704. doi: 10.1210/endo.140.8.6878. [DOI] [PubMed] [Google Scholar]
- 43.Flores-Morales A, Greenhalgh CJ, Norstedt G, Rico-Bautista E. Negative regulation of growth hormone receptor signaling. Mol Endocrinol. 2006;20:241–53. doi: 10.1210/me.2005-0170. [DOI] [PubMed] [Google Scholar]
- 44.Metcalf D, Greenhalgh CJ, Viney E, Willson TA, Starr R, Nicola NA, Hilton DJ, Alexander WS. Gigantism in mice lacking suppressor of cytokine signalling-2. Nature. 2000;405:1069–73. doi: 10.1038/35016611. [DOI] [PubMed] [Google Scholar]
- 45.Rico-Bautista E, Greenhalgh CJ, Tollet-Egnell P, Hilton DJ, Alexander WS, Norstedt G, Flores-Morales A. Suppressor of cytokine signaling-2 deficiency induces molecular and metabolic changes that partially overlap with growth hormone-dependent effects. Mol Endocrinol. 2005;19:781–93. doi: 10.1210/me.2004-0040. [DOI] [PubMed] [Google Scholar]
- 46.Piessevaux J, Lavens D, Montoye T, Wauman J, Catteeuw D, Vandekerckhove J, Belsham D, Peelman F, Tavernier J. Functional cross-modulation between SOCS proteins can stimulate cytokine signaling. J Biol Chem. 2006;281:32953–66. doi: 10.1074/jbc.M600776200. [DOI] [PubMed] [Google Scholar]
- 47.Greenhalgh CJ, Bertolino P, Asa SL, Metcalf D, Corbin JE, Adams TE, Davey HW, Nicola NA, Hilton DJ, Alexander WS. Growth enhancement in suppressor of cytokine signaling 2 (SOCS-2)-deficient mice is dependent on signal transducer and activator of transcription 5b (STAT5b) Mol Endocrinol. 2002;16:1394–406. doi: 10.1210/mend.16.6.0845. [DOI] [PubMed] [Google Scholar]
- 48.Greenhalgh CJ, Metcalf D, Thaus AL, Corbin JE, Uren R, Morgan PO, Fabri LJ, Zhang JG, Martin HM, Willson TA, Billestrup N, Nicola NA, Baca M, Alexander WS, Hilton DJ. Biological evidence that SOCS-2 can act either as an enhancer or suppressor of growth hormone signaling. J Biol Chem. 2002;277:40181–4. doi: 10.1074/jbc.C200450200. [DOI] [PubMed] [Google Scholar]
- 49.Ridderstrale M, Amstrup J, Hilton DJ, Billestrup N, Tornqvist H. SOCS-3 is involved in the downregulation of the acute insulin-like effects of growth hormone in rat adipocytes by inhibition of Jak2/IRS-1 signaling. Horm Metab Res. 2003;35:169–77. doi: 10.1055/s-2003-39077. [DOI] [PubMed] [Google Scholar]
- 50.Pezet A, Favre H, Kelly PA, Edery M. Inhibition and restoration of prolactin signal transduction by suppressors of cytokine signaling. J Biol Chem. 1999;274:24497–502. doi: 10.1074/jbc.274.35.24497. [DOI] [PubMed] [Google Scholar]
- 51.Dogusan Z, Hooghe-Peters EL, Berus D, Velkeniers B, Hooghe R. Expression of SOCS genes in normal and leukemic human leukocytes stimulated by prolactin, growth hormone and cytokines. J Neuroimmunol. 2000;109:34–9. doi: 10.1016/s0165-5728(00)00300-3. [DOI] [PubMed] [Google Scholar]
- 52.Dey BR, Spence SL, Nissley P, Furlanetto RW. Interaction of human suppressor of cytokine signaling (SOCS)-2 with the insulin-like growth factor-I receptor. J Biol Chem. 1998;273:24095–101. doi: 10.1074/jbc.273.37.24095. [DOI] [PubMed] [Google Scholar]
- 53.Spangenburg EE. SOCS-3 induces myoblast differentiation. J Biol Chem. 2005;280:10749–58. doi: 10.1074/jbc.M410604200. [DOI] [PubMed] [Google Scholar]
