Abstract
Our objective was to provide a comprehensive review of the current knowledge regarding pregnancy and hepatitis B virus (HBV) or hepatitis C virus (HCV) infection as well as recent efforts to reduce the rate of mother-to-child transmission (MTCT). Maternal infection with either HBV or HCV has been linked to adverse pregnancy and birth outcomes, including MTCT. MTCT for HBV has been reduced to approximately 5% overall in countries including the US that have instituted postpartum neonatal HBV vaccination and immunoprophylaxis with hepatitis B immune globulin. However, the rate of transmission of HBV to newborns is nearly 30% when maternal HBV levels are greater than 200 000 IU ml−1 (>6 log10 copies ml−1). For these patients, new guidelines from the European Association for the Study of the Liver (EASL) and the Asian Pacific Association for the Study of the Liver (APASL) indicate that, in addition to neonatal vaccination and immunoprophylaxis, treating with antiviral agents such as tenofovir disoproxil fumarate or telbivudine during pregnancy beginning at 32 weeks of gestation is safe and effective in preventing MTCT. In contrast to HBV, no therapeutic agents are yet available or recommended to further decrease the risk of MTCT of HCV, which remains 3 to 10%. HCV MTCT can be minimized by avoiding fetal scalp electrodes and birth trauma whenever possible. Young women with HCV should be referred for treatment post delivery, and neonates should be closely followed to rule out infection. New, better-tolerated treatment regimens for HCV are now available, which should improve outcomes for all infected individuals.
INTRODUCTION
Hepatitis B virus (HBV) and hepatitis C virus (HCV) are acquired by contaminated blood product exposure, sexual activity or perinatal transmission. Although the prevalence of HBV is relatively low in the US (0.4%), with approximately one million Americans chronically infected by HBV,1 it is more prevalent in East Asia (8%)2 (China 2 to 18%, Taiwan 2 to 19% and Hong Kong 4 to 10%, depending on the region),3 Southeast Asia (>6%)2 (Indonesia 2 to 9%, Thailand 1 to 25% and India 1 to 66%, depending on the region)3 and sub-Saharan Africa (8 to 12%).2 Both Tropical Latin America and Central Latin America have had a decrease in HBV prevalence since 1990 (to 1.6% in 2005).2 HCV is the most common chronic blood-borne infection in the US, affecting nearly four million Americans. Women of childbearing age have a 1 to 2% incidence of chronic HCV infection, with higher rates in those with risk factors such as intravenous drug use.4 Pregnancy in patients with chronic HBV or HCV is associated with mother-to-child transmission (MTCT) and may be associated with increased maternal and fetal complications. In this review, we discuss the relationship between HBV/HCV infection and adverse pregnancy outcomes. Also included is a perspective on the current strategies to decrease the rate of MTCT. The published literature was searched through MEDLINE and ClinicalTrials using search terms hepatitis and pregnancy. The 107 studies cited represent the consensus regarding management of HBV and HCV in pregnancy.
Epidemiology of chronic hepatitis B and chronic hepatitis C in pregnancy
In a large population-based study from Florida involving nearly 1.7 million pregnant women, the prevalence of HBV was approximately 27 times higher among Asian-Americans and 5 times higher among African-Americans as compared with whites. Conversely, prevalence rates for HCV were highest among white women.5 There is an increased incidence of HIV infection in pregnant women with chronic HBV or HCV infection.5,6 Moreover, high-risk behaviors such as smoking, alcohol abuse and drug abuse are increased in pregnant women with HBV or HCV infection.6
Pregnancy outcomes associated with HBV or HCV infection
Several large population studies indicate that there is increased risk for preterm birth (odds ratio 1.4; 11.5% vs 7.9%, P<0.001), low birth weight (<2500 g) (odds ratio 1.39; 10.4% vs 7.8%, P = 0.009), premature rupture of membranes (8.9% vs 6.9%, P = 0.026), gestational diabetes (13.2% vs 8.8%, P<0.02) and congenital abnormalities (odds ratio 1.55; 7.2% vs 5.1%, P = 0.01) in pregnancies associated with maternal HBV or HCV infection (Table 1).5–12 Maternal chronic HCV infection is also associated with cholestasis of pregnancy,7,13,14 neonatal narcotic withdrawal syndrome7 and neonatal intensive care unit admission.5,7,12
Table 1.
Pregnancy outcomes with HBV and HCV
| HBV and HCV |
| Preterm birth |
| Low birth weight |
| Premature rupture of membranes |
| Gestational diabetes |
| Possible small increase in congenital anomalies |
| HCV |
| Cholestasis of pregnancy |
| NICU admission |
| Neonatal abstinence syndrome |
A confounding factor that limits interpretation of these studies is exposure to illicit drugs during the prenatal period, especially heroin, methadone and amphetamines,5,7 which are independently associated with low birth weight, preterm birth, congenital anomalies and other adverse neonatal outcomes.7,15 Two of the largest studies showing adverse outcomes associated with HBV or HCV included drug abuse, alcohol abuse and tobacco use in the multivariate statistical analyses.5,7 Nonetheless, although pregnancies complicated by HBV or HCV are clearly associated with adverse maternal and fetal outcomes, it is not as evident if the etiology of these events are mediated by the viral infection, by other confounding factors, or by a combination of factors.
HEPATITIS B IN PREGNANCY
In the US, the prevalence of chronic HBV infection in pregnancy is 0.2 to 6%, with rates varying by race and ethnicity.10,16 In a study of pregnant women from four urban US areas, Asian-American women had the highest prevalence of chronic HBV infection (6%), followed by blacks (1%), whites (0.6%) and Hispanics (0.14%).16 Newborn infants acquiring HBV infection by perinatal transmission have a greater than 95% chance of becoming chronic HBV carriers.17–19 Therefore, it is very important to institute maximally effective measures to prevent MTCT.
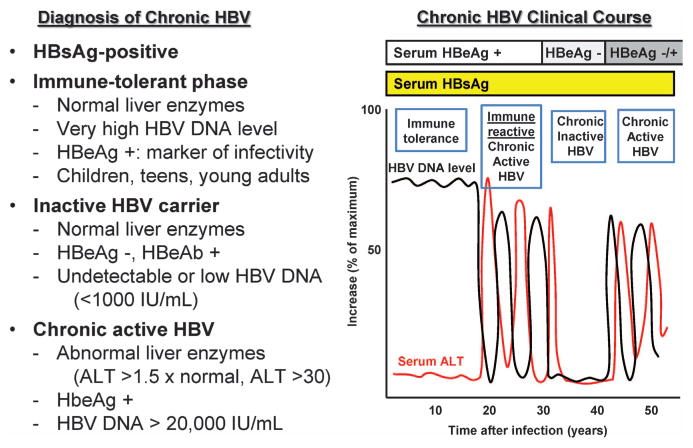
Diagnosis of chronic hepatitis B
Chronic HBV infection is diagnosed by the presence of hepatitis B surface antigen (HBsAg) in serum for longer than 6 months (Table 2 and Figure 1).20 Hepatitis B e antigen (HBeAg) is a marker of active viral replication and infectivity. Immune-tolerant chronic HBV patients have normal serum aspartate transaminase (AST) and alanine transaminase (ALT) levels, but have very high HBV DNA levels (>10 million IU ml −1; >6 × 7 log10 copies ml−1); they typically are children, teenagers or young adults. Inactive HBV carriers are HBsAg-positive, HBeAg-negative, hepatitis B e antibody positive, with undetectable or low (<1000 IU ml −1;<6 × 3 log10 copies ml −1) HBV DNA levels and normal liver function tests. Patients with chronic active HBV infection have increased AST and ALT levels, may be positive for HBeAg and have HBV DNA levels over 20 000 IU ml−1 (>5 log10 copies ml−1).
Table 2.
Diagnosis of hepatitis B
| Serologic markers | Clinical significance |
|---|---|
| HBcAb IgM (Hepatitis B core antibody IgM) | Acute infection |
| HBeAg (Hepatitis B e antigen) | High infectivity |
| HBeAb (Hepatitis B e antibody) | Low infectivity |
| HBsAb (Hepatitis B surface antibody) | Immunity |
| HBcAb IgG and HBsAg | Chronic infection |
| HBcAb IgG and HBsAb | Resolved infection |
| HBV DNA level (May be undetectable in chronic inactive HBV infection) | Acute or chronic infection |
Abbreviations: HBV, hepatitis B virus.
Figure 1.
Phases of chronic HBV infection. In the immune-tolerant phase of chronic HBV infection, ALT levels are normal whereas HBV DNA levels are markedly elevated. In the immune-reactive or chronic active phases, ALT and HBV DNA levels are elevated. In chonic inactive phases of HBV infection, ALT and HBV DNA levels are decreased; <1000 IU ml−1 is equivalent to <6 × 3 log10 copies ml−1; >200 000 IU ml−1 is>6 log10 copies ml−1.
