Abstract
BACKGROUND
We sought to determine the maximum tolerated dose (MTD) of gemcitabine given concurrently with preoperative, fixed-dose external-beam radiation therapy (EBRT) for patients with resectable, high-risk extremity and trunk soft tissue sarcomas (STS).
METHODS
Gemcitabine was administered on days 1, 8, 22, 29, 43, and 50 with EBRT (50 Gy, 25 fractions over 5 weeks). The gemcitabine MTD was determined using a toxicity severity weight method (TSWM), incorporating 6 toxicity types. The TSWM is a Bayesian procedure that chooses each cohort’s dose to have posterior mean total toxicity burden closest to a predetermined clinician-defined target. Clinicopathologic and outcome data were also collected.
RESULTS
Thirty-six patients completed the study. Using the TSWM, the gemcitabine MTD was 700 mg/m2. At this dose level, 4 patients (24%) experienced grade 4 toxicity; no toxicity-related deaths occurred. All tumors were resected with microscopically negative margins. Pathologic responses of >90% tumor necrosis were achieved in 17 patients (47%); 14 (39%) had complete responses. With a median follow-up of 6.2 years, the 5-year locoregional recurrence-free survival, distant metastasis-free survival, and overall survival rates were 85%, 80%, and 86%, respectively.
CONCLUSIONS
The TSWM combines data from qualitatively different toxicities and can be used to determine the MTD for a drug given as part of multimodality treatment. Neoadjuvant gemcitabine plus radiation therapy is feasible and safe in patients with high-risk extremity and trunk STS. Major pathologic responses can be achieved and after complete resection, long-term clinical outcomes are encouraging.
Keywords: neoadjuvant, gemcitabine, radiation, sarcoma, toxicity
INTRODUCTION
Soft tissue sarcomas (STS) account for only 1% of adult solid tumors;1 however, in affected patients, these tumors can often cause significant morbidity and death. While STS can develop anywhere in the body, the majority of tumors (70%) occur in the extremities or trunk. In patients with small (T1 or ≤ 5 cm) and low-grade tumors, surgical resection alone typically provides sufficient disease control; however, in patients with large (T2 or > 5 cm) or intermediate- to high-grade tumors, multimodality treatment is critical as these tumors have a high risk for both locoregional and distant recurrence after surgical resection alone. Several options for multimodality treatment are available, but the optimal regimen and appropriate sequence of treatments are currently unresolved.
Gemcitabine is an antimetabolite drug often used in combination with docetaxel as second-line systemic therapy against advanced STS.2,3 When used as a single agent, gemcitabine has a reported objective response rate of 8–18%4, 5 and for leiomyosarcoma specifically, 14–19%.6 Gemcitabine, both as a single agent and in combination with docetaxel, also appears to have activity against malignant fibrous histiocytoma, now known as undifferentiated pleomorphic sarcoma.5 In addition, gemcitabine has potent radiosensitizing properties:7 in fact, favorable clinical outcomes with gemcitabine plus radiation therapy have been reported for patients with advanced head and neck, pancreatic, and lung cancers.7–10 Preclinical data from cell lines and xenograft models also suggest that gemcitabine may have efficacy as a radiosensitizer specifically for STS.11,12
For localized, high-risk solid tumors, our institution has had a longstanding interest in preoperative or neoadjuvant therapy, which offers several advantages, including the ability to monitor primary tumor response in vivo and control potential sites of locoregional and distant microscopic disease upfront.13,14 The safety and efficacy of neoadjuvant gemcitabine plus radiation therapy in patients with STS is unknown. We performed a phase I trial to determine the maximum tolerated dose (MTD) of gemcitabine when combined with radiation therapy in the neoadjuvant setting for patients with high-risk extremity or trunk STS. To determine the MTD of gemcitabine, we used a novel, sequentially adaptive dose-finding method that incorporated multiple toxicity types with assigned severity weights15 rather than a traditional phase I trial designs, which characterizes toxicity as a single binary variable. Clinicopathologic and long-term outcome data for our study patients were also analyzed.
METHODS
This study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center. Written informed consent was obtained from all patients before enrollment in the trial and receipt of treatment. This study was registered in the Clinical Trials.gov database with the identifier NCT02046304.