- 54.De Sepulveda P, Okkenhaug K, Rose JL, Hawley RG, Dubreuil P, Rottapel R. Socs1 binds to multiple signalling proteins and suppresses steel factor-dependent proliferation. Embo J. 1999;18:904–15. doi: 10.1093/emboj/18.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bayle J, Letard S, Frank R, Dubreuil P, De Sepulveda P. Suppressor of cytokine signaling 6 associates with KIT and regulates KIT receptor signaling. J Biol Chem. 2004;279:12249–59. doi: 10.1074/jbc.M313381200. [DOI] [PubMed] [Google Scholar]
- 56.Badolato R, Bond HM, Valerio G, Petrella A, Morrone G, Waters MJ, Venuta S, Tenore A. Differential expression of surface membrane growth hormone receptor on human peripheral blood lymphocytes detected by dual fluorochrome flow cytometry. J Clin Endocrinol Metab. 1994;79:984–90. doi: 10.1210/jcem.79.4.7962309. [DOI] [PubMed] [Google Scholar]
- 57.Gagnerault MC, Postel-Vinay MC, Dardenne M. Expression of growth hormone receptors in murine lymphoid cells analyzed by flow cytofluorometry. Endocrinology. 1996;137:1719–26. doi: 10.1210/endo.137.5.8612507. [DOI] [PubMed] [Google Scholar]
- 58.Dardenne M, Mello-Coelho V, Gagnerault MC, Postel-Vinay MC. Growth hormone receptors and immunocompetent cells. Ann N Y Acad Sci. 1998;840:510–7. doi: 10.1111/j.1749-6632.1998.tb09589.x. [DOI] [PubMed] [Google Scholar]
- 59.Hull KL, Thiagarajah A, Harvey S. Cellular localization of growth hormone receptors/binding proteins in immune tissues. Cell Tissue Res. 1996;286:69–80. doi: 10.1007/s004410050676. [DOI] [PubMed] [Google Scholar]
- 60.Chen HT, Schuler LA, Schultz RD. Growth hormone receptor and regulation of gene expression in fetal lymphoid cells. Mol Cell Endocrinol. 1998;137:21–9. doi: 10.1016/s0303-7207(97)00230-x. [DOI] [PubMed] [Google Scholar]
- 61.Derfalvi B, Szalai C, Mandi Y, Kiraly A, Falus A. Growth hormone receptor gene expression on human lymphocytic and monocytic cell lines. Cell Biol Int. 1998;22:849–53. doi: 10.1006/cbir.1998.0324. [DOI] [PubMed] [Google Scholar]
- 62.Pankov YA. Growth hormone and a partial mediator of its biological action, insulin-like growth factor I. Biochemistry (Mosc) 1999;64:1–7. [PubMed] [Google Scholar]
- 63.Yamada M, Hato F, Kinoshita Y, Tominaga K, Tsuji Y. The indirect participation of growth hormone in the thymocyte proliferation system. Cell Mol Biol (Noisy-le-grand) 1994;40:111–21. [PubMed] [Google Scholar]
- 64.Sabharwal P, Varma S. Growth hormone synthesized and secreted by human thymocytes acts via insulin-like growth factor I as an autocrine and paracrine growth factor. J Clin Endocrinol Metab. 1996;81:2663–9. doi: 10.1210/jcem.81.7.8675594. [DOI] [PubMed] [Google Scholar]
- 65.Dobashi H, Sato M, Tanaka T, Tokuda M, Ishida T. Growth hormone restores glucocorticoid-induced T cell suppression. Faseb J. 2001;15:1861–3. doi: 10.1096/fj.00-0702fje. [DOI] [PubMed] [Google Scholar]
- 66.Mitsunaka H, Dobashi H, Sato M, Tanaka T, Kitanaka A, Yamaoka G, Tokuda M, Matoba K, Hiraishi T, Ishida T. Growth hormone prevents Fas-induced apoptosis in lymphocytes through modulation of Bcl-2 and caspase-3. Neuroimmunomodulation. 2001;9:256–62. doi: 10.1159/000054288. [DOI] [PubMed] [Google Scholar]
- 67.Lempereur L, Brambilla D, Scoto GM, D’Alcamo M, Goffin V, Crosta L, Palmucci T, Rampello L, Bernardini R, Cantarella G. Growth hormone protects human lymphocytes from irradiation-induced cell death. Br J Pharmacol. 2003;138:1411–6. doi: 10.1038/sj.bjp.0705173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arnold RE, Weigent DA. The inhibition of apoptosis in EL4 lymphoma cells overexpressing growth hormone. Neuroimmunomodulation. 2004;11:149–59. doi: 10.1159/000076764. [DOI] [PubMed] [Google Scholar]