Screening for chronic HBV infection
The Centers for Disease Control (CDC) recommends that all pregnant women should be screened for the presence of HBsAg at diagnosis of pregnancy (Figure 2).21 Repeat screening should be considered in HBsAg-negative women with risk factors for HBV infection (Asian, drug use, sexual exposure, incarceration, abnormal ALT) on admission for delivery.
Figure 2.
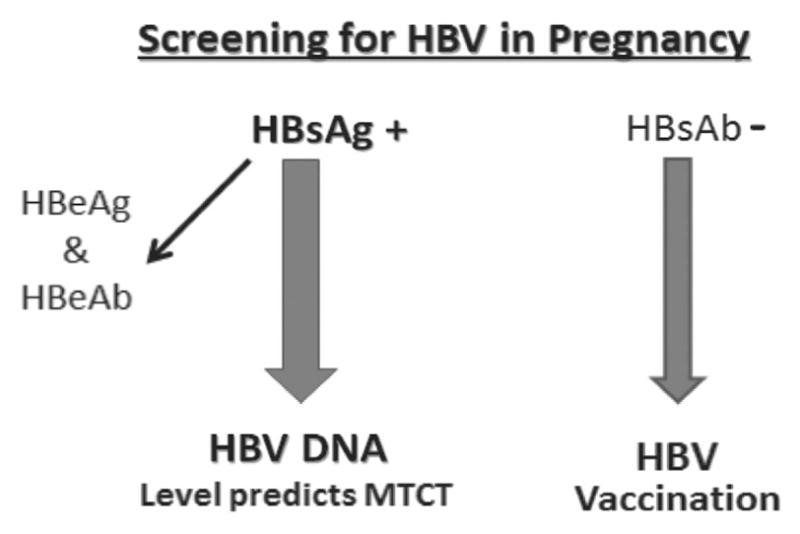
HBsAg is checked in all pregnant women. HBsAg-positive patients should have HBV DNA level and HBeAg checked. We recommend checking HBsAb and immunizing HBsAb-negative patients.
HBsAg-positive patients should be checked for the presence of HBeAg, hepatitis B e antibody and for HBV DNA level. High HBV DNA levels and HBeAg-positivity are associated with increased risk for MTCT of HBV infection.22 Neonates of all HBsAg-positive mothers should receive immunoprophylaxis treatment with hepatitis B immune globulin (HBIG) and HBV vaccine at delivery to decrease MTCT of HBV infection.23
Pregnancy is not a contraindication for vaccination to HBV.24 Therefore, pregnant women who are not immune to HBV should be vaccinated, because premature delivery may be increased if acute hepatitis B is acquired in the last trimester and because MTCT occurs in over 60% of pregnancies associated with acute HBV infection at or near term.24,25
Impact of pregnancy on chronic HBV infection
Pregnancy is well tolerated by women with chronic hepatitis B infection.26 HBV DNA levels may increase during pregnancy in association with a decrease in ALT levels, consistent with an HBV tolerance phase, followed by a post-partum decline in HBV DNA level that is associated with increased ALT levels and active hepatitis, consistent with a post-partum reconstitution of the immune system. This post-partum HBV reactivation may be associated with HBeAg seroconversion (i.e., clearance of HBeAg, development of hepatitis B e antibody positivity, decline in HBV DNA levels and normalization of ALT level) in 12.5 to 17% of patients.27–29
Mother-to-child-transmission of HBV infection
It is estimated that 30 to 40% of chronic HBV infections in the US are a result of perinatal transmission or early childhood infection.30 The most important risk factor for MTCT of HBV infection is a maternal level of HBV DNA>200 000 IU ml−1 (>6 log10 copies ml −1).22,31–35 Prior to neonatal prophylaxis with HBIG and HBV vaccination (see detailed description in the following section), the risk of perinatal transmission of HBV infection ranged from 10 to 40%, with 40 to 70% of those infants remaining chronically infected.36 The risk of MTCT was more than 90% when mothers had high HBV DNA levels and were HBeAg-positive (indicative of active viral replication and infectivity), and almost all of these infected infants became chronic HBsAg carriers.17–19 Young women in the immune-tolerant phase of chronic HBV infection are at high risk (up to 30%) for MTCT of HBV infection, regardless of neonatal immunoprophylaxis with HBIG and HBV vaccine.22,31,33,34,37,38 In contrast, chronic HBV infection occurred in fewer than 10% of infants of HBeAg-negative mothers.39 Other risk factors for MTCT of HBV include threatened preterm labor, prolonged labor and prior failure of immunoprophylaxis in siblings.31
MTCT of HBV can occur at three stages of pregnancy: intrauterine, intra-partum or post-partum. MTCT of HBV infection is thought to occur predominantly at or after birth based on the high protective efficacy of immunoprophylaxis. Intrauterine MTCT of HBV is reported to occur in 10 to 16% of pregnancies and probably accounts for the small percentage of infants who do not respond to immunoprophylaxis treatment for HBV at birth.40–42 Intrauterine transplacental transmission due to leakage of maternal blood can occur during threatened abortion.43,44 The risk of transmission of HBV from amniocentesis is low; in one study, the rate of MTCT did not significantly differ between women with HBV who underwent amniocentesis from those who did not undergo amniocentesis (9 vs 11%).45 The effect of other invasive procedures during pregnancy (chorionic villus sampling, cordocentesis, fetal surgery) on the risk of HBV transmission is unknown. No association between forceps or vacuum extraction during delivery and risk of HBV transmission has been demonstrated.46
Prevention of MTCT of HBV infection
Successes and failures of immunoprophylaxis: HBIG and HBV vaccination at birth
Current standard of care for the prevention of MTCT of HBV infection is treatment of the newborn with HBIG and HBV vaccination (Table 3). At-risk neonates who received HBV vaccine alone at birth had a 26 to 36% chance of MTCT of HBV infection,47 whereas administration of HBIG alone at birth decreased the rate of perinatal HBV transmission to 15 to 20%.23 If HBIG and the HBV vaccine are administered to the neonate of an HBsAg-positive mother within 12 h of delivery, approximately 5% of infants become chronic HBV carriers, a reduction in MTCT of almost 90%.47,48 Overall, the use of HBIG and HBV vaccine has reduced MTCT to 5 to 10%.31,47,49,50
Table 3.
Immunoprophylaxis of neonates with HBIG+HBV vaccine is superior to monotherapy with either agent alone for prevention of MTCT of HBV infection
| HBV vaccine | HBIG | HBIG+HBV vaccine | |
|---|---|---|---|
| MTCT of HBV | 26–36% | 15–20% | 5–10%a |
The Centers for Disease Control and Prevention (CDC) and the Advisory Committee on Immunization Practices (ACIP) recommend administration of HBV vaccine and HBIG to at-risk infants within 12 h of delivery, followed by completion of the hepatitis B vaccine series within the first year of life.23 Newborns of mothers with unknown HBsAg status at the time of birth should receive the HBV vaccine within 12 h of birth; if the mother is found to be HBsAg-positive, the infant should receive HBIG as soon as possible (within 7 days of birth).
The 5% of children who develop chronic hepatitis B infection despite immunoprophylaxis either fail to receive the full regimen of HBV vaccination, fail to develop hepatitis B surface antibody (HBsAb) or are born to mothers with very high levels of HBV DNA (>200 000 IU ml −1 or 6 log10 copies ml−1)22 or who are HBeAg-positive.50 Despite immunoprophylaxis, HBV is still transmitted from 8 to 30% of mothers with high levels of HBV DNA and HBeAg positivity.31,33,37,38 For example, a recent Chinese study demonstrated a dose-dependent correlation between maternal pre-delivery HBV DNA levels and rate of immunoprophylaxis failure.35 All infants who failed immunoprophylaxis were born to HBeAg-positive mothers with HBV DNA levels ≥6 log10 copies ml −1 (≥ 200 000 IU ml −1). In a meta-analysis from the Netherlands, the only factor that significantly affected the efficacy of immunoprophylaxis was the maternal HBV DNA level. There was 100% efficacy with HBV DNA less than 150 pg ml −1 (~107 IU ml −1; 6 × 7 log10 copies ml −1), but only 68% efficacy with HBV DNA levels greater than 150 pg ml−1,22 which is consistent with another report that found a 25 to 50% rate of MTCT of HBV infection with maternal HBV DNA levels over 150 pg ml −1.51 Finally, in an Iranian study of infants who received HBIG and HBV vaccine at birth, the rate of HBV infection was 1.5% in infants born to women who were HBeAg-negative and 18% for infants born to women who were HBeAg-positive.50 HBV DNA levels were not analyzed.