To be eligible for the trial, patients had to have resectable (measurable or non-measurable), grade 2 or 3 (intermediate- to high-grade) STS of an extremity or trunk, with histologic verification at MD Anderson. At the time of study enrollment, patients were also required to have a Zubrod performance status of 0 or 1; absolute neutrophil count > 1500 cells/μL; platelet count ≥ 100,000/mL; serum creatinine ≤ 1.8 mg/dL; liver transaminases ≤ 3 times the upper limit of normal; and total bilirubin ≤ 1.5 mg/dL. Patients were excluded if they had received prior radiation therapy in the area of the primary tumor or if the anticipated radiation field would include the perineum, scrotum, or vaginal introitus. Prior systemic chemotherapy was allowed with a washout period of at least three weeks and confirmed normal blood counts prior to enrolling in our study.
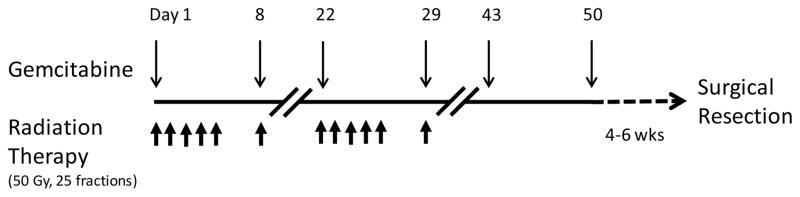
Study patients received neoadjuvant gemcitabine (Eli Lilly and Company; Indianapolis, Indiana, USA) at doses ranging from 400 to 700 mg/m2 in combination with fixed-dose external-beam radiation therapy (EBRT) for a total dose of 50 Gray (Gy) (Figure 1). Gemcitabine was given as an intravenous infusion of 10 mg/m2/min at the assigned dose on days 1, 8, 22, 29, 43, and 50. Radiation therapy was given in 25 fractions over 5 weeks. When given on the same day, gemcitabine was given 4 hours before radiation therapy. All of the patients were treated with 3D conformal planning with CT based simulation and 3D computer planning. No effort was made to spare the skin. Gross tumor volume was treated with a 2 cm radial margin and a 5 cm proximal and distal margin. Four to six weeks following the last dose of gemcitabine, patients underwent complete surgical resection of their primary tumor. Patients with microscopically positive margins underwent re-resection to achieve negative margins. Representative sections of resected tumors were prepared using standard hematoxylin and eosin staining. The percentage of tumor necrosis for each case was then scored by an experienced soft tissue pathologist, who was blinded to the details of the patient’s study treatment.
Figure 1.
Treatment schema for phase I trial of neoadjuvant gemcitabine with concurrent external-beam radiation therapy in high-risk extremity and trunk soft tissue sarcoma. Gy, gray.
The gemcitabine dose for each successive patient cohort was determined using the sequentially adaptive Bayesian toxicity severity weight method (TSWM) of Bekele and Thall.15 This method bases dose selection on Total Toxicity Burden (TTB). To compute TTB, before the trial, each grade of each type of toxicity is assigned a severity weight based on its relative clinical importance (Table 1), which quantifies the oncologists’ experiences dealing with multiple toxicities in the clinic. The observed TTB of a patient is defined as the sum of the weights of all toxicities experienced by the patient. The mean TTB of each dose is defined as the probability weighted average of all severities of all grades of all toxicities. The scientific goal of the trial is to estimate these probabilities based on the observed dose-toxicity data, and the clinical goal is to determine a “best” dose based on mean TTB. To do this, when designing the trial, a target TTB is determined by showing the clinicians a set of toxicity combinations and, for each combination, asking whether they would escalate, repeat the previous dose, or de-escalate for the next cohort. The mean of the TTBs of the combinations for which the answer is “repeat” is used as the target TTB in the trial. During trial conduct, each successive patient cohort is treated with the “best” dose having posterior mean TTB, under the Bayesian model, closest to the numerical target TTB. In the Gemcitabine trial, the target TTB was 3.04. Six gemcitabine-related toxicity types were continually assessed up to 6 weeks for each patient to provide the basis for the TSWM dose-finding algorithm. To our knowledge, this was the first phase I trial to use the TTB-based dose-finding method of Bekele and Thall.15 The idea of TTB and related methods have been discussed in “Design for Clinical Trials” (textbook, edited by David Harrington, 2002: Chapter 1) and by Ezzalfani, et al.22
TABLE 1.