- 69.Timsit J, Savino W, Safieh B, Chanson P, Gagnerault MC, Bach JF, Dardenne M. Growth hormone and insulin-like growth factor-I stimulate hormonal function and proliferation of thymic epithelial cells. J Clin Endocrinol Metab. 1992;75:183–8. doi: 10.1210/jcem.75.1.1619008. [DOI] [PubMed] [Google Scholar]
- 70.Tsuji Y, Kinoshita Y, Hato F, Tominaga K, Yoshida K. The in vitro proliferation of thymus epithelial cells stimulated with growth hormone and insulin-like growth factor-I. Cell Mol Biol (Noisy-le-grand) 1994;40:1135–42. [PubMed] [Google Scholar]
- 71.de Mello Coelho V, Villa-Verde DM, Farias-de-Oliveira DA, de Brito JM, Dardenne M, Savino W. Functional insulin-like growth factor-1/insulin-like growth factor-1 receptor-mediated circuit in human and murine thymic epithelial cells. Neuroendocrinology. 2002;75:139–50. doi: 10.1159/000048230. [DOI] [PubMed] [Google Scholar]
- 72.Aiuti A, Tavian M, Cipponi A, Ficara F, Zappone E, Hoxie J, Peault B, Bordignon C. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol. 1999;29:1823–31. doi: 10.1002/(SICI)1521-4141(199906)29:06<1823::AID-IMMU1823>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 73.Smaniotto S, de Mello-Coelho V, Villa-Verde DM, Pleau JM, Postel-Vinay MC, Dardenne M, Savino W. Growth hormone modulates thymocyte development in vivo through a combined action of laminin and CXC chemokine ligand 12. Endocrinology. 2005;146:3005–17. doi: 10.1210/en.2004-0709. [DOI] [PubMed] [Google Scholar]
- 74.Garzon R, Soriano SF, Rodriguez-Frade JM, Gomez L, Martin de Ana A, Sanchez-Gomez M, Martinez AC, Mellado M. CXCR4-mediated suppressor of cytokine signaling up-regulation inactivates growth hormone function. J Biol Chem. 2004;279:44460–6. doi: 10.1074/jbc.M408010200. [DOI] [PubMed] [Google Scholar]
- 75.Zaitseva MB, Lee S, Rabin RL, Tiffany HL, Farber JM, Peden KW, Murphy PM, Golding H. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J Immunol. 1998;161:3103–13. [PubMed] [Google Scholar]
- 76.Vila-Coro AJ, Rodriguez-Frade JM, Martin De Ana A, Moreno-Ortiz MC, Martinez AC, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. Faseb J. 1999;13:1699–710. [PubMed] [Google Scholar]
- 77.Soriano SF, Hernanz-Falcon P, Rodriguez-Frade JM, De Ana AM, Garzon R, Carvalho-Pinto C, Vila-Coro AJ, Zaballos A, Balomenos D, Martinez AC, Mellado M. Functional inactivation of CXC chemokine receptor 4-mediated responses through SOCS3 up-regulation. J Exp Med. 2002;196:311–21. doi: 10.1084/jem.20012041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pello OM, del Moreno-Ortiz CM, Rodriguez-Frade JM, Martinez-Munoz L, Lucas D, Gomez L, Lucas P, Samper E, Aracil M, Martinez C, Bernad A, Mellado M. SOCS up-regulation mobilizes autologous stem cells through CXCR4 blockade. Blood. 2006;108:3928–37. doi: 10.1182/blood-2006-02-006353. [DOI] [PubMed] [Google Scholar]
- 79.Heim MH. The Jak-STAT pathway: cytokine signalling from the receptor to the nucleus. J Recept Signal Transduct Res. 1999;19:75–120. doi: 10.3109/10799899909036638. [DOI] [PubMed] [Google Scholar]
- 80.Mayo KE, Godfrey PA, Suhr ST, Kulik DJ, Rahal JO. Growth hormone-releasing hormone: synthesis and signaling. Recent Prog Horm Res. 1995;50:35–73. doi: 10.1016/b978-0-12-571150-0.50007-x. [DOI] [PubMed] [Google Scholar]