Antiviral therapy for HBV in pregnancy
The significant rate of immunoprophylaxis failure in neonates of women with high HBV DNA levels led to the suggestion that antiviral therapy during the last trimester of pregnancy could decrease MTCT by reducing the level of HBV DNA at the time of delivery. To date, several antivirals have been examined, all of which are nucleos(t)ide analogues. In this section, we summarize those data.
Lamivudine, a cytosine analogue that acts as a nucleoside HBV reverse transcriptase inhibitor and thus is a potent replication inhibitor, produces a median 97% reduction in HBV DNA levels after 2 weeks.52 Multiple randomized controlled trials34,53,54 and two meta-analyses54,55 have demonstrated that lamivudine therapy significantly decreases the likelihood of MTCT of HBV infection and is safe for the mother and newborn. The most recent meta-analysis included 15 randomized controlled trials with 1693 HBV carrier mothers and demonstrated that lamivudine treatment beginning at week 28 of pregnancy significantly decreased MTCT of HBV infection (relative risk 0.33 to 0.43); efficacy was dependent on a decrease in maternal HBV DNA levels to less than 6 log10 copies ml −1 (200 000 IU ml−1).56 A randomized controlled Chinese trial with lamivudine 100 mg per day in HBeAg-positive mothers with HBV DNA greater than 6 log10 copies ml −1 in the third trimester of pregnancy demonstrated a significant reduction in immunoprophylaxis failure (18 vs 39%).34 Starting lamivudine therapy at week 32 of pregnancy has been suggested to achieve a sufficient reduction in HBV DNA level in case of an early delivery.31
Although lamivudine is a pregnancy category C medication (based on studies in animals showing an adverse effect on the fetus), there are insufficient well-controlled studies in humans. However, potential benefits may warrant the use of the drug in pregnant women despite potential risks. In actuality, the safety profile of lamivudine during pregnancy in women has been reported.31 The Antiviral Pregnancy Registry documents an extensive experience with use of lamivudine during pregnancy, with no evidence for teratogenicity or adverse effects (www.apregistry.com). However, lamivudine is no longer a first-line option for long-term treatment of non-pregnant patients with chronic HBV because of the high rate of lamivudine-resistance (occurring in 15% of patients per year).57 Lamivudine has been replaced by tenofovir disoproxil fumarate (TDF) or entecavir for treatment of chronic active HBV infection in non-pregnant patients.20
TDF may also be the preferred antiviral for HBV infection in pregnancy given its potency, safety profile and better resistance profile than lamivudine.58 TDF is a pregnancy category B medication; it has been found to be safe in animal models, but with limited data in humans. There are no prospective studies published for the use of TDF in pregnant women with HBV mono-infection; however, it has been safely used in 1731 pregnant women with HIV (some with HBV co-infection), and the rate of birth defects does not significantly differ from pregnancies not exposed to TDF.31 Given that TDF, 300 mg daily, is a first-line treatment for chronic active HBV infection, its use during the third trimester to prevent HBV transmission is an appropriate option for mothers who need long-term treatment after delivery,20,31 such as patients with chronic active HBV infection, AST or ALT levels greater than 1.5 to 2 times normal, and an HBV DNA level over 20 000 IU ml −1 (5 log10 copies ml−1).20
Telbivudine is another category B anti-HBV agent. Recent prospective studies demonstrated the efficacy of telbivudine 600 mg per day in preventing MTCT when used during the second or third trimesters in HBeAg-positive mothers with HBV DNA>200 000 IU ml −1 (>6 log10 copies ml −1).53,59 In the controlled trial by Han et al.,53 the incidence of perinatal transmission of HBV infection was significantly lower in infants who completed follow-up born to the telbivudine-treated mothers than to controls (0 vs 8%). No differences in maternal adverse events or fetal congenital deformities were observed at 28 weeks after birth.
The safety of these antiviral agents is established by the Antiviral Pregnancy Registry, which has tracked spontaneously reported maternal and fetal outcomes in women receiving oral nucleoside drugs since 1989. As of 31 January 2008, 9889 pregnancies were reported during which the mother had received an oral nucleoside analogue.60 The overall prevalence of birth defects in infants exposed to any antiretroviral agent during the first trimester of 3.0 per 100 live births (117 of 3951), or in any trimester of 2.8 per 100 live births (261 of 9400), was not significantly different from that reported in the general US population of 2.72 per 100 live births. Only infants exposed to the anti-HIV medication didanosine had a significantly higher rate of birth defects than expected. The prevalence of birth defects with lamivudine exposure in the first trimester (3.1%, 85 of 2784) and with TDF (2.2%, 11 of 491) were similar to population controls. The safety data for telbivudine in the Antiviral Pregnancy Registry are limited.31
Because of limited data on secretion of antiviral agents into human breast milk, breast feeding is not recommended by the makers of the nucleos(t)ide analogues if antiviral therapy is continued after delivery. There are scanty data on secretion of TDF or its metabolite, tenofovir, into animal or human breast milk. A single small human study found that small amounts of tenofovir, but not TDF, are present in breast milk of HIV-1-infected women taking TDF, representing 0.03% of the proposed oral HIV-prevention dose of tenofovir for infants.61 Infant exposure to tenofovir through breastfeeding may be negligible because pharmacologically, TDF is converted into its metabolite, tenofovir, prior to excretion of tenofovir into breast milk, and tenofovir is not absorbed by the adult gastrointestinal tract.62 Therefore, patients should be counseled regarding the scarcity of information on TDF and breastfeeding, as well as the known benefits of breastfeeding, to make an informed breastfeeding decision.
There are a few reports of lactic acidosis and hepatic steatosis in pregnant patients receiving nucleos(t)ide analogues, so monitoring of liver enzymes and electrolytes is recommended.10 Postpartum flares of hepatitis may occur after stopping lamivudine in patients who receive it during the last 4 weeks of pregnancy.31,34 For this reason, liver enzymes should be monitored after delivery.
Published guidelines for use of antiviral therapy for HBV in pregnancy
Recent clinical practice guidelines from the European Association for the Study of the Liver (EASL) address antiviral treatment in pregnancy for women with chronic HBV infection (Table 4).58 It is suggested that women with mild liver disease and low HBV DNA levels (chronic inactive HBV infection) complete pregnancy before antiviral treatment is considered; that women with moderate liver disease and no cirrhosis (chronic active HBV infection) undergo antiviral treatment and discontinue treatment before pregnancy if there is a viral response; that women with advanced liver disease (cirrhosis) receive antiviral treatment before, during and after pregnancy; and that women with mild liver disease and very high HBV DNA levels (immune-tolerant chronic HBV infection) receive a category B anti-viral agent (TDF or telbivudine) in the last trimester of pregnancy.58 The Asian Pacific Association for the Study of the Liver (APASL) also recommends prophylactic antiviral treatment in pregnant women with high levels of viremia.63
Table 4.
European Association for Study of the Liver (EASL) recommendation for antiviral therapy for HBV-infected women who desire pregnancya
| Mild liver disease, low viremia (chronic inactive HBV) | → Pregnancy before treatment |
| Moderate liver disease, no cirrhosis (chronic active HBV) | → Treatment before pregnancy; if responds, stop treatment before pregnancy |
| Advanced liver disease (advanced fibrosis-cirrhosis) | → Treatment before, during and after pregnancy |
| Mild liver disease, very high viremia (immunotolerant) | → Treatment in last trimester with a ‘B’ category drug with post-partum discontinuation |
Abbreviations: HBV, hepatitis B virus.
Adapted from the EASL Clinical Practice Guidelines, Ref. 58.
One of the most commonly cited American clinical guidelines for management of chronic hepatitis B, authored and updated by Keefe et al.,20 states ‘data from clinical studies indicate that women with chronic hepatitis B who have HBV DNA levels>107 copies ml −1 and elevated ALT levels, or who have had an HBsAg-positive child, are candidates for antiviral therapy because of the increased risk for transmission to the newborn.’