Toxicities by grade and elicited consensus severity weight used for adaptive dose-finding.
| Type of Toxicity | Grade | Elicited Consensus Severity Weight |
|---|---|---|
| Myelosuppression without fever | 3 | 1.0 |
| 4 | 1.5 | |
| Myelosuppression with fever | 3 | 5.0 |
| 4 | 6.0 | |
| Dermatitis | 3 | 2.5 |
| 4 | 6.0 | |
| Hepatotoxicity | 2 | 2.0 |
| 3 | 3.0 | |
| 4 | 6.0 | |
| Nausea / Vomiting | 3 | 1.5 |
| 4 | 2.0 | |
| Fatigue | 3 | 0.5 |
| 4 | 1.0 |
Locoregional recurrence-free survival (LRFS) and distant metastasis-free survival (DMFS) were measured from the date of surgery to the date of locoregional or distant recurrence, respectively. Overall survival (OS) was measured from the date of surgery to the date of death. Unadjusted event time distributions were estimated using the method of Kaplan and Meier.16
Comparisons between patient groups were made using the log-rank test.17 A Bayesian multivariate regression model18 was fit for TTB with the assumption that each time-to-event variable followed a parametric distribution. All statistical analyses were conducted in R [R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.] 3.0.2 with the following R packages: MASS v(7.3–29), lattice_v(0.20–29), survival v(2.37–7), R2WinBUGS v(2.1–19) and BRugs v(0.8–3). For frequentist tests, a p-value < 0.05 is considered significant. For Bayesian inferences, values of the posterior probabilities Pr( >0|data) > 0.95 or >0.99 may be considered to show significant or highly significant positive association, respectively, of the covariate with response. Values < 0.05 or < 0.01 correspond to significant or highly significant negative association of the covariate with response. Values near 0.50 correspond to no association with response.
RESULTS
Forty-one patients were enrolled in our study; 36 (88%) of them completed the study and had evaluable data. Among those who did not complete the study, one patient dropped out due to toxicity and the remaining patients withdrew consent for non-toxicity reasons.
Patient, disease, and treatment characteristics are summarized (Table 2). The study patients were relatively young, and the majority had primary disease of the lower extremity. Although most tumors were large (T2 or > 5 cm), five patients (14%) did have a tumor size of 0. These patients, enrolled for toxicity assessment, had pre-study resection with microscopic positive margins and then received neoadjuvant gemcitabine and EBRT followed by resection to negative margins. The most common histology was undifferentiated pleomorphic or spindle cell pathology, followed by myxoid liposarcoma. For the latter group, 2 out of 7 patients had round cell transformation.
TABLE 2.
Summary Statistics of Patient Demographic and Clinical Characteristics
| N | % | ||
|---|---|---|---|
| Age | |||
| Mean (SD) | 49.4 (15.8) | ||
| Median | 53.5 | ||
| Min-Max | 19.0–77.0 | ||
| Disease status at study enrollment | |||
| Primary | 32 | 88.9 | |
| Recurrent | 4 | 11.1 | |
| Pathology | |||
| Undifferentiated pleomorphic or spindle cell sarcoma / unclassified | 20 | 55.6 | |
| Myxoid liposarcoma | 7 | 19.4 | |
| Synovial sarcoma | 5 | 13.9 | |
| Myxofibrosarcoma | 4 | 11.1 | |
| Tumor location | |||
| Upper extremity | 8 | 22.2 | |
| Lower extremity | 25 | 69.4 | |
| Trunk | 3 | 8.3 | |
| Tumor size (cm) | |||
| Mean (SD) | 7.5 (5.3) | ||
| Median | 6.6 | ||
| Min-Max | 0.0–20.0 | ||
| Tumor grade | |||
| 2 | 12 | 33.3 | |
| 3 | 24 | 66.7 | |
| Pre-study chemotherapy | |||
| Yes | 23 | 63.9 | |
| No | 13 | 36.1 | |
| Gemcitabine dose (mg/m2) | |||
| 400 | 4 | 11.1 | |
| 500 | 4 | 11.1 | |
| 600 | 11 | 30.6 | |
| 700 | 17 | 47.2 | |
| TTB | |||
| Mean (SD) | 4.2 (3.3) | ||
| Median | 3.0 | ||
| Min-Max | 0.0–12.0 | ||
Abbreviations: SD, standard deviation; TTB, total toxicity burden.