- 81.Dialynas E, Brown-Borg H, Bartke A. Immune function in transgenic mice overexpressing growth hormone (GH) releasing hormone, GH or GH antagonist. Proc Soc Exp Biol Med. 1999;221:178–83. doi: 10.1046/j.1525-1373.1999.d01-73.x. [DOI] [PubMed] [Google Scholar]
- 82.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–7. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 83.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 84.Arvat E, Di Vito L, Broglio F, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E. Preliminary evidence that Ghrelin, the natural GH secretagogue (GHS)-receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest. 2000;23:493–5. doi: 10.1007/BF03343763. [DOI] [PubMed] [Google Scholar]
- 85.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 86.Smith RG, Jiang H, Sun Y. Developments in ghrelin biology and potential clinical relevance. Trends Endocrinol Metab. 2005;16:436–42. doi: 10.1016/j.tem.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 87.Smith RG, Griffin PR, Xu Y, Smith AG, Liu K, Calacay J, Feighner SD, Pong C, Leong D, Pomes A, Cheng K, Van der Ploeg LH, Howard AD, Schaeffer J, Leonard RJ. Adenosine: A partial agonist of the growth hormone secretagogue receptor. Biochem Biophys Res Commun. 2000;276:1306–13. doi: 10.1006/bbrc.2000.3610. [DOI] [PubMed] [Google Scholar]
- 88.Carreira MC, Camina JP, Smith RG, Casanueva FF. Agonist-specific coupling of growth hormone secretagogue receptor type 1a to different intracellular signaling systems. Role of adenosine. Neuroendocrinology. 2004;79:13–25. doi: 10.1159/000076042. [DOI] [PubMed] [Google Scholar]
- 89.Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159:1029–37. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C, Weintraub NL. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–6. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- 92.Granado M, Priego T, Martin AI, Villanua MA, Lopez-Calderon A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab. 2005;288:E486–92. doi: 10.1152/ajpendo.00196.2004. [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–20. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 94.Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat Med. 2005;11:892–8. doi: 10.1038/nm1269. [DOI] [PubMed] [Google Scholar]
- 95.Takagi H, Sanada T, Minoda Y, Yoshimura A. Regulation of cytokine and toll-like receptor signaling by SOCS family genes. Nippon Rinsho. 2004;62:2189–96. [PubMed] [Google Scholar]
- 96.Baetz A, Frey M, Heeg K, Dalpke AH. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. J Biol Chem. 2004;279:54708–15. doi: 10.1074/jbc.M410992200. [DOI] [PubMed] [Google Scholar]
- 97.Dixit VD, Yang H, Sun Y, Weeraratna AT, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117(10):2778–90. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taub DD. Novel Connections between the Neuroendocrine and Immune Systems: The Ghrelin Immunoregulatory Network. Vitam Horm. 2007;77:325–46. doi: 10.1016/S0083-6729(06)77014-5. [DOI] [PubMed] [Google Scholar]
- 99.De Pascalis B, Bianchi A, Satta MA, Lupascu A, Mentella MC, Leo D, Fiore F, Fedeli P, Pontecorvi A, Pola P, Melina D, Gasbarrini A, De Marinis L, Armuzzi A. Growth hormone in inflammatory bowel disease. Eur Rev Med Pharmacol Sci. 2006;10:13–6. [PubMed] [Google Scholar]