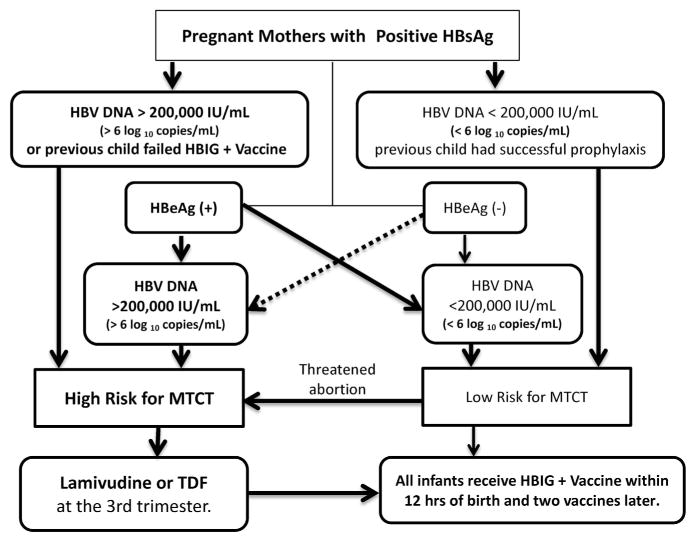
A recent publication proposed ‘an algorithm for risk assessment and patient management that is based on a review of the literature and the opinion of a panel of physicians with expertise in preventing MTCT’. The authors recommended that pregnant women with chronic HBV infection who are at high risk for MTCT (because of HBV DNA levels >200 000 IU ml −1 or >6 log10 copies ml−1, a previous child failed HBIG and HBV vaccine, or who threaten abortion or premature delivery) receive antiviral treatment. The recommended antiviral treatment is lamivudine or telbivudine, or TDF for those with chronic active hepatitis B, beginning in the third trimester of pregnancy.31 (Figure 3)
Figure 3.
Pregnant women with HBV DNA levels >200 000 IU ml−1 (>6 log10 copies ml−1), or any HBsAg-positive woman with a threatened abortion, are at high risk for MTCT and should receive antiviral treatment in the third trimester.31
Cesarean section for prevention of intrapartum HBV transmission
There has been little evidence that cesarean delivery prevents HBV transmission, and current guidelines do not recommend cesarean section to decrease the risk of MTCT in pregnant women with chronic HBV infection.21 Cesarean section would have to be performed before the onset of labor or before the rupture of membranes to be effective. A significant reduction in immuno-prophylaxis failure with elective cesarean section for highly viremic mothers was supported by a systematic review and meta-analysis of four randomized trials involving 789 patients.64 However, it was stated that ‘the conclusions of this review must be considered with great caution because of the high risk of bias in each included study (graded C).’ Pan et al., 65 analyzed data from 1409 infants born through vaginal delivery, elective cesarean section or urgent cesarean section to HBsAg-positive mothers who completed appropriate immunization against HBV. HBV infection was transmitted to a smaller percentage of infants born by elective cesarean section (1.4%) than by vaginal delivery (3.4%, P<0.032) or urgent cesarean section (4.2%, P<0.020). Urgent cesarean section had no effect on vertical transmission compared with vaginal delivery (P = 0.593), whereas infants born by elective cesarean section had a significantly lower rate of vertical transmission than those born by non-elective cesarean section (1.4 vs 36%, P = 0.17). Women with HBV DNA levels<6 log10 copies ml−1 did not transmit the infection to their infants, regardless of the method of delivery, and there were no differences in maternal or infant morbidity and mortality among the groups. The authors conclude that elective cesarean sections for HBeAg-positive mothers with levels of HBV DNA ≥6 log10 copies ml−1 could reduce vertical HBV transmission.
Prevention of MTCT of HBV in the postpartum period: breastfeeding is safe
Although breast milk contains HBsAg,66 breastfeeding does not increase the risk of MTCT of HBV. A 1975 study reported a 53% rate of HBV transmission in breastfed infants vs 60% in formula-fed infants.67 Multiple subsequent studies have shown breastfeeding to be safe for children with mothers with chronic HBV infection. Therefore, the American Academy of Pediatrics states that breastfeeding is not contraindicated.68 According to the prescribing information, use of lamivudine or TDF is not recommended during breastfeeding, though as discussed above, patients should be counseled regarding the scarcity of information on TDF and breastfeeding, as well as the known benefits of breastfeeding, to make an informed breastfeeding decision.
Postnatal follow-up of mother and infant
HBV-infected mothers should be referred to a Hepatology Clinic for evaluation and follow-up. Therapy is considered for individuals infected with HBV who have elevated liver function tests and viral levels greater than 20 000 IU ml −1 (5 log10 copies ml −1). Post-vaccination testing of the infant for HBsAg and HBsAb should be performed after completion of the vaccination series at 9 to 18 months. Testing performed before 9 months can detect HBsAb from HBIG administered during infancy and can miss late HBV infection.
HEPATITIS C IN PREGNANCY
Chronic HCV infection is a major public health problem in the US, accounting for most cases of viral hepatitis in adults and affecting 1 to 2% of the population.69 Most young women with chronic hepatitis C have no signs or symptoms of liver disease. The prevalence of HCV infection among women of childbearing age in the US is approximately 1%.70 Prevalence is increased among pregnant women with specific risk factors: intravenous drug use, inhaled drug use, transfusions prior to 1992, homemade tattoos, and HIV infection. The prevalence of HCV infection in pregnant women with intravenous drug use reaches 70 to 95%.71
Screening and diagnosis of chronic HCV infection
Routine screening of pregnant women for chronic HCV infection is not recommended because treatment of HCV infection is currently not recommended during pregnancy.72,73 This recommendation may change in the future as new therapies for HCV come on line, as discussed below. However, at the present time, screening for HCV infection is recommended for the subset of pregnant women with risk factors for HCV exposure74 (Table 5) because (i) HCV infection is transmitted to neonates, and (ii) postnatal treatment of women with hepatitis C is highly effective.
Table 5.
Indications for HCV screening in pregnancy
| Exposure to blood products before 1992 |
| History of intravenous drug use |
| Dialysis patients |
| HIV or HBV infection |
| Sexual partners of people with HIV, HBV or HCV |
| History of body piercing or tattoos |
| Organ transplant before 1992 |
| Unexplained elevation of AST or ALT |
| Involved in in vitro fertilization from anonymous donors |
| History of incarceration |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; HBV, hepatitis B virus, HCV, hepatitis C virus.
We recommend that all pregnant women with risk factors for hepatitis C, or with abnormal transaminase levels, undergo screening for antibody to HCV. Polymerase chain reaction (PCR) for detection and quantitation of HCV RNA should be performed if HCV antibody is positive.7 Only 60 to 70% of young women who are HCV antibody-positive will have active infection with detectable HCV RNA by PCR;75 30 to 40% of young women exposed to HCV will spontaneously clear the virus and have no chronic HCV infection. Women who are PCR-positive for HCV RNA are at risk for MTCT, and infants born to these mothers should be screened for HCV infection.
Effect of pregnancy on chronic hepatitis C
Most women with chronic hepatitis C do not develop signs or symptoms of liver disease during pregnancy, and improvement in serum AST and ALT levels may occur.76 For example, in a series that included 266 HCV RNA-positive pregnant women, the proportion of those with an elevated ALT level decreased from 56% at the beginning of pregnancy to 7% by the third trimester.76 However, a rise in HCV RNA levels may be noted during the third trimester.77 The improvement of serum ALT levels despite an increase in HCV RNA levels may be due to a pregnancy-related decrease in the immune response to HCV.78
Effect of HCV on pregnancy
As previously noted, after adjusting for sociodemographic variables, large population studies suggest that rates of preterm birth, low birth weight, premature rupture of membranes, gestational diabetes and congenital anomalies are increased with chronic hepatitis C.5–8,12 Maternal HCV infection is also associated with cholestasis of pregnancy, neonatal abstinence syndrome and neonatal ICU admission.5,7,12–14
Cholestasis of pregnancy
Cholestasis of pregnancy (pruritus with elevated serum bile acid levels) has been associated with HCV infection in several studies.7,13,14 For example, in a large study from New Mexico, cholestasis of pregnancy occurred in 10 of 159 HCV antibody-reactive patients (6.3%), but in none of the 141 HCV antibody-nonreactive patients.7 In addition, all of the patients with cholestasis were Hispanic, with a 9.3% incidence of cholestasis of pregnancy in Hispanic HCV antibody-reactive pregnant women (10/108). These data are consistent with the reported increased prevalence of cholestasis of pregnancy in Latino populations.79 Two other studies have demonstrated an association between HCV infection and cholestasis of pregnancy; 15.9% of HCV antibody-positive pregnant women developed cholestasis in one study,13 while the other found that cholestasis developed in 20.3% of HCV RNA-positive pregnant women.14
Preterm birth
Large population studies indicate that chronic HCV infection is associated with preterm birth.5,8 However, other factors may influence pregnancy outcomes in HCV patients. Drug abuse, alcohol abuse and tobacco use were included in the multivariate statistical analysis in the study by Connell.5 On the other hand, in the New Mexico study, the incidence of preterm delivery, defined as delivery before 37 completed weeks of gestation, was significantly increased in HCV antibody-positive pregnancies (24.5% compared with 14.9%); however, when multivariate regression was used to adjust for methadone use, tobacco use and prior preterm delivery, there was no significant difference.7
Neonatal abstinence syndrome
In the New Mexico study, 84 of 95 neonates (88.4%) born to HCV antibody-positive mothers on methadone had neonatal abstinence syndrome that required weaning, whereas only 12 of 33 neonates (36.4%) born to HCV antibody-negative mothers on similar methadone doses required weaning (P = 0.001).7 Pediatricians should be aware of the high risk of methadone withdrawal for infants born to HCV antibody-positive mothers on methadone.