Tumor size is defined as the largest tumor dimension at diagnosis. Total toxicity burden (TTB) is a weighted average based on observed adverse events and pre-assigned weights.
The most common toxicities observed during the study were myelosuppression without fever (17 patients, 47%), followed by dermatitis (12 patients, 33%) and hepatitis (10 patients, 28%) (Supplemental Table 1). These toxicities were mostly grade 3. Only 3 patients (8%) developed toxicities assigned the highest severity weight (6.0 = most clinical importance); all of whom had grade 4 dermatitis. No patients developed grade 4 myelosuppression with fever or grade 4 hepatitis. No deaths occurred as a result of treatment toxicity during the course of the study.
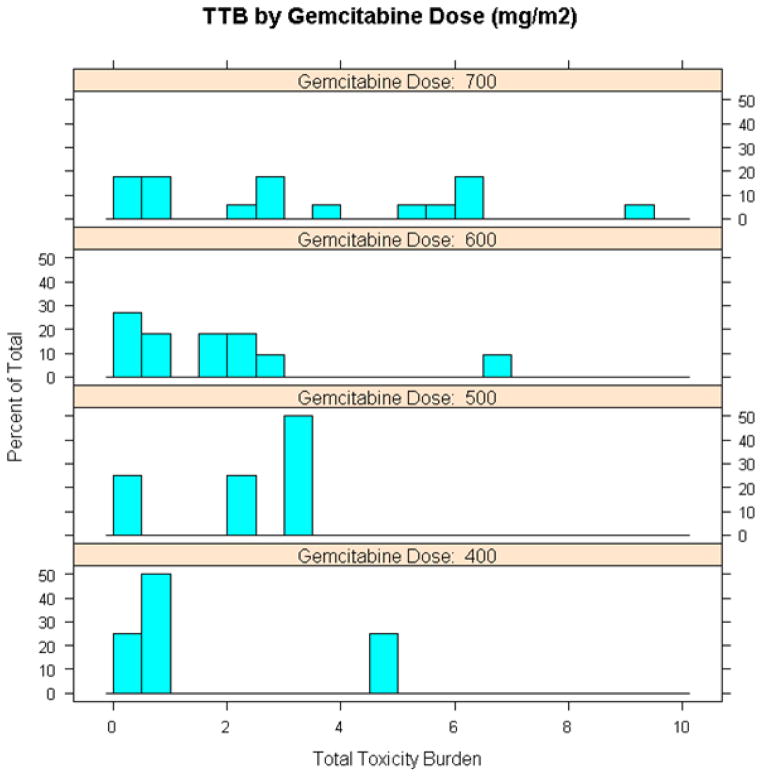
Using the TSWM, we determined that the MTD of gemcitabine when given with 50 Gy of EBRT was 700 mg/m2. At this dose level, the majority of toxicities observed were grade 3 or lower, and only 4 (24%) of 17 patients experienced grade 4 toxicities (2 had myelosuppression without fever and 2 had dermatitis) (Supplemental Table 1). In general, higher TTBs were observed in patients who had received 700 mg/m2 of gemcitabine compared to those in the lower dose cohorts (Figure 2). In fact, by regression modeling, gemcitabine dose of 700 mg/m2 was the only covariate that was significantly associated with higher TTB (Supplemental Table 2). Patient age, tumor size, tumor grade, receipt of pre-study chemotherapy, and tumor location (upper versus lower extremity versus trunk) did not appear to impact TTB.
Figure 2.
Distribution of total toxicity burden (TTB) at each gemcitabine dose level.
All 36 patients who completed neoadjuvant gemcitabine and EBRT were able to undergo complete tumor resection with microscopically negative margins. All resections consisted of wide local excision, and no patients required amputation. In collaboration with plastic and reconstructive surgeons, wound closure consisted of skin grafting and/or rotational or free flap coverage in 20 patients (56%); the remaining patients had simple primary closure. Only 2 patients (6% of total), both with lower extremity tumors in the 700 mg/m2 cohort (2/17, 12%), developed major postoperative wound complications (seroma requiring percutaneous drainage catheter placement and seroma, wound infection requiring hospitalization). Neither of these patients required reoperation for their wound complications. Eleven additional patients (31%) had minor wound complications that did not require procedural intervention or hospitalization. The majority of patients (23, 64%) had no wound complications.
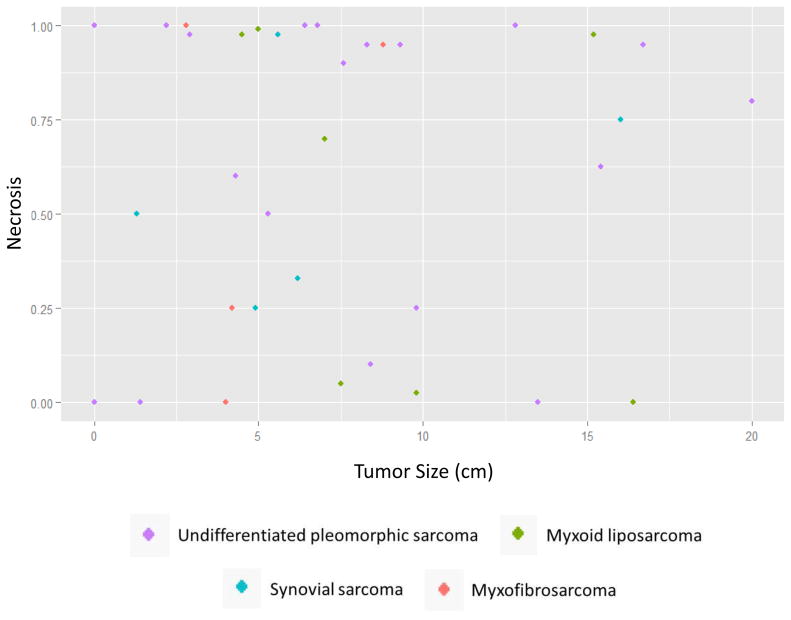
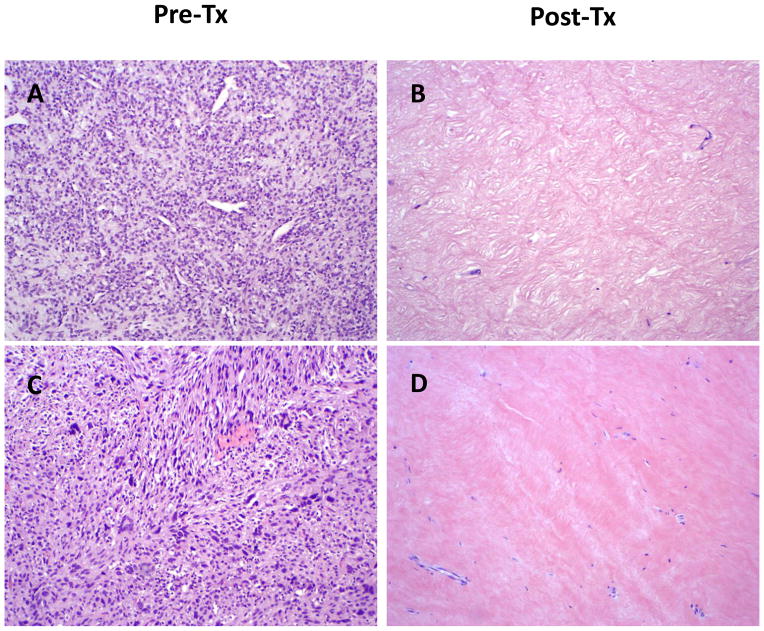
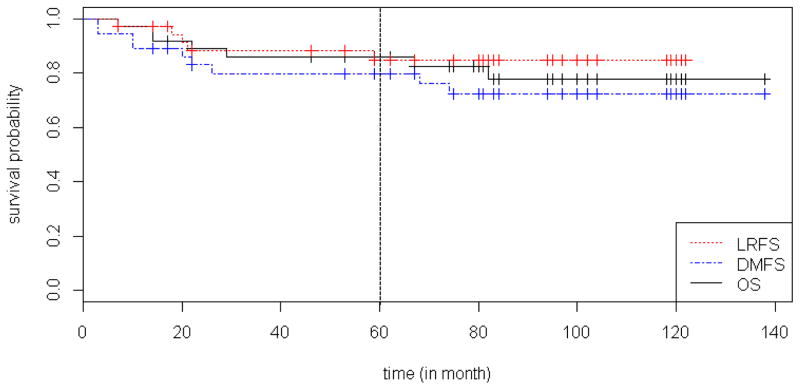
Resected tumors were subjected to microscopic examination. A major pathologic response, defined as > 90% tumor necrosis, was found in the resected specimens of 17 patients (47%). Fourteen of these patients (82% or 39% of total) experienced a complete pathologic response, defined as ≥95% tumor necrosis. A scatterplot of the degree of necrosis versus tumor size is shown (Figure 3). Pre- and post-therapy histology from 2 representative cases are shown (Figure 4). Occurrence of a major pathologic response was not associated with gemcitabine dose and was observed at all dose levels (Supplemental Table 3). With a median follow-up of 6.2 years, the 5-year LRFS rate was 85%, DMFS rate was 80%, and OS rate was 86% (Figure 5).