MTCT of HCV infection
MTCT of HCV occurs in 3 to 10% of pregnancies complicated by maternal HCV infection71,80 and is the leading cause of pediatric chronic HCV infection.80 Risk factors for perinatal HCV transmission include higher levels of HCV viremia HIV-HCV co-infection, prolonged rupture of membranes and invasive fetal monitoring (Table 6).
Table 6.
MTCT of HCV
| Occurs in 3–10% of HCV-infected mothers. |
| Is leading cause of pediatric chronic HCV. |
| Risk factors |
| Level of HCV viremia (>600 000 IU ml−1) |
| HIV-HCV co-infection: increased fourfold |
| Prolonged rupture of membranes (>6 h) |
| Invasive fetal monitoring, scalp electrodes |
| C-section does not decrease risk. |
Abbreviations: HCV, hepatitis C virus; MTCT, mother to child transmission.
High levels of HCV viremia
Many observational studies indicate that HCV transmission occurs only when women are positive for HCV RNA by PCR testing, and is more likely when mothers have a high HCV RNA level at the time of delivery.71,80,81 A recent study found that an HCV RNA level over 600 000 IU ml−1 was associated with an increase in MTCT of the virus.82
Though the rate of MTCT of HCV infection is not effected by HCV genotype, the rate of HCV chronicity may be higher for infants with HCV genotype 1 than for those with other genotypes, because of less frequent spontaneous viral clearance.82,83 Recent studies also indicate that there is a relationship between the interleukin 28B (IL28B) genotype and spontaneous clearance of HCV.84 The IL28B genotype of mother and child does not influence MTCT of HCV infection; however, 83% of infants with the CC genotype exhibited spontaneous HCV clearance vs only 22% of the children with a non-CC genotype.82
HIV-HCV co-infection
Maternal co-infection with HIV and HCV is associated with a higher risk of HCV vertical transmission. In HIV-infected pregnant women, the seroprevalence of HCV is 17 to 54%.85 The risk of HCV transmission is approximately threefold higher in infants born to women co-infected with HCV and HIV,86,87 ranging from 8.7 to 19%, with lower rates of MTCT associated with highly active antiretroviral therapy.71 In a large cohort study, HIV co-infected women receiving highly active antiretroviral therapy were no more likely to transmit HCV than those without HIV.76
Prolonged rupture of membranes and invasive fetal monitoring
Rupture of membranes greater than 6 h may increase the risk of MTCT of HCV infection, so it is recommended that the second stage of labor be kept short.74,88 Invasive monitoring of the fetus during labor with a scalp electrode, or exposing the infant to maternal blood infected by HCV because of vaginal or perineal laceration during vaginal delivery, increases the risk of perinatal transmission of HCV.88,89 Thus, avoidance of internal fetal monitoring in HCV-infected women is recommended.74,88
Cesarean section does not prevent HCV transmission
Delivery by cesarean section does not reduce the risk of transmission of HCV from HCV-positive, HIV-negative mothers; consensus statements and guidelines do not recommend cesarean section for these patients.74,90,91 However, observational studies have been under-powered and have not distinguished between elective pre-labor cesarean section and emergency cesarean section after the onset of labor.92 Many of the cesarean sections in observational studies occurred in HIV co-infected women and/or during labor after rupture of amniotic membranes.71,75,92 One study has suggested that HCV transmission may be reduced if infants are delivered by cesarean section prior to rupture of membranes.93 In contrast, a recent meta-analysis of eight studies with 641 mother–infant pairs94 and the large European Paediatric HCV Network study91 suggest that cesarean section does not decrease perinatal HCV transmission from HCV RNA-positive, HIV-negative mothers to infants. There are no randomized controlled trials of cesarean section vs vaginal delivery for preventing MTCT of HCV infection.
Breastfeeding and HCV transmission
There is no evidence that breastfeeding is a risk for MTCT of HCV infection.90 In one study, none of 76 samples of breast milk from HCV antibody-positive mothers contained HCV RNA, while 60% of the mothers tested had HCV viremia.95 Other studies show no evidence of transmission with breastfeeding; either similar rates of infection are observed in breastfed and bottle-fed infants, or no viral transmission is documented.96–98 The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics support breastfeeding by HCV-infected mothers.99
Prevention of MTCT of HCV infection
Until recently, standard HCV treatment has been with pegylated-interferon and ribavirin. Although young women generally respond well to treatment, ribavirin is considered teratogenic (pregnancy category X), and interferon has been associated with intrauterine growth restriction (pregnancy category C). Thus, treatment is not recommended for use in pregnancy or as a prophylactic in newborns. As such, there has been a critical need for new treatment options that do not have these adverse effects.
For patients who are not pregnant, antiviral therapy for HCV has become less toxic and more efficacious. Until recently, recommendations for HCV treatment were pegylated interferon plus ribavirin for genotype 2 or 3 HCV infection, and a three-drug regimen (pegylated interferon injection with oral ribavirin plus a protease inhibitor such as boceprevir or telaprevir) for genotype 1 HCV infection. However, the Food and Drug Administration has recently approved a new once/day protease inhibitor, simeprevir, as well as sofosbuvir, an oral HCV polymerase inhibitor. These interferon-free regimens have been in use since January 2014, but have not been studied for use during pregnancy.100–104 The question of HCV treatment with these new medications during pregnancy, now not recommended, will surface. Treatment of HCV-infected pregnant women might be envisioned in the future if non-teratogenic regimens are developed, which could further reduce the up to 10% risk of MTCT and the long-term health burden of HCV infection resulting from vertical transmission.
Diagnosis of HCV infection in the newborn
Infants born to women with chronic hepatitis C have maternal anti-HCV antibodies that can be detected until 12 to 15 months of life. Chronic hepatitis C infection in infancy is diagnosed by the presence of HCV RNA by PCR testing at 3 to 6 months of age or by detectable HCV antibody at age 18 months. Therefore, appropriate infant follow-up after birth for testing is needed to detect HCV MTCT.
Postnatal follow-up of mothers
Postnatal follow-up for chronic hepatitis C is particularly important because the new generation of oral anti-HCV medications, used for 12 weeks for genotypes 1 or 2 HCV and for 24 weeks for genotype 3 HCV, can achieve sustained viral response rates over 90%.105,106 These patients should be referred for treatment of hepatitis C once they have completed breastfeeding.
HBV AND HCV IN PREGNANCY: SUMMARY AND RECOMMENDED GUIDELINES FOR TREATMENT
Antiviral therapy with TDF or telbivudine beginning at 32 weeks of gestation should be strongly considered for women with high HBV DNA levels (>200 000 IU ml−1 or 6 log10 copies ml−1) to decrease the rate of MTCT of HBV infection (Level A) (Table 7). All infants born to HBsAg-positive mothers should receive HBIG and HBV vaccine as early as possible, no later than 12 h after birth (Level A). Newborns of mothers with unknown hepatitis B status should also receive immunoprophylaxis (Level C). The vaccine series should be completed according to recommended schedules.107 Current guidelines do not recommend elective cesarean delivery for mothers with chronic HBV infection (Level B); however, a recent nonrandomized study showed elective cesarean section may decrease vertical transmission of HBV if the HBV DNA level is >20 million IU ml −1 (> 6 log10 copies ml−1) at term. 65 Breast feeding does not increase MTCT of HBV and is not contraindicated (Level B), though breast feeding is not recommended during maternal antiviral therapy by drug manufacturers (Level C).
Table 7.
Grading system for recommendations
| Level of evidence | Description |
|---|---|
| Level A | Data derived from multiple randomized clinical trials or meta-analyses. |
| Level B | Data derived from a single randomized trial, or nonrandomized studies. |
| Level C | Only consensus opinion of experts, case studies, or standard-of-care. |
We recommend that all pregnant women with risk factors for hepatitis C or with abnormal ALT levels undergo screening for antibodies to HCV; if positive, PCR for quantitation of HCV RNA level should be performed (Level B). Women who are PCR-positive for HCV are at risk for MTCT of HCV, and infants born to these mothers should be screened for HCV infection (Level B). Women with HCV infection are at an increased risk for cholestasis of pregnancy (Level B). HCV-infected women on methadone have an increased risk for preterm birth, while their infants have a high incidence of neonatal withdrawal syndrome (Level C). Unlike HBV, patients are typically not treated for HCV during pregnancy. However, young women are ideal candidates for treatment of HCV infection in the postpartum period, with a greater than 90% chance of resolving the infection with less toxic and more efficacious new regimens (Level A).