Figure 3.
Scatterplot of degree of necrosis versus tumor size. Tumor histology is denoted by color.
Figure 4.
Histology from representative cases showing pathologic complete response (≥95% tumor necrosis). (A, B) Primary myxoid liposarcoma of the left thigh; gemcitabine dose: 600 mg/m2. (C, D) Primary high-grade, spindle cell sarcoma of the right knee area; gemcitabine dose: 700 mg/m2. 100X magnification for all images. Pre-Tx, pre-treatment; Post-Tx, post-treatment.
Figure 5.
Kaplan-Meier survival curves for all study patients. The dotted vertical line represents 5-year mark. LRFS, locoregional recurrence-free survival; DMFS, distant metastasis-free survival; OS, overall survival.
DISCUSSION
For patients with high-risk extremity and trunk STS, multimodality therapy is critical for adequate locoregional and distant disease control. To our knowledge, the combination of gemcitabine and EBRT has never previously been evaluated in the neoadjuvant setting for patients with high risk STS. In the current phase I trial, we determined that the MTD for gemcitabine is 700 mg/m2 when given concurrently with fixed-dose (50 Gy) EBRT as neoadjuvant therapy. At this dose level, the toxicity profile and major wound complication rate are acceptable.
MTD determination in our study was achieved through utilization of a novel, adaptive dose-finding method--the TSWM. We believe that this method accurately reflects clinical decision-making during the conduct of a phase I trial. The TSWM acknowledges the fact that not all toxicities have equal clinical importance and that patients may experience more than one toxicity during the study period. Data from several qualitatively different toxicities are combined to determine the MTD. Since the technique’s initial statistical description in 2004,15 several other groups have proposed similar methods for toxicity assessment in phase I trials. Yuan et al. transformed toxicity grades into a numerical score and incorporated this into a continual reassessment method.19 Chen et al. devised a normalized equivalent toxicity score that also employs a subjective ranking of toxicity grades but includes additional toxicities as a decimal portion of the overall score.20 Lee et al. reported a toxicity burden score similar to our description.21 Ezzalfani et al. used a Euclidean norm of toxicity weights rather than arithmetic sum to define a total toxicity profile.22 All of these methods, including the TSWM, require close collaboration between clinicians and statisticians, with specification of which toxicities are relevant to a given trial and group of patients and predetermination of an acceptable target for total toxicity. To our knowledge, our study is the first to prospectively implement the TSWM in an actual clinical trial and moreover, to use a TSWM for MTD determination of a drug given as part of multimodality treatment.
The TSWM does come with important caveats. First, it is a relatively labor-intensive design compared to traditional dose-finding methods, and therefore, it may not appeal to some clinicians. Second, the TSWM applied in this trial did not incorporate less severe (grade 1 or 2, “sub-dose-limiting”) toxicities, which can still be clinically significantly and affect dose escalation (e.g., as an indicator of higher probability for experiencing subsequent dose-limiting toxicity). Assigning these lower severity weights and including them in the TTB, however, is straightforward. Third, our TTB definition did not account for the duration or reversibility of a given toxicity. Fourth, and particularly relevant for combination treatment strategies that include radiation therapy, late toxicities were not assessed in the TSWM. Nonetheless, we feel that the TSWM is a useful tool and much more clinically relevant than the traditional MTD determinations based on a binary toxicity indicator, used in most phase I trials.