Acknowledgments
This work was partially supported the Department of Obstetrics and Gynecology Research Development and Faculty Development Funds at the University of Iowa.
Footnotes
CONFLICT OF INTEREST
Kimberly K. Leslie and Kristina W. Thiel are cofounders of Immortagen, L.L.C. The authors declare no conflict of interest.
References
- 1.Sorrell MF, Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, et al. National Institutes of Health Consensus Development Conference Statement: management of hepatitis B. Ann Intern Med. 2009;150(2):104–110. doi: 10.7326/0003-4819-150-2-200901200-00100. [DOI] [PubMed] [Google Scholar]
- 2.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 3.Merrill RM, Hunter BD. Seroprevalence of markers for hepatitis B viral infection. Int J Infect Dis. 2011;15(2):E78–E121. doi: 10.1016/j.ijid.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Rustgi VK. The epidemiology of hepatitis C infection in the United States. J Gastroenterol. 2007;42(7):513–521. doi: 10.1007/s00535-007-2064-6. [DOI] [PubMed] [Google Scholar]
- 5.Connell LE, Salihu HM, Salemi JL, August EM, Weldeselasse H, Mbah AK. Maternal hepatitis B and hepatitis C carrier status and perinatal outcomes. Liver Int. 2011;31(8):1163–1170. doi: 10.1111/j.1478-3231.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- 6.Reddick KL, Jhaveri R, Gandhi M, James AH, Swamy GK. Pregnancy outcomes associated with viral hepatitis. J Viral Hepat. 2011;18(7):e394–e398. doi: 10.1111/j.1365-2893.2011.01436.x. [DOI] [PubMed] [Google Scholar]
- 7.Berkley EM, Leslie KK, Arora S, Qualls C, Dunkelberg JC. Chronic hepatitis C in pregnancy. Obstet Gynecol. 2008;112(2 Pt 1):304–310. doi: 10.1097/AOG.0b013e318180a4f3. [DOI] [PubMed] [Google Scholar]
- 8.Safir A, Levy A, Sikuler E, Sheiner E. Maternal hepatitis B virus or hepatitis C virus carrier status as an independent risk factor for adverse perinatal outcome. Liver Int. 2010;30(5):765–770. doi: 10.1111/j.1478-3231.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 9.Tse KY, Ho LF, Lao T. The impact of maternal HBsAg carrier status on pregnancy outcomes: a case-control study. J Hepatol. 2005;43(5):771–775. doi: 10.1016/j.jhep.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Gambarin-Gelwan M. Hepatitis B in pregnancy. Clin Liver Dis. 2007;11(4):945–963. x. doi: 10.1016/j.cld.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Lao TT, Chan BC, Leung WC, Ho LF, Tse KY. Maternal hepatitis B infection and gestational diabetes mellitus. J Hepatol. 2007;47(1):46–50. doi: 10.1016/j.jhep.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Pergam SA, Wang CC, Gardella CM, Sandison TG, Phipps WT, Hawes SE. Pregnancy complications associated with hepatitis C: data from a 2003–2005 Washington state birth cohort. Am J Obstet Gynecol. 2008;199(1):38, e31–e39. doi: 10.1016/j.ajog.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Locatelli A, Roncaglia N, Arreghini A, Bellini P, Vergani P, Ghidini A. Hepatitis C virus infection is associated with a higher incidence of cholestasis of pregnancy. Br J Obstet Gynaecol. 1999;106(5):498–500. doi: 10.1111/j.1471-0528.1999.tb08305.x. [DOI] [PubMed] [Google Scholar]
- 14.Paternoster DM, Fabris F, Palu G, Santarossa C, Bracciante R, Snijders D, et al. Intra-hepatic cholestasis of pregnancy in hepatitis C virus infection. Acta Obstet Gynecol Scand. 2002;81(2):99–103. [PubMed] [Google Scholar]
- 15.Ludlow JP, Evans SF, Hulse G. Obstetric and perinatal outcomes in pregnancies associated with illicit substance abuse. Aust N Z J Obstet Gynaecol. 2004;44(4):302–306. doi: 10.1111/j.1479-828X.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 16.Euler GL, Wooten KG, Baughman AL, Williams WW. Hepatitis B surface antigen prevalence among pregnant women in urban areas: implications for testing, reporting, and preventing perinatal transmission. Pediatrics. 2003;111(5 Part 2):1192–1197. [PubMed] [Google Scholar]
- 17.Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol. 1977;105(2):94–98. doi: 10.1093/oxfordjournals.aje.a112370. [DOI] [PubMed] [Google Scholar]
- 18.Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med. 1976;294(14):746–749. doi: 10.1056/NEJM197604012941402. [DOI] [PubMed] [Google Scholar]
- 19.Stevens CE, Neurath RA, Beasley RP, Szmuness W. HBeAg and anti-HBe detection by radioimmunoassay: correlation with vertical transmission of hepatitis B virus in Taiwan. J Med Virol. 1979;3(3):237–241. doi: 10.1002/jmv.1890030310. [DOI] [PubMed] [Google Scholar]
- 20.Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6(12):1315–1341. doi: 10.1016/j.cgh.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 21.ACOG educational bulletin. Viral hepatitis in pregnancy. Number 248, July 1998 (replaces No. 174, November 1992) American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1998;63(2):195–202. [PubMed] [Google Scholar]
- 22.del Canho R, Grosheide PM, Schalm SW, de Vries RR, Heijtink RA. Failure of neonatal hepatitis B vaccination: the role of HBV-DNA levels in hepatitis B carrier mothers and HLA antigens in neonates. J Hepatol. 1994;20(4):483–486. doi: 10.1016/s0168-8278(05)80494-5. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Assessing completeness of perinatal hepatitis B virus infection reporting through comparison of immunization program and surveillance data--United States. MMWR Morb Mortal Wkly Rep. 2011;60(13):410–413. [PubMed] [Google Scholar]
- 24.Levy M, Koren G. Hepatitis B vaccine in pregnancy: maternal and fetal safety. Am J Perinatol. 1991;8(3):227–232. doi: 10.1055/s-2007-999384. [DOI] [PubMed] [Google Scholar]
- 25.Ornoy A, Tenenbaum A. Pregnancy outcome following infections by coxsackie, echo, measles, mumps, hepatitis, polio and encephalitis viruses. Reprod Toxicol. 2006;21(4):446–457. doi: 10.1016/j.reprotox.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Rawal BK, Parida S, Watkins RP, Ghosh P, Smith H. Symptomatic reactivation of hepatitis B in pregnancy. Lancet. 1991;337(8737):364. doi: 10.1016/0140-6736(91)90993-y. [DOI] [PubMed] [Google Scholar]
- 27.Lin HH, Chen PJ, Chen DS, Sung JL, Yang KH, Young YC, et al. Postpartum subsidence of hepatitis B viral replication in HBeAg-positive carrier mothers. J Med Virol. 1989;29(1):1–6. doi: 10.1002/jmv.1890290102. [DOI] [PubMed] [Google Scholar]
- 28.Lin HH, Wu WY, Kao JH, Chen DS. Hepatitis B post-partum e antigen clearance in hepatitis B carrier mothers: Correlation with viral characteristics. J Gastroenterol Hepatol. 2006;21(3):605–609. doi: 10.1111/j.1440-1746.2006.04198.x. [DOI] [PubMed] [Google Scholar]
- 29.ter Borg MJ, Leemans WF, de Man RA, Janssen HL. Exacerbation of chronic hepatitis B infection after delivery. J Viral Hepat. 2008;15(1):37–41. doi: 10.1111/j.1365-2893.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- 30.Libbus MK, Phillips LM. Public health management of perinatal hepatitis B virus. Public Health Nurs. 2009;26(4):353–361. doi: 10.1111/j.1525-1446.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 31.Pan CQ, Duan ZP, Bhamidimarri KR, Zou HB, Liang XF, Li J, et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol. 2012;10(5):452–459. doi: 10.1016/j.cgh.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 32.van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2003;10(4):294–297. doi: 10.1046/j.1365-2893.2003.00440.x. [DOI] [PubMed] [Google Scholar]
- 33.Xu DZ, Yan YP, Choi BC, Xu JQ, Men K, Zhang JX, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67(1):20–26. doi: 10.1002/jmv.2187. [DOI] [PubMed] [Google Scholar]
- 34.Xu WM, Cui YT, Wang L, Yang H, Liang ZQ, Li XM, et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. J Viral Hepat. 2009;16(2):94–103. doi: 10.1111/j.1365-2893.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- 35.Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat. 2012;19(2):e18–e25. doi: 10.1111/j.1365-2893.2011.01492.x. [DOI] [PubMed] [Google Scholar]
- 36.Alter MJ. Epidemiology of hepatitis B in Europe and worldwide. J Hepatol. 2003;39(Suppl 1):S64–S69. doi: 10.1016/s0168-8278(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 37.Wang JS, Chen H, Zhu QR. Transformation of hepatitis B serologic markers in babies born to hepatitis B surface antigen positive mothers. World J Gastroenterol. 2005;11(23):3582–3585. doi: 10.3748/wjg.v11.i23.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu ZY, Duan SC, Margolis HS, Purcell RH, Ou-Yang PY, Coleman PJ, et al. Long-term efficacy of active postexposure immunization of infants for prevention of hepatitis B virus infection. United States-People’s Republic of China Study Group on Hepatitis B. J Infect Dis. 1995;171(1):54–60. doi: 10.1093/infdis/171.1.54. [DOI] [PubMed] [Google Scholar]
- 39.Krugman S. Hepatitis B virus and the neonate. Ann NY Acad Sci. 1988;549:129–134. doi: 10.1111/j.1749-6632.1988.tb23965.x. [DOI] [PubMed] [Google Scholar]
- 40.Beasley RP, Hwang LY, Stevens CE, Lin CC, Hsieh FJ, Wang KY, et al. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology. 1983;3(2):135–141. doi: 10.1002/hep.1840030201. [DOI] [PubMed] [Google Scholar]
- 41.Schalm SW, Mazel JA, de Gast GC, Heijtink RA, Botman MJ, Banffer JR, et al. Prevention of hepatitis B infection in newborns through mass screening and delayed vaccination of all infants of mothers with hepatitis B surface antigen. Pediatrics. 1989;83(6):1041–1048. [PubMed] [Google Scholar]
- 42.Stevens CE, Toy PT, Tong MJ, Taylor PE, Vyas GN, Nair PV, et al. Perinatal hepatitis B virus transmission in the United States. Prevention by passive-active immunization. JAMA. 1985;253(12):1740–1745. [PubMed] [Google Scholar]
- 43.Lin HH, Lee TY, Chen DS, Sung JL, Ohto H, Etoh T, et al. Transplacental leakage of HBeAg-positive maternal blood as the most likely route in causing intrauterine infection with hepatitis B virus. J Pediatr. 1987;111(6 Pt 1):877–881. doi: 10.1016/s0022-3476(87)80210-x. [DOI] [PubMed] [Google Scholar]
- 44.Ohto H, Lin HH, Kawana T, Etoh T, Tohyama H. Intrauterine transmission of hepatitis B virus is closely related to placental leakage. J Med Virol. 1987;21(1):1–6. doi: 10.1002/jmv.1890210102. [DOI] [PubMed] [Google Scholar]
- 45.Ko TM, Tseng LH, Chang MH, Chen DS, Hsieh FJ, Chuang SM, et al. Amniocentesis in mothers who are hepatitis B virus carriers does not expose the infant to an increased risk of hepatitis B virus infection. Arch Gynecol Obstet. 1994;255(1):25–30. doi: 10.1007/BF02390671. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Zhu Q, Zhang X. Effect of delivery mode on maternal-infant transmission of hepatitis B virus by immunoprophylaxis. Chin Med J (Engl) 2002;115(10):1510–1512. [PubMed] [Google Scholar]
- 47.Andre FE, Zuckerman AJ. Review: protective efficacy of hepatitis B vaccines in neonates. J Med Virol. 1994;44(2):144–151. doi: 10.1002/jmv.1890440206. [DOI] [PubMed] [Google Scholar]
- 48.Stevens CE, Taylor PE, Tong MJ, Toy PT, Vyas GN, Nair PV, et al. Yeast-recombinant hepatitis B vaccine. Efficacy with hepatitis B immune globulin in prevention of perinatal hepatitis B virus transmission. JAMA. 1987;257(19):2612–2616. doi: 10.1001/jama.257.19.2612. [DOI] [PubMed] [Google Scholar]
- 49.Noto H, Terao T, Ryou S, Hirose Y, Yoshida T, Ookubo H, et al. Combined passive and active immunoprophylaxis for preventing perinatal transmission of the hepatitis B virus carrier state in Shizuoka, Japan during 1980–1994. J Gastroenterol Hepatol. 2003;18(8):943–949. doi: 10.1046/j.1440-1746.2003.03092.x. [DOI] [PubMed] [Google Scholar]
- 50.Soleimani Amiri MJ, Hasanjani Roushan MR, Baiany M, Taheri H, Hasanjani Roushan M. Outcomes of passive-active immunoprophylaxis given to infants of mothers infected with hepatitis B virus in Babol, Iran. J Clin Virol. 2010;49(4):283–285. doi: 10.1016/j.jcv.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Ip HM, Lelie PN, Wong VC, Kuhns MC, Reesink HW. Prevention of hepatitis B virus carrier state in infants according to maternal serum levels of HBV DNA. Lancet. 1989;1(8635):406–410. doi: 10.1016/s0140-6736(89)90003-2. [DOI] [PubMed] [Google Scholar]
- 52.Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339(2):61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 53.Han GR, Cao MK, Zhao W, Jiang HX, Wang CM, Bai SF, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011;55(6):1215–1221. doi: 10.1016/j.jhep.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 54.Shi Z, Yang Y, Ma L, Li X, Schreiber A. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116(1):147–159. doi: 10.1097/AOG.0b013e3181e45951. [DOI] [PubMed] [Google Scholar]
- 55.Su GG, Pan KH, Zhao NF, Fang SH, Yang DH, Zhou Y. Efficacy and safety of lamivudine treatment for chronic hepatitis B in pregnancy. World J Gastroenterol. 2004;10(6):910–912. doi: 10.3748/wjg.v10.i6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han L, Zhang HW, Xie JX, Zhang Q, Wang HY, Cao GW. A meta-analysis of lamivudine for interruption of mother-to-child transmission of hepatitis B virus. World J Gastroenterol. 2011;17(38):4321–4333. doi: 10.3748/wjg.v17.i38.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peters MG. Special populations with hepatitis B virus infection. Hepatology. 2009;49(5 Suppl):S146–S155. doi: 10.1002/hep.22965. [DOI] [PubMed] [Google Scholar]
- 58.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Pan CQ, Han GR, Jiang HX, Zhao W, Cao MK, Wang CM, et al. Telbivudine prevents vertical transmission from HBeAg-positive women with chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(5):520–526. doi: 10.1016/j.cgh.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 60.Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology. 2009;49(5 Suppl):S185–S195. doi: 10.1002/hep.22885. [DOI] [PubMed] [Google Scholar]
- 61.Benaboud S, Pruvost A, Coffie PA, Ekouevi DK, Urien S, Arrive E, et al. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d’Ivoire, in the ANRS 12109 TEmAA Study, Step 2. Antimicrob Agents Chemother. 2011;55(3):1315–1317. doi: 10.1128/AAC.00514-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pan CQ, Mi LJ, Bunchorntavakul C, Karsdon J, Huang WM, Singhvi G, et al. Tenofovir disoproxil fumarate for prevention of vertical transmission of hepatitis B virus infection by highly viremic pregnant women: a case series. Dig Dis Sci. 2012;57(9):2423–2429. doi: 10.1007/s10620-012-2187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liaw YF, Kao JH, Piratvisuth T, Chan HLY, Chien RN, Liu CJ, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6(3):531–561. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 64.Yang J, Zeng XM, Men YL, Zhao LS. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis B virus--a systematic review. Virol J. 2008;5:100. doi: 10.1186/1743-422X-5-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pan CQ, Zou HB, Chen Y, Zhang XH, Zhang H, Li J, et al. Cesarean section reduces perinatal transmission of hepatitis B virus infection from hepatitis B surface antigen-positive women to their infants. Clini Gastroenterol Hepatol. 2013;11(10):1349–1355. doi: 10.1016/j.cgh.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 66.Wong VC, Lee AK, Ip HM. Transmission of hepatitis B antigens from symptom free carrier mothers to the fetus and the infant. Br J Obstet Gynaecol. 1980;87(11):958–965. doi: 10.1111/j.1471-0528.1980.tb04458.x. [DOI] [PubMed] [Google Scholar]
- 67.Beasley RP, Stevens CE, Shiao IS, Meng HC. Evidence against breastfeeding as a mechanism for vertical transmission of hepatitis B. Lancet. 1975;2(7938):740–741. doi: 10.1016/s0140-6736(75)90724-2. [DOI] [PubMed] [Google Scholar]