Although the primary objective of our study was toxicity assessment and MTD determination, we also analyzed clinical outcomes, including tumor response. Histologic examination of resected tumors demonstrated that almost half of study patients (47%) had major pathologic response. Moreover, pathologic complete response, defined as ≥95% tumor necrosis, was observed in 39% of study patients. These results compare very favorably to reported pathologic complete response rates with radiation therapy alone (8–10%) and with the combination of doxorubicin and radiation therapy (9–14%).23–26 Similar pathologic complete response rates have been reported previously with neoadjuvant doxorubicin, ifosfamide and radiation therapy (48%) and more recently, with neoadjuvant sorafenib, either with radiation therapy alone (38%) or in combination with chemoradiation (44%).25, 27–28 Pathologic complete response in STS has been shown to be significantly associated with improved distant recurrence free survival,24 but to our knowledge has not been reported for progression free or overall survival.
In the current study, patients with smaller tumor size did appear to have better response to neoadjuvant gemcitabine and EBRT (Figure 3, Supplemental Table 3). By histology, however, the distribution of patients with myxoid liposarcoma, a typically radiosensitive subtype, and undifferentiated pleomorphic sarcoma, a typically non-radiosensitive subtype, appears to be relatively balanced between responders and nonresponders (Figure 3).
It is important to note that in the current study, while patients with prior radiation therapy were excluded, the enrolled patients frequently received systemic chemotherapy before our study therapy (23 patients, 64%). The most majority of these patients received a doxorubicin-based regimen (Supplemental Table 4). Receipt of prior systemic chemotherapy was not associated with higher toxicity from our neoadjuvant therapy regimen (Supplemental Table 2) but was associated with greater pathologic response (Supplemental Table 3). Although age alone was not associated with pathologic response, interestingly older patients who received preoperative chemotherapy on average had a higher response rate. Seven patients (19%) also received further adjuvant chemotherapy off study for disease progression (Supplemental Table 5).
In summary, the current phase I trial demonstrates that a TSWM incorporating multiple toxicity types is a useful method to help guide MTD determination for a drug given as part of multimodality treatment. Gemcitabine combined with EBRT in the neoadjuvant setting for patients with resectable, high-risk extremity and trunk STS appears to be feasible and safe with an MTD of 700 mg/m2. Major pathologic responses, including complete responses, can be achieved in a substantial proportion of patients. After complete surgical resection, locoregional and distant disease control rates as well as overall survival are encouraging (5 year LRFS, DMFS, OS all > 80%). Further evaluation of this treatment’s efficacy in a phase II trial is definitely warranted.
Supplementary Material
Acknowledgments
Funding: Eli Lilly and Company
Footnotes
Financial Disclosures: None
The work presented in this article was completed at The University of Texas MD Anderson Cancer Center, Houston, Texas.
References
- 1.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–11. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 2.Penel N, Van Glabbeke M, Marreaud S, Ouali M, Blay JY, Hohenberger P. Testing new regimens in patients with advanced soft tissue sarcoma: analysis of publications from the last 10 years. Ann Oncol. 2011;22:1266–72. doi: 10.1093/annonc/mdq608. [DOI] [PubMed] [Google Scholar]
- 3.Hensley ML. Update on gemcitabine and docetaxel combination therapy for primary and metastatic sarcomas. Curr Opin Oncol. 2010;22:356–61. doi: 10.1097/CCO.0b013e32833aafef. [DOI] [PubMed] [Google Scholar]
- 4.Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol. 2001;19:3483–9. doi: 10.1200/JCO.2001.19.15.3483. [DOI] [PubMed] [Google Scholar]
- 5.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002. J Clin Oncol. 2007;25:2755–63. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 6.Pautier P, Floquet A, Penel N, et al. Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: a Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study) Oncologist. 2012;17:1213–20. doi: 10.1634/theoncologist.2011-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pauwels B, Korst AE, Lardon F, Vermorken JB. Combined modality therapy of gemcitabine and radiation. Oncologist. 2005;10:34–51. doi: 10.1634/theoncologist.10-1-34. [DOI] [PubMed] [Google Scholar]
- 8.El Deen DA, Toson EA, El Morsy SM. Gemcitabine-based induction chemotherapy and concurrent with radiation in advanced head and neck cancer. Med Oncol. 2012;29:3367–73. doi: 10.1007/s12032-012-0269-x. [DOI] [PubMed] [Google Scholar]
- 9.Kim EJ, Ben-Josef E, Herman JM, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer. 2013;119:2692–700. doi: 10.1002/cncr.28117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mornex F, Girard N. Gemcitabine and radiation therapy in non-small cell lung cancer: state of the art. Ann Oncol. 2006;17:1743–7. doi: 10.1093/annonc/mdl117. [DOI] [PubMed] [Google Scholar]
- 11.Milas L, Fujii T, Hunter N, et al. Enhancement of tumor radioresponse in vivo by gemcitabine. Cancer Res. 1999;59:107–14. [PubMed] [Google Scholar]
- 12.Murphy JD, Lucas DR, Somnay YR, Hamstra DA, Ray ME. Gemcitabine-mediated radiosensitization of human soft tissue sarcoma. Transl Oncol. 2008;1:50–6. doi: 10.1593/tlo.07121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pisters PW, Ballo MT, Patel SR. Preoperative chemoradiation treatment strategies for localized sarcoma. Ann Surg Oncol. 2002;9:535–42. doi: 10.1007/BF02573888. [DOI] [PubMed] [Google Scholar]
- 14.Reynoso D, Subbiah V, Trent JC, et al. Neoadjuvant treatment of soft-tissue sarcoma: a multimodality approach. J Surg Oncol. 2010;101:327–33. doi: 10.1002/jso.21481. [DOI] [PubMed] [Google Scholar]
- 15.Bekele BN, Thall PF. Dose-finding based on multiple toxicities in a soft tissue sarcoma trial. J Am Stat Assoc. 2004;99:26–35. [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 17.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 18.Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian Data Analysis. New York: Chapman & Hall/CRC; 2004. [Google Scholar]
- 19.Yuan Z, Chappell R, Bailey H. The continual reassessment method for multiple toxicity grades: a Bayesian quasi-likelihood approach. Biometrics. 2007;63:173–9. doi: 10.1111/j.1541-0420.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Krailo MD, Azen SP, Tighiouart M. A novel toxicity scoring system treating toxicity response as a quasi-continuous variable in Phase I clinical trials. Contemp Clin Trials. 2010;31:473–82. doi: 10.1016/j.cct.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SM, Hershman DL, Martin P, Leonard JP, Cheung YK. Toxicity burden score: a novel approach to summarize multiple toxic effects. Ann Oncol. 2012;23:537–41. doi: 10.1093/annonc/mdr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezzalfani M, Zohar S, Qin R, Mandrekar SJ, Deley MC. Dose-finding designs using a novel quasi-continuous endpoint for multiple toxicities. Stat Med. 2013;32:2728–46. doi: 10.1002/sim.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canter RJ, Martinez SR, Tamurian RM, et al. Radiographic and histologic response to neoadjuvant radiotherapy in patients with soft tissue sarcoma. Ann Surg Oncol. 2010;17:2578–84. doi: 10.1245/s10434-010-1156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911–5. [PMC free article] [PubMed] [Google Scholar]
- 25.Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: A predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203–9. doi: 10.1200/JCO.2001.19.13.3203. [DOI] [PubMed] [Google Scholar]
- 26.Pisters PW, Patel SR, Prieto VG, et al. Phase I trial of preoperative doxorubicin-based concurrent chemoradiation and surgical resection for localized extremity and body wall soft tissue sarcomas. J Clin Oncol. 2004;22:3375–80. doi: 10.1200/JCO.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Canter RJ, Borys D, Olusanya A, et al. Phase I trial of neoadjuvant conformal radiotherapy plus sorafenib for patients with locally advanced soft tissue sarcoma of the extremity. Ann Surg Oncol. 2014;21:1616–23. doi: 10.1245/s10434-014-3543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer JM, Perlewitz KS, Hayden JB, et al. Phase I trial of preoperative chemoradiation plus sorafenib for high-risk extremity soft tissue sarcomas with dynamic contrast-enhanced MRI correlates. Clin Cancer Res. 2013;19:6902–11. doi: 10.1158/1078-0432.CCR-13-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.