- 68.Gartner LM, Morton J, Lawrence RA, Naylor AJ, O’Hare D, Schanler RJ, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 69.Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26(3 Suppl 1):62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 70.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 71.Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34(2):223–229. doi: 10.1053/jhep.2001.25885. [DOI] [PubMed] [Google Scholar]
- 72.Jonas MM. Hepatitis C infection in children. N Engl J Med. 1999;341(12):912–913. doi: 10.1056/NEJM199909163411210. [DOI] [PubMed] [Google Scholar]
- 73.Zanetti AR, Tanzi E, Newell ML. Mother-to-infant transmission of hepatitis C virus. J Hepatol. 1999;31(Suppl 1):96–100. doi: 10.1016/s0168-8278(99)80383-3. [DOI] [PubMed] [Google Scholar]
- 74.National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002--June 10–12, 2002. Hepatology. 2002;36(5 Suppl 1):S3–20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 75.Roberts EA, Yeung L. Maternal-infant transmission of hepatitis C virus infection. Hepatology. 2002;36(5 Suppl 1):S106–S113. doi: 10.1053/jhep.2002.36792. [DOI] [PubMed] [Google Scholar]
- 76.Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31(3):751–755. doi: 10.1002/hep.510310328. [DOI] [PubMed] [Google Scholar]
- 77.Gervais A, Bacq Y, Bernuau J, Martinot M, Auperin A, Boyer N, et al. Decrease in serum ALT and increase in serum HCV RNA during pregnancy in women with chronic hepatitis C. J Hepatol. 2000;32(2):293–299. doi: 10.1016/s0168-8278(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 78.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 79.Lee RH, Goodwin TM, Greenspoon J, Incerpi M. The prevalence of intrahepatic cholestasis of pregnancy in a primarily Latina Los Angeles population. J Perinatol. 2006;26(9):527–532. doi: 10.1038/sj.jp.7211545. [DOI] [PubMed] [Google Scholar]
- 80.Ohto H, Terazawa S, Sasaki N, Hino K, Ishiwata C, Kako M, et al. Transmission of hepatitis C virus from mothers to infants. The Vertical Transmission of Hepatitis C Virus Collaborative Study Group. N Engl J Med. 1994;330(11):744–750. doi: 10.1056/NEJM199403173301103. [DOI] [PubMed] [Google Scholar]
- 81.Ferrero S, Lungaro P, Bruzzone BM, Gotta C, Bentivoglio G, Ragni N. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990–2000) Acta Obstet Gynecol Scand. 2003;82(3):229–234. doi: 10.1034/j.1600-0412.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- 82.Ruiz-Extremera A, Munoz-Gamez JA, Salmeron-Ruiz MA, de Rueda PM, Quiles-Perez R, Gila-Medina A, et al. Genetic variation in interleukin 28B with respect to vertical transmission of hepatitis C virus and spontaneous clearance in HCV-infected children. Hepatology. 2011;53(6):1830–1838. doi: 10.1002/hep.24298. [DOI] [PubMed] [Google Scholar]
- 83.Bortolotti F, Verucchi G, Camma C, Cabibbo G, Zancan L, Indolfi G, et al. Long-term course of chronic hepatitis C in children: from viral clearance to end-stage liver disease. Gastroenterology. 2008;134(7):1900–1907. doi: 10.1053/j.gastro.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 84.Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461(7265):798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thomas DL, Villano SA, Riester KA, Hershow R, Mofenson LM, Landesman SH, et al. Perinatal transmission of hepatitis C virus from human immunodeficiency virus type 1-infected mothers. Women and Infants Transmission Study. J Infect Dis. 1998;177(6):1480–1488. doi: 10.1086/515315. [DOI] [PubMed] [Google Scholar]
- 86.Thaler MM, Park CK, Landers DV, Wara DW, Houghton M, Veereman-Wauters G, et al. Vertical transmission of hepatitis C virus. Lancet. 1991;338(8758):17–18. doi: 10.1016/0140-6736(91)90006-b. [DOI] [PubMed] [Google Scholar]
- 87.Zanetti AR, Tanzi E, Paccagnini S, Principi N, Pizzocolo G, Caccamo ML, et al. Mother-to-infant transmission of hepatitis C virus. Lombardy Study Group on Vertical HCV Transmission. Lancet. 1995;345(8945):289–291. doi: 10.1016/s0140-6736(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 88.Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, Wurtzel H, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192(11):1880–1889. doi: 10.1086/497701. [DOI] [PubMed] [Google Scholar]
- 89.Steininger C, Kundi M, Jatzko G, Kiss H, Lischka A, Holzmann H. Increased risk of mother-to-infant transmission of hepatitis C virus by intrapartum infantile exposure to maternal blood. J Infect Dis. 2003;187(3):345–351. doi: 10.1086/367704. [DOI] [PubMed] [Google Scholar]
- 90.Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1–39. [PubMed] [Google Scholar]
- 91.European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. European Paediatric Hepatitis C Virus Network. BJOG. 2001;108(4):371–377. [PubMed] [Google Scholar]
- 92.McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database Syst Rev. 2006;(4):CD005546. doi: 10.1002/14651858.CD005546.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gibb DM, Goodall RL, Dunn DT, Healy M, Neave P, Cafferkey M, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356(9233):904–907. doi: 10.1016/s0140-6736(00)02681-7. [DOI] [PubMed] [Google Scholar]
- 94.Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, Alavian SM. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283(2):255–260. doi: 10.1007/s00404-010-1588-9. [DOI] [PubMed] [Google Scholar]
- 95.Polywka S, Schroter M, Feucht HH, Zollner B, Laufs R. Low risk of vertical transmission of hepatitis C virus by breast milk. Clin Infect Dis. 1999;29(5):1327–1329. doi: 10.1086/313473. [DOI] [PubMed] [Google Scholar]
- 96.Kumar RM, Shahul S. Role of breast-feeding in transmission of hepatitis C virus to infants of HCV-infected mothers. J Hepatol. 1998;29(2):191–197. doi: 10.1016/s0168-8278(98)80003-2. [DOI] [PubMed] [Google Scholar]
- 97.Resti M, Azzari C, Mannelli F, Moriondo M, Novembre E, de Martino M, et al. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Tuscany Study Group on Hepatitis C Virus Infection. BMJ. 1998;317(7156):437–441. doi: 10.1136/bmj.317.7156.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thomas SL, Newell ML, Peckham CS, Ades AE, Hall AJ. A review of hepatitis C virus (HCV) vertical transmission: risks of transmission to infants born to mothers with and without HCV viraemia or human immunodeficiency virus infection. Int J Epidemiol. 1998;27(1):108–117. doi: 10.1093/ije/27.1.108. [DOI] [PubMed] [Google Scholar]
- 99.ACOG committee opinion. Breastfeeding and the risk of hepatitis C virus transmission. Number 220, August 1999. Committee on Obstetric Practice. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1999;66(3):307–308. [PubMed] [Google Scholar]
- 100.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 101.Jesudian AB, de Jong YP, Jacobson IM. Emerging therapeutic targets for hepatitis C virus infection. Clin Gastroenterol Hepatol. 2013;11(6):612–619. e611. doi: 10.1016/j.cgh.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 102.Schmidt WN, Nelson DR, Pawlotsky JM, Sherman KE, Thomas DL, Chung RT. Direct-acting antiviral agents and the path to interferon independence. Clin Gastroenterol Hepatol. 2013;12(5):728–737. doi: 10.1016/j.cgh.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 104.Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson DR, Zeuzem S, et al. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med. 2014;370(3):222–232. doi: 10.1056/NEJMoa1306227. [DOI] [PubMed] [Google Scholar]
- 105.Schmidt WN, Nelson DR, Pawlotsky JM, Sherman KE, Thomas DL, Chung RT. Direct-acting antiviral agents and the path to interferon independence. Clin Gastroenterol Hepatol. 2014;12(5):728–737. doi: 10.1016/j.cgh.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sulkowski MS. Daclatasvir plus Sofosbuvir for Previously Treated or Untreated Chronic HCV Infection. New Engl J Med. 2014;370(15):1469–1469. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 107.Poland GA, Jacobson RM. Clinical practice: prevention of hepatitis B with the hepatitis B vaccine. N Engl J Med. 2004;351(27):2832–2838. doi: 10.1056/NEJMcp041507. [DOI] [PubMed] [Google Scholar]